Published online Feb 27, 2024. doi: 10.4240/wjgs.v16.i2.438
Peer-review started: October 15, 2023
First decision: December 6, 2023
Revised: December 18, 2023
Accepted: January 15, 2024
Article in press: January 15, 2024
Published online: February 27, 2024
Processing time: 133 Days and 10.9 Hours
The neutrophil-to-lymphocyte ratio (NLR), a composite inflammatory biomarker, is associated with the prognosis in patients with colorectal tumors. However, whether the NLR can be used as a predictor of symptomatic postoperative ana
To assess the role of the NLR in predicting the occurrence of symptomatic AL after surgery in elderly patients with colon cancer.
Data from elderly colon cancer patients who underwent elective radical colectomy with anastomosis at three centers between 2018 and 2022 were retrospectively analyzed. Receiver operating characteristic curve analysis was performed to determine the best predictive cutoff value for the NLR. Twenty-two covariates were matched using a 1:1 propensity score matching method, and univariate and multivariate logistic regression analyses were used to determine risk factors for the development of postoperative AL.
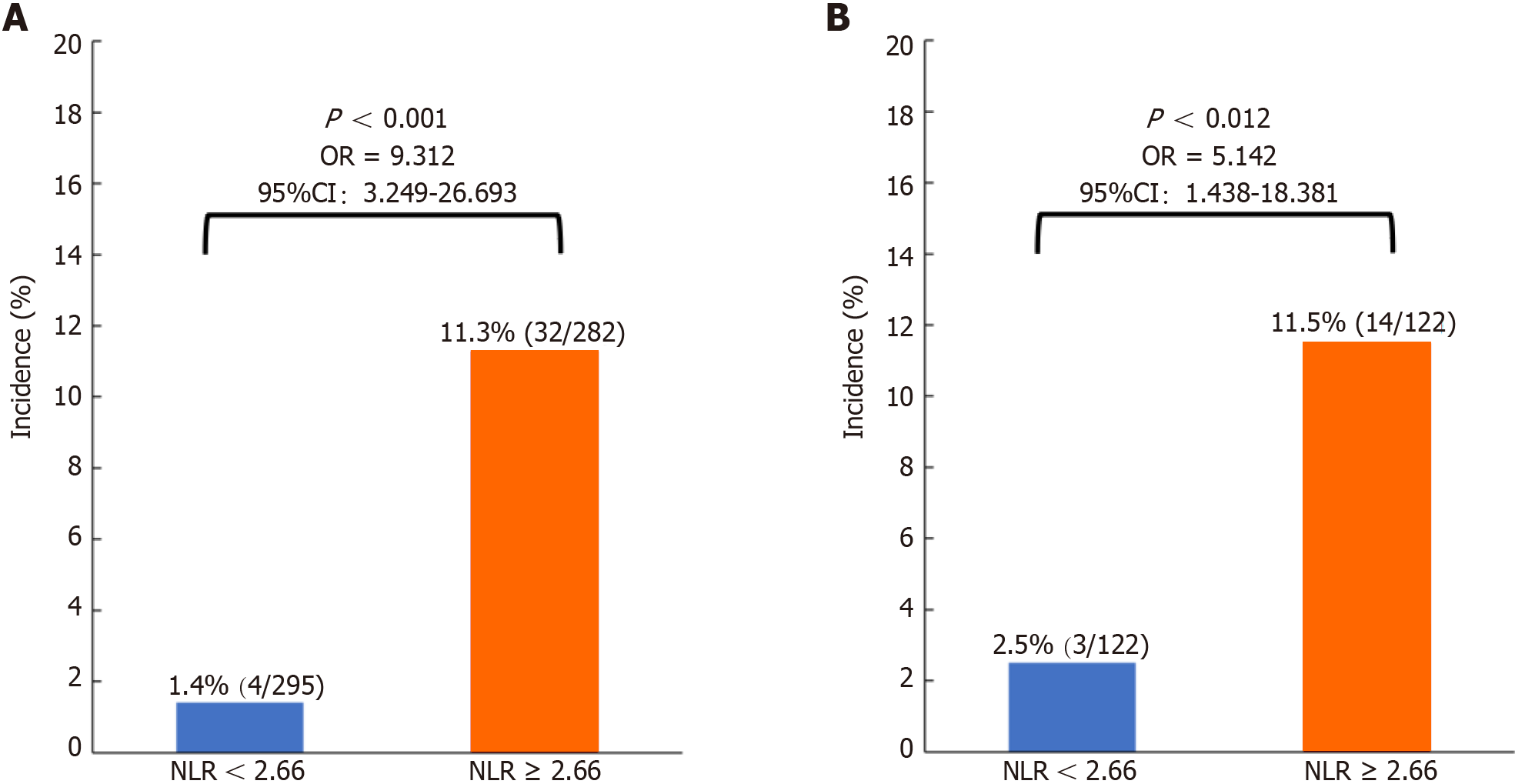
Of the 577 patients included, 36 (6.2%) had symptomatic AL. The optimal cutoff value of the NLR for predicting AL was 2.66. After propensity score matching, the incidence of AL was significantly greater in the ≥ 2.66 NLR subgroup than in the < 2.66 NLR subgroup (11.5% vs 2.5%; P = 0.012). Univariate logistic regression analysis revealed statistically significant correlations between blood transfusion intraoperatively and within 2 d postoperatively, preoperative albumin concentration, preoperative prognostic nutritional index, and preoperative NLR and AL occurrence (P < 0.05); multivariate logistic regression analysis revealed that an NLR ≥ 2.66 [odds ratio (OR) = 5.51; 95% confidence interval (CI): 1.50-20.26; P = 0.010] and blood transfusion intraoperatively and within 2 d postoperatively (OR = 2.52; 95%CI: 0.88-7.25; P = 0.049) were risk factors for the occurrence of symptomatic AL.
A preoperative NLR ≥ 2.66 and blood transfusion intraoperatively and within 2 d postoperatively are associated with a higher incidence of postoperative symptomatic AL in elderly patients with colon cancer. The preoperative NLR has predictive value for postoperative symptomatic AL after elective surgery in elderly patients with colon cancer.
Core Tip: The relationship between the preoperative neutrophil-to-lymphocyte ratio (NLR) and postoperative symptomatic anastomotic leakage (AL) in elderly patients with colon cancer was investigated in this study primarily. The findings showed that a higher preoperative NLR corresponded to a greater incidence of postoperative symptomatic AL. Moreover, in elderly patients with colon cancer after elective surgery, the preoperative NLR may serve as a predictor of postoperative symptomatic AL.
- Citation: Wang CY, Li XL, Ma XL, Yang XF, Liu YY, Yu YJ. Preoperative neutrophil-to-lymphocyte ratio predicts symptomatic anastomotic leakage in elderly colon cancer patients: Multicenter propensity score-matched analysis. World J Gastrointest Surg 2024; 16(2): 438-450
- URL: https://www.wjgnet.com/1948-9366/full/v16/i2/438.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i2.438
Older patients with colon cancer are becoming more common as the global elderly population continues to grow[1]. Increasing age also increases the risk of complications during and after colorectal surgery. Anastomotic leakage (AL) is a serious complication after colorectal surgery and may increase the risk of postoperative death in elderly patients[2] and severely affect the prognosis. Therefore, early prediction of the probability of AL occurrence and timely preventive measures are important for ensuring surgical safety and patient prognosis.
In recent years, various inflammatory response markers, such as the C-reactive protein-to-albumin ratio, platelet-to-lymphocyte ratio, and neutrophil-to-lymphocyte ratio (NLR), have been shown to be highly useful for predicting postoperative complications and prognosis in patients with colorectal cancer[3-5]. The effect of elevated peripheral blood NLRs on postoperative complications, cancer prognosis, and patient survival related to colorectal cancer has been confirmed by previous studies[6-9]. However, there are few data related to the role of the NLR in predicting the postoperative occurrence of AL in elderly patients with colon cancer. Therefore, in this study, we evaluated the role of the preoperative NLR as a predictive marker for symptomatic AL after radical resection in elderly patients with colon cancer.
Clinical data from elderly patients (≥ 65 years old) with primary colon cancer who underwent radical colon cancer surgery at the First Hospital of Lanzhou University, the Second Hospital of Lanzhou University, or Gansu Provincial People’s Hospital from January 2018 to December 2022 were retrospectively collected. The clinicopathologic characteristics of these patients included the following: Basic information including sex, age, body mass index (BMI), smoking status, history of abdominal surgery, nonsteroidal drug use, hypertension, diabetes mellitus, pulmonary disease, and neoadjuvant chemotherapy; surgical information including the American Society of Anesthesiologists (ASA) score, surgical approach (open/laparoscopic/robotic), anastomotic approach (end-to-end/end-to-lateral/lateral-to-lateral), and blood transfusion intraoperatively and within 2 d postoperatively; tumor pathological characteristics including tumor location, T-stage, N-stage, and tumor diameter; and serological examination including preoperative neutrophil count, preoperative lymphocyte count, preoperative hemoglobin, preoperative serum albumin (ALB) concentration, and carcinoembryonic antigen (CEA). The relevant preoperative serological indices were collected within 1 wk before surgery. The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Medical Ethics Committee of the First Hospital of Lanzhou University (leading center of the study, approval No. LDYYLL-2023-363). The requirement for informed consent was waived by the Medical Ethics Committee of the First Hospital of Lanzhou University due to the retrospective nature of the study and the absence of any risk.
The diagnosis of AL included three main aspects: Clinical symptoms, drainage fluid characteristics, and abdominal computed tomography (CT) scans. Patients with clinical symptoms (such as fever) and signs of peritonitis underwent CT for further diagnosis. AL was classified as asymptomatic (grade A) or symptomatic (grades B and C) based on severity and clinical signs. Symptomatic AL required aggressive therapeutic intervention (such as drainage and irrigation, antibiotics, and endoscopic surgery) or secondary surgical treatment and was diagnosed if one of the following manifestations was present: (1) Abnormal fluid such as pus and intestinal contents draining from the postoperative abdominal drainage tube; or (2) Fluid or gas collection around the anastomosis seen on abdominal CT or contrast enema. The follow-up for AL was within 15 d postoperatively. NLR was calculated as neutrophil count (109/L)/lymphocyte count (109/L), and prognostic nutritional index (PNI) was calculated as serum ALB (g/L) + 5 × lymphocyte count (109/L).
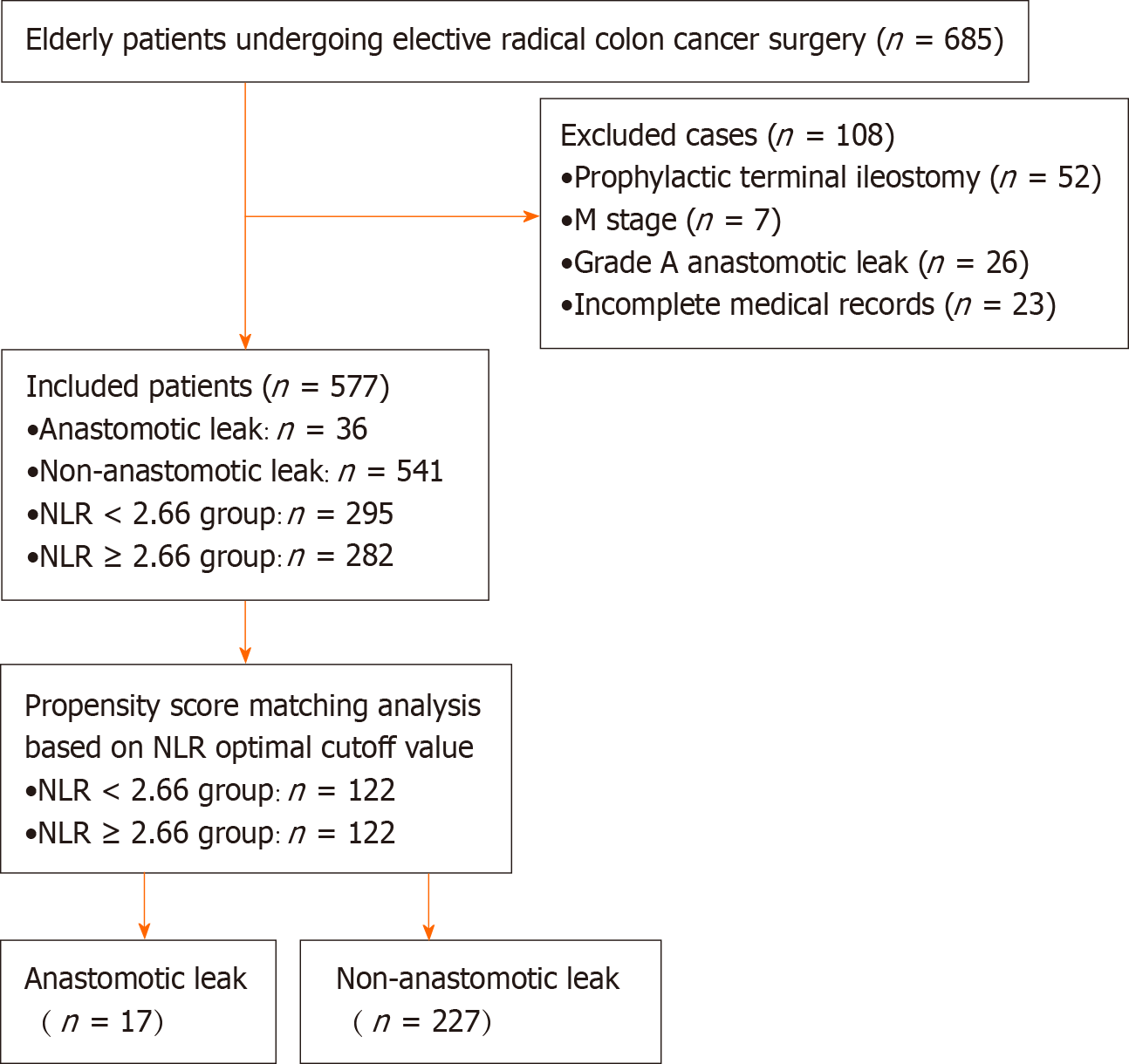
The inclusion criteria were as follows: ≥ 65 years of age, pathologically confirmed primary colon cancer, elective radical resection of colon cancer with one-stage anastomosis, and complete clinical and pathological data. The exclusion criteria were as follows: Patients with grade A AL without specific clinical management, patients with intraoperative prophylactic stoma, patients with postoperative pathologic results showing M1 stage, and patients with incomplete postope
A database was created in Excel, a propensity score-matched (PSM) analysis was performed with R-Studio (2023.03.1 + 446), and the data analysis was performed with SPSS 26.0 software, with the significance set at P < 0.05. In all cohorts, the normality of the continuous variables was assessed by the Shapiro-Wilcoxon test, and continuous variables with a normal distribution are presented as the mean ± SD. Continuous data not conforming to a normal distribution are presented as the median (interquartile range) and were further analyzed by the independent Student’s t test or Mann-Whitney U test. Categorical variables are expressed as frequencies (percentages), and comparisons were made using the χ2 test, Fisher’s exact test, or rank sum test. Receiver operating characteristic (ROC) curve analysis was used to identify the best critical value for the NLR prediction of AL.
A 1:1 PSM analysis was performed using the nearest neighbor method. The caliper value was set to 0.2 times the standard deviation of the propensity score. To reduce selection bias and confounding factors, logistic regression analysis was used to calculate propensity scores for age, sex, BMI, smoking history, abdominal surgery history, nonsteroidal drug use, hypertension, diabetes mellitus, lung disease, neoadjuvant chemotherapy, tumor location, ASA classification, surgical approach, anastomosis, blood transfusion intraoperatively and within 2 d postoperatively, T stage, N stage, tumor diameter, CEA, preoperative hemoglobin, preoperative ALB, and preoperative PNI. The standardized mean difference (SMD) was measured to determine the balance between the two groups before and after propensity score matching. An SMD of < 0.2 indicated that an adequate balance was achieved between the two groups. After matching, covariates with P < 0.05 in the univariate logistic regression analysis were entered into the multivariate logistic regression analysis to determine the risk factors for the occurrence of postoperative symptomatic AL.
The study population enrollment process is shown in Figure 1. A total of 577 patients were ultimately included in the present study, 36 of whom (6.2%) developed symptomatic AL. The basic, preoperative, intraoperative, and postoperative data of the patients are shown in Table 1. Sex, blood transfusion intraoperatively and within 2 d postoperatively, N stage, preoperative ALB level, preoperative NLR, and preoperative PNI were significantly different between the AL group and the group without AL (P < 0.05).
| Variable | All patients (n = 577) | Non-AL patients (n = 541) | AL patients (n = 36) | Statistical value | P value |
| Gender | χ2 = 6.189 | 0.013 | |||
| Female | 276 (47.8) | 266 (49.2) | 10 (27.8) | ||
| Male | 301 (52.2) | 275 (50.8) | 26 (72.2) | ||
| Age (yr) | 71.0 (10.0) | 71.0 (10.0) | 72.0 (8.5) | Z = -1.005 | 0.315 |
| BMI (kg/m2) | 22.81 ± 3.43 | 22.79 ± 3.44 | 23.16 ± 3.25 | t = -0.627 | 0.531 |
| Smoking | χ2 = 0.866 | 0.352 | |||
| No | 393 (68.1) | 371 (68.6) | 22 (61.1) | ||
| Yes | 184 (31.9) | 170 (31.4) | 14 (38.9) | ||
| History of abdominal surgery | χ2 = 0.086 | 0.770 | |||
| No | 413 (71.6) | 388 (71.7) | 25 (69.4) | ||
| Yes | 164 (28.4) | 153 (28.3) | 11 (30.6) | ||
| Nonsteroidal drug use | χ2 = 0.000 | 1.000 | |||
| No | 524 (90.8) | 491 (90.8) | 33 (91.7) | ||
| Yes | 53 (9.2) | 50 (9.2) | 3 (8.3) | ||
| Lung disease | χ2 = 0.061 | 0.804 | |||
| No | 316 (54.8) | 297 (54.9) | 19 (52.8) | ||
| Yes | 261 (45.2) | 244 (45.1) | 17 (47.2) | ||
| Hypertension | χ2 = 2.362 | 0.124 | |||
| No | 381 (66.0) | 353 (65.3) | 28 (77.8) | ||
| Yes | 196 (34.0) | 188 (34.7) | 8 (22.2) | ||
| Diabetes mellitus | χ2 = 1.203 | 0.273 | |||
| No | 486 (84.2) | 458 (84.7) | 28 (77.8) | ||
| Yes | 91 (15.8) | 83 (15.3) | 8 (22.2) | ||
| Neoadjuvant chemotherapy | -1 | 0.124 | |||
| No | 567 (98.3) | 533 (98.5) | 34 (94.4) | ||
| Yes | 10 (1.7) | 8 (1.5) | 2 (5.6) | ||
| Tumor location | -1 | 0.422 | |||
| Right hemicolon | 276 (47.8) | 261 (48.2) | 15 (41.7) | ||
| Transverse colon | 29 (5.0) | 28 (5.2) | 1 (2.8) | ||
| Left hemicolon | 64 (11.1) | 57 (10.5) | 7 (19.4) | ||
| Sigmoid colon | 208 (36.1) | 195 (36.1) | 13 (36.1) | ||
| ASA classification | χ2 = 1.389 | 0.499 | |||
| 1 | 9 (1.6) | 9 (1.7) | 0 (0.0) | ||
| 2 | 429 (74.4) | 404 (74.7) | 25 (69.4) | ||
| 3 | 139 (24.0) | 128 (23.6) | 11 (30.6) | ||
| Surgical approach | χ2 = 4.110 | 0.128 | |||
| Open | 171 (29.6) | 155 (28.7) | 16 (44.4) | ||
| Laparoscopic | 354 (61.4) | 337 (62.2) | 17 (47.2) | ||
| Robotic | 52 (9.0) | 49 (9.1) | 3 (8.4) | ||
| Anastomosis | χ2 = 0.055 | 0.973 | |||
| End-to-end anastomosis | 198 (34.3) | 185 (34.2) | 13 (36.1) | ||
| End-lateral anastomosis | 248 (43.0) | 233 (43.1) | 15 (41.7) | ||
| Lateral anastomosis | 131 (22.7) | 123 (22.7) | 8 (22.2) | ||
| Blood transfusion intraoperatively and within 2 d postoperatively | χ2 = 20.869 | < 0.001 | |||
| No | 427 (74.0) | 412 (76.2) | 15 (41.7) | ||
| Yes | 150 (26.0) | 129 (23.8) | 21 (58.3) | ||
| T stage | -1 | 0.444 | |||
| 1 | 36 (6.2) | 36 (6.7) | 0 (0.0) | ||
| 2 | 60 (10.4) | 57 (10.5) | 3 (8.3) | ||
| 3 | 371 (64.3) | 346 (64.0) | 25 (69.4) | ||
| 4 | 110 (19.1) | 102 (18.8) | 8 (22.3) | ||
| N stage | χ2 = 7.778 | 0.020 | |||
| 0 | 355 (61.5) | 340 (62.9) | 15 (41.7) | ||
| 1 | 138 (23.9) | 127 (23.5) | 11 (30.6) | ||
| 2 | 84 (14.6) | 74 (13.7) | 9 (27.7) | ||
| Tumor diameter (cm) | 4.0 (3.0) | 4.0 (3.0) | 4.75 (2.6) | Z = -0.660 | 0.509 |
| CEA (ng/mL) | 3.78 (5.99) | 3.66 (5.82) | 5.11 (10.02) | Z = -1.468 | 0.142 |
| Preoperative hemoglobin (g/L) | 119.0 (40.0) | 119.0 (40.0) | 118.5 (32.75) | Z = -0.476 | 0.634 |
| Preoperative albumin (g/L) | 38.23 ± 4.69 | 38.40 ± 4.69 | 35.68 ± 3.81 | t = 4.076 | < 0.001 |
| Preoperative NLR | 2.62 (1.93) | 2.55 (1.91) | 3.36 (1.70) | Z = -3.688 | < 0.001 |
| Preoperative PNI | 45.58 ± 5.94 | 45.86 ± 5.95 | 41.41 ± 3.94 | t = 6.325 | < 0.001 |
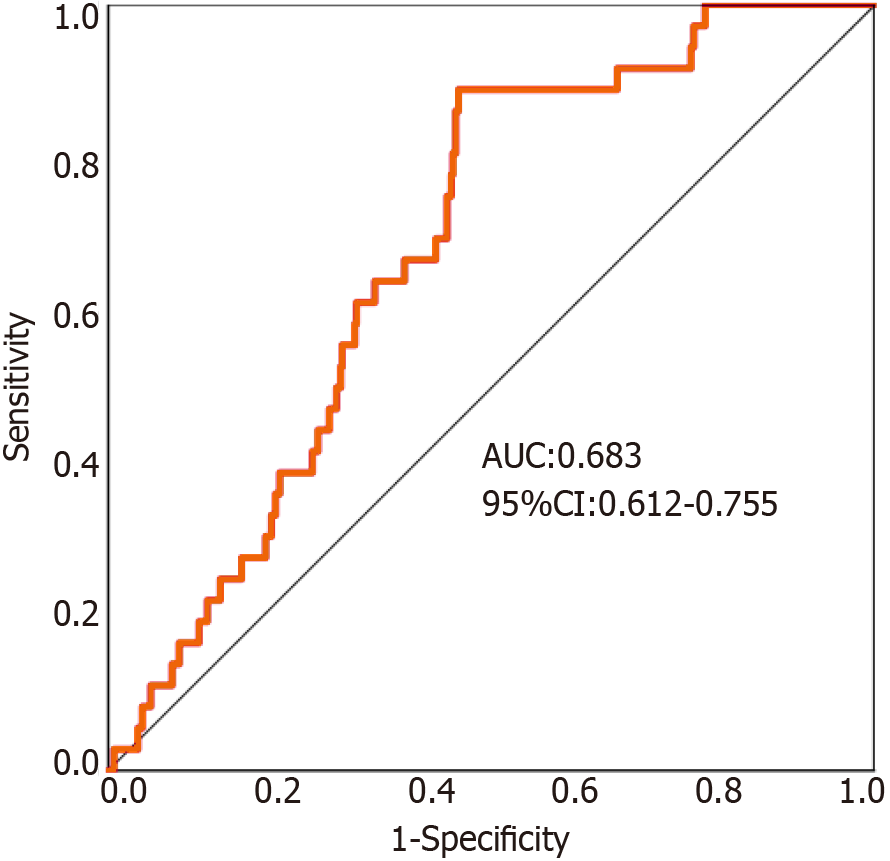
The continuous variable NLR was used as the test variable, and the dichotomous variable AL was used as the outcome variable. ROC curve analysis revealed that the area under the curve for the preoperative NLR was 0.683 [95% confidence interval (CI): 0.612-0.755]. For all patients, the sensitivity (0.861), specificity (0.543), and Youden index (0.405) of the NLR in predicting the occurrence of postoperative symptomatic AL in elderly patients undergoing radical resection for colon cancer were highest when the NLR was 2.66 (Figure 2).
Of the 577 patients, 295 (51.1%) had an NLR < 2.66, and 282 (48.9%) had an NLR ≥ 2.66 (Table 2). After 1:1 PSM, 122 matched pairs were generated, and patients were divided into NLR < 2.66 (n = 122) and NLR ≥ 2.66 (n = 122) groups (Table 2). All covariates were well balanced with an SMD less than 0.2, and the difference between the NLR < 2.66 and NLR ≥ 2.66 groups was not statistically significant (Table 2). Before (1.4% vs 11.3%, P < 0.001) (Figure 3A) and after (2.5% vs 11.5%, P = 0.012) (Figure 3B) PSM, the incidence of symptomatic AL was significantly lower in the NLR < 2.66 subgroup than in the NLR ≥ 2.66 subgroup.
| Variable | Before propensity score matching | After propensity score matching | ||||||
| NLR < 2.66 (n = 295) | NLR ≥ 2.66 (n = 282) | SMD | P value | NLR < 2.66 (n = 122) | NLR ≥ 2.66 (n = 122) | SMD | P value | |
| Gender | 0.237 | 0.005 | 0.131 | 0.305 | ||||
| Female | 158 (53.6) | 118 (41.8) | 55 (45.1) | 63 (51.6) | ||||
| Male | 137 (46.4) | 164 (58.2) | 67 (54.9) | 59 (48.4) | ||||
| Age (yr) | 70.0 (9.0) | 72.0 (9.0) | 0.232 | 0.004 | 70.0 (9.8) | 71.0 (7.8) | 0.032 | 0.722 |
| BMI (kg/m2) | 23.20 ± 3.49 | 22.40 ± 3.31 | 0.241 | 0.005 | 22.51 ± 3.20 | 22.67 ± 3.08 | 0.052 | 0.689 |
| Smoking | 0.230 | 0.004 | 0.119 | 0.118 | ||||
| No | 217 (73.6) | 176 (62.4) | 82 (67.2) | 93 (76.2) | ||||
| Yes | 78 (26.4) | 106 (37.6) | 40 (32.8) | 29 (23.8) | ||||
| History of abdominal surgery | 0.065 | 0.443 | 0.076 | 0.560 | ||||
| No | 207 (70.2) | 206 (73.1) | 88 (72.1) | 92 (75.4) | ||||
| Yes | 88 (29.8) | 76 (26.9) | 34 (27.9) | 30 (24.6) | ||||
| Nonsteroidal drug use | 0.022 | 0.795 | 0.063 | 0.641 | ||||
| No | 267 (90.5) | 257 (91.1) | 111 (91.0) | 113 (92.6) | ||||
| Yes | 28 (9.5) | 25 (8.9) | 11 (9.0) | 9 (7.4) | ||||
| Lung disease | 0.214 | 0.010 | 0.117 | 0.364 | ||||
| No | 177 (60.0) | 139 (49.3) | 67 (54.9) | 74 (60.7) | ||||
| Yes | 118 (40.0) | 143 (50.7) | 55 (45.1) | 48 (39.3) | ||||
| Hypertension | 0.047 | 0.573 | 0.018 | 0.891 | ||||
| No | 198 (67.1) | 183 (64.9) | 82 (67.2) | 83 (68.0) | ||||
| Yes | 97 (32.9) | 99 (35.1) | 40 (32.8) | 39 (32.0) | ||||
| Diabetes mellitus | 0.100 | 0.207 | 0.022 | 0.864 | ||||
| No | 254 (86.1) | 232 (82.3) | 101 (82.8) | 102 (83.6) | ||||
| Yes | 41 (13.9) | 50 (17.7) | 21 (17.2) | 20 (16.4) | ||||
| Neoadjuvant chemotherapy | 0.128 | 0.376 | 0.065 | 1.000 | ||||
| No | 288 (97.6) | 279 (98.9) | 119 (97.5) | 120 (98.4) | ||||
| Yes | 7 (2.4) | 3 (1.1) | 3 (2.5) | 2 (1.6) | ||||
| Tumor location | 0.210 | < 0.001 | 0.045 | 0.211 | ||||
| Right hemicolon | 126 (42.7) | 150 (53.2) | 59 (48.4) | 59 (48.4) | ||||
| Transverse colon | 8 (2.7) | 21 (7.5) | 2 (1.6) | 8 (6.6) | ||||
| Left hemicolon | 30 (10.2) | 34 (12.1) | 17 (13.9) | 19 (15.5) | ||||
| Sigmoid colon | 131 (44.4) | 77 (27.2) | 44 (36.1) | 36 (29.5) | ||||
| ASA classification | 0.118 | 0.147 | 0.096 | 0.272 | ||||
| 1 | 6 (2.0) | 3 (1.1) | 0 (0.0) | 3 (2.5) | ||||
| 2 | 227 (77.0) | 202 (71.6) | 98 (80.3) | 93 (76.2) | ||||
| 3 | 62 (21.0) | 77 (27.3) | 24 (19.7) | 26 (21.3) | ||||
| Surgical approach | 0.279 | < 0.001 | 0.099 | 0.330 | ||||
| Open | 65 (22.0) | 106 (37.6) | 37 (30.3) | 36 (29.5) | ||||
| Laparoscopic | 201 (68.2) | 153 (54.3) | 76 (62.3) | 70 (57.4) | ||||
| Robotic | 29 (9.8) | 23 (8.1) | 9 (7.4) | 16 (13.1) | ||||
| Anastomosis | 0.194 | 0.078 | 0.091 | 0.786 | ||||
| End-to-end anastomosis | 114 (38.6) | 84 (29.8) | 40 (32.8) | 35 (28.7) | ||||
| End-lateral anastomosis | 117 (39.7) | 131 (46.5) | 52 (42.6) | 55 (45.1) | ||||
| Lateral anastomosis | 64 (21.7) | 67 (23.7) | 30 (24.6) | 32 (26.2) | ||||
| Blood transfusion intraoperatively and within 2 d postoperatively | 0.318 | < 0.001 | 0.000 | 1.000 | ||||
| No | 240 (81.3) | 187 (66.3) | 91 (74.6) | 91 (74.6) | ||||
| Yes | 55 (18.7) | 95 (33.7) | 31 (25.4) | 31 (25.4) | ||||
| T stage | 0.339 | < 0.001 | 0.066 | 0.944 | ||||
| 1 | 27 (9.2) | 9 (3.2) | 6 (4.9) | 8 (6.6) | ||||
| 2 | 36 (12.2) | 24 (8.5) | 11 (9.0) | 12 (9.8) | ||||
| 3 | 197 (66.8) | 174 (61.7) | 85 (69.7) | 82 (67.2) | ||||
| 4 | 35 (11.8) | 75 (26.6) | 20 (16.4) | 20 (16.4) | ||||
| N stage | 0.147 | 0.191 | 0.116 | 0.655 | ||||
| 0 | 192 (65.1) | 163 (57.8) | 75 (61.5) | 68 (55.7) | ||||
| 1 | 63 (21.4) | 75 (26.6) | 29 (23.8) | 34 (27.9) | ||||
| 2 | 40 (13.5) | 44 (15.6) | 18 (14.7) | 20 (16.4) | ||||
| Tumor diameter (cm) | 4.00 (2.00) | 5.00 (2.50) | 0.427 | < 0.001 | 4.00 (3.0) | 4.75 (3.0) | 0.051 | 0.824 |
| CEA (ng/mL) | 3.33 (4.74) | 4.32 (6.97) | 0.107 | 0.008 | 3.52 (5.57) | 4.21 (5.59) | 0.118 | 0.560 |
| Preoperative hemoglobin (g/L) | 126.00 (39.00) | 109.50 (37.00) | 0.332 | < 0.001 | 119.5 (43.5) | 117.5 (43.5) | 0.035 | 0.711 |
| Preoperative albumin (g/L) | 39.00 ± 4.16 | 37.42 ± 5.06 | 0.311 | < 0.001 | 38.34 ± 4.29 | 38.29 ± 4.77 | 0.009 | 0.944 |
| Preoperative PNI | 48.02 ± 5.22 | 43.04 ± 5.58 | 0.892 | < 0.001 | 45.31 ± 4.99 | 45.16 ± 5.26 | 0.030 | 0.812 |
According to the univariate logistic regression analysis, blood transfusion intraoperatively and within 2 d postoperatively, preoperative ALB concentration, preoperative PNI, and preoperative NLR were associated with the occurrence of postoperative AL (Table 3). Multivariate logistic regression analysis revealed that intraoperative blood transfusion and postoperative blood transfusion within 2 d [odds ratio (OR) = 2.52; 95%CI: 0.88-7.25; P = 0.049] and a high NLR (≥ 2.66) (OR = 5.51; 95%CI: 1.50-20.26; P = 0.010) were significantly associated with the occurrence of symptomatic AL after radical resection for colon cancer in elderly individuals.
| Variable | Univariate analysis | Multivariate analysis | |||
| OR (95%CI) | P value | OR (95%CI) | P value | ||
| Gender | |||||
| Female1 | |||||
| Male | 1.37 (0.50-3.72) | 0.540 | |||
| Age (yr) | 1.00 (0.91-1.09) | 0.949 | |||
| BMI (kg/m2) | 0.98 (0.84-1.15) | 0.812 | |||
| Smoking | |||||
| No1 | |||||
| Yes | 0.77 (0.24-2.44) | 0.653 | |||
| History of abdominal surgery | |||||
| No1 | |||||
| Yes | 1.19 (0.40-3.51) | 0.757 | |||
| Nonsteroidal drug use | |||||
| No1 | |||||
| Yes | 1.55 (0.33-7.31) | 0.581 | |||
| Lung disease | |||||
| No1 | |||||
| Yes | 0.73 (0.26-2.05) | 0.551 | |||
| Hypertension | |||||
| No1 | |||||
| Yes | 0.86 (0.29-2.54) | 0.787 | |||
| Diabetes mellitus | |||||
| No1 | |||||
| Yes | 1.07 (0.29-3.89) | 0.923 | |||
| Neoadjuvant chemotherapy | |||||
| No1 | |||||
| Yes | 3.48 (0.37-33.03) | 0.277 | |||
| Tumor location | |||||
| Right hemicolon1 | |||||
| Transverse colon | 1.53 (0.17-13.61) | 0.704 | |||
| Left hemicolon | 1.25 (0.31-4.98) | 0.752 | |||
| Sigmoid colon | 0.92 (0.29-2.91) | 0.883 | |||
| ASA classification | |||||
| < 21 | |||||
| 3 | 1.211 (0.377-3.887) | 0.748 | |||
| Surgical approach | |||||
| Open1 | |||||
| Laparoscopic | 0.65 (0.22-1.94) | 0.438 | |||
| Robotic | 1.52 (0.35-6.60) | 0.574 | |||
| Anastomosis | |||||
| End-to-end anastomosis1 | |||||
| End-lateral anastomosis | 1.43 (0.42-4.95) | 0.568 | |||
| Lateral anastomosis | 1.56 (0.40-6.07) | 0.523 | |||
| Blood transfusion intraoperatively and within 2 d postoperatively | |||||
| No1 | |||||
| Yes | 2.85 (1.05-7.74) | 0.040 | 2.52 (0.88-7.25) | 0.049 | |
| T stage | |||||
| ≤ 21 | |||||
| ≥ 3 | 0.822 (0.224-3.014) | 0.768 | |||
| N stage | |||||
| 01 | |||||
| 1 | 1.28 (0.41-4.00) | 0.667 | |||
| 2 | 1.28 (0.33-4.97) | 0.725 | |||
| Tumor diameter (cm) | 1.01 (0.81-1.26) | 0.931 | |||
| CEA (ng/mL) | 1.00 (0.99-1.01) | 0.636 | |||
| Preoperative hemoglobin (g/L) | 1.01 (0.99-1.03) | 0.327 | |||
| Preoperative albumin (g/L) | 0.85 (0.75-0.97) | 0.013 | 0.99 (0.73-1.35) | 0.946 | |
| Preoperative PNI | 0.86 (0.76-0.96) | 0.010 | 0.88 (0.66-1.17) | 0.380 | |
| Preoperative NLR | |||||
| NLR < 2.661 | |||||
| NLR ≥ 2.66 | 5.14 (1.44-18.38) | 0.012 | 5.51 (1.50-20.26) | 0.010 | |
The main finding of this study was that the incidence of postoperative symptomatic AL in 577 elderly patients who underwent radical colon cancer resection was 6.2%. The optimal cutoff value of the preoperative NLR for predicting symptomatic AL after radical colon cancer resection was determined by the ROC curve to be 2.66. After PSM, the incidence of AL was significantly greater in the ≥ 2.66 NLR subgroup than in the < 2.66 NLR subgroup, and a preope
When inflammation and infection occur, neutrophils rapidly migrate to the site of inflammation, responding first to tissue damage and playing a key role in the host’s resistance to infection[10]. Elevated peripheral blood neutrophil levels are characteristic of pathogenesis and are a hallmark of the inflammatory response. However, persistent neutrophilic infiltration is a hallmark of chronic inflammation and leads to tissue damage[11]. Furthermore, in one study, the number of lymphocytes involved in the immune state decreased with the progression of inflammatory disease, and this increase in lymphocyte count was a cause of decreased cellular immune function[12]. That is, cell-mediated immune responses are strongly dependent on lymphocytes. The NLR is an easily accessible marker identified as a good indicator of systemic inflammatory status in the general population[13]. The NLR has a better ability to predict systemic inflammation and prognosis than does the leukocyte, neutrophil, or lymphocyte count alone[14,15].
Previous studies have suggested that the post-colorectal AL may be associated with the NLR. First, Josse et al[16] reported that a preoperative NLR ≥ 2.3 was significantly associated with postoperative complication rates and that the incidence of AL increased non-significantly. Subsequently, Miyakita et al[17] showed that a preoperative NLR ≥ 2.21 was significantly associated with postoperative AL in patients with rectal cancer (OR = 4.51; P = 0.0329). In addition, Paliogiannis et al[9] showed that a higher NLR was associated with the occurrence of postoperative AL in patients undergoing elective colorectal surgery. According to our findings, high preoperative NLRs are a risk factor for the development of symptomatic AL after radical resection in elderly patients with colon cancer. The OR for the occurrence of symptomatic AL in the high NLR subgroup (≥ 2.66) was 5.51, indicating that the preoperative NLR may help to predict the risk of postoperative symptomatic AL in elderly patients with colon cancer before surgery, which could allow patients to take effective preventive measures. However, the predictive role of inflammatory indicators should be validated in larger clinical studies. In particular, for more effective prevention of AL occurrence, prevention methods should be explored through interventional prospective studies with risk stratification based on preoperative NLR values.
This study revealed that blood transfusion intraoperatively and within 2 d postoperatively was associated with the occurrence of postoperative symptomatic AL, which is consistent with previous findings[18,19]. Most of the transfusions were due to excessive surgical blood loss or severe anemia. Hemoglobin is associated with blood flow and oxygenation at the anastomotic margin and is an important factor in anastomotic healing. However, blood transfusion may lead to impaired blood rheology[20], which adversely affects microcirculation; transfusion may also induce immunosuppression[21], which increases the risk of infectious diseases around the anastomosis. Therefore, careful blood transfusion intraoperatively and within 2 d postoperatively may be an intervention to reduce the occurrence of postoperative AL in elderly patients undergoing radical colon cancer resection.
This study has several limitations. First, because this study was retrospective, we could not assess all the covariates that might have influenced the analysis. Therefore, our study may be affected to some extent by unavoidable selection bias. However, we performed a PSM analysis to minimize bias. Second, preoperative NLR values may be influenced by patients’ infectious comorbidities, and there is no standard optimal cutoff value for the NLR. Therefore, these findings should be interpreted with caution. Third, further studies are needed to validate the relationship between the preoperative NLR and other postsurgical symptomatic ALs, as these parameters have not been specifically evaluated.
Symptomatic AL is associated with a high preoperative NLR and blood transfusion intraoperatively and within 2 d postoperatively. In elderly patients who undergo radical colon cancer resection, a preoperative NLR ≥ 2.66 is significantly associated with the likelihood of postoperative symptomatic AL occurrence. We recommend the preoperative use of the NLR as a predictive marker for the risk of AL for better preoperative evaluation and selection of the best surgical and care plan for elderly patients undergoing elective radical colon cancer resection.
The neutrophil-to-lymphocyte ratio (NLR) is a complex inflammatory biomarker that is associated with prognosis in patients with colorectal tumors. However, it is unclear whether NLR can be used as a predictor of postoperative symptomatic anastomotic leakage (AL) in elderly colon cancer patients.
The discovery of biomarkers able to predict AL early after colorectal surgery would bring consistent advantages in the management and outcomes of this complication. NLR is a low-cost, easy-to-perform, and widely available index. Here, we aimed to investigate the NLR as an early available predictive marker for AL.
To assess the role of preoperative NLR in predicting the development of symptomatic AL after surgery in elderly patients with colon cancer by using propensity score matched (PSM) analysis.
We used a retrospective analysis to examine data from elderly colon cancer patients admitted between January 2018 and December 2022 at three large medical centers. The best predictive cutoff value for NLR was determined using the receiver operating characteristic curve. All covariates were matched using a 1:1 PSM method, and finally, all variables were analyzed using univariate and multivariate logistic regression analyses to determine the correlation between NLR and the occurrence of postoperative AL and other associated risk factors.
Among 577 patients, 36 (6.2%) experienced symptomatic AL. The optimal NLR cutoff for predicting AL was 2.66. After propensity score matching, the incidence of AL was significantly higher in the NLR ≥ 2.66 subgroup compared to the NLR < 2.66 subgroup (11.5% vs 2.5%; P = 0.012). Univariate logistic regression analysis showed significant differences in blood transfusion intraoperatively and within 2 d postoperatively, preoperative albumin concentration, preoperative prognostic nutritional index, and preoperative NLR regarding AL occurrence (P < 0.05); multivariate logistic regression analysis identified NLR ≥ 2.66 [odds ratio (OR) = 5.51; 95% confidence interval (CI): 1.50-20.26; P = 0.010] and blood transfusion intraoperatively and within 2 d postoperatively (OR = 2.52; 95%CI: 0.88-7.25; P = 0.049) as risk factors for symptomatic AL occurrence.
High preoperative NLR (≥ 2.66) and intraoperative, as well as postoperative (within 2 d), blood transfusions are associated with increased postoperative symptomatic AL in elderly colon cancer patients. Preoperative NLR serves as a predictor for postoperative symptomatic AL after elective surgery for elderly colon cancer patients.
In the future, we plan to further confirm the clinical applicability of NLR using a prospective randomized controlled trial approach.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jamali R, Iran S-Editor: Wang JJ L-Editor: Wang TQ P-Editor:Zhao YQ
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64628] [Article Influence: 16157.0] [Reference Citation Analysis (176)] |
| 2. | Foppa C, Ng SC, Montorsi M, Spinelli A. Anastomotic leak in colorectal cancer patients: New insights and perspectives. Eur J Surg Oncol. 2020;46:943-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 3. | Liao CK, Yu YL, Lin YC, Hsu YJ, Chern YJ, Chiang JM, You JF. Prognostic value of the C-reactive protein to albumin ratio in colorectal cancer: an updated systematic review and meta-analysis. World J Surg Oncol. 2021;19:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 4. | Chen W, Xin S, Xu B. Value Research of NLR, PLR, and RDW in Prognostic Assessment of Patients with Colorectal Cancer. J Healthc Eng. 2022;2022:7971415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | Xia LJ, Li W, Zhai JC, Yan CW, Chen JB, Yang H. Significance of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio and prognostic nutritional index for predicting clinical outcomes in T1-2 rectal cancer. BMC Cancer. 2020;20:208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 6. | Zhao X, Zhou Y, Liu B, Shen Y, Qian J, Zhang X, Zhao H. Preoperative Neutrophil-Lymphocyte Ratio (NLR)-Binding Fibrinogen-Albumin Ratio (FAR) Is Superior to Platelet-Lymphocyte Ratio (PLR)-Binding Fibrinogen-Albumin Ratio (FAR) and Lymphocyte-Monocyte (LMR)-Binding Fibrinogen-Albumin Ratio (FAR) as Predictors of Survival in Surgical Patients with Colorectal Adenocarcinoma. Med Sci Monit. 2023;29:e939442. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Mazaki J, Katsumata K, Kasahara K, Tago T, Wada T, Kuwabara H, Enomoto M, Ishizaki T, Nagakawa Y, Tsuchida A. Neutrophil-to-lymphocyte ratio is a prognostic factor for colon cancer: a propensity score analysis. BMC Cancer. 2020;20:922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 8. | Tan F, Xu K, Qi X, Gao P, Liu M, Yao Z, Zhang N, Yang H, Zhang C, Xing J, Cui M, Su X. Neutrophil-to-Lymphocyte Ratio as an Early Predictor of Symptomatic Anastomotic Leakage in Patients after Rectal Cancer Surgery: A Propensity Score-Matched Analysis. J Pers Med. 2022;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Paliogiannis P, Deidda S, Maslyankov S, Paycheva T, Farag A, Mashhour A, Misiakos E, Papakonstantinou D, Mik M, Losinska J, Scognamillo F, Sanna F, Feo CF, Cherchi G, Xidas A, Zinellu A, Restivo A, Zorcolo L. Blood cell count indexes as predictors of anastomotic leakage in elective colorectal surgery: a multicenter study on 1432 patients. World J Surg Oncol. 2020;18:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Fine N, Tasevski N, McCulloch CA, Tenenbaum HC, Glogauer M. The Neutrophil: Constant Defender and First Responder. Front Immunol. 2020;11:571085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 11. | Oliveira-Costa KM, Menezes GB, Paula Neto HA. Neutrophil accumulation within tissues: A damage x healing dichotomy. Biomed Pharmacother. 2022;145:112422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Bergmann CB, Beckmann N, Salyer CE, Crisologo PA, Nomellini V, Caldwell CC. Lymphocyte Immunosuppression and Dysfunction Contributing to Persistent Inflammation, Immunosuppression, and Catabolism Syndrome (PICS). Shock. 2021;55:723-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Imtiaz F, Shafique K, Mirza SS, Ayoob Z, Vart P, Rao S. Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int Arch Med. 2012;5:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 391] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 14. | Kumarasamy C, Sabarimurugan S, Madurantakam RM, Lakhotiya K, Samiappan S, Baxi S, Nachimuthu R, Gothandam KM, Jayaraj R. Prognostic significance of blood inflammatory biomarkers NLR, PLR, and LMR in cancer-A protocol for systematic review and meta-analysis. Medicine (Baltimore). 2019;98:e14834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 15. | Bhat T, Teli S, Rijal J, Bhat H, Raza M, Khoueiry G, Meghani M, Akhtar M, Costantino T. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. 2013;11:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 485] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 16. | Josse JM, Cleghorn MC, Ramji KM, Jiang H, Elnahas A, Jackson TD, Okrainec A, Quereshy FA. The neutrophil-to-lymphocyte ratio predicts major perioperative complications in patients undergoing colorectal surgery. Colorectal Dis. 2016;18:O236-O242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Miyakita H, Sadahiro S, Saito G, Okada K, Tanaka A, Suzuki T. Risk scores as useful predictors of perioperative complications in patients with rectal cancer who received radical surgery. Int J Clin Oncol. 2017;22:324-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Marinello FG, Baguena G, Lucas E, Frasson M, Hervás D, Flor-Lorente B, Esclapez P, Espí A, García-Granero E. Anastomotic leakage after colon cancer resection: does the individual surgeon matter? Colorectal Dis. 2016;18:562-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Kang J, Kim H, Park H, Lee B, Lee KY. Risk factors and economic burden of postoperative anastomotic leakage related events in patients who underwent surgeries for colorectal cancer. PLoS One. 2022;17:e0267950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Nagababu E, Scott AV, Johnson DJ, Dwyer IM, Lipsitz JA, Barodka VM, Berkowitz DE, Frank SM. Oxidative stress and rheologic properties of stored red blood cells before and after transfusion to surgical patients. Transfusion. 2016;56:1101-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Fragkou PC, Torrance HD, Pearse RM, Ackland GL, Prowle JR, Owen HC, Hinds CJ, O'Dwyer MJ. Perioperative blood transfusion is associated with a gene transcription profile characteristic of immunosuppression: a prospective cohort study. Crit Care. 2014;18:541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |