Published online May 27, 2024. doi: 10.4240/wjgs.v16.i5.1344
Revised: April 6, 2024
Accepted: April 15, 2024
Published online: May 27, 2024
Processing time: 116 Days and 3.5 Hours
Preoperative serum tumor markers not only play a role in the auxiliary diagnosis and postoperative monitoring in colorectal cancer (CRC), but also have been found to have potential prognostic value.
To analyze whether preoperative serum tumor markers, including carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9), affect the prog
This was a retrospective study conducted in a single center. Patients with non
Eventually, 3526 postoperative patients with nonmetastatic CRC were included in the study. There were 2473 patients at the development site and 1056 patients at the validation site. Age (P < 0.01, HR = 1.042, 95%CI = 1.033-1.051), tumor node metastasis (TNM) classification (P < 0.01, HR = 1.938, 95%CI = 1.665-2.255), preoperative CEA (P = 0.001, HR = 1.393, 95%CI = 1.137-1.707) and CA19-9 (P < 0.01, HR = 1.948, 95%CI = 1.614-2.438) levels were considered independent prog
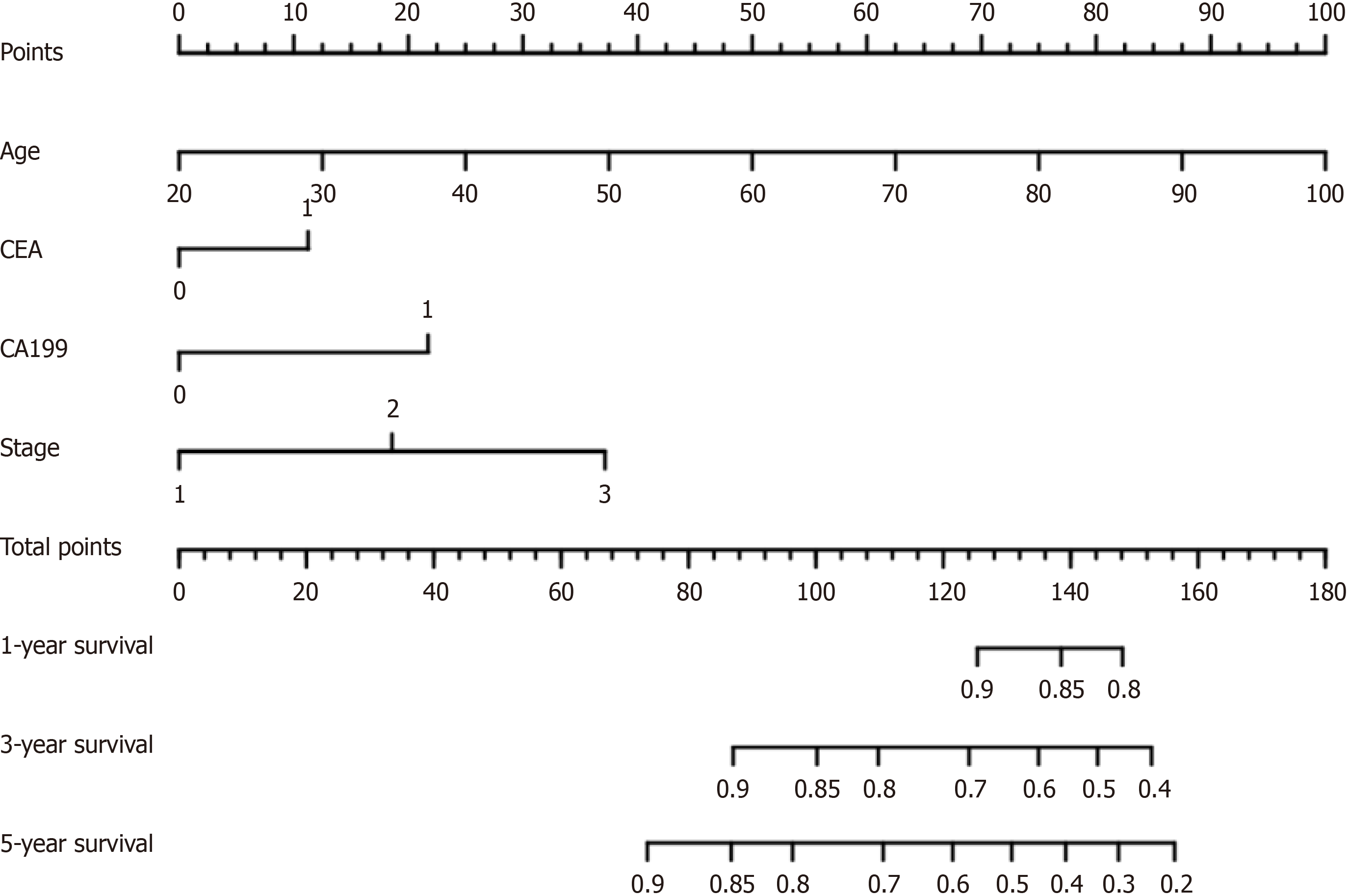
We successfully constructed a nomogram model based on age, TNM stage, preoperative CEA, and CA19-9 levels to evaluate the overall survival of patients with nonmetastatic CRC.
Core Tip: The tumor markers carcinoembryonic antigen and carbohydrate antigen 19-9 are often used in the auxiliary diagnosis and postoperative monitoring of colorectal patients, but their role in prognosis needs to be further explored. Here, we retrospectively analyzed the clinical data and pathological characteristics of 3526 patients with nonmetastatic colorectal cancer (CRC), confirmed the negative correlation between preoperative serum tumor marker levels and prognosis, and established a nomogram model to evaluate the prognosis of CRC patients.
- Citation: Diao YH, Rao SQ, Shu XP, Cheng Y, Tan C, Wang LJ, Peng D. Prognostic prediction model of colorectal cancer based on preoperative serum tumor markers. World J Gastrointest Surg 2024; 16(5): 1344-1353
- URL: https://www.wjgnet.com/1948-9366/full/v16/i5/1344.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i5.1344
Colorectal cancer (CRC) is the third most common cancer worldwide[1,2]. Although the 5-year survival rate of CRC patients has improved due to continuous improvements in screening, chemoradiotherapy, immunotherapy, metastasis resection and other treatment measures[3,4], CRC is still the second leading cause of cancer death worldwide, accounting for 10% of all cancer deaths[5,6].
At present, the prognosis prediction and treatment decision of patients with CRC depend on traditional tumor node metastasis (TNM) staging (according to the degree of tumor invasion, lymph node status, and distant metastasis status)[7]. However, as the clinical outcomes of patients with the same stage of CRC vary greatly, it is often inaccurate to judge the prognosis by TNM staging alone, especially for patients with nonmetastatic CRC[8,9]. Therefore, it is necessary to identify biomarkers to help judge the prognosis of patients with CRC.
As serum tumor markers, carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) are often used for postoperative monitoring and auxiliary diagnosis of CRC. At present, CEA is the most commonly used biomarker for CRC. In addition to preoperative monitoring, monitoring every 3-6 months after surgery is recommended by guidelines[10,11]. Although there is some controversy regarding the clinical benefit of CA19-9, it is still considered useful for monitoring disease progression in CRC patients without elevated CEA[12]. Many studies have shown that the pre
Due to the existence of tumor heterogeneity, it is impractical to predict the prognosis of patients with CRC with a single factor. Therefore, this study combined tumor markers with TNM stage and clinical characteristics of patients to construct a prediction model for the prognosis of CRC patients using a nomogram in hopes of elucidating further prog
In this retrospective study, we enrolled 3529 patients with nonmetastatic CRC who underwent surgical resection at the Department of Gastrointestinal Surgery, the First Affiliated Hospital of Chongqing Medical University, from January 2011 to January 2020. The inclusion criteria were as follows: (1) Aged ≥ 18 years; (2) diagnosed with primary CRC for the first time; (3) underwent radical surgery; and (4) had TNM stage I-III disease. The exclusion criteria were as follows: (1) Previous diagnosis of any malignant tumor; (2) distant metastasis at initial diagnosis of CRC; and (3) lack of clinical pa
Clinical and pathological variables were obtained from 3526 selected patients, including age, sex, body mass index (BMI), smoking history, drinking history, hypertension status, type 2 diabetes mellitus (T2DM) status, chronic heart disease status, tumor location, tumor size, TNM stage, surgical time, and the levels of the preoperative serum tumor markers CEA, CA19-9, and alpha-fetoprotein. Serum tumor markers were measured within one week before surgery, and normal and abnormal levels were distinguished based on the test results.
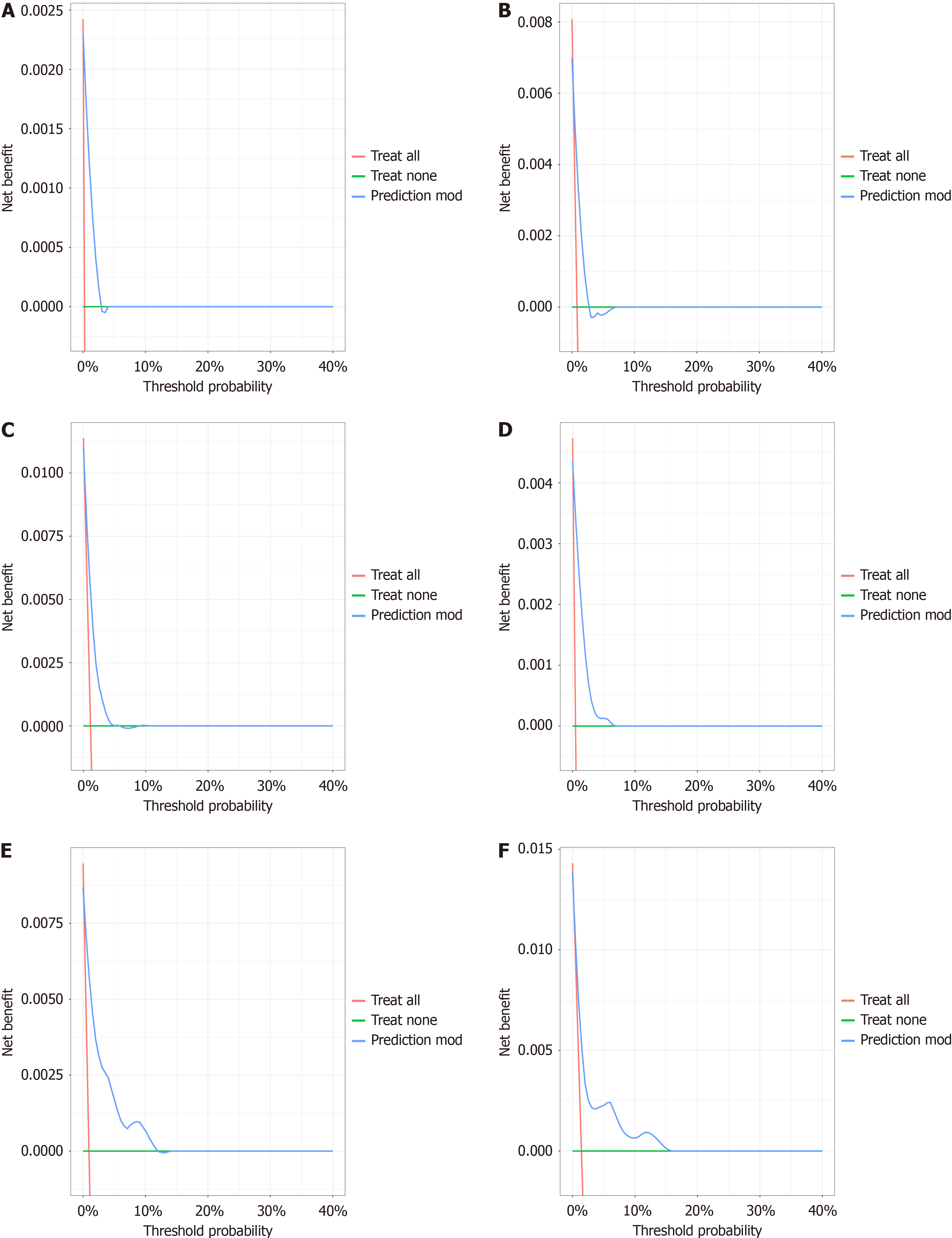
The independent sample t test was used to analyze the differences between groups for continuous variables, and the chi-square test or Fisher's exact test was used to analyze the differences between groups for categorical variables. Univariate analysis was used to test the relationships between various prognostic predictors and overall survival (OS). Variables with P values less than 0.05 in univariate analysis were used for Cox proportional hazard analysis. The nomogram model was constructed with R software 4.1.2, and then the newly established nomogram was evaluated in the validation site group. The ratio of participants in the development site group to those in the validation site group was 7:3. The areas under the curve (AUCs) and decision curve analysis (DCA)[19] were used to evaluate the performance of the nomogram model, and the accuracy of the model was further evaluated by comparing the predicted results with the actual ob
According to the inclusion criteria, 3529 patients were ultimately enrolled. Patients were randomized at a 7:3 ratio, with 2473 patients assigned to the development site group and 1056 to the validation site group (Table 1). In the development site group, males accounted for 58.4%, females accounted for 41.6%, and the average age was 63.1 years. Moreover, 47.3% of patients had colon cancer, and 60.1% of the patients had a tumor larger than 5 cm. Among them, 649 (25.1%) had hypertension, and 311 (11.5%) had T2DM. According to the TNM classification, 18.9% of patients were in stage I, 39.9% were in stage II, and 35.6% were in stage III. Preoperative CEA and CA19-9 levels were elevated in 36.8% and 20.2% of the patients, respectively.
| Characteristics | Development (2473) | Validation (1056) | P value |
| Age, yr | 63.1 ± 12.0 | 61.7 ± 12.3 | 0.002a |
| Sex | 0.930 | ||
| Male | 1448 (58.4) | 620 (59.1) | |
| Female | 1025 (41.6) | 436 (40.9) | |
| BMI, kg/m2 | 22.6 ± 3.2 | 22.8 ± 3.2 | 0.180 |
| Smoking | 942 (37.0) | 395 (37.9) | 0.700 |
| Drinking | 767 (30.8) | 310 (29.8) | 0.327 |
| Hypertension | 649 (25.1) | 257 (25.4) | 0.235 |
| T2DM | 311 (11.5) | 123 (11.5) | 0.442 |
| CHD | 98 (4.3) | 52 (4.8) | 0.195 |
| Tumor location | 0.217 | ||
| Colon | 1166 (47.3) | 582 (46.9) | |
| Rectum | 1307 (52.7) | 474 (53.1) | |
| TNM stage | 0.802 | ||
| I | 490 (18.9) | 215 (18.4) | |
| II | 1067 (39.9) | 443 (40.4) | |
| III | 916 (35.6) | 398 (36.6) | |
| Tumor size | 0.705 | ||
| < 5 cm | 1442 (60.1) | 623 (60.9) | |
| ≥ 5 cm | 1031 (39.9) | 433 (39.1) | |
| CEA | 0.053 | ||
| Normal | 1562 (63.2) | 703 (66.6) | |
| Abnormal | 911 (36.8) | 353 (33.4) | |
| CA199 | 0.823 | ||
| Normal | 1973 (79.8) | 839 (79.5) | |
| Abnormal | 500 (20.2) | 217 (20.5) | |
| AFP | 0.105 | ||
| Normal | 2366 (95.7) | 997 (94.4) | |
| Abnormal | 107 (4.3) | 59 (5.6) | |
| Surgical time, min | 224.5 ± 78.3 | 224.6 ± 78.3 | 0.967 |
To predict the prognosis of CRC, univariate and Cox analyses were performed (Table 2). According to univariate analysis, age (P < 0.01, HR = 1.045, 95%CI = 1.037-1.055), BMI (P = 0.001, HR = 0.952, 95%CI = 0.924-0.981), tumor size (P < 0.01, HR = 1.426, 95%CI = 1.184-1.718), tumor stage (P < 0.01, HR = 2.105, 95%CI = 1.817-2.438), and preoperative CEA (P < 0.01, HR = 2.185, 95%CI = 1.812-2.633) and CA19-9 (P < 0.01, HR = 2.646, 95%CI = 2.185-3.204) levels all showed highly significant differences. Unhealthy lifestyle habits, such as smoking (P = 0.706, HR = 0.964, 95%CI = 0.795-1.168) and drinking (P = 0.248, HR = 0.884, 95%CI = 0.718-1.089), were not significantly associated with OS, and chronic diseases, such as hypertension (P = 0.493, HR = 0.926, 95%CI = 0.743-1.154) and T2DM (P = 0.134, HR = 1.231, 95%CI = 0.938-1.617), were also not associated with OS.
| Risk factors | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age (yr) | 1.045 (1.037-1.055) | < 0.01a | 1.042 (1.033-1.051) | < 0.01a |
| Sex (male/female) | 0.931 (0.770-1.126) | 0.461 | ||
| BMI (kg/m2) | 0.952 (0.924-0.981) | 0.001a | 0.980 (0.952-1.008) | 0.161 |
| T2DM (yes/no) | 1.231 (0.938-1.617) | 0.134 | ||
| Tumor location (colon/ rectum) | 1.179 (0.979-1.421) | 0.083 | ||
| Tumor stage (III/II/I) | 2.105 (1.817-2.438) | < 0.01a | 1.938 (1.665-2.255) | < 0.01a |
| Smoking (yes/no) | 0.964 (0.795-1.168) | 0.706 | ||
| Drinking (yes/no) | 0.884 (0.718-1.089) | 0.248 | ||
| Hypertension (yes/no) | 0.926 (0.743-1.154) | 0.493 | ||
| Tumor size (≥ 5 cm/< 5 cm) | 1.426 (1.184-1.718) | < 0.01a | 1.117 (0.923-1.351) | 0.255 |
| CEA (abnormal/normal) | 2.185 (1.812-2.633) | < 0.01a | 1.393 (1.137-1.707) | 0.001a |
| AFP (abnormal/normal) | 1.108 (0.715-1.718) | 0.645 | ||
| CA-199 (abnormal/normal) | 2.646 (2.185-3.204) | < 0.01a | 1.984 (1.614-2.438) | < 0.01a |
| Surgical time, min | 1.001 (1.000-1.002) | 0.069 | ||
Next, we included variables with significant differences in the univariate analysis in the Cox analysis, which revealed that age (P < 0.01, HR = 1.042, 95%CI = 1.033-1.051), tumor stage (P < 0.01, HR = 1.938, 95%CI = 1.665-2.255), preoperative CEA (P = 0.001, HR = 1.393, 95%CI = 1.137-1.707) and CA19-9 (P < 0.01, HR = 1.948, 95%CI = 1.614-2.438) levels were independent risk factors for the prognosis of patients with CRC. A nomogram based on the Cox regression model was established (Figure 1). The score of each factor was obtained according to the patient's own condition, and the total score was obtained by adding the four scores. Then, the prognosis of patients with nonmetastatic CRC was estimated according to the total score.
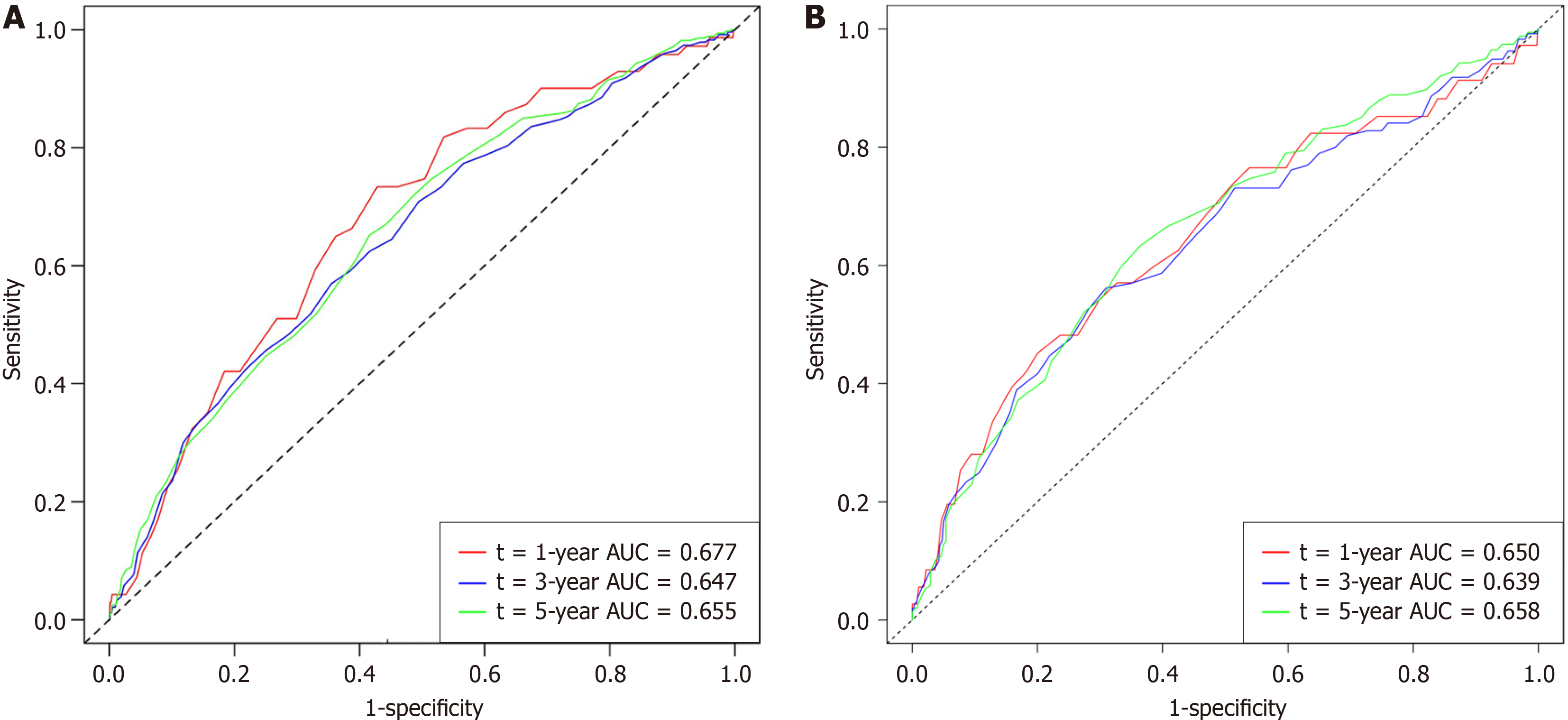
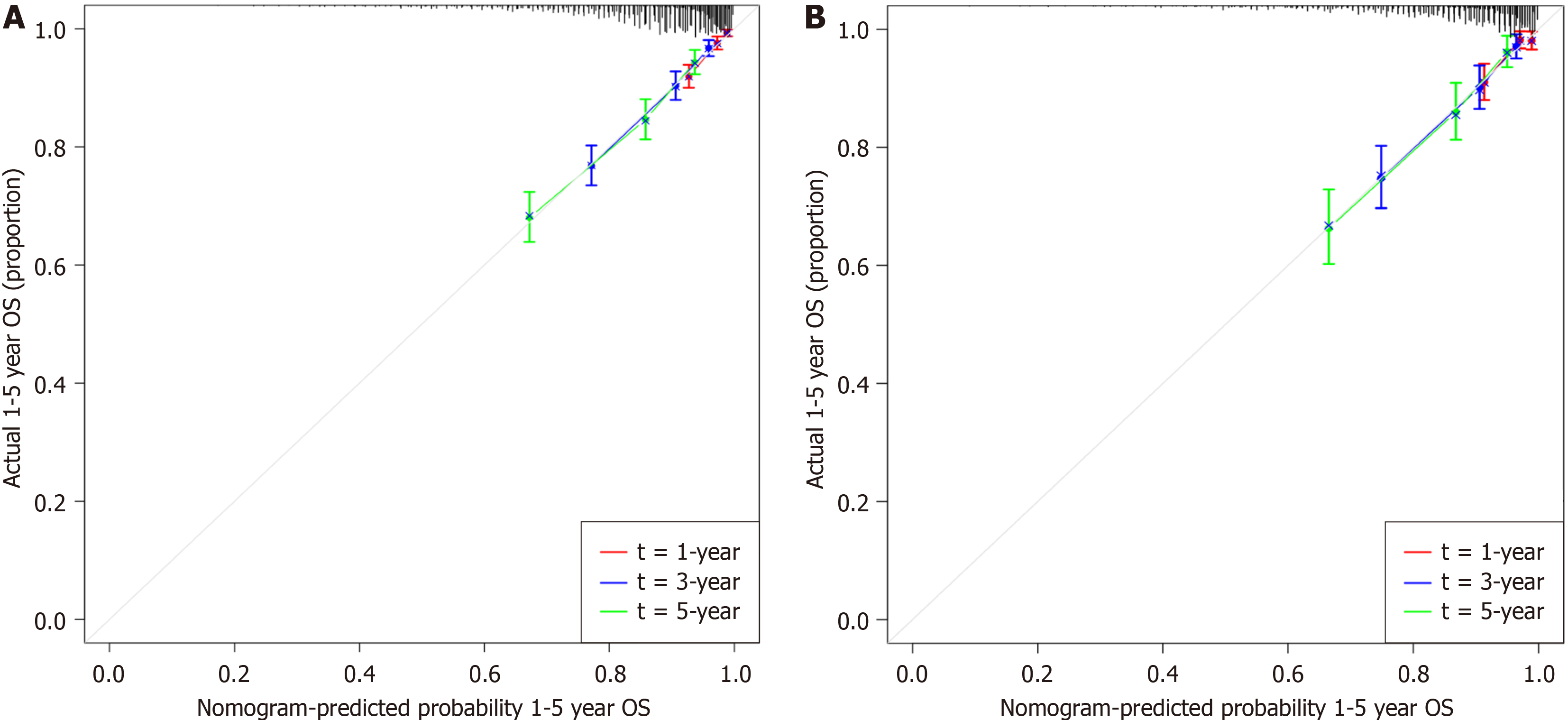
To verify whether the nomogram was applicable to other datasets, we conducted a validation study using data from 1056 CRC patients at the validation site. Time-dependent receiver operating characteristic curves for the OS-associated nomo
Relying only on the traditional TNM stage to judge the prognosis of patients with CRC[22], especially patients with nonmetastatic CRC, is difficult, and additional influencing factors should be considered[23-25]. Over the past two decades, many molecular biomarkers of CRC have been extensively investigated, but serum tumor markers remain the most commonly used. CEA and CA19-9, which are readily available serum tumor markers, are widely used in the diag
In this study, we attempted to establish a nomogram including serum tumor markers combined with traditional TNM staging to improve prognosis prediction in patients with CRC. A total of 15 variables were included in the study, and four variables (age, TNM stage, preoperative CEA level and CA19-9 level) were ultimately included in the nomogram. BMI and tumor size were also considered to be associated with prognostic outcome in the univariate analysis, but the asso
Consistent with the results of other studies[34-37], we found that the CEA level was an independent predictor of survival. Compared with normal levels of CEA, elevated preoperative CEA resulted in a 62% increased risk of death[38]. The guidelines also recommend CEA as an effective predictor of OS[39,40]. Notably, previous reports have shown that the significance of postoperative CEA measurements depends on preoperative CEA levels. Almost all patients with high preoperative CEA levels had increased CEA levels at the time of CRC recurrence, but this increase was rarely observed in patients with normal preoperative CEA levels[17,41]. Therefore, we selected the preoperative CEA level as a prognostic predictor. In contrast, previous guidelines did not recommend the use of CA19-9 to assess prognosis[40,42]. However, similar to our findings, several recent studies have also demonstrated the prognostic value of CA19-9[31,43], especially in CRC patients with normal preoperative CEA levels[12,44]. Furthermore, some studies have reported that the combined assessment of preoperative serum CEA and CA19-9 may enhance the diagnostic prediction and prognosis prediction of CRC patients[45].
In our study, we successfully established a novel prognostic model for patients with nonmetastatic CRC. Compared with previous studies, our sample size was quite large, and after internal validation, our prediction model showed good performance. However, the current study has several limitations. First, our study was retrospective and was conducted at a single center, which might have caused selection bias. Second, while we performed internal validation of the prediction model, it would have been better if external validation could have been performed to verify whether our findings were generally applicable.
Our study demonstrated the prognostic impact of the tumor markers CEA and CA19-9 and established a more accurate and practical nomogram model for predicting the prognosis of patients with nonmetastatic CRC.
We acknowledge all the authors whose publications are referred in our article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Soldera J, Brazil S-Editor: Qu XL L-Editor: A P-Editor: Xu ZH
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64636] [Article Influence: 16159.0] [Reference Citation Analysis (176)] |
| 2. | Kang B, Zhao ZQ, Liu XY, Cheng YX, Tao W, Wei ZQ, Peng D. Effect of hypoalbuminemia on short-term outcomes after colorectal cancer surgery: A propensity score matching analysis. Front Nutr. 2022;9:925086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 3. | Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 520] [Cited by in RCA: 907] [Article Influence: 113.4] [Reference Citation Analysis (2)] |
| 4. | Smith RJ, Bryant RG. Metal substitutions incarbonic anhydrase: a halide ion probe study. Biochem Biophys Res Commun. 1975;66:1281-1286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 5. | Liu XR, Liu F, Li ZW, Liu XY, Zhang W, Peng D. The risk of postoperative complications is higher in stage I-III colorectal cancer patients with previous abdominal surgery: a propensity score matching analysis. Clin Transl Oncol. 2023;25:3471-3478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 3024] [Article Influence: 504.0] [Reference Citation Analysis (3)] |
| 7. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6462] [Article Influence: 430.8] [Reference Citation Analysis (0)] |
| 8. | Lea D, Håland S, Hagland HR, Søreide K. Accuracy of TNM staging in colorectal cancer: a review of current culprits, the modern role of morphology and stepping-stones for improvements in the molecular era. Scand J Gastroenterol. 2014;49:1153-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Li J, Guo BC, Sun LR, Wang JW, Fu XH, Zhang SZ, Poston G, Ding KF. TNM staging of colorectal cancer should be reconsidered by T stage weighting. World J Gastroenterol. 2014;20:5104-5112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (2)] |
| 10. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Johung KL, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Gregory KM, Gurski LA. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:329-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 959] [Article Influence: 239.8] [Reference Citation Analysis (16)] |
| 11. | Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Jeck W, Johung KL, Kirilcuk N, Krishnamurthi S, Maratt JK, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stotsky-Himelfarb E, Tavakkoli A, Willett CG, Gregory K, Gurski L. Rectal Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:1139-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 409] [Article Influence: 136.3] [Reference Citation Analysis (0)] |
| 12. | Becerra AZ, Probst CP, Tejani MA, Aquina CT, González MG, Hensley BJ, Noyes K, Monson JR, Fleming FJ. Evaluating the Prognostic Role of Elevated Preoperative Carcinoembryonic Antigen Levels in Colon Cancer Patients: Results from the National Cancer Database. Ann Surg Oncol. 2016;23:1554-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 13. | Das V, Kalita J, Pal M. Predictive and prognostic biomarkers in colorectal cancer: A systematic review of recent advances and challenges. Biomed Pharmacother. 2017;87:8-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 186] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 14. | Baqar AR, Wilkins S, Staples M, Angus Lee CH, Oliva K, McMurrick P. The role of preoperative CEA in the management of colorectal cancer: A cohort study from two cancer centres. Int J Surg. 2019;64:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Yang XQ, Chen C, Wang FB, Peng CW, Li Y. Preoperative serum carcinoembryonic antigen, carbohydrate antigen19-9 and carbohydrate antigen 125 as prognostic factors for recurrence-free survival in colorectal cancer. Asian Pac J Cancer Prev. 2011;12:1251-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Yakabe T, Nakafusa Y, Sumi K, Miyoshi A, Kitajima Y, Sato S, Noshiro H, Miyazaki K. Clinical significance of CEA and CA19-9 in postoperative follow-up of colorectal cancer. Ann Surg Oncol. 2010;17:2349-2356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Gao Y, Wang J, Zhou Y, Sheng S, Qian SY, Huo X. Evaluation of Serum CEA, CA19-9, CA72-4, CA125 and Ferritin as Diagnostic Markers and Factors of Clinical Parameters for Colorectal Cancer. Sci Rep. 2018;8:2732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 200] [Article Influence: 28.6] [Reference Citation Analysis (1)] |
| 18. | Lin Z, Li Y, Wu J, Zheng H, Yang C. Nomogram for prediction of prolonged postoperative ileus after colorectal resection. BMC Cancer. 2022;22:1273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 19. | Kerr KF, Brown MD, Zhu K, Janes H. Assessing the Clinical Impact of Risk Prediction Models With Decision Curves: Guidance for Correct Interpretation and Appropriate Use. J Clin Oncol. 2016;34:2534-2540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 457] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 20. | Fitzgerald M, Saville BR, Lewis RJ. Decision curve analysis. JAMA. 2015;313:409-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 532] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 21. | Schlechter BL, Ng K. Colorectal Cancer: Advancing Science, Improving Access, and Refining Therapy. Hematol Oncol Clin North Am. 2022;36:xiii-xxiv. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Delattre JF, Selcen Oguz Erdogan A, Cohen R, Shi Q, Emile JF, Taieb J, Tabernero J, André T, Meyerhardt JA, Nagtegaal ID, Svrcek M. A comprehensive overview of tumour deposits in colorectal cancer: Towards a next TNM classification. Cancer Treat Rev. 2022;103:102325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 23. | Bagaria B, Sood S, Sharma R, Lalwani S. Comparative study of CEA and CA19-9 in esophageal, gastric and colon cancers individually and in combination (ROC curve analysis). Cancer Biol Med. 2013;10:148-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 24. | Ma Y, Lu P, Liang X, Wei S. Models Based on Dynamic Clinicopathological Indices for Predicting Prognosis During the Perioperative Period for Patients with Colorectal Cancer. J Inflamm Res. 2021;14:1591-1601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Park CH, Lizarraga AD, Lee YT, Yoon KJ, Yoo TK. Increased Carcinoembryonic Antigen (CEA) Level Is Highly Associated with Low Skeletal Muscle Mass in Asymptomatic Adults: A Population-Based Study. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 26. | Lee TH, Kim JS, Baek SJ, Kwak JM, Kim J. Diagnostic Accuracy of Carcinoembryonic Antigen (CEA) in Detecting Colorectal Cancer Recurrence Depending on Its Preoperative Level. J Gastrointest Surg. 2023;27:1694-1701. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Takagawa R, Fujii S, Ohta M, Nagano Y, Kunisaki C, Yamagishi S, Osada S, Ichikawa Y, Shimada H. Preoperative serum carcinoembryonic antigen level as a predictive factor of recurrence after curative resection of colorectal cancer. Ann Surg Oncol. 2008;15:3433-3439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 28. | Wang WS, Lin JK, Chiou TJ, Liu JH, Fan FS, Yen CC, Lin TC, Jiang JK, Yang SH, Wang HS, Chen PM. Preoperative carcinoembryonic antigen level as an independent prognostic factor in colorectal cancer: Taiwan experience. Jpn J Clin Oncol. 2000;30:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Wiratkapun S, Kraemer M, Seow-Choen F, Ho YH, Eu KW. High preoperative serum carcinoembryonic antigen predicts metastatic recurrence in potentially curative colonic cancer: results of a five-year study. Dis Colon Rectum. 2001;44:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Hou S, Jing J, Wang Y, Du L, Tian B, Xu X, Sun T, Shi Y. Evaluation of Clinical Diagnostic and Prognostic Value of Preoperative Serum Carcinoembryonic Antigen, CA19-9, and CA24-2 for Colorectal Cancer. Altern Ther Health Med. 2023;29:192-197. [PubMed] [DOI] [Full Text] |
| 31. | Park JS, Choi GS, Jang YS, Jun SH, Kang H. Influence of obesity on the serum carcinoembryonic antigen value in patients with colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:2461-2468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Bell S, Kong JC, Wale R, Staples M, Oliva K, Wilkins S, Mc Murrick P, Warrier SK. The effect of increasing body mass index on laparoscopic surgery for colon and rectal cancer. Colorectal Dis. 2018;20:778-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | Huh JW, Oh BR, Kim HR, Kim YJ. Preoperative carcinoembryonic antigen level as an independent prognostic factor in potentially curative colon cancer. J Surg Oncol. 2010;101:396-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Spindler BA, Bergquist JR, Thiels CA, Habermann EB, Kelley SR, Larson DW, Mathis KL. Incorporation of CEA Improves Risk Stratification in Stage II Colon Cancer. J Gastrointest Surg. 2017;21:770-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Kim CG, Ahn JB, Jung M, Beom SH, Heo SJ, Kim JH, Kim YJ, Kim NK, Min BS, Koom WS, Kim H, Roh YH, Ma BG, Shin SJ. Preoperative Serum Carcinoembryonic Antigen Level as a Prognostic Factor for Recurrence and Survival After Curative Resection Followed by Adjuvant Chemotherapy in Stage III Colon Cancer. Ann Surg Oncol. 2017;24:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 36. | Zhou S, Sheng N, Ren J, He Q, Zhang Y, Gong J, Wang Z. Clinical Significance of and Predictive Risk Factors for the Postoperative Elevation of Carcinoembryonic Antigen in Patients With Non-Metastatic Colorectal Cancer. Front Oncol. 2021;11:741309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Hall C, Clarke L, Pal A, Buchwald P, Eglinton T, Wakeman C, Frizelle F. A Review of the Role of Carcinoembryonic Antigen in Clinical Practice. Ann Coloproctol. 2019;35:294-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 137] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 38. | Duffy MJ, van Dalen A, Haglund C, Hansson L, Holinski-Feder E, Klapdor R, Lamerz R, Peltomaki P, Sturgeon C, Topolcan O. Tumour markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines for clinical use. Eur J Cancer. 2007;43:1348-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 342] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 39. | Bolocan A, Ion D, Ciocan DN, Paduraru DN. Prognostic and predictive factors in colorectal cancer. Chirurgia (Bucur). 2012;107:555-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Körner H, Söreide K, Stokkeland PJ, Söreide JA. Diagnostic accuracy of serum-carcinoembryonic antigen in recurrent colorectal cancer: a receiver operating characteristic curve analysis. Ann Surg Oncol. 2007;14:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF, Bast RC Jr; ASCO. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313-5327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1111] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 42. | Li C, Zhang D, Pang X, Pu H, Lei M, Fan B, Lv J, You D, Li Z, Zhang T. Trajectories of Perioperative Serum Tumor Markers and Colorectal Cancer Outcomes: A Retrospective, Multicenter Longitudinal Cohort Study. EBioMedicine. 2021;74:103706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 43. | Li Z, Zhu H, Pang X, Mao Y, Yi X, Li C, Lei M, Cheng X, Liang L, Wu J, Ding Y, Yang J, Sun Y, Zhang T, You D, Liu Z. Preoperative serum CA19-9 should be routinely measured in the colorectal patients with preoperative normal serum CEA: a multicenter retrospective cohort study. BMC Cancer. 2022;22:962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 44. | Ogata S, Fujita F, Fujiyoshi K, Sudou T, Yoshida T, Koushi K, Murotani K, Yamauchi S, Sugihara K, Akagi Y. Prognostic Value of Preoperative Carcinoembryonic Antigen and Carbohydrate Antigen 19-9 Levels for Adjuvant Chemotherapy in Stage II Colorectal Cancer: A Nationwide Multicenter Retrospective Study. J Anus Rectum Colon. 2022;6:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 45. | Lakemeyer L, Sander S, Wittau M, Henne-Bruns D, Kornmann M, Lemke J. Diagnostic and Prognostic Value of CEA and CA19-9 in Colorectal Cancer. Diseases. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |