Published online Jun 27, 2024. doi: 10.4240/wjgs.v16.i6.1537
Revised: April 29, 2024
Accepted: May 16, 2024
Published online: June 27, 2024
Processing time: 185 Days and 16.7 Hours
The optimal extent of lymphadenectomy in esophageal squamous cell carcinoma (ESCC) patients remained debatable.
To explore the ideal number of cleared lymph nodes in ESCC patients undergoing upfront surgery.
In this retrospective, propensity score-matched study, we included 1042 ESCC patients who underwent esophagectomy from November 2008 and October 2019. Patients who underwent neoadjuvant therapy were excluded. We collected pa
Among the included 1042 patients, two cohorts: ≤ 21 (n = 664) and > 21 NRLN (n = 378) were identified. The final prognostic model included four variables: T stage, N, venous thrombus, and the number of removed lymph nodes. Among them, NRLN > 21 was determined as an independent prognosticator after surgery for esophageal cancer (hazards regression = 0.66, 95% confidence interval: 0.50-0.87, P = 0.004). A nomogram was created based on the regression coefficients of the variables in the final model. In the training cohort, the predictive model dis
NRLN > 21 was an independent prognostic factor after ESCC surgery. The combination of NRLN and RPLN may provide a reference for adjuvant chemotherapy use in potential beneficiaries.
Core Tip: This study delineates the prognostic value of the number of lymph nodes removed during esophagectomy in esophageal squamous cell carcinoma (ESCC) patients, highlighting that a count greater than 21 significantly improves survival outcomes. It introduces a novel prognostic model, incorporating lymph node count with clinical variables, and proposes a nuanced approach to post-operative adjuvant chemotherapy based on lymph node ratio. These insights affirm the importance of extensive lymphadenectomy in ESCC and offer a refined strategy for tailoring adjuvant treatment, thereby enhancing personalized patient care.
- Citation: Tang JM, Huang SJ, Chen QB, Wu HS, Qiao GB. Optimal extent of lymphadenectomy improves prognosis and guides adjuvant chemotherapy in esophageal cancer: A propensity score-matched analysis. World J Gastrointest Surg 2024; 16(6): 1537-1547
- URL: https://www.wjgnet.com/1948-9366/full/v16/i6/1537.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i6.1537
Esophageal cancer (EC) is one of the deadliest cancers in the world[1], and most of the cases are esophageal squamous cell carcinoma (ESCC). Benefiting from multimodality treatment, the mortality rate of EC is decreasing year by year[2,3]. Neoadjuvant therapy followed by surgery is the current standard treatment option for patients with locally advanced ESCC[4]. Despite advances in clinical research, the significance of the number of lymph nodes removed in clinical practice is still unclear. On one hand, performing a more comprehensive lymphadenectomy could lead to more precise staging, which in turn may enhance postoperative treatment guidance and improve disease-specific survival rates[5], on the other hand, extensive lymphadenectomy is associated with more postoperative complications in the short term[6]. A study shows that removing more lymph nodes increases the risk of chylothorax, which makes it more difficult for thoracic surgeons to manage post-operative events[7]. The National Comprehensive Cancer Network currently recommends that at least 15 lymph nodes be resected during lymphadenectomy[8]. However, the proper upper limit of lymph node resection remains unclear for preventing complications of excessive surgery. Consequently, investigating the optimal number of lymph node resection is essential to strike a balance between survival benefits and potential complications. In the present study, we studied the appropriate number of lymph node resections for specific patients to ensure survival benefits and reduce postoperative complications. We tried to obtain the extent of lymph node resection for a better prognosis and provide a reference for the thoracic surgeon to perform lymphadenectomy.
This multicenter database contains specific information on patients’ clinicopathological features, information regarding lymph nodes including the total number of resected lymph nodes, and pathologically diagnosed positive lymph nodes. The naming of the lymph node stations was based on the 11th Japanese Classification of Esophageal Cancer[9].
Between November 2008 and October 2019, 1821 patients with EC who underwent esophagectomy at the First Affiliated Hospital of Shantou University Medical College and Guangdong Provincial People’s Hospital were eligible for further selection. In this retrospective cohort study, the inclusion criteria included: (1) Pathologically confirmed diagnosis of ESCC; (2) Thoracic EC; (3) Underwent lymph nodes resection; and (4) No history of other cancers. 1470 patients met the inclusion criteria. Patients with a lack of lymph node information (n = 364), lack of follow-up information (n = 29), positive resection margins (n = 22), and death within one month after surgery (n = 13) were excluded. Eventually, a total of 1042 patients were enrolled in this study. All clinical characteristics and pathological data were retrieved from medical records.
The preoperative workup included upper gastrointestinal endoscopy to confirm the diagnosis of EC; chest computed tomography or positron emission tomography-computed tomography reveal tumor and lymph node features.
The patients underwent a right or left transthoracic esophagectomy with lymphadenectomy. Resection of lymph nodes was performed with standard lymphadenectomy, extended lymphadenectomy, and total lymphadenectomy. Standard 2-field lymphadenectomy is defined as an extent that covers the entire posterior mediastinum and includes the resection of lymph nodes in the abdomen, along the celiac trunk, common hepatic and splenic arteries, and those along the lesser curvature of the stomach and in the lesser omentum; extended 2-field lymphadenectomy includes all lymph nodes addressed in the standard 2-field, with additional clearance of the nodes in the right paratracheal gutter; total 2-field lymphadenectomy expands upon the extended 2-field resection by also removing the lymph nodes in the left paratracheal gutter. Among the included patients, 519 received standard 2-field lymphadenectomy, 335 received extended 2-field lymphadenectomy, and 188 received total 2-field lymphadenectomy. Pathological staging was assigned to each patient following the eighth edition of the tumor-node-metastasis (TNM) staging system released by the American Joint Committee on Cancer[10]. T staging was based on the depth of tumor invasion, and N staging was categorized by the number of regional positive lymph nodes.
