Published online Feb 27, 2024. doi: 10.4240/wjgs.v16.i2.463
Peer-review started: December 12, 2023
First decision: January 2, 2024
Revised: January 4, 2024
Accepted: January 30, 2024
Article in press: January 30, 2024
Published online: February 27, 2024
Processing time: 75 Days and 6.4 Hours
Colon cancer (CC) has a high incidence rate. Radical resection is the main treatment method for CC; however, liver metastasis (LM) often occurs post-surgery. The liver contains both innate and adaptive immune cells that monitor and remove abnormal cells and pathogens. Before LM, tumor cells secrete cytokines and exosomes to adjust the immune microenvironment of the liver, thus forming an inhibitory immune microenvironment for colonization by circulating tumor cells. This indicates that the immune state of patients with CC plays a crucial role in the occurrence and progression of LM.
To observe and analyze the relationship between immune status and expression of tumor factors in patients with LM of CC, and to provide a scientific interven
A retrospective analysis was performed. The baseline data of 100 patients with CC and 100 patients with CC who suffered from postoperative LM and were admitted to our hospital from May 2021 to May 2023 were included in the non-occurrence and occurrence groups, respectively. The immune status of the pa
Compared with the non-occurrence group, the expression of serum carcinoembryonic antigen (CEA), CA19-9, CA242, CA72-4 and CA50 in patients in the occurrence group were significantly higher, while the expression of CD3+, CD4+, CD8+, natural killer (NK) and CD4+/CD25 in patients in the occurrence group were significantly lower (P < 0.05). No significant difference was observed in other baseline data between groups (P > 0.05). Multivariate logistic regression model analysis revealed that the expressions of CEA, CA19-9, CA242, CA72-4, CA50, CD3+, CD4+, CD8+, NK, and CD4+/CD25 were associated with the LM in patients with CC. High expressions of serum CEA, CA19-9, CA242, CA72-4 and CA50, and low expressions of CD3+, CD4+, CD8+, NK, and CD4+/CD25 in patients with CC were risk factors for LM (OR > 1, P < 0.05). The receiver operating characteristic curve showed that the area under curve for CEA, CA19-9, CA242, CA72-4, CA50, CD3+, CD4+, CD8+, NK, and CD4+/CD25 in the prediction of LM in patients with CC were all > 0.80, with a high predictive value.
The expression of tumor factors and immune state-related indices in patients with CC is closely associated with the occurrence of LM.
Core Tip: Postoperative liver metastasis (LM) in patients with colon cancer (CC) leads to a poor prognosis; therefore, monitoring the risk factors that affect postoperative LM in patients with CC is crucial to improving their prognosis. The anti-tumor immune system of the body includes cellular immunity mediated by T cells and their subsets. An imbalance in the proportion of these cells or abnormalities in their function lead to a disordered immune system and a variety of immune-mediated diseases. Therefore, a correlation between postoperative LM and immune function may occur in patients with CC.
- Citation: Xiong L, Liu FC. Immune function status of postoperative patients with colon cancer for predicting liver metastasis. World J Gastrointest Surg 2024; 16(2): 463-470
- URL: https://www.wjgnet.com/1948-9366/full/v16/i2/463.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i2.463
The incidence of colon cancer (CC) is high in China, and the liver is the main organ involved in CC metastasis. A previous report showed that approximately 20% and 25% of patients with CC develop simultaneous liver metastasis (LM) and LM after radical surgery, respectively. Some important factors may lead to a poor prognosis and an increased risk of LM in patients with CC. Therefore, exploring these factors is important for guiding advanced intervention and promoting patient prognosis[1-3]. The microenvironment before tumor metastasis is favorable for tumor cell colonization at a distance, and its formation is completely determined by the interaction between the primary lesion and the metastatic organ. Macrophages, hepatocytes, and neutrophils in the liver have significant tumor-promoting functions during cancer cell metastasis. Tumor cells in the primary lesion interfere with the liver microenvironment through many pathways, such as the portal vein system, in the early stage, resulting in an environment conducive to tumor cell colonization and growth[4,5]. The liver contains congenital and adaptive immune cells that monitor and eliminate abnormal cells and pathogens. The innate immune system of the liver includes natural killer (NK) cells, monocytes, and liver macrophages, which maintain the balance between immune activation and tolerance. Before LM, tumor cells secrete cytokines and exosomes to regulate the liver immune microenvironment, thereby forming an inhibitory immune microenvironment for colonization by circulating tumor cells. Notably, the immune state of CC patients plays a crucial role in the occurrence and progression of LM[6-8]. The clinical studies on the relationship between immune state-related factors and LM in patients with CC are limited. Therefore, this study focused on the immune state of patients with LM of CC and the related expression of tumor factors and analyzed their relationship with the occurrence of LM of CC to provide a scientific intervention to promote the patient prognosis.
Baseline data of 100 patients with CC who were admitted to our hospital from May 2021 to May 2023 were included in the non-occurrence group using retrospective analysis. This group included 58 males and 42 females with an age range of 42 to 70 years, with an average of 62.25 ± 3.75 years. The baseline data of 100 patients with CC with postoperative LM treated in the same period were included in the occurrence group. This group included 61 males and 39 females with an age range of 40 to 73 years, with an average of 63.02 ± 3.88 years.
Inclusion criteria: The diagnosis of CC was performed by referring to the Chinese Protocol of Diagnosis and Treatment of Colorectal Cancer (2020 edition)[9] and confirmed using a case puncture or surgical pathology examination. LM of CC was based on relevant evidence in the China guideline for diagnosis and comprehensive treatment of colorectal liver metastases (version 2023)[10] and was confirmed based on clinical signs, manifestations, imaging, and pathological examination. Clinical manifestations included changes in stool traits, changes in defecation habits, abdominal discomfort or pain, and palpable masses. Additionally, CT scan and B-type ultrasound imaging analyses were performed. Pathological diagnosis for LM of CC was confirmed through a case puncture or surgical pathology. All operations related to CC were successfully performed in our hospital. The baseline data and laboratory tests of the patients were properly preserved.
Exclusion criteria: Patients with other site metastases, liver and kidney failure, CC recurrence, other intestinal diseases, mental history or antipsychotic medication (resulting in decreased compliance), or LM before surgery were excluded.
Baseline data collection method: The baseline data of the patients, including sex, age, and tumor stage, were analyzed.
Detection methods of laboratory indicators: Collecting five milliliters of fasting peripheral venous blood of each patient, and serum was collected after high-speed centrifugation. Immune-related factors, including NK cells, CD3+, CD4+, CD8+, and CD4+/CD25 in the serum, were detected using Agilent flow cytometers (NovoCyte Quanteon). Tumor factors, including serum carcinoembryonic antigen (CEA), CA242, CA72-4, CA19-9, and CA50 levels, were detected using chemiluminescence kits (Abbott, China).
Statistical analyses were performed using SPSS 24.0 software. All measurement data were subjected to the Shapiro–Wilk normality test. Data conforming to a normal distribution were expressed as mean ± SD, and comparisons between groups were performed using an independent sample t test. The countable data were expressed as % using the χ2 test. Logistic regression analysis was used to observe the correlation between each indicator and patients with LM of CC. The receiver operating characteristic (ROC) curve of the patients was drawn, and the value of the main indicators for predicting LM in patients with CC after surgery was tested. The area under curve (AUC) ≤ 0.50 represented no predictive value, 0.50 < AUC ≤ 0.70 represented low predictive value, 0.70 < AUC ≤ 0.90 represented medium predictive value, and AUC >0.90 represented high predictive value. P < 0.05 indicated that the difference was statistically significant.
Compared with the non-occurrence group, the expression of serum CEA, CA19-9, CA242, CA72-4 and CA50 in patients in the occurrence group were significantly higher, while the expression of CD3+, CD4+, CD8+, NK and CD4+/CD25 in patients in the occurrence group were significantly lower (P < 0.05). No significant difference was observed in other baseline data between the groups (P > 0.05; Table 1).
| Data | Occurrence group (n = 100) | Non-occurrence group (n = 100) | Statistical values | P value | |
| Sex | Male | 61 (61.00) | 58 (58.00) | χ2 = 0.187 | 0.666 |
| Female | 39 (39.00) | 42 (42.00) | |||
| Age | 63.02 ± 3.88 (49-71) | 62.25 ± 3.75 (53-71) | t = 1.427 | 0.155 | |
| Tumor site | Right hemicolon | 40 (40.00) | 45 (45.00) | χ2 = 0.512 | 0.475 |
| Left hemicolon | 60 (60.00) | 55 (55.00) | |||
| Tumor typing | Infiltration type | 22 (22.00) | 30 (30.00) | χ2 = 0.189 | 0.389 |
| Ulcer type | 35 (35.00) | 30 (30.00) | |||
| Uplift type | 45 (45.00) | 40 (40.00) | |||
| CEA (μg/L) | 8.95 ± 1.30 | 5.25 ± 1.52 | t = 18.499 | < 0.001 | |
| CA19-9 (IU/mL) | 42.25 ± 8.25 | 22.56 ± 4.58 | t = 20.867 | < 0.001 | |
| CA242 (U/mL) | 142.25 ± 20.52 | 85.65 ± 15.05 | t = 17.055 | < 0.001 | |
| CA72-4 (U/mL) | 55.85 ± 10.58 | 23.25 ± 5.28 | t = 27.570 | < 0.001 | |
| CA50 (U/mL) | 42.25 ± 6.25 | 32.15 ± 4.20 | t = 13.413 | < 0.001 | |
| CD3+ (%) | 53.45 ± 5.52 | 67.50 ± 8.40 | t = 13.978 | < 0.001 | |
| CD4+ (%) | 26.58 ± 3.45 | 37.35 ± 4.10 | t = 20.100 | < 0.001 | |
| CD8+ (%) | 24.52 ± 4.10 | 33.02 ± 6.50 | t = 11.060 | < 0.001 | |
| NK (%) | 31.25 ± 3.85 | 45.20 ± 5.15 | t = 21.695 | < 0.001 | |
| CD4+/CD25 (%) | 5.10 ± 2.15 | 9.52 ± 3.56 | t = 10.628 | < 0.001 | |
The levels of serum CEA, CA19-9, CA242, CA72-4, CA50, CD3+, CD4+, CD8+, NK, and CD4+/CD25 in patients with CC were considered the covariates, and LM was considered the dependent variable (1 = occurred and 0 = not occurred). A multivariate logistic regression model analysis showed that the expression of CEA, CA19-9, CA242, CA72-4, CA50, CD3+, CD4+, CD8+, NK, and CD4+/CD25 was associated with LM in patients with CC. High expressions of serum CEA, CA19-9, CA242, CA72-4 and CA50, and low expressions of CD3+, CD4+, CD8+, NK, and CD4+/CD25 in patients with CC were risk factors for LM (OR > 1, P < 0.05; Table 2).
| Variable | B | SE | Walls | P value | OR value | 95% confidence interval | |
| Upper limit | Lower limit | ||||||
| Constant | -14.183 | 2.230 | 40.440 | < 0.001 | 0.000 | - | - |
| CEA | 1.953 | 0.299 | 42.755 | < 0.001 | 7.051 | 3.926 | 12.662 |
| CA19-9 | 0.483 | 0.081 | 35.206 | < 0.001 | 1.620 | 1.382 | 1.900 |
| CA242 | 0.163 | 0.027 | 37.738 | < 0.001 | 1.177 | 1.117 | 1.240 |
| CA72-4 | 0.630 | 0.178 | 12.509 | < 0.001 | 1.878 | 1.324 | 2.662 |
| CA50 | 0.367 | 0.051 | 51.721 | < 0.001 | 1.443 | 1.306 | 1.595 |
| CD3+ | 0.318 | 0.045 | 49.250 | < 0.001 | 1.374 | 1.258 | 1.502 |
| CD4+ | 0.85 | 0.127 | 40.082 | < 0.001 | 2.236 | 1.743 | 2.869 |
| CD8+ | 0.255 | 0.084 | 9.257 | < 0.001 | 1.291 | 1.095 | 1.521 |
| NK | 0.582 | 0.111 | 27.604 | < 0.001 | 1.790 | 1.440 | 2.224 |
| CD4+/CD25 | 0.476 | 0.091 | 27.525 | < 0.001 | 1.610 | 1.348 | 1.923 |
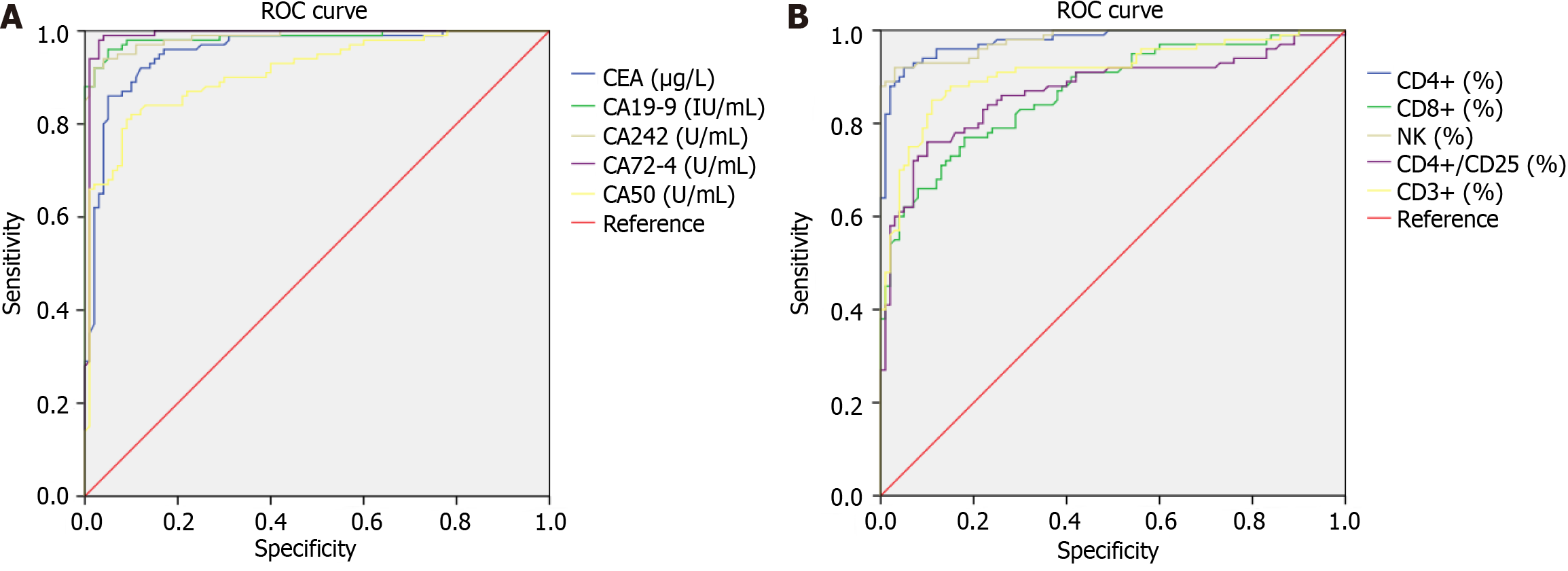
The levels of serum CEA, CA19-9, CA242, CA72-4, CA50, CD3+, CD4+, CD8+, NK, and CD4+/CD25 in patients with CC were considered test variables, and LM was considered the state variable (1 = occurred, 0 = not occurred). Further, ROC curves were drawn (Figure 1). The results indicated that the AUC of CEA, CA19-9, CA242, CA72-4, CA50, CD3+, CD4+, CD8+, NK, and CD4+/CD25 for predicting LM in patients with CC were all >0.80, with a high prediction value (Table 3).
| Indicator | AUC | 95% CI for AUC | Standard error | P value | Cut-off value | Sensitivity | Specificity |
| CEA | 0.956 | 0.927-0.984 | 0.014 | < 0.001 | 4.170 | 0.990 | 0.770 |
| CA19-9 | 0.986 | 0.972-1.000 | 0.007 | < 0.001 | 21.820 | 0.990 | 0.640 |
| CA242 | 0.987 | 0.976-1.000 | 0.006 | < 0.001 | 89.915 | 0.990 | 0.420 |
| CA72-4 | 0.990 | 0.976-1.000 | 0.007 | < 0.001 | 28.625 | 0.990 | 0.150 |
| CA50 | 0.912 | 0.872-0.952 | 0.020 | < 0.001 | 28.985 | 0.990 | 0.780 |
| CD3+ | 0.912 | 0.870-0.954 | 0.021 | < 0.001 | 46.925 | 0.990 | 0.900 |
| CD4+ | 0.978 | 0.962-0.995 | 0.008 | < 0.001 | 26.725 | 0.990 | 0.480 |
| CD8+ | 0.869 | 0.820-0.917 | 0.025 | < 0.001 | 19.290 | 0.990 | 0.900 |
| NK | 0.980 | 0.965-0.995 | 0.008 | < 0.001 | 32.430 | 0.990 | 0.370 |
| CD4+/CD25 | 0.870 | 0.818-0.922 | 0.027 | < 0.001 | 0.015 | 0.990 | 0.990 |
Cellular carcinogenesis is a dynamic process. During different periods, different stimulation and agonist factors work together or in sequence to induce disease and accelerate disease progression. Notably, the immune state of the body is also one of the stimulation factors. The anti-tumor immune system mainly involves cellular immunity mediated by T cells and their subsets. An imbalance in the proportion of these cells or abnormalities in their function lead to a disordered immune system and a variety of immune-mediated diseases[11-13]. Therefore, the immune status of patients with cachexia is critical.
Our results indicated that the levels of serum CEA, CA19-9, CA242, CA72-4 and CA50 in patients in the occurrence group were higher than those in the non-occurrence group, while the expression of CD3+, CD4+, CD8+, NK and CD4+/CD25 in patients in the occurrence group were lower than those in the non-occurrence group. A multivariate logistic regression model indicated that the expression of CEA, CA19-9, CA242, CA72-4, CA50, CD3+, CD4+, CD8+, NK, and CD4+/CD25 was associated with LM in patients with CC. High expressions of serum CEA, CA19-9, CA242, CA72-4 and CA50, and low expressions of CD3+, CD4+, CD8+, NK, and CD4+/CD25 in patients with CC were risk factors for LM, indicating that these indicators can be used to predict LM in patients with CC. Tumor markers are macromolecular proteins synthesized and secreted by tumor lesions during their occurrence and progression. They can exist in the nuclei, cytoplasm, and cytomembranes of tumor cells and can be detected after their entry into the blood, such as CEA, CA19-9, and CA242[14]. CEA is present on the surface of cancer cells differentiated from endodermal cells, 90% of which are involved in cell adhesion in colorectal cancer. CEA expression is higher in malignant tumors than in normal tissues, thus playing a crucial role in the occurrence, development, and prognosis of colorectal, pancreatic, and liver cancers[15]. CA19-9 is a mucin-type glycoprotein antigen that is mainly isolated from colorectal cancer tissues at an early stage and can accelerate the distant metastasis of cancer cells by mediating the adhesion of cachexia cells to vascular endothelial cells[16]. CA72-4 is a high-molecular-weight glycoprotein antigen, and its expression is extremely low in healthy conditions; usually, < 6 U/mL in the serum. When cancer lesions occur in the digestive system of the body, the expression of CA72-4 increases rapidly and continues thereafter[17]. CA50 is a sialic acid ester and a protein that cannot be detected in normal tissues. With cell deterioration, increased activity of glycosylation enzymes, and changes in glycosyl structure on the cell surface, CA50 has been confirmed to be closely related to the occurrence and progress of the colorectal and pancreatic cancers[18]. CA242 belongs to the sialylated mucin lipid antigen family and can identify the sialylated sugars shed by malignant tumor cells of many organs into the serum[19]. CC occurrence, progression, and prognosis are closely associated with the immune status of the body. T lymphocytes, the main effector cells of cellular immunity, are pivotal in controlling the growth and reproduction of tumor cells[20]. CD3+ and CD4+ cells assist in humoral immunity by mediating immune responses and stimulating the anti-tumor effects of NK cells, cytotoxic T cells, and B lymphocytes. CD8+ cells are cytotoxic T cells with high killing activity that negatively regulate immune responses. NK cells are widely distributed in peripheral lymphocytes and blood and are congenital effector cells. They can help the body prevent the invasion of external viruses and bacteria and remove tumor cells to achieve innate immunity[21]. CD4+/CD25 cells exhibit an immunosuppressive effect mainly by secreting cytokines. These cells function as CD4+ and CD8+ T cells secreting their factors, which can inhibit the anti-tumor effect in vivo and assist the body in immune escape from malignant tumors. Notably, the expression and function of these cells help to maintain the normal immune ability of the body[22].
To explore the relationship between the above tumor factors, immune state-related indicators, and LM in patients with CC, the ROC curve was drawn in this study. The results indicated that the AUC of CEA, CA19-9, CA242, CA72-4, CA50, CD3+, CD4+, CD8+, NK, and CD4+/CD25 for the prediction of LM in patients with CC were all > 0.80, with a high prediction value. This indicates that these indicators could be used clinically for the assessment of LM in patients with CC. However, the sample size of this study was limited, and a retrospective analysis was performed. Further, limitations in the inclusion of relevant indicators also exist. Therefore, the credibility of the study needs to be verified using a larger sample size.
The expression of tumor factors and immune state-related indicators in patients with CC is closely associated with LM. Clinical intervention for indicators with abnormal expression can be analyzed in a timely manner by detecting the expression of tumor factors and immune state-related indicators to reduce the risk of LM in patients with CC.
Colon cancer (CC) has a high incidence. Liver metastasis (LM) easily occurs after radical resection of CC. The liver maintains a congenital adaptive immune system. Liver metastases after CC surgery significantly affect the patient’s prognosis. Before LM, tumor cells secrete cytokines and exosomes to regulate the liver immune microenvironment and form an inhibitory immune microenvironment for the colonization of circulating tumor cells. Therefore, the immune function in patients after CC surgery is associated with LM.
LM after CC surgery seriously affects patient prognosis, and identifying the risk factors that affect LM after CC surgery is crucial for its early prevention and improving patient prognosis.
To observe the expression of immune function factors in patients with LM of CC and explore their correlation with LM.
Analysis of variance and logistic regression analysis.
The expressions of serum carcinoembryonic antigen (CEA), CA19-9, CA242, CA72-4 and CA50 in patients in the occurrence group were significantly higher compared with the non-occurrence group, while the expression of CD3+, CD4+, CD8+, natural killer (NK) and CD4+/CD25 in patients in the occurrence group were significantly lower compared with the non-occurrence group (P < 0.05). Multivariate logistic regression indicated that the expressions of CEA, CA19-9, CA242, CA72-4, CA50, CD3+, CD4+, CD8+, NK, and CD4+/CD25 were associated with LM in patients with CC. High expressions of serum CEA, CA19-9, CA242, CA72-4 and CA50, and low expressions of CD3+, CD4+, CD8+, NK, and CD4+/CD25 in patients with CC were risk factors for LM (OR > 1, P < 0.05). The ROC curve indicated that the AUC of CEA, CA19-9, CA242, CA72-4, CA50, CD3+, CD4+, CD8+, NK, and CD4+/CD25 in the prediction of LM in patients with CC were all > 0.80, with a high predictive value.
The expression of tumor factors and immune state-related indices in patients with CC is closely associated with the occurrence of LM.
Postoperative LM in patients with CC is related to many factors, including the immune system, which plays a crucial role in the body's resistance to tumors. When the immune system is destroyed or becomes dysfunctional, the immune state of the body is disordered, leading to the occurrence of diseases caused by immune disorders, including LM. Therefore, a correlation between postoperative LM and immune function has been speculated in patients with CC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bhosale PB, South Korea S-Editor: Yan JP L-Editor: A P-Editor: Zhao S
| 1. | Christou N, Bergen ES, Canton C, Le Malicot K, Di Bartolomeo M, Galli F, Labianca R, Shi Q, Alberts SR, Goldberg RM, Lepage C, Sinicrope FA, Taieb J. Impact of diabetes and metformin use on recurrence and outcome in stage II-III colon cancer patients-A pooled analysis of three adjuvant trials. Eur J Cancer. 2022;166:100-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Taylor AS, Liu N, Fang JM, Panarelli N, Zhao L, Cheng J, Gopal P, Hammer S, Sun J, Appelman H, Westerhoff M. Cribriform colon cancer: a morphological growth pattern associated with extramural venous invasion, nodal metastases and microsatellite stability. J Clin Pathol. 2022;75:483-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Shaib WL, Zakka KM, Jiang R, Yan M, Alese OB, Akce M, Wu C, Behera M, El-Rayes BF. Survival outcome of adjuvant chemotherapy in deficient mismatch repair stage III colon cancer. Cancer. 2020;126:4136-4147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Stewart L, Indukuri VV, Charepalli V, Chrisfield BJ, Anantheswaran RC, Lambert JD, Vanamala JKP. Comparative effects of vacuum or conventional frying on the polyphenol chemistry and in vitro colon cancer stem cell inhibitory activity of purple-flesh potatoes. J Food Sci. 2022;87:3260-3267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Lin YZ, Cheng HH, Huang SC, Chang SC, Lan YT. Comparison of two-stage and three-stage surgery for obstructing left-sided colon cancer. ANZ J Surg. 2022;92:1466-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Jiang X, Cao G, Gao G, Wang W, Zhao J, Gao C. Triptolide decreases tumor-associated macrophages infiltration and M2 polarization to remodel colon cancer immune microenvironment via inhibiting tumor-derived CXCL12. J Cell Physiol. 2021;236:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Day GL, Bryan ML, Northrup SA, Lyles DS, Westcott MM, Stewart JH 4th. Immune Effects of M51R Vesicular Stomatitis Virus Treatment of Carcinomatosis From Colon Cancer. J Surg Res. 2020;245:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Youn B, Trikalinos NA, Mor V, Wilson IB, Dahabreh IJ. Real-world use and survival outcomes of immune checkpoint inhibitors in older adults with non-small cell lung cancer. Cancer. 2020;126:978-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 9. | National Health Commission of the People’s Republic of China. Chinese Protocol of Diagnosis and Treatment of Colorectal Cancer (2020 edition). Zhonghua Waike Zazhi. 2020;58:561-585. [RCA] [DOI] [Full Text] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 10. | Chinese College of Surgeons; Section of Gastrointestinal Surgery, Branch of Surgery, Chinese Medical Association; Section of Colorectal Surgery, Branch of Surgery, Chinese Medical Association; Colorectal Cancer Professional Committee, Chinese Anti⁃Cancer Association; Colorectal Cancer Professional Committee, Chinese Medical Doctor Association; Colorectal Cancer Expert Committee, Chinese Society of Clinical Oncology; Chinese Society of Colon & Rectal Surgeons, Chinese College of Surgeons, Chinese Medical Doctor Association; Metastasis Research Committee, Anorectal Branch of Chinese Medical Doctor Association; Section of Colorectal Oncology, Oncology Branch, Chinese Medical Association; Branch of Metastatic Tumor Therapy, China International Exchange and Promotive Association for Medical and Health Care; Branch of Colorectal Disease, China International Exchange and Promotive Association for Medical and Health Care. [China guideline for diagnosis and comprehensive treatment of colorectal liver metastases (version 2023)]. Zhonghua Wei Chang Wai Ke Za Zhi. 2023;26:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Lee H, Sha D, Foster NR, Shi Q, Alberts SR, Smyrk TC, Sinicrope FA. Analysis of tumor microenvironmental features to refine prognosis by T, N risk group in patients with stage III colon cancer (NCCTG N0147) (Alliance). Ann Oncol. 2020;31:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Liu Z, Xie Y, Xiong Y, Liu S, Qiu C, Zhu Z, Mao H, Yu M, Wang X. TLR 7/8 agonist reverses oxaliplatin resistance in colorectal cancer via directing the myeloid-derived suppressor cells to tumoricidal M1-macrophages. Cancer Lett. 2020;469:173-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 13. | Chow Z, Gan T, Chen Q, Huang B, Schoenberg N, Dignan M, Evers BM, Bhakta AS. Nonadherence to Standard of Care for Locally Advanced Colon Cancer as a Contributory Factor for High Mortality Rates in Kentucky. J Am Coll Surg. 2020;230:428-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Gillis C, Richer L, Fenton TR, Gramlich L, Keller H, Culos-Reed SN, Sajobi TT, Awasthi R, Carli F. Colorectal cancer patients with malnutrition suffer poor physical and mental health before surgery. Surgery. 2021;170:841-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Lu C, Klement JD, Yang D, Albers T, Lebedyeva IO, Waller JL, Liu K. SUV39H1 regulates human colon carcinoma apoptosis and cell cycle to promote tumor growth. Cancer Lett. 2020;476:87-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Zhao M, Wang Y, Jiang C, Wang Q, Mi J, Zhang Y, Zuo L, Geng Z, Song X, Ge S, Li J, Wen H, Wang J, Wang Z, Su F. miR-107 regulates the effect of MCM7 on the proliferation and apoptosis of colorectal cancer via the PAK2 pathway. Biochem Pharmacol. 2021;190:114610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Song B, Li H, Guo S, Yang T, Li L, Cao L, Wang J. LINC00882 Plays a Tumor-promoter Role in Colorectal Cancer by Targeting miR-3619-5p to Up-regulate CTNNB1. Arch Med Res. 2022;53:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 18. | Giannini R, Zucchelli G, Giordano M, Ugolini C, Moretto R, Ambryszewska K, Leonardi M, Sensi E, Morano F, Pietrantonio F, Cremolini C, Falcone A, Fontanini G. Immune Profiling of Deficient Mismatch Repair Colorectal Cancer Tumor Microenvironment Reveals Different Levels of Immune System Activation. J Mol Diagn. 2020;22:685-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Clarke N, Kearney PM, Gallagher P, McNamara D, O'Morain CA, Sharp L. Negative emotions and cancer fatalism are independently associated with uptake of Faecal Immunochemical Test-based colorectal cancer screening: Results from a population-based study. Prev Med. 2021;145:106430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Long J, He Q, Yin Y, Lei X, Li Z, Zhu W. The effect of miRNA and autophagy on colorectal cancer. Cell Prolif. 2020;53:e12900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 21. | Fischer J, Walker LC, Robinson BA, Frizelle FA, Church JM, Eglinton TW. Clinical implications of the genetics of sporadic colorectal cancer. ANZ J Surg. 2019;89:1224-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Granito A, Muratori L, Lalanne C, Quarneti C, Ferri S, Guidi M, Lenzi M, Muratori P. Hepatocellular carcinoma in viral and autoimmune liver diseases: Role of CD4+ CD25+ Foxp3+ regulatory T cells in the immune microenvironment. World J Gastroenterol. 2021;27:2994-3009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 122] [Cited by in RCA: 131] [Article Influence: 32.8] [Reference Citation Analysis (1)] |