Published online Feb 27, 2024. doi: 10.4240/wjgs.v16.i2.289
Peer-review started: December 1, 2023
First decision: December 7, 2023
Revised: December 19, 2023
Accepted: January 15, 2024
Article in press: January 15, 2024
Published online: February 27, 2024
Processing time: 86 Days and 8.9 Hours
Phospholipase A2 (PLA2) enzymes are pivotal in various biological processes, such as lipid mediator production, membrane remodeling, bioenergetics, and maintaining the body surface barrier. Notably, these enzymes play a significant role in the development of diverse tumors.
To systematically and comprehensively explore the expression of the PLA2 family genes and their potential implications in cholangiocarcinoma (CCA).
We conducted an analysis of five CCA datasets from The Cancer Genome Atlas and the Gene Expression Omnibus. The study identified differentially expressed genes between tumor tissues and adjacent normal tissues, with a focus on PLA2G2A and PLA2G12B. Gene Set Enrichment Analysis was utilized to pinpoint associated pathways. Moreover, relevant hub genes and microRNAs for PLA2G2A and PLA2G12B were predicted, and their correlation with the pro
PLA2G2A and PLA2G12B were discerned as differentially expressed in CCA, manifesting significant variations in expression levels in urine and serum between CCA patients and healthy individuals. Elevated expression of PLA2G2A was correlated with poorer overall survival in CCA patients. Additionally, the study delineated pathways and miRNAs associated with these genes.
Our findings suggest that PLA2G2A and PLA2G12B may serve as novel potential diagnostic and prognostic markers for CCA. The increased levels of these genes in biological fluids could be employed as non-invasive markers for CCA, and their expression levels are indicative of prognosis, underscoring their potential utility in clinical settings.
Core Tip: This study reveals significant findings in cholangiocarcinoma (CCA) research, focusing on the roles of PLA2G2A and PLA2G12B enzymes. Key discoveries include the elevated expression of these enzymes in CCA, their involvement in carcinogenic pathways, and their potential as diagnostic and prognostic biomarkers. Notably, PLA2G2A’s expression correlates with poor survival outcomes in CCA patients. Additionally, the study highlights the prognostic value of associated microRNAs in serum and urine, offering new insights into non-invasive biomarkers for CCA. The research underscores the need for further validation and exploration of these findings in clinical settings.
- Citation: Qiu C, Xiang YK, Da XB, Zhang HL, Kong XY, Hou NZ, Zhang C, Tian FZ, Yang YL. Phospholipase A2 enzymes PLA2G2A and PLA2G12B as potential diagnostic and prognostic biomarkers in cholangiocarcinoma. World J Gastrointest Surg 2024; 16(2): 289-306
- URL: https://www.wjgnet.com/1948-9366/full/v16/i2/289.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i2.289
Cholangiocarcinoma (CCA) represents a complex spectrum of highly heterogeneous malignancies originating within the biliary tract. Its global incidence has been progressively increasing, presently constituting approximately 15% of all primary hepatic malignancies and about 3% of gastrointestinal cancers[1]. CCA is stratified into three primary subtypes: Intrahepatic CCA (iCCA), perihilar CCA (pCCA), and distal CCA (dCCA)[2]. Statistically, the incidence of iCCA in the United States has shown a continuous upward trajectory from 1973 to 2012[3]. The current management strategy for CCA integrates a combination of systemic chemotherapy, targeted radiotherapy, and surgical intervention[4]. Recent advancements in understanding the molecular intricacies of CCA, including its tumor microenvironment, have been pivotal in shedding light on its pathogenesis, the mechanisms underlying drug resistance, and in identifying promising new targets for therapeutic intervention. These insights are critical in a landscape where the complex biology of CCA continues to challenge effective treatment and patient outcomes.
The phospholipase A2 (PLA2) enzyme family, characterized by its ability to hydrolyze the sn-2 acyl bond of glycerophospholipids, plays a crucial role in a variety of cellular processes including lipid metabolism, inflammation, and cell proliferation[5,6]. The PLA2 superfamily consists of 16 groups of structurally and functionally diverse enzymes[7]. PLA2 enzymes not only play a critical role in the production of lipid mediators but also play a critical role in membrane remodeling, bioenergetics, and body surface barriers, thus participating in many biological events. Therefore, disorders of lipid metabolism regulated by PLA2 are commonly associated with a variety of diseases[8-10]. In this study, we discussed 5 main types of PLA enzymes types of PLA2 enzymes include the secreted (sPLA2), cytosolic (cPLA2), calcium-independent (iPLA2), platelet-activating factor acetylhydrolase (PAF-AH), also known as lipoprotein-associated PLA2 (Lp-PLA2), lysosomal PLA2 (LPLA2)[9].
The objective of this research was to conduct a thorough and systematic examination of the gene expression of the PLA2 family, with an emphasis on unraveling their potential roles in CCA. To achieve this, we meticulously collected clinical data, expression profiles, and copy number variations of CCA patients from two robust databases: The Cancer Genome Atlas (TCGA) and the Gene Expression Omnibus (GEO). Initially, our analysis focused on identifying statistically significant differentially expressed genes (DEGs) between normal and tumor tissues in CCA patients, using data from five distinct datasets. This approach enabled us to pinpoint potential genetic markers, specifically within the PLA2 family. Notably, PLA2G2A and PLA2G12B exhibited differential expression between normal and tumor samples [P < 0.05 and |log2 fold change (FC)| > 1]. Subsequent Gene Set Enrichment Analysis (GSEA) suggested their involvement in key Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. Further, we delved into the copy number variations of PLA2G2A and PLA2G12B in CCA cases to discern the impact of genetic and epigenetic alterations on gene expression. The pathways related to PLA2G2A and PLA2G12B were extensively explored through GSEA, which also facilitated the prediction of associated hub genes and microRNAs (miRNAs), Lastly, the relationship between patients’ overall survival (OS) and clinicopathological characteristics was rigorously evaluated using univariate Cox regression analysis. The prognostic significance of PLA2G2A and PLA2G12B was further substantiated through the construction of survivorship curves and the application of the Cox proportional hazards model.
In conclusion, our study employed a suite of sophisticated bioinformatics tools to meticulously investigate the distinctive expression patterns and the multifaceted prognostic significance of PLA2G2A and PLA2G12B in CCA. This comprehensive analysis has not only shed light on the unique roles of these genes in CCA but also paved the way for their potential application in enhancing the diagnostic accuracy and therapeutic strategies for CCA patients. Our findings underscore the importance of these biomarkers in the clinical landscape of CCA and suggest new avenues for future research aimed at improving patient outcomes.
Download the clinical data of CCA patients from the TCGA database (https://portal.gdc.cancer.gov) and five datasets (GSE107943, GSE132305, GSE26566, GSE32225, and GSE76311) of the GEO dataset (https://www.ncbi.nlm.nih.gov/geo). miRNA profiles in serum and urine extracellular vesicles (EVs) of patients with CCA, Cholangitis [primary sclerosing cholangitis (PSC)], and normal control (GSE144521). miRNA blood serum samples were extracted from CCA patients, cholelithiasis patients, and healthy subjects (GSE85589).
cBioportal (https://www.cbioportal.org/) is a bioinformatics tool developed for comprehensive genomics analysis[11]. Meanwhile, the cBioPortal for Cancer Genomics provides visualization, analysis, and downloading of large-scale cancer genomics datasets[12,13].
TargetScan (v7.0) (http://www.targetscan.org/vert_71/) provides an option to sort the predicted targets of mammalian miRNAs. There are two sorts, A: According to Cumulative Weighted Context++ Score, which is based on the predicted inhibitory effect, B: According to evolution conservative targeting probability ranks the Pct scores of the longest 3-UTR subtype[14]. MiRDB is an online database for miRNA target prediction and functional annotation (http://mirdb.org/). The bioinformatics tool MirTarget is used to predict all targets in MiRDB. MirTarget is developed by analyzing thousands of miRNA-target interactions from high-throughput sequencing experiments. For each miRNA target pair, the database script scans the miRNA pre-selected binding site and generates the target feature predicted by mirTarget. The prediction result is an annotation table of all miRNA target pairs, including the target’s prediction score and miRNA sequence[15,16]. The two databases have their advantages and disadvantages. Therefore, we selected the cross-genes in the two databases after screening as the predicted miRNA.
We screened the PLA2G2A and PLA2G12B-related hub genes. We then uploaded these genes to the David website (https://david.ncifcrf.gov/) to perform Genes Ontology (GO) functional enrichment analysis, with P < 0.05 as the cutoff value.
Univariate and multivariate Cox regression analysis was performed on PLA2 family members to evaluate the OS of patients: hazard ratio (HR) and 95% confidence interval (95%CI). The paired t test was used to compare the tumor and adjacent normal tissues (NAT) in GEO and TCGA-CCA datasets. Statistics software version 23.0 from SPSS Inc. (Chicago, IL, United States) was used. A two-tailed P < 0.05 was considered significant for all tests.
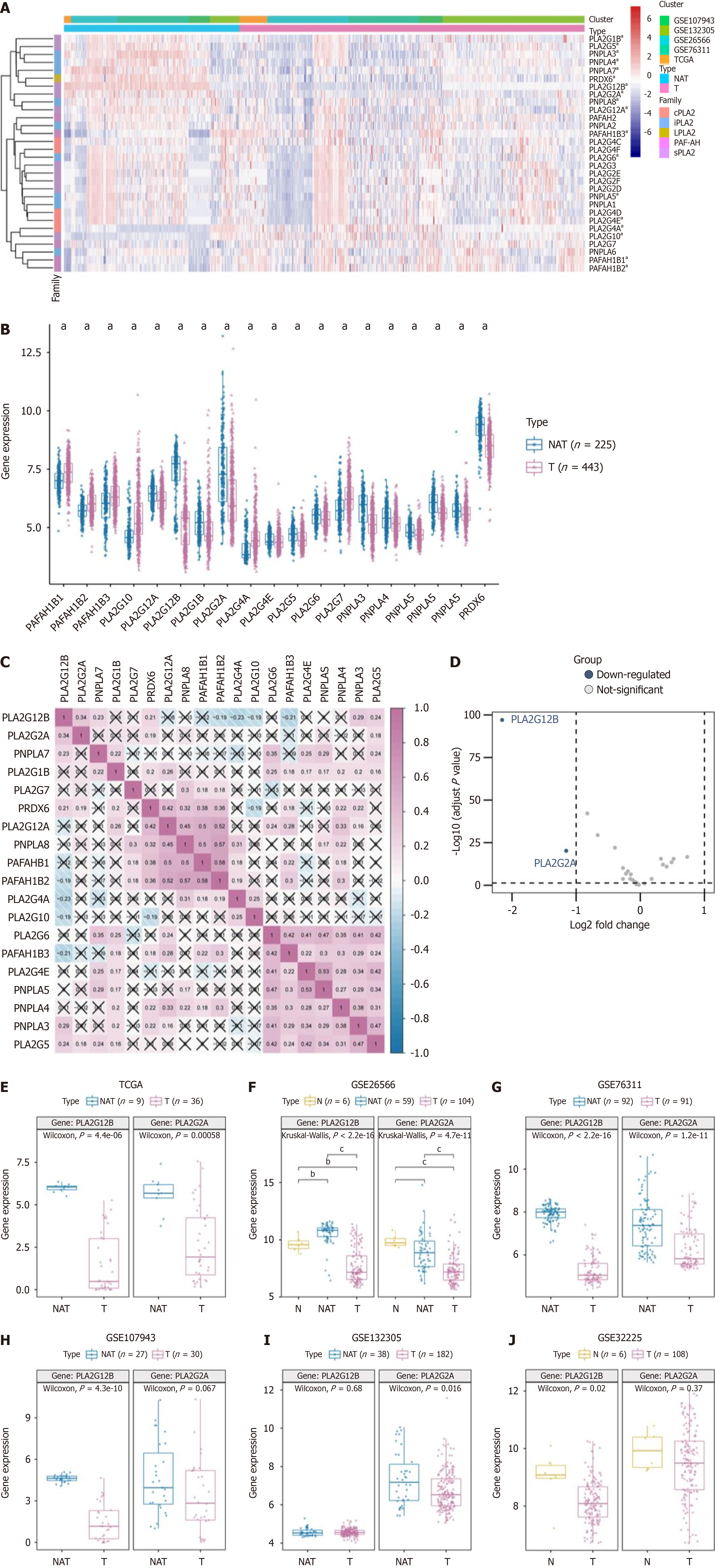
In our investigation, we incorporated five distinct datasets, comprising tumor (T) samples (n = 443) and NAT samples (n = 225) in CCA. Utilizing heatmap visualization, we identified significant differential expression in nineteen PLA2 family genes, encompassing subfamilies sPLA2, cPLA2, iPLA2, PAF-AH, Lp-PLA2, and LPLA2. These differential expressions were statistically significant (P < 0.05) (Figure 1A). Further analysis was conducted on the expression of these 19 DEGs in both T and NAT groups, which was visually represented (Figure 1B). Additionally, we examined the inter-gene correlations and networks, noting significant and positive correlations among the genes. Positive and negative correlations were indicated in red and blue, respectively, with cell numbers representing Spearman correlation coefficients (Figure 1C).
In the T group, genes such as PLA2G12A, PLA2G12B, PLA2G1B, PLA2G2A, PLA2G4E, PLA2G5, Patatin like phospholipase domain containing protein 3 (PNPLA3), PNPLA4, PNPLA7, PNPLA8, and PRDX6 were predominantly down-regulated, while others were up-regulated. To delve deeper into the potential molecular function of the PLA2 gene family in CCA and tumor progression, we compared the DEGs between high and low PLA2 gene expression groups, with a cutoff criterion set as log2FC > 1, P < 0.05. Among these, two genes were notably down-regulated (Figure 1D). The expression levels of PLA2G2A and PLA2G12B across different datasets were further depicted through box diagrams (Wilcoxon signed-rank test, all P < 0.05, Figure 1E-J).
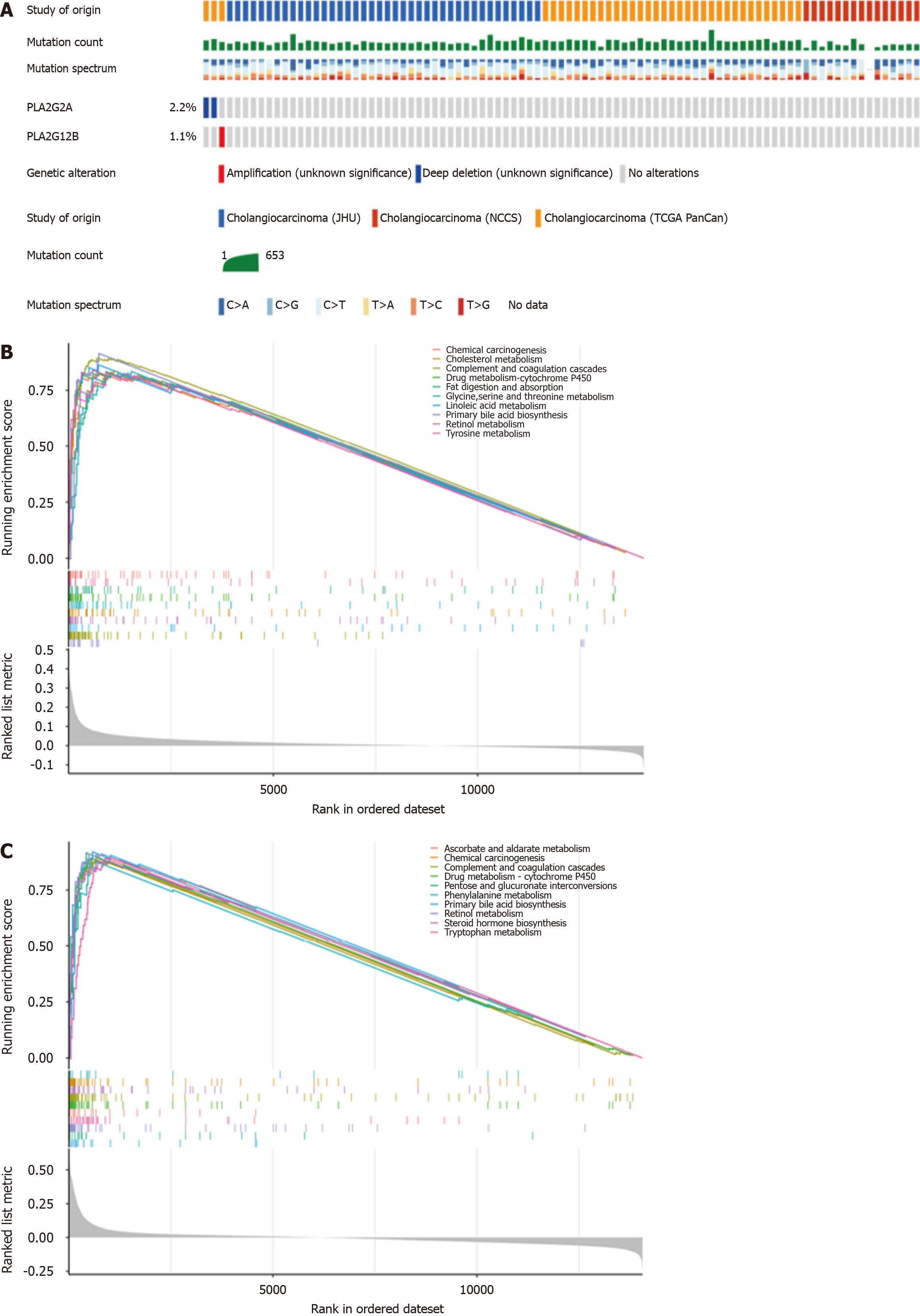
We analyzed the genetic alterations in PLA2G12B and PLA2G2A, considering mutation and copy number alteration data from various CCA studies. A total of 91 samples were obtained from three studies from TCGA, JHC, and the National Cancer Centre of Singapore, among which 2.2% samples involved deep deletion of PLA2G12B, 1.1% samples involved amplification of PLA2G2A (Figure 2A). For a further comprehensive exploration of functions of the PLA2 gene family in CCA, we selected “PLA2G12B and PLA2G2A gene sets” to perform GSEA. The GSEA results included: Cancer-related terms such as chemical carcinogenesis, cholesterol metabolism, ascorbate, and alternate metabolism, complement and coagulation cascades, fat digestion and absorption, phenylalanine metabolism, drug metabolism - cytochrome P450, steroid hormone biosynthesis, pentose and glucuronate interconversions (Figure 2B and C). Taken together, these results suggested that PLA2G12B and PLA2G2B might be linked with tumor development, metabolism in CCA.
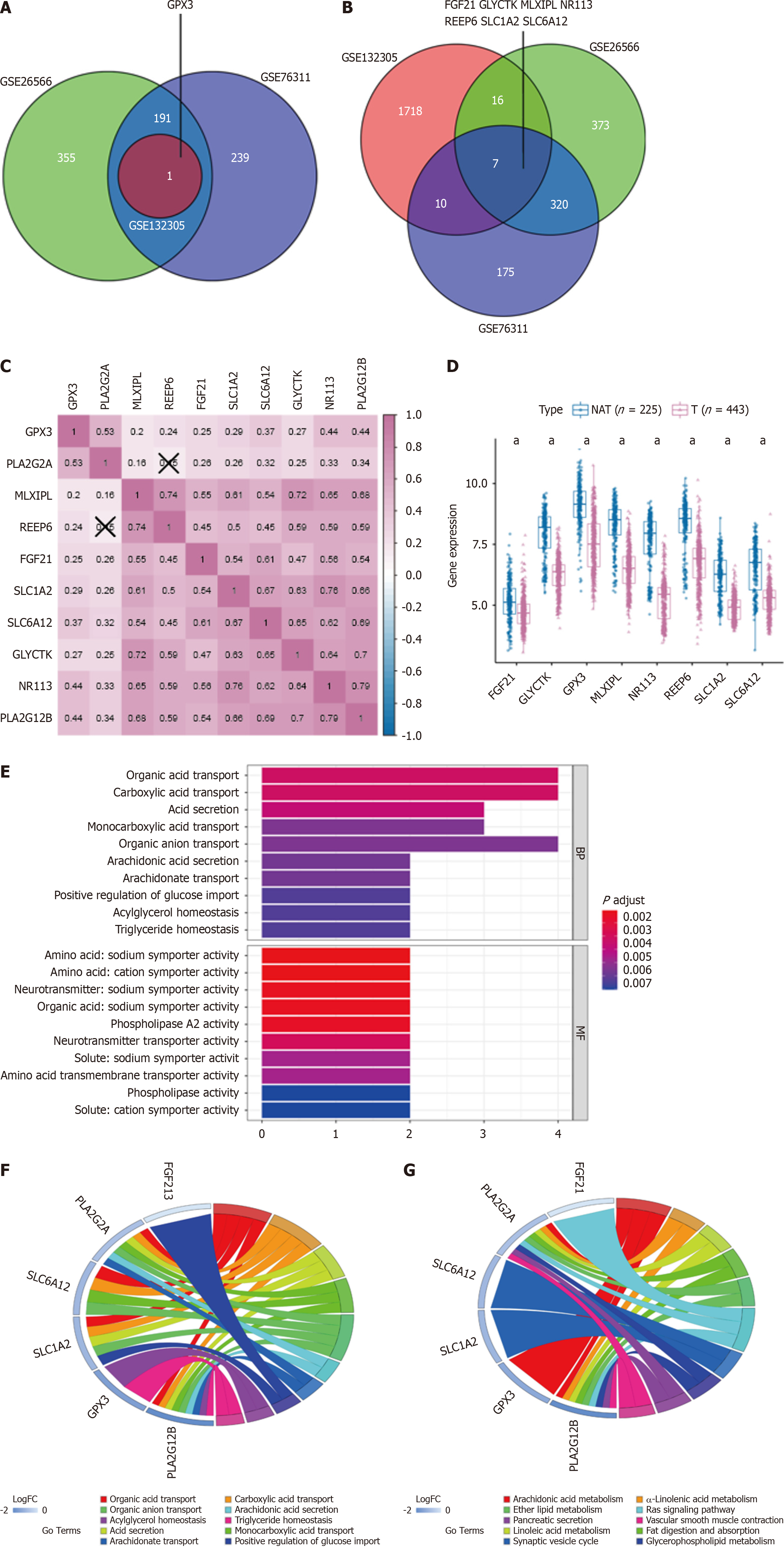
Utilizing the GEO gene dataset, we identified hub genes related to the carcinogenesis and progression of PLA2G2A and PLA2G12B. Notable genes included GPX3, FGF21, GLYTK, MLXIPL, NR113, REEP6, SLC1A2, and SLC6A12, all showing log2FC > 1, P < 0.05 (Figure 3A and B). Soon afterward, correlation analysis of hub genes, PLA2G2A and PLA2G12B was carried out. The number in the small square represents the Spearman correlation coefficient, and the value represents the correlation degree (Figure 3C). Figure 3D shows the differential expression of the relevant hub genes between CCA tumors and NAT in all data sets. A box plot was used to show the distribution of gene expression levels, and the Wilcoxon test was used to evaluate the statistical significance of differential expression. The results showed that the relevant hub genes were down-regulated in the CCA group. To better understand the role of hub genes in the occurrence and development of CCA disease, GO and KEGG pathway enrichment analysis were applied to discover the function of these relevant hub genes (the cut-off criterion was set as log2FC > 1, P < 0.05). GO analysis showed that the biological processes involved in these relevant hub genes were organic acid transport, carboxylic acid transport, acid secretion. The molecular function, involved in these relevant hub genes were amino acid: Sodium symporter activity, amino acid: Cation symporter activity, neurotransmitter: Sodium symporter activity, organic acid: Sodium symporter activity, and PLA2 activity (Figure 3E). This was followed by a DAVID analysis of proteins for the enrichment of the GO and KEGG functional pathways. We selected 10 GO terms respectively (Figure 3F). which included organic acid transport, carboxylic acid transport, organic anion transport, arachidonic acid secretion, acylglycerol homeostasis, triglyceride homeostasisacid, acid secretion, monocarboxylic acid transport, arachidonate transport, positive regulation of glucose import. Moreover, KEGG pathway analysis identified arachidonic acid metabolism, alpha-Linolenic acid metabolism, ether lipid metabolism, Ras signaling pathway, pancreatic secretion, vascular smooth muscle contraction, linoleic acid metabolism, fat digestion and absorption, synaptic vesicle cycle, glycerophospholipid metabolism (Figure 3G).
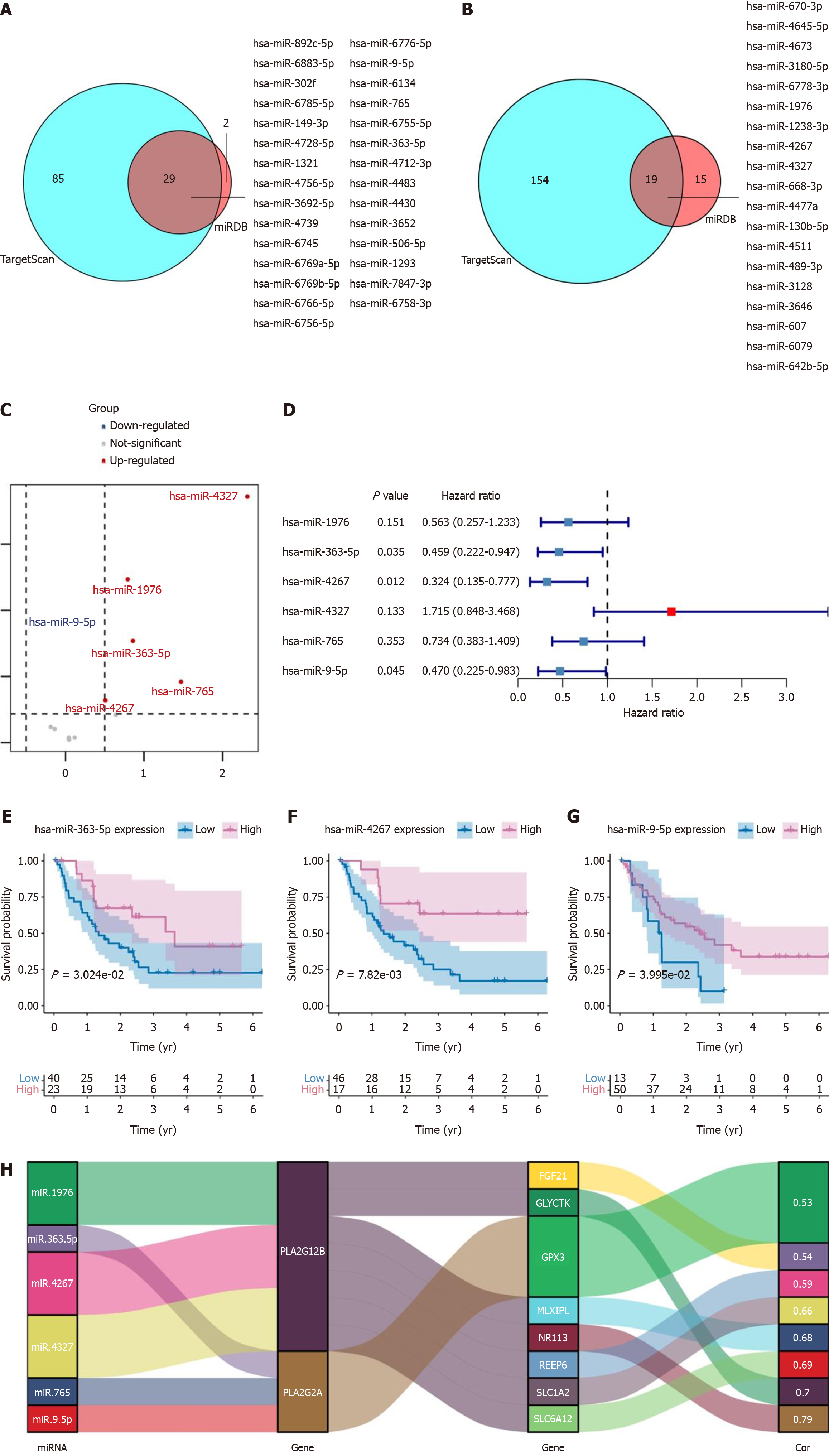
miRNAs are small endogenous RNAs that regulate post-transcriptional silencing of target genes. A single miRNA molecule can target hundreds of mRNAs and influence the expression of many genes involved in functional interaction pathways. Certain miRNAs can be secreted into the blood as free miRNAs. They can be detected in serum as a highly stable form. In exploring the role of miRNAs in post-transcriptional gene regulation, we identified specific miRNA targets for PLA2G2A and PLA2G12B. Using TargetScan and MiRDB databases, we obtained 29 intersecting miRNA targets for the PLA2G2A gene and 19 for the PLA2G12B gene (Figure 4A and B). An expression microarray series, GSE53870, containing CCA tumor and non-tumor samples was downloaded from the GEO database. This series, containing 1094 miRNA probes from 63 CCA patients and 9 normal intrahepatic bile ducts, facilitated the construction of a miRNA expression profile. Differential expression of these miRNAs between tumor and normal samples was analyzed using the “edgeR” package, with a log2FC > 0.5 (Figure 4C). Subsequently, six predicted miRNA genes were analyzed further, with the P value and HR of selected genes determined via univariable Cox regression analysis (P < 0.05) (Figure 4D). Additional survival analyses were conducted for hsa-miR-363-5p, hsa-miR-4267, and hsa-miR-9-5p, showing a better prognosis and survival in the high expression group (Figure 4E-G). We also employed the “ggalluvialR” package for data visualization analysis of miRNA targets, PLA2G2A, PLA2G12B, and hub gene network data flow, presented in a Sankey diagram. This analysis demonstrated correlations between PLA2G2A, PLA2G12B, and related concentrator genes (Figure 4H).
To clarify the expression levels of PLA2G2A and PLA2G12B in serum and urine of CCA patients, we utilized the GSE14521 expression microarray series from the GEO database. This series included CCA tumor samples, PSC samples, and non-tumor (N) samples. Total RNA was obtained from normal bile duct cells (NHC) and tumor bile duct cells (EGI1 and TFK1) cell lines, as well as from EVs isolated from serum, urine, and bile duct cell supernatant. The analysis revealed that PLA2G12B expression in serum EVs was significantly higher in the CCA and PSC groups compared to the normal group (P = 0.015). Similarly, PLA2G2A expression was significantly higher in the CCA group compared to the PSC group (P = 0.015). Additionally, PLA2G2A expression in urinary EVs was significantly higher in both the CCA and PSC groups compared to the normal group (P = 0.013). These data were analyzed using the Kruskal-Wallis test (Figure 5A and B).
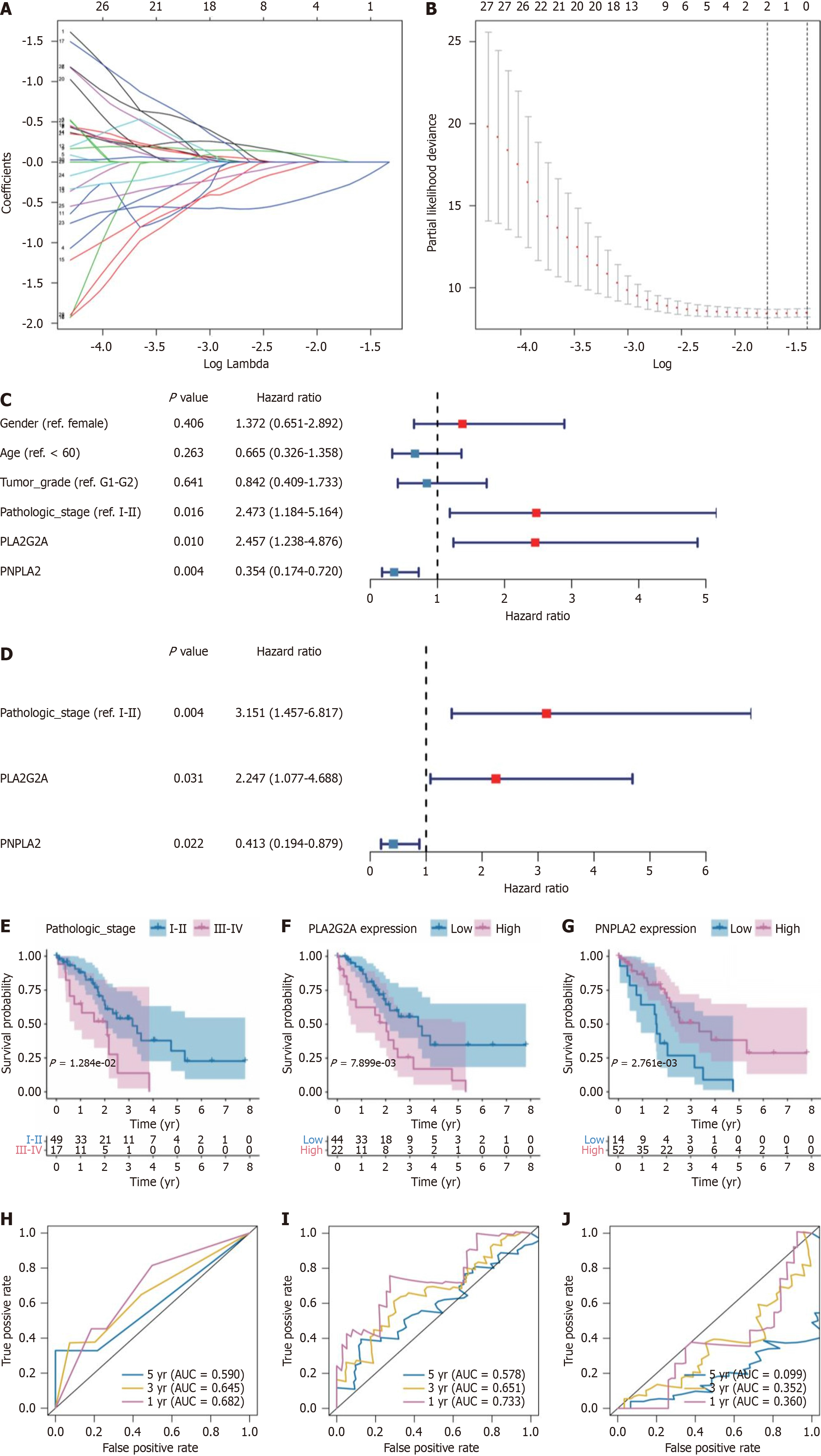
To further elucidate miRNA targets linked to PLA2G2A and PLA2G12B, we utilized the GSE85589 microarray series. This dataset was stratified into two cohorts: Intrahepatic cholangiocarcinoma (ICC) and a normal control group. We performed a detailed analysis of the differential expression of miRNAs between these groups using a volcano plot methodology. The criteria for significant differential expression were set at a log2FC greater than 0.1 and adjusted P values less than 0.05. Our analysis revealed an up-regulation in the expression of has-miR-3128, has-miR-4712-3p, and has-miR-1238-3p, and a down-regulation of has-miR-4739, has-miR-149-3p, and has-miR-607 (Figure 5C). To validate these findings, we examined the expression of these six miRNAs within a GSE data cohort comprising serum samples from 101 ICC patients, 10 cholelithiasis patients (Ch), and 19 healthy subjects (N). To verify the expression of the six miRNA in the GSE data cohort, which included serum samples from 101 ICC patients, 10 cholelithiasis patients (Ch), and 19 healthy subjects (N). The box diagram showed that the expression of has-miR-4749, has-miR-149-3p in Ch and N groups was significantly higher than that of the ICC group (P = 0.015, P = 0.0054). The expression of gene has-miR-3128 was the highest in the Ch group and significantly higher than that in the N group (Kruskal Wallis test) (Figure 5D). The area under the curve (AUC) values were calculated to gauge the accuracy of each potential biomarker. In the context of serum EVs, PLA2G12B presented an AUC of 0.861 when differentiating CCA from the normal group (Figure 5E), and an AUC of 0.917 when contrasting CCA against the PSC group (Figure 5F). In urinary Evs, PLA2G2A demonstrated an AUC of 0.878, underscoring its significance in separating CCA from the normal group (Figure 5G). Further analysis within the GSE85589 dataset revealed that has-miR-3128, has-miR-4712-3p, has-miR-1238-3p, has-miR-4739, has-miR-149-3p, and has-miR-607 exhibited varying AUC values, indicating their respective capacities to serve as reliable biomarkers. Notably, has-miR-1238-3p showed a promising AUC of 0.653 in discriminating CCA from the normal group (Figure 5H), and an AUC of 0.572 when differentiating between CCA and Ch (Figure 5I). Furthermore, the application of Least Absolute Shrinkage and Selection Operator (LASSO) regression analysis was crucial in pinpointing six key genes: Has-miR-3128, has-miR-4712-3p, has-miR-1238-3p, has-miR-4739, has-miR-149-3p, and has-miR-607. The LASSO method, a regularization technique designed to enhance the model’s predictive accuracy while preventing overfitting, identified these genes by shrinking less relevant coefficients to zero, effectively selecting the most robust set of predictive variables (Figure 6A and B). The coefficient profile plot against the log (λ) sequence depicted in Figure 6A illustrates the trajectories of these coefficients as the regularization parameter λ is tuned, which is instrumental in model selection. The optimal λ value was selected based on the point where the model achieved the best balance between complexity and fit, as demonstrated by the partial likelihood deviance shown in Figure 6B. The red dots represent the partial likelihood deviance values across a range of λ values, and the gray lines indicate the standard error, highlighting the precision of the model at each point. This analysis solidified the prognostic utility of the identified miRNAs, substantiating their roles as independent prognostic factors for the condition under study.
We selected 30 CCA patients with complete clinical data from GSE107943 for further analysis. Clinical data mainly includes patient’s gender, gender, survival, pathological diagnosis, etc. We performed univariate (Figure 6D) and multivariate (Figure 6C) Cox regression analyses to ascertain the independence of the Clinical features as a prognostic factor for other features, such as gender, age, tumor-grade, pathologic-stage, PLA2G2A, and PNPLA2. The results showed that pathologic-stage, PLA2G2A, and PNPLA2 were independent risk factors for prognosis. Then, by drawing a survival curve to clarify the pathological grade, the expression of PLA2G2A and PNPLA2 affect the prognostic survival. As indicated by the results, the higher the pathological stage of CCA, the lower the prognosis and survival rate (P = 1.284e-02) (Figure 6E). It also shows that PLA2G2A overexpression was significantly associated with a poorer prognosis
PLA2 hydrolyze the sn-2 acyl bond of glycerophospholipids to release lysophospholipids and most polyunsaturated free fatty acids. The two substances released are widely involved in physiological processes such as cell proliferation, survival, apoptosis, inflammation, and carcinogenesis[17]. PLA2G2A is a member of the PLA family. Recent studies have shown that elevated serum levels of PLA2G2A were found in prostate cancer patients and associated with increased tumor grade in literature[18]. Related studies have also pointed out that chronic hepatitis B, hepatocellular carcinoma, and the level of PLA2G2A in the patient’s serum are significantly higher than those of normal people[19]. Dong et al[20] pointed out that perfluorodecanoicacid inhibits cell senescence by inhibiting the expression of PLA2G2A protein and increases the proliferation ability of gastric cancer cells. Inhibiting the expression of PLA2G2A is a new molecular mechanism engaged in regulating tumor proliferation. In the research of non-tumor diseases, some scholars also believe that PLA2G2A polymorphisms are involved in the risk of developing metabolic syndrome and type 2 diabetes mellitus and are associated with subclinical atherosclerosis in this group of patients[21]. However, the roles of most PLA2 family members and their regulatory mechanisms in CCA cancer remain unclear. In recent years, next-generation sequencing technology has made significant progress and is widely used in the field of oncology[22]. Consequently, we adopted a collection of bioinformatics methods to analyze the RNA sequence data of CCA tissues to provide a general and comprehensive analysis of PLA2 family genes in CCA.
Next, we investigated the enrichment of PLA2G2A and PL2G12B in key pathways, suggesting that the two have the same enrichment results in carcinogenic, cholesterol, lipid metabolism, and cytochrome P40 metabolic pathways. In addition, by searching for hub genes through 5 sets of GEO sequencing cohort data, we also screened the CCA group with significantly down-regulated related genes and performed GO and KEGG pathway enrichment analysis. The results indicated that the significantly enriched in arachidonic acid metabolism, RAS signaling pathway, and monocarboxylic acid transport, they have been considered as important cancer-promoting factors in tumor progression, as well as new targets for the prevention and treatment of tumors[23-27].
Another important aspect of this research is the prediction of miRNA molecules related to PLA2G2A and PLA2G12B, which, for now, have not been extensively studied. Use the selected miRNA molecules in the GSE sequencing data queue to confirm the differentially expressed miRNA according to the CCA group and the normal control group. At the same time, in virtue of the Cox regression analysis to verify the HR and P value, determine the difference has-miR-363-5p, has-miR-4267, and has-miR-9-5p molecules, and evaluate their prognostic ability. The results demonstrated that miRNA associated with PLA2G2A and PLA2G12B were associated with prognostic survival in CCA patients, and the survival performance of the high expression group was worse than that of the low expression group. This finding aligns with our observations in CCA, where specific miRNAs associated with PLA2G2A and PLA2G12B demonstrate significant prognostic value. The differential expression of these miRNAs in serum and urine of CCA patients further emphasizes their potential as non-invasive diagnostic tools. Immediately afterward, we further paid attention to the expression of PLA2G2A and PLA2G12B in serum and urine EVs of CCA patients. many studies indicated that miRNAs stably existed in various body fluids, including serum, plasma, saliva, and urine. and they can transmit signals between cells and regulate intracellular gene expression. The use of exosomal miRNA as a new non-invasive biomarker has potential advantages[28-30]. In the past two decades, miRNAs have been pivotal in cancer research, serving as oncogenic or tumor-suppressive indicators. They have emerged as promising diagnostic and prognostic markers in cancer, leading to numerous clinical trials. Despite initial challenges in miRNA therapy, advancements in synthetic RNA and delivery technologies are paving the way for their potential as next-generation cancer treatments[31]. Jana et al[32] found in the latest study that miR-216b induction may be useful for sensitizing tumors to chemotherapy and radiotherapy, the expression of miR-216b may be associated with better overall and disease-free survival and might be potentially represent a prognostic biomarker for in multiple carcinomas. The current version of miRBase lists over 2600 mature human miRNAs, with a significant portion experimentally validated. These miRNAs, crucial in gene regulation, are implicated in complex cellular processes, including breast cancer metastasis. Petri and Klinge[33] highlighted the dual role of miRNA in cancer progression, with some promoting metastasis and others inhibiting metastasis. The study of miRNA’s impact on key metastatic pathways and their regulation in breast cancer emphasizes the need for more detailed research to understand their potential in therapeutic development[33]. It was found that the expression of PLA2G12B in the serum of the CCA group and PSC group was significantly higher than that of the normal group, but this phenomenon was not found in urine. However, PLA2G2A was still highly expressed in the serum and urine of CCA patients. After that, the potential differences in serum miRNA molecules between the ICC group and the normal control group were determined: Has-miR-3128, has-miR-4712-3p, has-miR-1238-3p, has-miR-4739, has-miR-149-3p, and has-miR-607 further confirmed that they were associated with biliary carcinoma. Use the selected miRNA molecules in the GSE sequencing data queue to confirm the differentially expressed miRNA according to the CCA and the normal control group. The consequence showed that the expressions of hsa-miR-4739 and hsa-miR-149-3p in the N group and the Ch group were significantly higher than those in the ICC group. eventually, the AUC values derived from the ROC curves serve as a testament to the discriminative efficiency of these biomarkers. PLA2G2A’s significant AUC in serum EVs underscores its potential utility in distinguishing CCA from non-cancerous conditions and from other diseases such as PSC. The AUC of 0.861 when differentiating CCA from normal groups (Figure 6E) indicates a high predictive accuracy, which could be pivotal in early detection strategies. Carbohydrate antigen 199 was used as a reference to evaluate the AUC value of the miRNA prediction model. Furthermore, the spectrum of AUC values observed for various miRNAs in the GSE85589 dataset reaffirms the heterogeneity and complexity of CCA. The relatively high AUC of has-miR-1238-3p in discriminating between CCA and normal controls, as well as CCA and Ch groups, emphasizes its potential as a non-invasive biomarker. While the AUCs for some biomarkers did not achieve the near-perfect discriminatory power, they nonetheless contribute valuable information to the collective understanding of CCA’s biomarker landscape. These findings are particularly relevant in the context of improving diagnostic methodologies and refining prognostic assessments in CCA. The integration of these miRNAs and PLA2 enzymes into a biomarker panel could enhance the sensitivity and specificity of CCA diagnostics, potentially leading to more personalized and effective patient management.
PLA2G2A regulators have prognostic value in cancer are of great significance. When patients were also grouped according to clinicopathological variables, multivariate analysis can group patients according to clinicopathological variables. It can distinguish the prognostic risk of gender, age, tumor grade, pathologic stage, PLA2G2A, and PNPLA2 patients. Univariate analyses showed that pathologic stage, PLA2G2A, and PNPLA2 were significantly associated with OS.
The prognostic landscape of CCA is multifaceted, with molecular markers playing a pivotal role in forecasting patient outcomes. Our ROC curve analysis delineates the discriminative performance of pathological staging, PLA2G2A overexpression, and PNPLA2 overexpression in predicting 1, 3, and 5-year OS in CCA patients. The AUC values derived from these curves are indicative of the potential utility of these markers in clinical prognostication. For short-term survival, represented by the 1-year AUC, the markers demonstrate substantial predictive validity. However, as we extend the forecast to medium-term (3-year AUC) and long-term (5-year AUC) survival rates, the predictive power shows variation. The modest AUC of 0.590 for 5-year survival suggests a nuanced relationship between the evaluated markers and patient survival, which appears to diminish over time. This could be attributed to the complex interplay of genetic and environmental factors that influence disease progression and patient resilience over extended periods.
Interestingly, the AUCs for long-term survival presented in Figure 6J are notably low, raising questions about the reliability of these markers for long-term prognostic assessments in CCA. Such findings compel us to consider the temporal dynamics of biomarker efficacy. A marker that is predictive in the short term may not necessarily maintain its prognostic capability over the long term, underscoring the necessity for a dynamic prognostic model that adapts over time. Finally, our research evidence confirms that PLA2G2A of the PLA2 family is a cancer-related gene, but our study has some limitations. Firstly, our investigations into the role of PL2AG2A in tumors were based on data that was already existed in the GEO, TCGA, cBioPortal, TargetScanHuman, and MiRDB databases. However, we did not use our clinical samples to verify these outcomes. Secondly, we still need more sample sizes to confirm these results. Thirdly, we did not conduct in vivo and in vitro experiments to confirm the role of PLA2G2A in the arachidonic acid metabolism, RAS signaling pathway, and monocarboxylic acid transport, the need for larger, clinically diverse datasets and further validation through in vivo and in vitro studies remains. Such comprehensive studies are crucial to establish the full spectrum of PLA2G2A’s role in CCA and its potential utility in clinical settings. Hence, further studies are necessary to verify the role played by PLA2G2A in CCA.
In conclusion, our results suggest that PLA2G2A is a potential prognostic biomarker for CCA patients and is associated with the levels of arachidonic acid metabolism, RAS signaling pathway, and monocarboxylic acid transport. Relatively high levels of PLA2G2A in CCA may indicate a greater risk of tumor progression and close medical supervision will be necessary for such patients. while the studied markers show promise, particularly for near-term prognostication, their efficacy in predicting long-term survival in CCA is limited. This emphasizes the ongoing challenge in oncology to identify robust, time-resistant biomarkers. Future studies should aim to validate these findings and explore additional markers that may offer improved long-term prognostic power, thereby contributing to more personalized and effective patient management strategies.
The study delves into the complex nature of cholangiocarcinoma (CCA), a heterogeneous set of malignancies in the biliary tract. It acknowledges the growing global incidence of CCA, now accounting for a significant portion of primary liver and gastrointestinal cancers. The research examines the role of phospholipase A2 (PLA2) enzymes in CCA, focusing on the differential expression of PLA2G2A and PLA2G12B genes and their potential as prognostic markers. This comprehensive analysis aims to enhance diagnostic and therapeutic strategies for CCA, addressing the complex biology that challenges current treatment approaches.
This study focuses on CCA, a group of complex biliary tract cancers. It aims to unravel the roles of specific PLA2 enzymes, particularly PLA2G2A and PLA2G12B in CCA. The research addresses the challenge of understanding CCA’s diverse genetic landscape and its implications for treatment resistance. Solving these issues could lead to breakthroughs in targeted therapies, improving prognosis and patient outcomes in CCA. This could significantly advance the field, offering insights into the molecular underpinnings of CCA and guiding future research directions.
The main objective of this research was to investigate the roles of PLA2G2A and PLA2G12B in CCA, focusing on their expression patterns and prognostic significance. The study realized these objectives by analyzing extensive bioinformatics data and highlighted the potential of these genes as biomarkers for CCA. Achieving these objectives is significant for future research, as it opens avenues for more targeted therapeutic strategies, improves diagnostic accuracy, and contributes to a better understanding of CCA’s molecular biology, potentially leading to enhanced patient care and outcomes.
The study employs advanced bioinformatics and clinical data analysis to explore PLA2G2A and PLA2G12B genes in CCA. Using data from The Cancer Genome Atlas (TCGA) and the Gene Expression Omnibus (GEO), it integrates gene expression profiling, microRNA (miRNA) analysis, and statistical methods to understand the genes’ roles and prognostic value, offering novel insights for future cancer research and therapy.
Our study makes significant contributions to the understanding of CCA by examining the gene expression of the PLA2 family and its impact on CCA. We identified specific PLA2G2A and PLA2G12B genes that showed notable expression differences between normal and tumor tissues. These findings were further supported by our exploration of genetic alterations and pathway enrichment analyses. Additionally, we investigated the prognostic value of miRNAs related to these genes, providing new insights into their role in CCA.
This study introduces a comprehensive bioinformatics analysis of PLA2G2A and PLA2G12B genes in CCA, providing new insights into their roles in cancer progression and prognosis. The methods involve advanced data analysis from databases like TCGA and GEO, emphasizing the significance of these genes in CCA’s molecular pathways. This approach contributes to understanding CCA’s complex biology and highlights potential diagnostic and therapeutic targets.
Future research should focus on validating the roles of PLA2G2A and PLA2G12B in CCA using clinical samples, ex
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hegazy AA, Egypt S-Editor: Wang JJ L-Editor: A P-Editor: Zheng XM
| 1. | Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V, Carpino G, Andersen JB, Braconi C, Calvisi DF, Perugorria MJ, Fabris L, Boulter L, Macias RIR, Gaudio E, Alvaro D, Gradilone SA, Strazzabosco M, Marzioni M, Coulouarn C, Fouassier L, Raggi C, Invernizzi P, Mertens JC, Moncsek A, Rizvi S, Heimbach J, Koerkamp BG, Bruix J, Forner A, Bridgewater J, Valle JW, Gores GJ. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1555] [Cited by in RCA: 1549] [Article Influence: 309.8] [Reference Citation Analysis (0)] |
| 2. | Blechacz B. Cholangiocarcinoma: Current Knowledge and New Developments. Gut Liver. 2017;11:13-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 347] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 3. | Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist. 2016;21:594-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 563] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 4. | Doherty B, Nambudiri VE, Palmer WC. Update on the Diagnosis and Treatment of Cholangiocarcinoma. Curr Gastroenterol Rep. 2017;19:2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 5. | Murakami M. Novel functions of phospholipase A(2)s: Overview. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:763-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Mouchlis VD, Dennis EA. Phospholipase A(2) catalysis and lipid mediator lipidomics. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:766-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 7. | Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev. 2011;111:6130-6185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 874] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 8. | Murakami M, Sato H, Taketomi Y. Updating Phospholipase A(2) Biology. Biomolecules. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 9. | Sukocheva O, Menschikowski M, Hagelgans A, Yarla NS, Siegert G, Reddanna P, Bishayee A. Current insights into functions of phospholipase A2 receptor in normal and cancer cells: More questions than answers. Semin Cancer Biol. 2019;56:116-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Aloulou A, Rahier R, Arhab Y, Noiriel A, Abousalham A. Phospholipases: An Overview. Methods Mol Biol. 2018;1835:69-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 11. | Zeng Q, Sun S, Li Y, Li X, Li Z, Liang H. Identification of Therapeutic Targets and Prognostic Biomarkers Among CXC Chemokines in the Renal Cell Carcinoma Microenvironment. Front Oncol. 2019;9:1555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 12. | Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8187] [Cited by in RCA: 11256] [Article Influence: 938.0] [Reference Citation Analysis (0)] |
| 13. | Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9144] [Cited by in RCA: 12805] [Article Influence: 985.0] [Reference Citation Analysis (0)] |
| 14. | Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4013] [Cited by in RCA: 5416] [Article Influence: 541.6] [Reference Citation Analysis (0)] |
| 15. | Chen Y, Wang X. miRDB: an online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020;48:D127-D131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 855] [Cited by in RCA: 1997] [Article Influence: 399.4] [Reference Citation Analysis (0)] |
| 16. | Liu W, Wang X. Prediction of functional microRNA targets by integrative modeling of microRNA binding and target expression data. Genome Biol. 2019;20:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 444] [Cited by in RCA: 566] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 17. | Yung YC, Stoddard NC, Chun J. LPA receptor signaling: pharmacology, physiology, and pathophysiology. J Lipid Res. 2014;55:1192-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 565] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 18. | Ozturk K, Onal MS, Efiloglu O, Nikerel E, Yildirim A, Telci D. Association of 5'UTR polymorphism of secretory phospholipase A2 group IIA (PLA2G2A) gene with prostate cancer metastasis. Gene. 2020;742:144589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Zhu C, Song H, Shen B, Wu L, Liu F, Liu X. Promoting effect of hepatitis B virus on the expressoin of phospholipase A2 group IIA. Lipids Health Dis. 2017;16:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Dong T, Peng Y, Zhong N, Liu F, Zhang H, Xu M, Liu R, Han M, Tian X, Jia J, Chang LK, Guo LH, Liu S. Perfluorodecanoic acid (PFDA) promotes gastric cell proliferation via sPLA2-IIA. Oncotarget. 2017;8:50911-50920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Monroy-Muñoz IE, Angeles-Martinez J, Posadas-Sánchez R, Villarreal-Molina T, Alvarez-León E, Flores-Dominguez C, Cardoso-Saldaña G, Medina-Urrutia A, Juárez-Rojas JG, Posadas-Romero C, Alarcon GV. PLA2G2A polymorphisms are associated with metabolic syndrome and type 2 diabetes mellitus. Results from the genetics of atherosclerotic disease Mexican study. Immunobiology. 2017;222:967-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Kamps R, Brandão RD, Bosch BJ, Paulussen AD, Xanthoulea S, Blok MJ, Romano A. Next-Generation Sequencing in Oncology: Genetic Diagnosis, Risk Prediction and Cancer Classification. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 323] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 23. | Payen VL, Mina E, Van Hée VF, Porporato PE, Sonveaux P. Monocarboxylate transporters in cancer. Mol Metab. 2020;33:48-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 395] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 24. | Bae S, Kim MK, Kim HS, Moon YA. Arachidonic acid induces ER stress and apoptosis in HT-29 human colon cancer cells. Anim Cells Syst (Seoul). 2020;24:260-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Degirmenci U, Wang M, Hu J. Targeting Aberrant RAS/RAF/MEK/ERK Signaling for Cancer Therapy. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 380] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 26. | Pérez-Escuredo J, Van Hée VF, Sboarina M, Falces J, Payen VL, Pellerin L, Sonveaux P. Monocarboxylate transporters in the brain and in cancer. Biochim Biophys Acta. 2016;1863:2481-2497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 303] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 27. | Sausville LN, Williams SM, Pozzi A. Cytochrome P450 epoxygenases and cancer: A genetic and a molecular perspective. Pharmacol Ther. 2019;196:183-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Lapitz A, Arbelaiz A, O'Rourke CJ, Lavin JL, Casta A, Ibarra C, Jimeno JP, Santos-Laso A, Izquierdo-Sanchez L, Krawczyk M, Perugorria MJ, Jimenez-Aguero R, Sanchez-Campos A, Riaño I, Gónzalez E, Lammert F, Marzioni M, Macias RIR, Marin JJG, Karlsen TH, Bujanda L, Falcón-Pérez JM, Andersen JB, Aransay AM, Rodrigues PM, Banales JM. Patients with Cholangiocarcinoma Present Specific RNA Profiles in Serum and Urine Extracellular Vesicles Mirroring the Tumor Expression: Novel Liquid Biopsy Biomarkers for Disease Diagnosis. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 29. | Zhao Y, Song Y, Yao L, Song G, Teng C. Circulating microRNAs: Promising Biomarkers Involved in Several Cancers and Other Diseases. DNA Cell Biol. 2017;36:77-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Nedaeinia R, Manian M, Jazayeri MH, Ranjbar M, Salehi R, Sharifi M, Mohaghegh F, Goli M, Jahednia SH, Avan A, Ghayour-Mobarhan M. Circulating exosomes and exosomal microRNAs as biomarkers in gastrointestinal cancer. Cancer Gene Ther. 2017;24:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 31. | Kim T, Croce CM. MicroRNA: trends in clinical trials of cancer diagnosis and therapy strategies. Exp Mol Med. 2023;55:1314-1321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 191] [Article Influence: 95.5] [Reference Citation Analysis (0)] |
| 32. | Jana S, Krishna M, Singhal J, Horne D, Awasthi S, Salgia R, Singhal SS. Therapeutic targeting of miRNA-216b in cancer. Cancer Lett. 2020;484:16-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Petri BJ, Klinge CM. Regulation of breast cancer metastasis signaling by miRNAs. Cancer Metastasis Rev. 2020;39:837-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (0)] |