Patients were monitored every three months for the first two years post-esophagectomy and biannually for the subsequent three years. Follow-up continued until January 31, 2022, or until the patient’s death, with a median follow-up duration of 53.0 months. The primary endpoints were overall survival (OS) and disease-free survival (DFS) which were defined as the survival time after surgery and the time with no evidence of local or distant disease recurrence, respectively.
The ethics committee of the two hospitals approved our work (No. GDREC2019687H), and written consent was waived due to the retrospective nature of this study. The Declaration of Helsinki’s rules and regulations were followed when carrying out the study protocol.
The Student’s t-test was employed to analyze continuous variables, while the χ2 test or Fisher’s exact test was utilized for comparing categorical variables. The optimal cutoff values of the total lymph nodes number were determined by the “surv_cutpoint” function of the “survminer” R package (Supplementary Figure 1). We employed the Kaplan-Meier method and the log-rank test for univariate analysis, selecting variables with a P value less than 0.05 for inclusion in the multivariate analysis, which was performed using forward stepwise Cox proportional hazards regression. The prognostic model, developed from variables that were statistically significant in the multivariate analysis, was depicted using a nomogram. The performance of the predictive model was evaluated using operating characteristic curves (ROC) curve analysis and calibration curves, while decision curve analysis (DCA) was utilized to assess its clinical utility. Propensity score matching was used to compare the OS between the cohorts with different numbers of cleared lymph nodes. The variables age, sex, tumor stage, nodal stage, differentiation grade, venous thrombus, perineurial invasion, positive lymph node number, and positive lymph node ratio were matched. Using nearest neighbor-matching, a 1:1 match was conducted on the propensity score with a maximum caliper of 0.2 (Supplementary Table 1). All statistical analyses were performed by SPSS software (version 26.0; IBM Corp) and R software (version 4.0.0, R Foundation).
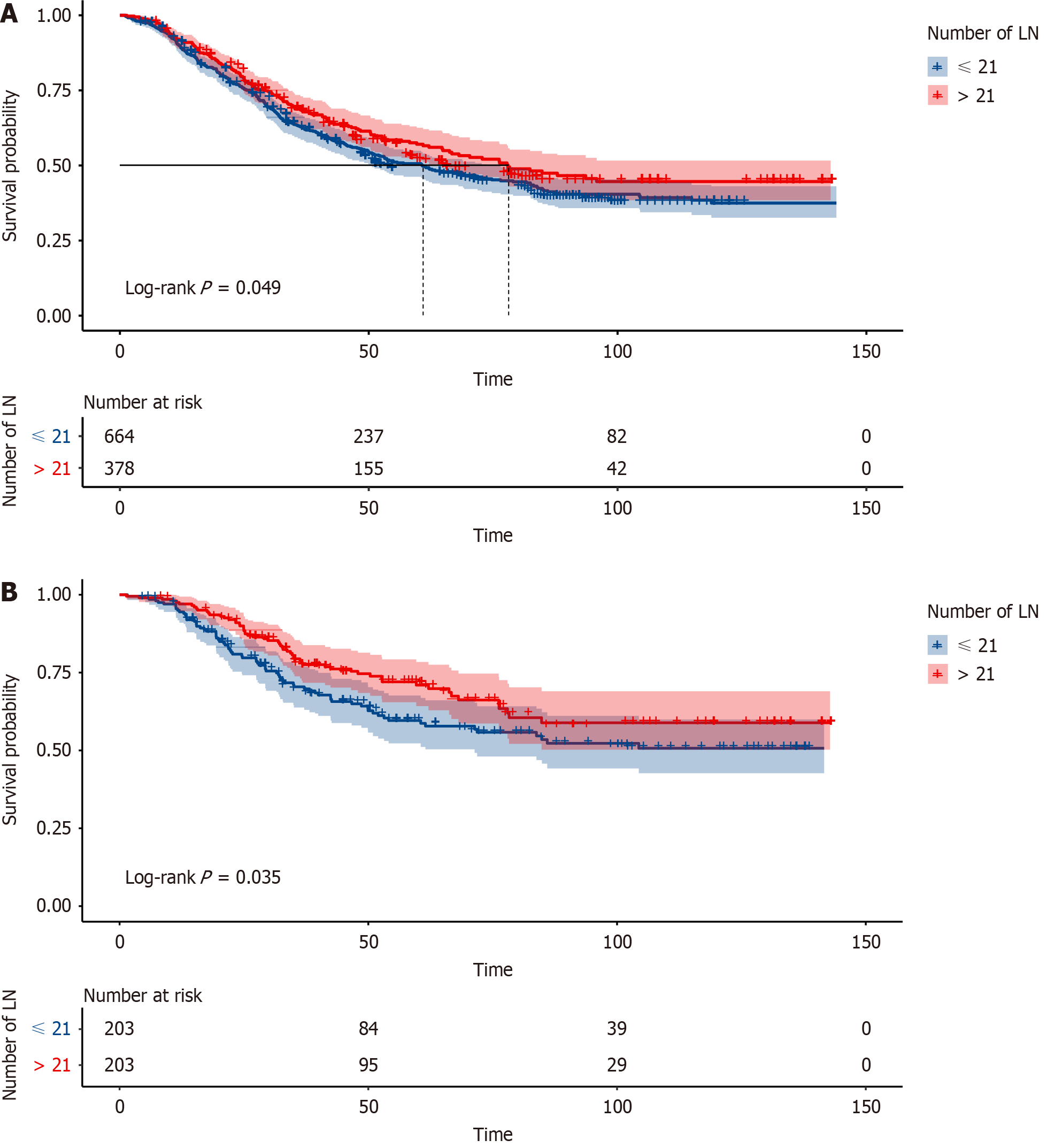
In the enrolled cohort of 1042 patients, their median age was 60 (interquartile range: 54-66) years and most of the patients were male (78.7%). More than half of the patients were at the T3 stage (46.7%). Most of the patients had moderately differentiated pathologic outcomes. Most patients were negative for venous thrombus (14.9% positive) and perineurial invasion (25.0% positive) (Table 1). 12 patients died within 90 d, with a 90-d mortality rate of 1.15%. 130 patients died within 1 year, with a 1-year mortality rate of 12.18%. Based on the optimal cutoff values of the total number of removed lymph nodes number, the patients were divided into two cohorts: ≤ 21 cohorts (n = 664) and > 21 cohorts (n = 378). Patients in > 21 cohorts were more likely to achieve a longer survival time at the follow-up (P = 0.049; Figure 1A). In the final result, 203 patients were matched well. Patients in > 21 cohorts continued to have better survival outcomes (P = 0.035; Figure 1B).
| Variables | Level | Overall |
| Number | 1042 | |
| Sex, n (%) | Male | 820 (78.7) |
| Female | 222 (21.3) | |
| Age (yr), median (IQR) | 60 (54, 66) | |
| T, n (%) | 1 | 98 (9.4) |
| 2 | 219 (21.0) | |
| 3 | 487 (46.7) | |
| 4 | 238 (22.8) | |
| N, n (%) | 0 | 612 (58.7) |
| 1 | 234 (22.5) | |
| 2 | 134 (12.9) | |
| 3 | 62 (6.0) | |
| G, n (%) | Well differentiated | 124 (11.9) |
| Moderate differentiated | 740 (71.0) | |
| Poor differentiated | 178 (17.1) | |
| Venous thrombus, n (%) | Negative | 887 (85.1) |
| Positive | 155 (14.9) | |
| Perineurial invasion, n (%) | Negative | 509 (75.0) |
| Positive | 170 (25.0) | |
| NPLN, mean (SD) | 1.17 (2.15) | |
| RPLN, mean (SD) | 0.06 (0.11) | |
| NRLN, mean (SD) | 19.67 (9.81) | |
| Adjuvant chemotherapy, n (%) | No | 599 (57.5) |
| Yes | 443 (42.5) | |
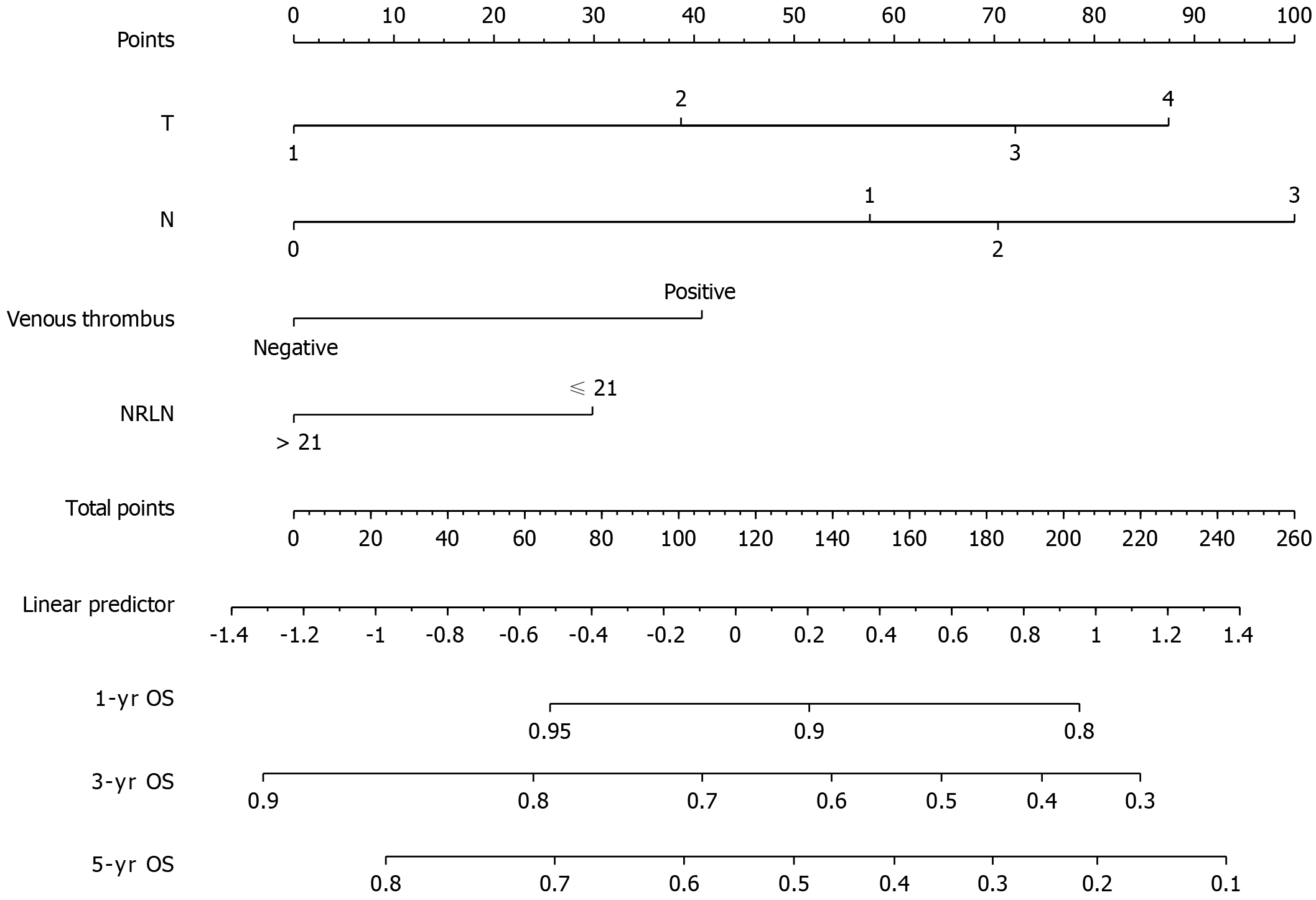
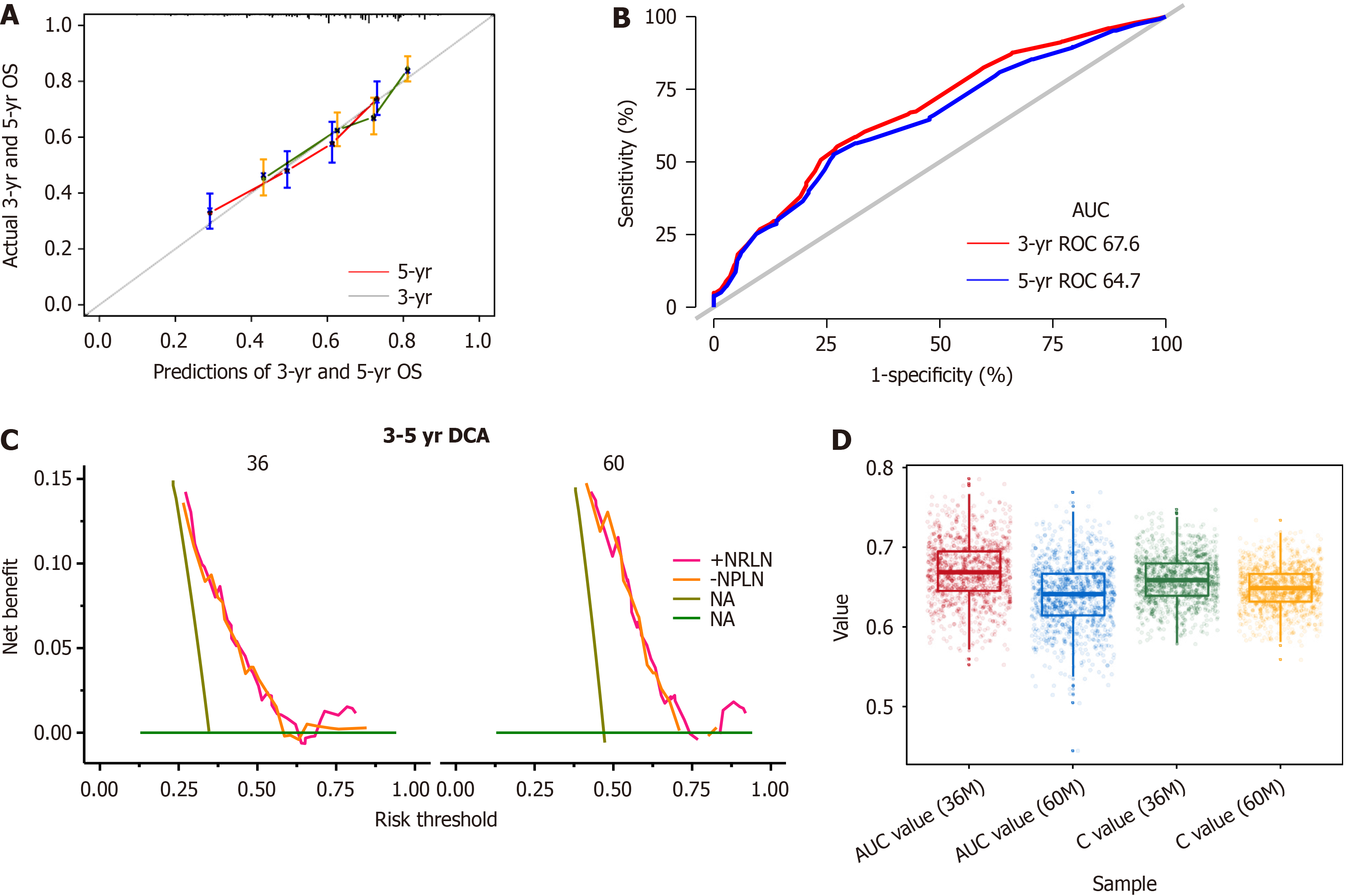
A univariate regression analysis was applied to the clinicopathological characteristics to determine which variables affected the prognosis (Table 2). There were statistically significant differences in G stage, N stage, T stage, venous thrombus [hazards regression (HR) = 2.1, 95% confidence interval (CI): 1.68-2.36, P < 0.001], perineurial Invasion (HR = 1.79, 95%CI: 1.41-2.29, P < 0.001), maximal tumor diameter (HR = 1.01, 95%CI: 1-1.01, P = 0.038), adjuvant chemotherapy (HR = 1.27, 95%CI: 1.06-1.52, P = 0.009) and the number of removed lymph nodes (> 21 vs ≤ 21, HR = 0.83, 95%CI: 0.68-1, P = 0.049). The forward stepwise Cox regression model for multivariate analysis included a univariate analysis of variables with significant differences. By excluding the interaction between variables, the final prognostic model included four variables: T stage, N, venous thrombus, and the number of removed lymph nodes (Table 3). The number of lymph nodes > 21 was identified as an independent favorable prognostic factor following EC surgery (HR = 0.66, 95%CI: 0.50-0.87, P = 0.004). A nomogram was created based on the regression coefficients of the variables in the final model (Figure 2). The model in the training cohort had an uncorrected 5-year OS C-index of 0.659 and a bootstrap-corrected 5-year OS C-index of 0.654. To assess the discriminatory ability of the predictive models, the calibration curve of the nomogram predicting 3-year and 5-year OS (Figure 3A) and 3-year OS and 5-year OS ROC were plotted with area under the ROC curves (AUCs) of 0.676 and 0.647 (Figure 3B), respectively. The clinical utility of the models was assessed using DCA (Figure 3C).
| Prognostic factors | P value | HR (95%CI) | |
| Age | 0.576 | 1 (0.99-1.01) | |
| Maximal tumor diameter | 0.038 | 1.01 (1-1.01) | |
| NRLN | 0.049 | 0.83 (0.68-1) | |
| Sex (ref male) | 0.634 | 0.95 (0.76-1.18) | |
| Tumor location (ref upper) | |||
| Middle | 0.065 | 0.71 (0.49-1.02) | |
| Lower | 0.051 | 0.65 (0.43-1) | |
| T (ref T1) | |||
| T2 | 0.028 | 1.78 (1.07-2.99) | |
| T3 | < 0.001 | 2.8 (1.74-4.52) | |
| T4 | < 0.001 | 2.71 (1.65-4.44) | |
| N (ref N0) | |||
| N1 | < 0.001 | 2.04 (1.64-2.53) | |
| N2 | < 0.001 | 2.35 (1.82-3.02) | |
| M3 | < 0.001 | 3.38 (2.45-4.65) | |
| G (ref G1) | |||
| G2 | 0.006 | 1.57 (1.14-2.15) | |
| G3 | 0.002 | 1.81 (1.25-2.61) | |
| Venous thrombus (ref negative) | Positive | < 0.001 | 2.1 (1.68-2.63) |
| Perineurial Invasion (ref negative) | Positive | < 0.001 | 1.79 (1.41-2.29) |
| Adjuvant chemotherapy (ref no) | Yes | 0.009 | 1.27 (1.06-1.52) |
| Prognostic factors | P value | HR (95%CI) | |
| Maximal tumor diameter | 0.667 | 1 (0.99-1.01) | |
| T (ref T1) | |||
| T2 | 0.055 | 2.04 (0.99-4.20) | |
| T3 | 0.002 | 3.09 (1.51-6.31) | |
| T4 | 0.005 | 7.44 (1.82-30.35) | |
| N (ref N0) | |||
| N1 | 0.077 | 1.35 (0.97-1.89) | |
| N2 | 0.075 | 1.46 (0.96-2.21) | |
| M3 | 0.002 | 1.99 (1.28-3.11) | |
| G (ref G1) | |||
| G2 | 0.103 | 1.42 (0.93-2.16) | |
| G3 | 0.387 | 1.25 (0.76-2.05) | |
| Venous thrombus (ref negative) | Positive | < 0.001 | 1.8 (1.34-2.42) |
| Perineurial invasion (ref negative) | Positive | 0.088 | 1.29 (0.96-1.71) |
| Adjuvant chemotherapy (ref no) | Yes | 0.738 | 0.95 (0.71-1.27) |
| NRLN (ref ≤ 21) | > 21 | 0.004 | 0.66 (0.50-0.87) |
A 5-fold internal cross-validation was conducted 200 times to protect against the influence of the random splits (Figure 3D).
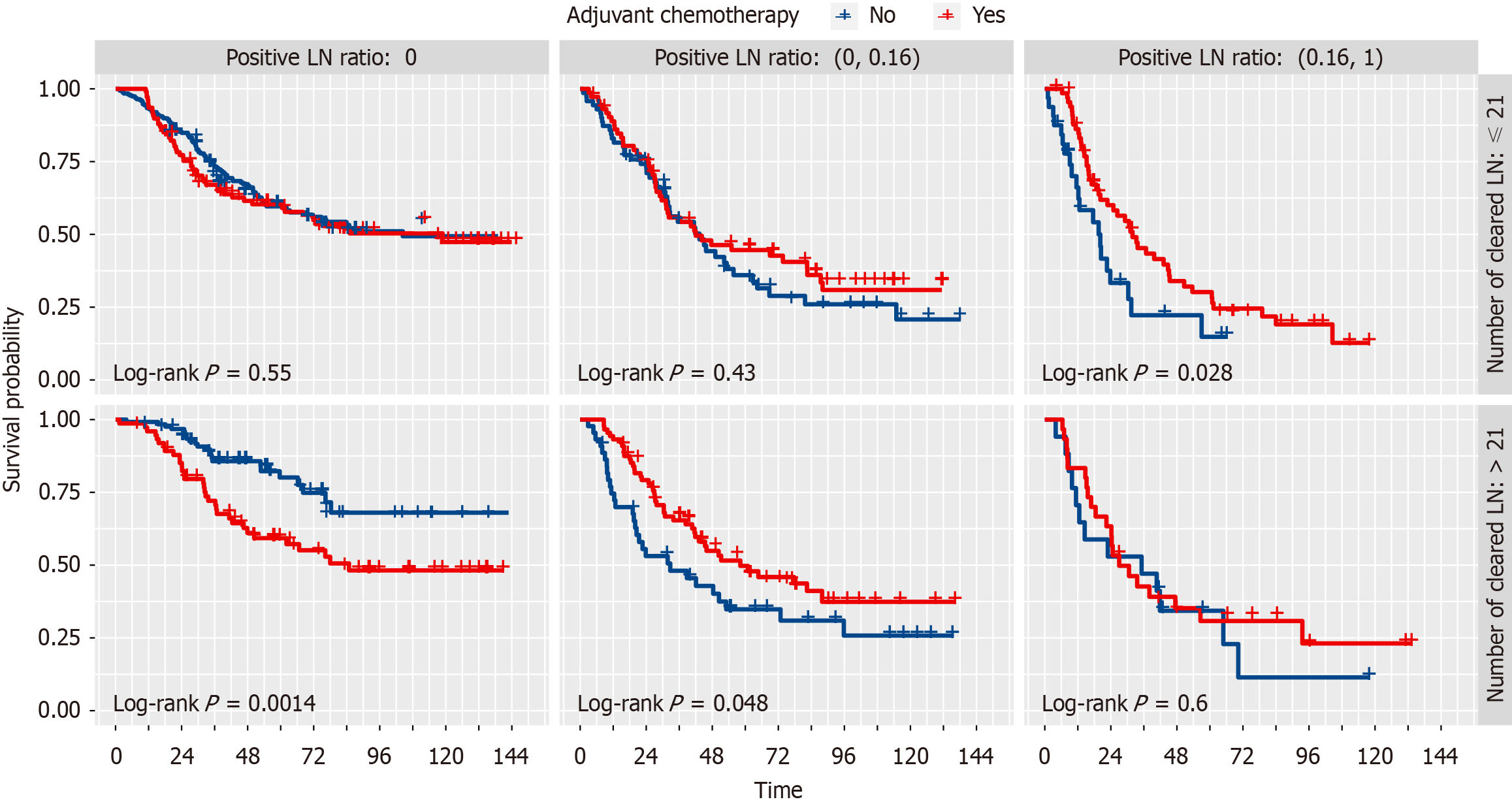
We used the Kaplan-Meier analysis to draw survival curves to further explore the stratified effect of number of resected lymph nodes (NRLN) and ratio of positive lymph nodes (RPLN) on adjuvant chemotherapy. The results found that a combination of NRLN and RPLN could identify the patients who underwent adjuvant chemotherapy and could receive a better prognosis (Figure 4).
Surgery is the foundation of the treatment of EC, and lymph node resection is an important part[11,12]. Nonetheless, the effect of the number of lymph nodes resected on survival remains uncertain. For years, researchers have argued whether adequate lymph node resection yields actual therapeutic benefit. The appropriate NRLN should be carefully selected to balance the potential survival benefit with lower postoperative morbidity. In this retrospective analysis, data from two thoracic surgical centers with radical esophagectomy were used to identify the role of NRLN in survival prognosis. Given the potential impact of neoadjuvant therapy on lymph node status, which could bias the results, patients with neoadjuvant therapy were excluded from this study.
Our results indicate that NRLN is an independent prognostic factor, with the survival of NRLN > 21 better than ≤ 21. By matching the propensity scores of clinicopathological information between the two groups, the differences remained. In this study, we observed a 90-d mortality rate of 1.15% and a 1-year mortality rate of 12.18%, reflecting the technical challenges and complexity of esophageal surgery. Specifically, patients with more than 21 lymph nodes removed exhibited improved survival metrics compared to the general cohort, suggesting that extensive lymphadenectomy might be associated with better medium-term survival outcomes despite its complexity. This comparison underscores the importance of surgical precision and comprehensive perioperative care in enhancing patient survival.
There is a rich longitudinal lymphatic network in the submucosa of the esophagus[13]. The extended lymph node resection removed potential micrometastases that were not detected by pathological examination[14]. Kamel et al[15] noted that patients with 20 or more lymph nodes removed experienced a 14% relative improvement in OS, and underwent esophagectomy following neoadjuvant chemoradiation. In contrast, this study was conducted on patients who did not receive neoadjuvant therapy. However, the results of the exploration are similar. This indicates that the NRLN is an important factor in improving postoperative survival.
The TNM staging system is now the most frequently applied tool for assessing patient outcomes. However, considerable variations in survival have been reported among individuals with the same clinical stage[16]. As a result, a more accurate and effective prognostic model is urgently required. Nomograms have long been used in oncology to evaluate a patient’s prognosis based on important clinical factors[17,18]. In this research, we constructed and internally verified a nomogram to predict postoperative survival time in patients with ESCC, and we discovered that our model had good performance in predictive accuracy. This tool was developed using independent prognostic factors for ESCC, including T stage, N stage, Venous Thrombus, and NRLN. The 3- and 5-year AUCs were 0.676 and 0.647, respectively. By combining several independent prognostic factors in a prognostic model, this nomogram scoring system is more accurate and convincing in predicting different patients, helping to identify different prognoses of ESCC patients and accurately predicting long-term survival. This scoring system is indicative of postoperative treatment strategy decisions for ESCC patients. The thoracic surgeon can easily predict OS rates based on the clinicopathological characteristics of a specific patient by visualization.
The effectiveness of adjuvant chemotherapy after ESCC has been hotly debated[19-24], and while some articles have reported on the survival benefits of adjuvant chemotherapy[25], there is still a lack of high-level evidence to identify specific groups of ESCC who would benefit from adjuvant chemotherapy, such as the phase III randomized controlled trials[8]. Our study stratified patients by the number of lymph nodes removed and the rate of positive lymph nodes and we were surprised to find that different subgroups responded differently to adjuvant chemotherapy. In the subgroup with positive postoperative pathological lymph nodes(pN+), adjuvant chemotherapy was beneficial in the subgroup with NRLN ≤ 21 and RPLN > 0.16. Similarly, adjuvant chemotherapy was beneficial in the subgroup with NRLN > 21 and RPLN ≤ 0.16. Consistent with our findings, Zheng et al[26] and Feng et al[27] suggested that postoperative adjuvant chemotherapy improves the OS of patients with resected ESCC with positive lymph nodes. However, our study also found that a subgroup of lymph node-positive patients with NRLN > 21 and RPLN > 0.16 did not benefit from adjuvant chemotherapy. A comprehensive treatment plan may be required for this group of patients.
Findings were also obtained in our analysis of subgroups with negative postoperative pathological lymph nodes (pN0). In the NRLN ≤ 21 subgroup, no significant differences in OS were found between the adjuvant-treated and non-adjuvant-treated groups. In the NRLN > 21 subgroup, patients treated with adjuvant chemotherapy obtained a worse prognosis. Adjuvant chemotherapy may do more harm than good to patients in this population. On the contrary, Deng et al[28] showed that adjuvant chemotherapy prolonged OS and DFS in ESCC patients with pN0 disease. Further research is needed to elucidate such differences.
Our study primarily investigates the prognostic value of the extent of lymphadenectomy in EC. While this focus is crucial, we also recognize the significant impact that perioperative care factors, such as advancements in anesthesia, pain management, and Enhanced Recovery After Surgery protocols, have on patient recovery and morbidity. The perioperative period in foregut cancer surgery is complex, with numerous elements influencing outcomes. In addition to these perioperative factors, nutritional status emerges as a critical component of patient management. The anatomical impacts of foregut cancers often lead to complications such as dysphagia or vomiting, directly impairing oral intake and con
One of the limitations of our retrospective analysis is the unavailability of certain prognostic variables which could potentially influence the outcomes. Due to the retrospective nature of our study, variables such as albumin levels, nutritional status, and liver function, which could significantly impact patient outcomes, were not included in our analysis. Moreover, in assessing the predictive performance of our model, it is important to recognize that the AUC value obtained is below 0.70, indicating moderate discriminative ability. This level of discrimination reflects a certain degree of uncertainty in the model’s predictive accuracy and presents a potential limitation to the robustness of our prognostic evaluations. Prospective validation with a more comprehensive dataset and potentially the integration of additional predictive variables may enhance the discriminative capacity of future models.
In conclusion, NRLN > 21 was an independent prognostic factor after ESCC surgery. We developed and validated a nomogram, which is useful for thoracic surgeons to assess the prognosis of different patients.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64125] [Article Influence: 16031.3] [Reference Citation Analysis (174)] |
| 2. | Liang H, Fan JH, Qiao YL. Epidemiology, etiology, and prevention of esophageal squamous cell carcinoma in China. Cancer Biol Med. 2017;14:33-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 248] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 3. | Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J. Cancer incidence and mortality in China, 2016. JNCC. 2022;2:1-9. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 852] [Cited by in RCA: 938] [Article Influence: 312.7] [Reference Citation Analysis (1)] |
| 4. | Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, Cuesta MA, Blaisse RJB, Busch ORC, Ten Kate FJW, Creemers GM, Punt CJA, Plukker JTM, Verheul HMW, Bilgen EJS, van Dekken H, van der Sangen MJC, Rozema T, Biermann K, Beukema JC, Piet AHM, van Rij CM, Reinders JG, Tilanus HW, Steyerberg EW, van der Gaast A; CROSS study group. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 1809] [Article Influence: 180.9] [Reference Citation Analysis (0)] |
| 5. | Peyre CG, Hagen JA, DeMeester SR, Altorki NK, Ancona E, Griffin SM, Hölscher A, Lerut T, Law S, Rice TW, Ruol A, van Lanschot JJ, Wong J, DeMeester TR. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg. 2008;248:549-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 388] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 6. | Koterazawa Y, Oshikiri T, Takiguchi G, Hasegawa H, Yamamoto M, Kanaji S, Yamashita K, Matsuda T, Nakamura T, Fujino Y, Tominaga M, Suzuki S, Kakeji Y. Prophylactic Cervical Lymph Node Dissection in Thoracoscopic Esophagectomy for Esophageal Cancer Increases Postoperative Complications and Does Not Improve Survival. Ann Surg Oncol. 2019;26:2899-2904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Sachdeva UM, Axtell AL, Kroese TE, Chang DC, Mathisen DJ, Morse CR. Contributing factors to lymph node recovery with esophagectomy by thoracic surgeons: an analysis of the Society of Thoracic Surgeons General Thoracic Surgery Database. Dis Esophagus. 2022;35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | National Comprehensive Cancer Network. Esophageal and Esophagogastric Junction Cancers (Version 3. 2024). [cited 25 April 2024]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf. |
| 9. | Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus. 2017;14:1-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 704] [Article Influence: 88.0] [Reference Citation Analysis (1)] |
| 10. | Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, Goldstraw P. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer. J Thorac Oncol. 2017;12:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 486] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 11. | Lagergren J, Mattsson F, Zylstra J, Chang F, Gossage J, Mason R, Lagergren P, Davies A. Extent of Lymphadenectomy and Prognosis After Esophageal Cancer Surgery. JAMA Surg. 2016;151:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 12. | van der Schaaf M, Johar A, Wijnhoven B, Lagergren P, Lagergren J. Extent of lymph node removal during esophageal cancer surgery and survival. J Natl Cancer Inst. 2015;107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Kumakura Y, Yokobori T, Yoshida T, Hara K, Sakai M, Sohda M, Miyazaki T, Yokoo H, Handa T, Oyama T, Yorifuji H, Kuwano H. Elucidation of the Anatomical Mechanism of Nodal Skip Metastasis in Superficial Thoracic Esophageal Squamous Cell Carcinoma. Ann Surg Oncol. 2018;25:1221-1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Shigeno T, Hoshino A, Matsunaga S, Shimano R, Ishibashi N, Shinohara H, Shiobara H, Tomii C, Saito K, Fujiwara N, Sato Y, Kawada K, Tokunaga M, Kinugasa Y. The impact of lymphadenectomy on lymph node recurrence after performing various treatments for esophageal squamous cell carcinoma. BMC Surg. 2022;22:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 15. | Kamel MK, Harrison S, Lee B, Port JL, Stiles BM, Altorki NK. Extended Lymphadenectomy Improves Survival After Induction Chemoradiation for Esophageal Cancer: A Propensity-Matched Analysis of the National Cancer Database. Ann Surg. 2023;277:e772-e776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Wang H, Chen Y, Pi G, Zhu Y, Yang S, Mei H, Lin Z, Zhang T. Validation and proposed modification of the 8th edition American Joint Committee on Cancer staging system for patients with esophageal neuroendocrine neoplasms: Evaluation of a revised lymph node classification. Oncol Lett. 2020;19:4122-4132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Zhu J, Han Y, Ni W, Chang X, Wu L, Wang Y, Jiang L, Tan Y, Xiao Z, Wang Q, Peng L. Nomogram-Based Survival Predictions and Treatment Recommendations for Locally Advanced Esophageal Squamous Cell Carcinoma Treated with Upfront Surgery. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 18. | Li X, Xing J, Li P, Xie S, Lin Q, Zhang Q, Zhang S. A nomogram and risk classification system predicting esophageal stricture after endoscopic submucosal dissection of a large area for early esophageal cancer. J Surg Oncol. 2023;127:568-577. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Zhou Z, Huang S, Ben X, Zhuang W, Hong L, Xie Z, Zhang D, Xie L, Zhou H, Tang J, Chen G, Wu H, Qiao G. A novel prognostic model: which group of esophageal squamous cell carcinoma patients could benefit from adjuvant chemotherapy. Ann Transl Med. 2022;10:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Sohda M, Saito H, Kuriyama K, Yoshida T, Kumakura Y, Honjyo H, Hara K, Ozawa D, Suzuki S, Tanaka N, Sakai M, Miyazaki T, Fukuchi M, Kuwano H. Post-esophagectomy Adjuvant Chemotherapy Benefits Esophageal Cancer Patients. In Vivo. 2019;33:501-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Pasquer A, Gronnier C, Renaud F, Duhamel A, Théreaux J, Carrere N, Gagniere J, Meunier B, Collet D, Mariette C. Impact of Adjuvant Chemotherapy on Patients with Lymph Node-Positive Esophageal Cancer who are primarily Treated with Surgery. Ann Surg Oncol. 2015;22 Suppl 3:S1340-S1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Qin RQ, Wen YS, Wang WP, Xi KX, Yu XY, Zhang LJ. The role of postoperative adjuvant chemotherapy for lymph node-positive esophageal squamous cell carcinoma: a propensity score matching analysis. Med Oncol. 2016;33:31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Wong AT, Shao M, Rineer J, Lee A, Schwartz D, Schreiber D. The Impact of Adjuvant Postoperative Radiation Therapy and Chemotherapy on Survival After Esophagectomy for Esophageal Carcinoma. Ann Surg. 2017;265:1146-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Zhao P, Yan W, Fu H, Lin Y, Chen KN. Efficacy of postoperative adjuvant chemotherapy for esophageal squamous cell carcinoma: A meta-analysis. Thorac Cancer. 2018;9:1048-1055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Burt BM, Groth SS, Sada YH, Farjah F, Cornwell L, Sugarbaker DJ, Massarweh NN. Utility of Adjuvant Chemotherapy After Neoadjuvant Chemoradiation and Esophagectomy for Esophageal Cancer. Ann Surg. 2017;266:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 26. | Zheng B, Chen M, Chen C, Xiao J, Cai B, Zhang S, Liang M, Zeng T, Chen H, Wu W, Xu G, Zheng W, Zhu Y. Adjuvant chemoradiotherapy for patients with pathologic node-positive esophageal cancer following radical resection is associated with improved survival. Ann Transl Med. 2020;8:1633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Feng SK, Liu XB, Xing WQ, Liu Y, Chen PN, Jiang D, Sun HB. Adjuvant Chemotherapy for Node-positive Esophageal Squamous Cell Carcinoma Improves Survival. Ann Thorac Surg. 2022;114:1205-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Deng X, He W, Jiang Y, Deng S, Mao T, Leng X, Luo Q, Zheng K, Han Y. The impact of adjuvant therapy on survival for node-negative esophageal squamous cell carcinoma: a propensity score-matched analysis. Ann Transl Med. 2021;9:998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |