Published online Dec 27, 2024. doi: 10.4240/wjgs.v16.i12.3818
Revised: September 5, 2024
Accepted: October 22, 2024
Published online: December 27, 2024
Processing time: 228 Days and 17.4 Hours
Acute gastrointestinal injury (AGI) is common in intensive care unit (ICU) and worsens the prognosis of critically ill patients. The four-point grading system proposed by the European Society of Intensive Care Medicine is subjective and lacks specificity. Therefore, a more objective method is required to evaluate and determine the grade of gastrointestinal dysfunction in this patient population. Digital continuous monitoring of bowel sounds and some biomarkers can change in gastrointestinal injuries. We aimed to develop a model of AGI using continuous monitoring of bowel sounds and biomarkers.
To develop a model to discriminate AGI by monitoring bowel sounds and biomarker indicators.
We conducted a prospective observational study with 75 patients in an ICU of a tertiary-care hospital to create a diagnostic model for AGI. We recorded their bowel sounds, assessed AGI grading, collected clinical data, and measured biomarkers. We evaluated the model using misjudgment probability and leave-one-out cross-validation.
Mean bowel sound rate and citrulline level are independent risk factors for AGI. Gastrin was identified as a risk factor for the severity of AGI. Other factors that correlated with AGI include mean bowel sound rate, amplitude, interval time, Sequential Organ Failure Assessment score, Acute Physiology and Chronic Health Evaluation II score, platelet count, total protein level, blood gas potential of hydrogen (pH), and bicarbonate (HCO3-) level. Two discriminant models were constructed with a misclassification probability of < 0.1. Leave-one-out cross-validation correctly classified 69.8% of the cases.
Our AGI diagnostic model represents a potentially effective approach for clinical AGI grading and holds promise as an objective diagnostic standard for AGI.
Core Tip: We developed a model to discriminate acute gastrointestinal injury (AGI) by continuous monitoring of bowel sounds and biomarker indicators. The study found that mean bowel sound rate and citrulline level are independent risk factors for AGI. Gastrin was identified as a risk factor for the severity of AGI. Two discriminant models were constructed with a misclassification probability of < 0.1. Our AGI diagnostic model represents a potentially effective approach for clinical AGI grading and holds promise as an objective diagnostic standard for AGI.
- Citation: Sun YH, Song YY, Sha S, Sun Q, Huang DC, Gao L, Li H, Shi QD. Diagnostic value of digital continuous bowel sounds in critically ill patients with acute gastrointestinal injury: A prospective observational study. World J Gastrointest Surg 2024; 16(12): 3818-3834
- URL: https://www.wjgnet.com/1948-9366/full/v16/i12/3818.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i12.3818
Gastrointestinal dysfunctions are highly prevalent among patients in the intensive care unit (ICU)[1] and are associated with a poor prognosis[2]. The hypothesis that the gut is the “engine” of multi-organ failure in critically ill patients has a long history[3,4]. In 2012, the European Society of Intensive Care Medicine (ESICM) introduced the term “acute gastrointestinal injury” (AGI) to describe gastrointestinal dysfunction resulting from acute illnesses in critically ill patients and proposed a four-point grading system to assess the severity of AGI[5]. However, this system is subjective and generalized, lacks specificity, and relies on clinical judgment rather than on objective measurements and laboratory results. Therefore, a more objective method for evaluating and determining the grade of gastrointestinal function must be developed.
Several innovative approaches have recently emerged for evaluating gastrointestinal function, such as digital bowel sound monitoring technology. This technology involves the recording, storage, transmission, and computer-assisted analysis of sound signals. Real-time online auscultation enables automatic identification and conversion of audible signals into visual data that can be comprehensively and objectively assessed without invasive procedures. This approach facilitates the quantitative analysis of changes in bowel sounds[6,7].
The diagnostic value of some serum biomarkers, such as citrulline (Cit) and intestinal fatty acid-binding protein (I-FABP), for gastrointestinal disorders has been recently investigated[8]. A reduced Cit level is considered a marker of loss of enterocyte mass and intestinal failure (IF)[9,10]. I-FABP has been reported as the most sensitive biomarker of intestinal ischemia[11-13]. This study aimed to construct an AGI grading decision model based on discriminant analysis using bowel sound characteristics, biomarkers, and other objective indicators combined with relevant clinical features to provide an objective method for identifying, assessing, diagnosing, and grading AGI in critically ill patients.
This study was approved by the Medical Ethics Committee of The First Affiliated Hospital of Xi’an Jiaotong University (approval No. XJTU1AF2021LSK-013). All patients or authorized individuals were provided complete study information, including their rights, potential benefits, and risks. Those who agreed to participate signed an informed consent form, and those who declined were excluded. This study is registered at ClinicalTrials.gov (NCT04769830).
Based on the ESICM recommendations for the definition of AGI, the patients were categorized into four grades according to disease severity: (1) Grade I: AGI was defined as the presence of risk factors for gastrointestinal dysfunction and failure, with a clear etiology, partial impairment of gastrointestinal function, and transient and self-limiting features; (2) Grade II: AGI was defined as gastrointestinal dysfunction that hinders the body’s ability to fully meet its needs for nutrients and water, without impacting the patient's overall health. This condition necessitates some intervention; (3) Grade III: AGI was defined as gastrointestinal dysfunction that does not resolve after intervention and does not improve the systemic condition; and (4) Grade IV: AGI was defined as gastrointestinal dysfunction with life-threatening distal organ dysfunction.
A prospective observational study was conducted to consecutively enroll adult patients admitted to the Department of Intensive Care Medicine, The First Affiliated Hospital of Xi'an Jiaotong University, from April 2021 to October 2021. After obtaining informed consent, 24 consecutive hours of bowel sounds were collected and analyzed on the first day of admission. Peripheral venous blood was collected to detect biomarkers and assess routine blood biochemical indices, disease severity scores, AGI grades, and other relevant clinical information. Data were statistically analyzed.
Adult patients consecutively admitted to the ICU from April 2021 to October 2021 were included. The inclusion criteria were as follows: (1) Age > 18 years; (2) Expected ICU stay > 24 hours; and (3) Voluntary participation in the study and signing of informed consent. The exclusion criteria were as follows: (1) Expected ICU stay < 24 hours and incomplete case data; (2) Pregnancy or lactation in women; (3) Severe cardiovascular disease and hemodynamic instability with risk of cardiac and respiratory arrest within a short period; (4) Refusal to participate or participation in another clinical trial; and (5) Re-admission to the ICU.
Demographic information, including sex, age, body mass index, relevant medical history, primary cause for ICU admission, and surgical details, was collected. On the first day of participation, the following data were collected from all patients: (1) Evaluation of AGI grading; (2) Compilation of data on bowel sound characteristics, including mean bowel sound rate, duration, amplitude, frequency, and interval of gastrointestinal sounds; (3) Examination of biomarkers and gastrointestinal hormones, including Cit, I-FABP, gastrin, and motilin (MTL); (4) Assessment of Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation II (APACHE II) scores; (5) Routine bioche
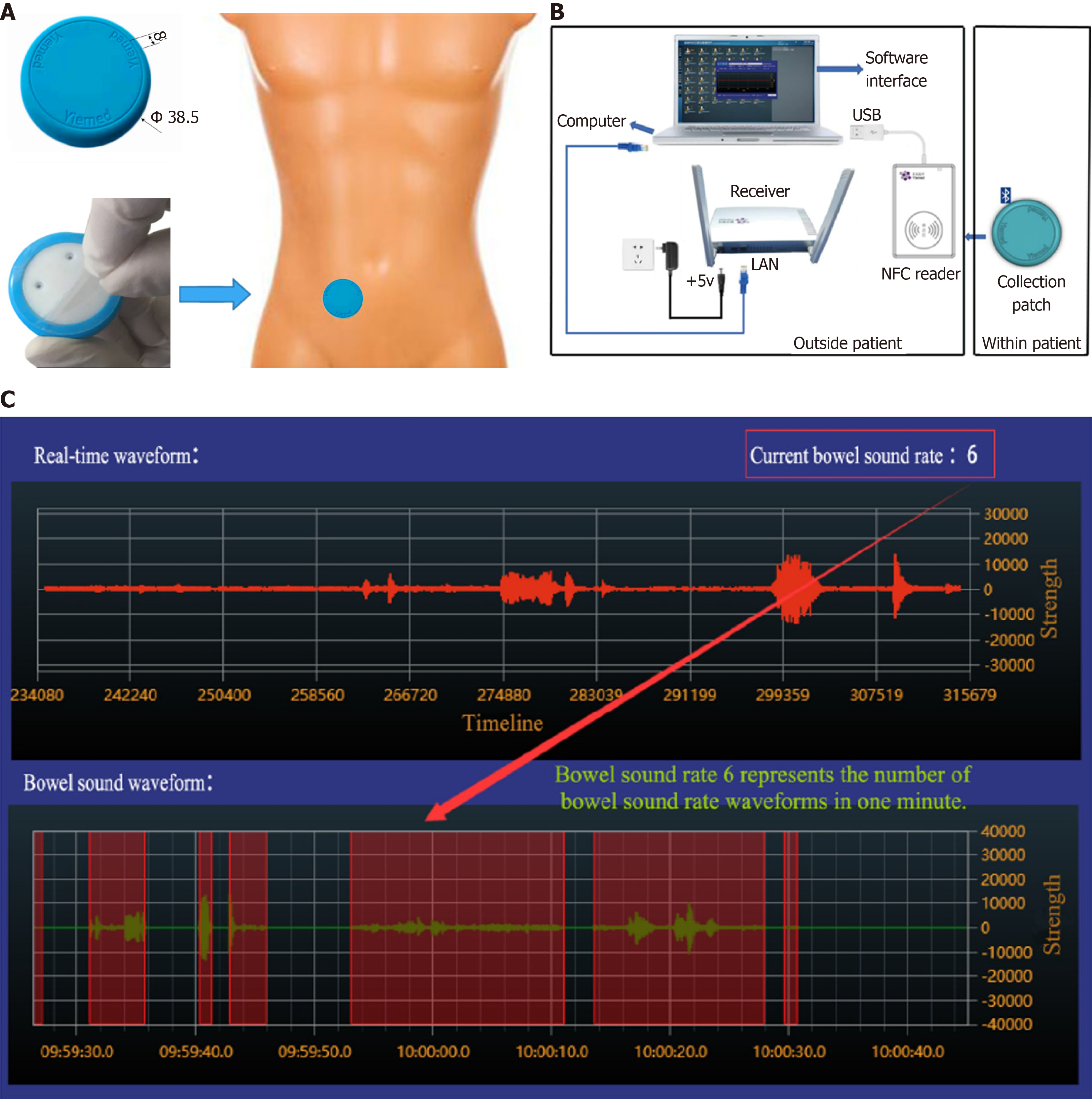
The YM-TYJL-01 continuous auscultation recorder from Shandong Yimai Medical Technology Co. (Shandong Province, China) was used (Figure 1). The system comprises a collection patch, receiver, Near Field Communication reader, and support software. By continuously collecting human bowel sounds, the continuous auscultation recorder enables wireless real-time transmission and storage combined with specialized software for recording and analysis. First, the acquisition patch utilizes micro-electromechanical system sensors to capture bowel sounds. The microprocessor embedded in the patch then transmits the data to the receiver via Bluetooth. The receiver then uploads the data to the server running the supporting software. The software stores the received data, records relevant information, and generates relevant monitoring indicators for continuous bowel sounds.
Serum Cit, I-FABP, MTL, and gastrin levels were measured using a double-antibody one-step sandwich ELISA kit (Shanghai Jijin Chemical Technology Co. Ltd., Shanghai, China). Samples, standard samples, and horseradish peroxidase-labeled detection antibodies were added sequentially to micropores coated with MTL antibodies, followed by thorough incubation and washing. The 3, 3´ , 5, 5´-Tetramethylbenzidine was catalytically converted with peroxidase to produce a blue color, followed by the addition of an acid to obtain the final yellow color. A positive correlation between the color and indicator was detected in the sample. The absorbance value was measured using an enzyme-labeling instrument at a wavelength of 450 nm, and the sample concentration was calculated. Other indicators were analyzed following the same procedure.
Quantitative variables are expressed as mean and standard deviation or as median and interquartile range. Categorical variables are expressed as frequency and percentage. Analysis of variance or the non-parametric rank test was used to assess observational indicators. Spearman’s rank correlation was performed to analyze the correlation between AGI classification and observation indices. Variables that were statistically significant (P < 0.05) based on univariate analysis were included in the multiple logistic regression analysis to determine the relationship between the variables and AGI and grading. The area under the receiver operating characteristic (ROC) curve (AUC) was determined to evaluate the diagnostic value of the variables for AGI. Based on the univariate analysis, statistically significant variables were included in the discriminant analysis, the discriminant function was constructed, and the accuracy and discriminant effect of the model was evaluated using misjudgment probability and leave-one-out cross-validation classification. Using Statistical Package for the Social Sciences version 26.0 (IBM Corp, Armonk, NY, United States) for data analysis, P < 0.05 was statistically significant. The statistical methods of this study were reviewed by Zeng LX from Department of Epidemiology and Health Statistics, School of Public Health, Xi'an Jiaotong University Health Science Center.
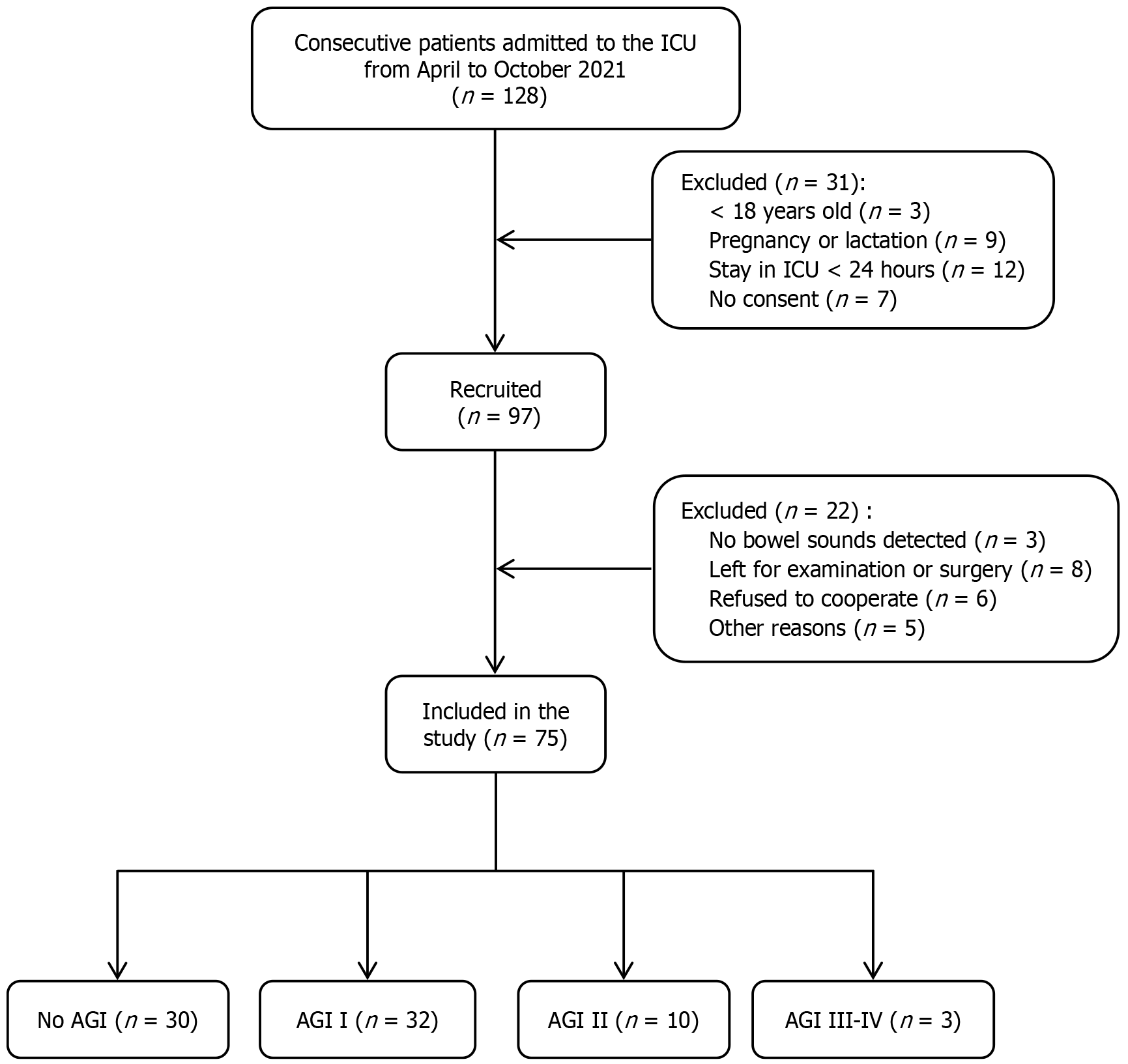
A total of 128 patients were enrolled in the cohort for screening during the study period; however, 31 of them did not meet the inclusion criteria for the following reasons: (1) The 3 patients were aged < 18 years old; (2) The 9 were pregnant or lactating; (3) The 12 stayed in the ICU for <24 hours; and (4) The 7 refused to participate in the study. In addition, among the patients who met the inclusion criteria, 22 could not be included in the evaluation and differentiation of AGI for various reasons: (1) The 3 patients exhibited no monitored bowel sounds; (2) The 8 had insufficient bowel sound monitoring and biomarker data due to going out for examination or operation; (3) The 6 refused to cooperate; and (4) The 5 were excluded from the final study for other reasons. Finally, 75 patients were included in the study, including 45 with AGI (60.0%): (1) 32 with grade I; (2) 10 with grade II; (3) 3 with grade III; and (4) 0 with grade IV AGI. The incidence of grade II AGI and above was 17.3% (13/75 patients). Figure 2 shows a flowchart of the participant enrollment process.
Of the 75 patients, 68.0% were men, with a median age of 64 years. Their median height, weight, and body mass index were 170 cm, 66 kg, and 22.86 kg/m2, respectively. On the first day of admission, the median APACHE II and SOFA scores were 19 and 8, respectively. Respiratory, cardiovascular, and urinary diseases were present in 60%, 29.3%, and 18.7% of the patients, respectively. Sepsis or septic shock (41.3%), multiple organ dysfunction syndrome (22.7%), postoperative symptoms (18.7%), post-cardiac arrest syndrome (6.7%), trauma or multiple injuries (6.7%), severe acute pancreatitis (5.3%), and hemorrhagic fever with renal syndrome (5.3%) were the primary reasons for ICU admission.
Out of the total, 76%, 9.3%, and 14.7% of patients underwent non-surgical, gastrointestinal, and non-gastrointestinal surgeries, respectively. Regarding nutritional support, 30 (40.0%), 18 (24.0%), and 3 (0.04%) patients received enteral, parenteral, and a combination of enteral and parenteral nutrition, respectively. Moreover, 36 (48%) patients received vasoactive drugs and invasive mechanical ventilation, and 16 (21.3%) received non-invasive mechanical ventilation. The median mechanical ventilation time was 4 days, the median ICU and hospital stays were 10 and 16 days, respectively, and the 28-day mortality rate was 33.3% (Table 1).
| Characteristics | Total, n = 75 |
| Male sex | 51 (68.0) |
| Age (years) | 64 (51, 74) |
| Body mass index (kg/m2) | 22.9 (19.3, 26.1) |
| Acute Physiology and Chronic Health Evaluation II score, points | 19 (15, 24) |
| Sequential Organ Failure Assessment score, points | 8 (5, 10) |
| Main reason for admission to ICU | |
| Respiratory system (severe pneumonia, acute respiratory distress syndrome, respiratory failure, immunosuppressive host pneumonia) | 45 (60.0) |
| Cardiovascular circulation (pulmonary embolism, shock, heart failure, disseminated intravascular coagulation) | 22 (29.3) |
| Urinary system (acute kidney injury) | 14 (18.7) |
| Sepsis/septic shock | 31 (41.3) |
| Multiple organ dysfunction syndrome | 17 (22.7) |
| Postoperative care | 14 (18.7) |
| Post-cardiopulmonary resuscitation | 5 (6.7) |
| Trauma/multiple injuries | 5 (6.7) |
| Severe acute pancreatitis | 4 (5.3) |
| Hemorrhagic fever with renal syndrome | 4 (5.3) |
| Others (immune checkpoint inhibitor-related damage, Japanese encephalitis, refeeding syndrome) | 3 (4.0) |
| Operation | |
| Gastrointestinal surgery | 7 (9.3) |
| Non-gastrointestinal surgery | 11 (14.7) |
| Non-operative medical treatment | 57 (76.0) |
| Nutrition | |
| Enteral nutrition | 30 (40.0) |
| Parenteral nutrition | 18 (24.0) |
| Enteral and parenteral nutrition | 3 (0.04) |
| Life support | |
| Vasoactive drugs | 36 (48.0) |
| Invasive mechanical ventilation | 36 (48.0) |
| Non-invasive mechanical ventilation | 16 (21.3) |
| Outcome | |
| Duration of mechanical ventilation (days) | 4 (0, 9) |
| ICU length of stay (days) | 10 (6, 17) |
| Hospital length of stay (days) | 16 (8, 26) |
| 28-day mortality | 25 (0.33) |
In this study, 45 patients were classified as AGI cases and 30 as non-AGI cases. As shown in Table 2, significant differences were noted in the: (1) Mean bowel sound rate, duration, amplitude, average frequency, and interval time; (2) Cit level; (3) SOFA score; (4) APACHE II score; (5) Interleukin (IL)-6 level; and (6) PH between patients with AGI and those without AGI (P < 0.05). Among these factors, the mean bowel sound rate, duration, amplitude, average frequency, and interval time, as well as the Cit level were significantly lower in patients with AGI than in patients without AGI, whereas the SOFA score, APACHE II score, IL-6 level, and pH were higher in patients with AGI than in those without AGI.
| Variables | Non-AGI (n = 30) | AGI (n = 45) | t/Z | P value |
| Mean bowel sound rate (counts per minute) | 4.343 ± 2.756 | 1.185 ± 1.441 | -5.352 | < 0.001b |
| Duration of bowel sounds (seconds) | 1.579 ± 1.049 | 0.534 ± 0.919 | -5.041 | < 0.001b |
| Amplitude (dB) | 0.121 ± 0.097 | 0.037 ± 0.076 | -5.029 | < 0.001b |
| Average frequency (Hz) | 708.559± 219.649 | 355.458± 238.318 | -5.094 | < 0.001b |
| Interval time (seconds) | 3.977 ± 1.410 | 2.098 ± 1.508 | -4.448 | <0.001b |
| Citrulline (μmol/L) | 19.29 ± 1.46 | 18.47 ± 1.68 | 2.165 | 0.034a |
| Intestinal fatty acid-binding protein (ng/L) | 467 ± 59 | 493 ± 60 | -1.863 | 0.067 |
| Gastrin (ng/L) | 79.45 ± 12.16 | 74.95 ± 12.87 | 1.493 | 0.140 |
| Motilin (ng/L) | 215.20 ± 37.58 | 211.73 ± 38.27 | 0.382 | 0.703 |
| Sequential Organ Failure Assessment score, points | 6 ± 3 | 9 ± 5 | -2.596 | 0.009b |
| Acute Physiology and Chronic Health Evaluation II score, points | 17 ± 6 | 22 ± 9 | -2.430 | 0.018a |
| Hemoglobin (g/L) | 98 ± 26 | 96 ± 21 | 0.418 | 0.678 |
| Platelets (109/L) | 161 ± 104 | 122 ± 89 | -1.553 | 0.120 |
| white blood cell (109/L) | 8.45 ± 3.36 | 10.08 ± 4.41 | -1.462 | 0.144 |
| Neutrophil count (109/L) | 7.32 ± 3.09 | 8.82 ± 436 | -1.697 | 0.094 |
| Percentage of central granulocyte count | 84.9 ± 7.9 | 88.6 ± 6.7 | -1.430 | 0.153 |
| C-reactive protein (mg/L) | 99.3 ± 72.7 | 104.4 ± 80.2 | -0.159 | 0.874 |
| procalcitonin (ng/mL) | 3.768 ± 7.037 | 7.250 ± 12.337 | -1.187 | 0.235 |
| Interleukin 6 (ng/mL) | 103.688 ± 256.797 | 166.78 ± 322.69 | -2.262 | 0.024a |
| Total protein (g/L) | 52.0 ± 6.7 | 49.8 ± 6.3 | 1.421 | 0.160 |
| Albumin (g/L) | 28.6 ± 3.6 | 27.7 ± 4.2 | 0.905 | 0.369 |
| Creatinine (µmol/L) | 115 ± 108 | 109 ± 82 | -0.108 | 0.914 |
| Blood urea nitrogen (mmol/L) | 11.25 ± 6.00 | 11.96 ± 8.16 | -0.132 | 0.895 |
| Serum sodium (mmol/L) | 140.28 ± 5.17 | 138.94 ± 4.90 | -1.070 | 0.284 |
| Serum potassium (mmol/L) | 4.09 ± 0.52 | 4.03 ± 0.42 | 0.509 | 0.613 |
| Serum calcium (mmol/L) | 2.07 ± 0.17 | 2.02 ± 0.15 | 1.397 | 0.167 |
| Serum phosphorus (mmol/L) | 0.94 ± 0.32 | 0.96 ± 0.43 | -0.053 | 0.958 |
| Arterial blood gas potential of hydrogen | 7.457 ± 0.060 | 7.422 ± 0.074 | 2.158 | 0.034a |
| Partial pressure of oxygen (mmHg) | 106.4 ± 37.8 | 105.5 ± 34.9 | -0.024 | 0.981 |
| Partial pressure of carbon dioxide (mmHg) | 36.8 ± 8.9 | 38.0 ± 8.1 | -0.591 | 0.556 |
| Bicarbonate (mmol/L) | 26.4 ± 5.6 | 24.6 ± 4.3 | 1.564 | 0.122 |
| Lactic acid (mmol/L) | 1.2 ± 0.8 | 1.5 ± 1.0 | -1.308 | 0.191 |
| Serum magnesium (mmol/L) | 0.95 ± 0.13 | 0.94 ± 0.13 | 0.186 | 0.853 |
| Body mass index (kg/m2) | 22.52 ± 4.25 | 23.19 ± 5.01 | -0.552 | 0.583 |
| Age (years) | 59 ± 16 | 62 ± 19 | -1.098 | 0.272 |
| Duration of mechanical ventilation (days) | 7 ± 16 | 8 ± 11 | -1.054 | 0.292 |
| Intensive care unit length of stay (days) | 16 ± 16 | 13 ± 12 | -0.774 | 0.439 |
| Hospital length of stay (days) | 19 ± 17 | 20 ± 16 | -0.292 | 0.770 |
To identify factors influencing AGI, the significant indices in the univariate analysis in Table 2 were further incorporated into multivariate binary logistic regression analysis. The results showed that mean bowel sound rate (95% CI: 0.225–0.642, P < 0.001) and Cit level (95%CI: 0.308–0.957, P = 0.035) were independent risk factors for AGI. The risk of AGI increased by 38.0% for every 1 time/minute reduction in mean bowel sound rate. For every 1 μmol/L reduction in Cit level, the risk of AGI increased by 54.3% (Table 3).
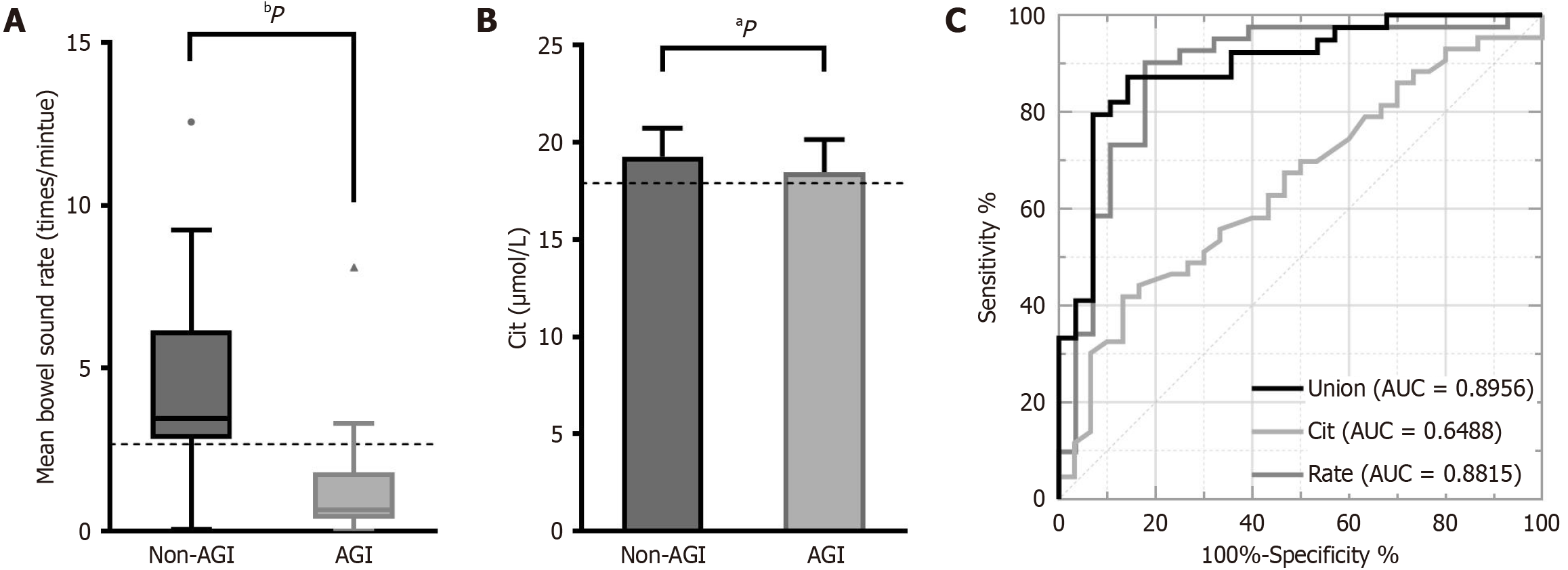
Furthermore, ROC curve analysis was conducted to evaluate the diagnostic value of the mean bowel sound rate and Cit level for AGI. As shown in Figure 3, the AUC of mean bowel sound rate and Cit level were 0.8815 and 0.6488, respectively, both of which were > 0.5, and had a P < 0.05, indicating that the mean bowel sound rate and Cit level have diagnostic value for AGI. AGI can be diagnosed when the mean bowel sound rate falls below 2.665 counts per minute, with a sensitivity of 90.2% and specificity of 82.1%, or when the Cit level is ≤ 17.91 μmol/L, with a sensitivity of 41.9% and specificity of 86.7%. The AUC for mean bowel sound rate combined with Cit level was 0.8956 (P < 0.05), indicating diagnostic value for AGI (Table 4).
According to the AGI grade, patients with AGI were divided into three groups: (1) AGI I (n = 30); (2) AGI II (n = 19); and (3) AGI III (n = 4) and AGI IV (n = 0). As shown in Table 5, significant differences were noted in gastrin levels and the length of hospital stay between these different AGI groups. Further, pairwise comparisons showed a significant difference in gastrin levels (P = 0.019) between the AGI I and AGI III groups. However, no significant difference was noted in the length of hospital stay among the three groups (P > 0.05) (Table 6). Multivariate logistic regression analysis was performed to examine the observation indexes showing statistical significance in the univariate analysis. In the parallel line test, χ² was 4.964 and the P-value was 0.084 (P > 0.05). The regression results demonstrated that gastrin (P = 0.020) had a significant influence on the severity of AGI (P < 0.05), b = 0.071, Wald χ2 = 5.417, and that gastrin was an independent risk factor for the severity of AGI. The length of hospital stay (P = 0.355) was not observed to be a factor affecting the severity of AGI (P > 0.05) (Table 7).
| Variables | AGI I (n = 32) | AGI II (n = 10) | AGI III-IV (n = 3) | PF/χ² |
| Mean bowel sound rate (counts per minute) | 1.308 ± 1.610 | 0.942 ± 0.994 | 0.717 ± 0.690 | 0.828 |
| Duration of bowel sounds (seconds) | 0.513 ± 0.795 | 0.719 ± 1.385 | 0.178 ± 0.180 | 0.719 |
| Amplitude (dB) | 0.032 ± 0.043 | 0.065 ± 0.144 | 0.011 ± 0.009 | 0.818 |
| Average frequency (Hz) | 366.215 ± 238.280 | 344.720 ± 256.841 | 287.276 ± 260.841 | 0.858 |
| Interval time (seconds) | 2.180 ± 1.554 | 1.851 ± 1.285 | 2.045 ± 2.171 | 0.854 |
| Citrulline (μmol/L) | 18.47 ± 1.68 | 18.53 ± 1.94 | 18.22 ± 0.88 | 0.973 |
| Intestinal fatty acid-binding protein (ng/L) | 489 ± 58 | 516 ± 61 | 467 ± 68 | 0.364 |
| Gastrin (ng/L) | 72.49 ± 12.11 | 77.07 ± 11.22 | 93.33 ± 13.01 | 0.019a |
| Motilin (ng/L) | 212.23 ± 38.97 | 218.42 ± 33.79 | 186.32 ± 46.98 | 0.459 |
| Sequential Organ Failure Assessment score, points | 9 ± 4 | 9 ± 6 | 12 ± 3 | 0.510 |
| Acute Physiology and Chronic Health Evaluation II score, points | 21 ± 8 | 27 ± 12 | 20 ± 5 | 0.240 |
| Hemoglobin (g/L) | 95 ± 21 | 96 ± 23 | 110 ± 8 | 0.509 |
| Platelets (109/L) | 138 ± 95 | 78 ± 52 | 67 ± 38 | 0.076 |
| White blood cell (109/L) | 10.58 ± 4.78 | 9.07 ± 2.41 | 7.01 ± 2.34 | 0.170 |
| Neutrophil count (109/L) | 9.56 ± 4.61 | 6.84 ± 3.03 | 6.16 ± 1.68 | 0.159 |
| Percentage of central granulocyte count | 89.5 ± 6.0 | 84.9 ± 8.3 | 89.3 ± 7.0 | 0.322 |
| C-reactive protein (mg/L) | 98.2 ± 77.6 | 107.3 ± 96.1 | 159.3 ± 50.3 | 0.410 |
| Procalcitonin (ng/mL) | 7.504 ± 14.053 | 4.888 ± 4.323 | 10.927 ± 7.004 | 0.897 |
| Interleukin 6 (ng/mL) | 164.07 ± 358.46 | 169.29 ± 232.63 | 187.21 ± 170.99 | 0.292 |
| Total protein (g/L) | 50.8 ± 6.3 | 47.1 ± 6.0 | 46.1 ± 4.808 | 0.171 |
| Albumin (g/L) | 28.0 ± 4.4 | 27.2 ± 3.8 | 25.7 ± 3.0 | 0.630 |
| Creatinine (µmol/L) | 111 ± 88 | 94 ± 66 | 131 ± 79 | 0.664 |
| Blood urea nitrogen (mmol/L) | 11.03 ± 6.90 | 10.64 ± 7.24 | 25.19 ± 13.52 | 0.141 |
| Serum sodium (mmol/L) | 138.51 ± 3.96 | 139.12 ± 7.87 | 142.91 ± 3.64 | 0.338 |
| Serum potassium (mmol/L) | 4.02 ± 0.44 | 4.08 ± 0.37 | 4.03 ± 0.46 | 0.791 |
| Serum calcium (mmol/L) | 2.04 ± 0.15 | 2.00 ± 0.16 | 1.92 ± 0.09 | 0.396 |
| Serum phosphorus (mmol/L) | 0.93 ± 0.40 | 1.02 ± 0.58 | 1.10 ± 0.12 | 0.472 |
| Arterial blood gas potential of hydrogen | 7.427 ± 0.075 | 7.397 ± 0.075 | 7.441 ± 0.074 | 0.520 |
| Partial pressure of oxygen (mmHg) | 106.5 ± 39.7 | 103.3 ± 15.7 | 101.7 ± 31.7 | 0.886 |
| Partial pressure of carbon dioxide (mmHg) | 39.0 ± 8.3 | 35.8 ± 7.4 | 34.6 ± 8.7 | 0.435 |
| Bicarbonate (mmol/L) | 25.6 ± 4.3 | 21.8 ± 3.4 | 23.1 ± 2.3 | 0.050 |
| Lactic acid (mmol/L) | 1.4 ± 1.0 | 1.6 ± 1.1 | 1.7 ± 1.3 | 0.722 |
| Serum magnesium (mmol/L) | 0.96 ± 0.14 | 0.91 ± 0.11 | 0.84 ± 0.19 | 0.345 |
| Body mass index (kg/m2) | 23.47 ± 4.96 | 22.07 ± 4.98 | 23.44 ± 7.21 | 0.813 |
| Duration of mechanical ventilation, days | 10 ± 12 | 3 ± 5 | 8 ± 11 | 0.132 |
| Intensive care unit length of stay (days) | 14 ± 13 | 10 ± 10 | 17 ± 11 | 0.552 |
| Hospital length of stay (days) | 21+16 | 11 ± 11 | 32 ± 22 | 0.040a |
| Age (years) | 62 ± 18 | 58 ± 23 | 73 ± 13 | 0.460 |
Univariate analysis: To evaluate differences between the non-AGI group and different AGI grade groups, univariate analysis was conducted. As shown in Table 8, significant differences were noted in the: (1) Mean bowel sound rate, duration, amplitude, average frequency, and interval time; (2) Gastrin level; (3) SOFA score; and (4) APACHE II score among the three groups (P < 0.05). Similarly, significant differences were noted in the mean bowel sound rate, amplitude, average frequency, and interval time between the non-AGI and AGI I groups and between the non-AGI and AGI II groups (P < 0.05). We also found significant differences in the duration of bowel sounds between the non-AGI group and the AGI I (P < 0.001), AGI II (P = 0.018), and AGI III (P = 0.043) groups. A significant difference was found in gastrin levels between the AGI I and AGI III groups (P = 0.034). A significant difference was noted in the APACHE II scores between the non-AGI and AGI II groups (P < 0.05) (Table 9).
| Variables | Non-AGI (n = 30) | AGI I (n = 32) | AGI II (n = 10) | AGI III-IV (n = 3) | PF/χ2 | Pr | rs |
| Mean bowel sound rate (counts per minute) | 4.343 ± 2.756 | 1.308 ± 1.610 | 0.942 ± 0.994 | 0.717 ± 0.690 | < 0.001b | < 0.001b | -0.621 |
| Duration of bowel sounds (seconds) | 1.579 ± 1.049 | 0.513 ± 0.795 | 0.719 ± 1.385 | 0.178 ± 0.180 | < 0.001b | < 0.001b | -0.572 |
| Amplitude (dB) | 0.121 ± 0.097 | 0.032 ± 0.043 | 0.065 ± 0.144 | 0.011 ± 0.009 | < 0.001b | < 0.001b | -0.568 |
| Average frequency (Hz) | 708.559 ± 219.649 | 366.215 ± 238.280 | 344.720 ± 256.841 | 287.276 ± 260.841 | < 0.001b | < 0.001b | -0.592 |
| Interval time (seconds) | 3.977 ± 1.410 | 2.180 ± 1.554 | 1.851 ± 1.285 | 2.045 ± 2.171 | < 0.001b | < 0.001b | -0.514 |
| Citrulline (μmol/L) | 19.29 ± 1.46 | 18.47 ± 1.68 | 18.53 ± 1.94 | 18.22 ± 0.88 | 0.212 | 0.044a | -0.237 |
| Intestinal fatty acid-binding protein (ng/L) | 467 ± 59 | 489 ± 58 | 516 ± 61 | 467 ± 68 | 0.145 | 0.106 | 0.191 |
| Gastrin (ng/L) | 79.45 ± 12.16 | 72.49 ± 12.11 | 77.07 ± 11.22 | 93.33 ± 13.01 | 0.016a | 0.651 | -0.054 |
| Motilin (ng/L) | 215.20 ± 37.58 | 212.23 ± 38.97 | 218.42 ± 33.79 | 186.32 ± 46.98 | 0.625 | 0.692 | -0.047 |
| Sequential Organ Failure Assessment score, points | 6 ± 3 | 9 ± 4 | 9 ± 6 | 12 ± 3 | 0.033a | 0.008b | 0.308 |
| Acute Physiology and Chronic Health Evaluation II score, points | 17 ± 6 | 21 ± 8 | 27 ± 12 | 20 ± 5 | 0.025a | 0.017a | 0.276 |
| Hemoglobin (g/L) | 98 ± 26 | 95 ± 21 | 96 ± 23 | 110 ± 8 | 0.733 | 0.890 | 0.017 |
| Platelets (109/L) | 161 ± 104 | 138 ± 95 | 78 ± 52 | 67 ± 38 | 0.087 | 0.025a | -0.266 |
| white blood cell (109/L) | 8.45 ± 3.36 | 10.58 ± 4.78 | 9.07 ± 2.41 | 7.01 ± 2.34 | 0.202 | 0.339 | 0.115 |
| Neutrophil count (109/L) | 7.32 ± 3.09 | 9.56 ± 4.61 | 6.84 ± 3.03 | 6.16 ± 1.68 | 0.110 | 0.544 | 0.073 |
| Percentage of central granulocyte count | 84.9 ± 7.9 | 89.5 ± 6.0 | 84.9 ± 8.3 | 89.3 ± 7.0 | 0.069 | 0.201 | 0.154 |
| C-reactive protein (mg/L) | 99.3 ± 72.7 | 98.2 ± 77.6 | 107.3 ± 96.1 | 159.3 ± 50.3 | 0.544 | 0.625 | 0.059 |
| Procalcitonin (ng/mL) | 3.768 ± 7.037 | 7.504 ± 14.053 | 4.888 ± 4.323 | 10.927 ± 7.004 | 0.150 | 0.073 | 0.215 |
| Interleukin 6 (ng/mL) | 103.68 ± 256.797 | 164.07 ± 358.46 | 169.29 ± 232.63 | 187.21 ± 170.99 | 0.066 | 0.007b | 0.322 |
| Total protein (g/L) | 52.0 ± 6.7 | 50.8 ± 6.3 | 47.1 ± 6.0 | 46.1 ± 4.808 | 0.124 | 0.024a | -0.267 |
| Albumin (g/L) | 28.6 ± 3.6 | 28.0 ± 4.4 | 27.2 ± 3.8 | 25.7 ± 3.0 | 0.604 | 0.186 | -0.159 |
| Creatinine (µmol/L) | 115 ± 108 | 111 ± 88 | 94 ± 66 | 131 ± 79 | 0.827 | 0.767 | 0.036 |
| Blood urea nitrogen (mmol/L) | 11.25 ± 6.00 | 11.03 ± 6.90 | 10.64 ± 7.24 | 25.19 ± 13.52 | 0.219 | 0.746 | 0.039 |
| Serum sodium (mmol/L) | 140.28 ± 5.17 | 138.51 ± 3.96 | 139.12 ± 7.87 | 142.91 ± 3.64 | 0.216 | 0.584 | -0.066 |
| Serum potassium (mmol/L) | 4.09 ± 0.52 | 4.02 ± 0.44 | 4.08 ± 0.37 | 4.03 ± 0.46 | 0.933 | 0.944 | -0.008 |
| Serum calcium (mmol/L) | 2.07 ± 0.17 | 2.04 ± 0.15 | 2.00 ± 0.16 | 1.92 ± 0.09 | 0.310 | 0.126 | -0.183 |
| Serum phosphorus (mmol/L) | 0.94 ± 0.32 | 0.93 ± 0.40 | 1.02 ± 0.58 | 1.10 ± 0.12 | 0.623 | 0.725 | 0.042 |
| Potential of hydrogen | 7.457 ± 0.060 | 7.427 ± 0.075 | 7.397 ± 0.075 | 7.441 ± 0.074 | 0.114 | 0.021a | -0.273 |
| Partial pressure of oxygen (mmHg) | 106.4 ± 37.8 | 106.5 ± 39.7 | 103.3 ± 15.7 | 101.7 ± 31.7 | 0.980 | 0.910 | 0.014 |
| Partial pressure of carbon dioxide (mmHg) | 36.8 ± 8.9 | 39.0 ± 8.3 | 35.8 ± 7.4 | 34.6 ± 8.7 | 0.590 | 0.907 | 0.014 |
| Bicarbonate (mmol/L) | 26.4 ± 5.6 | 25.6 ± 4.3 | 21.8 ± 3.4 | 23.1 ± 2.3 | 0.073 | 0.036a | -0.249 |
| Lactic acid (mmol/L) | 1.2 ± 0.8 | 1.4 ± 1.0 | 1.6 ± 1.1 | 1.7 ± 1.3 | 0.529 | 0.140 | 0.177 |
| Serum magnesium (mmol/L) | 0.95 ± 0.13 | 0.96 ± 0.14 | 0.91 ± 0.11 | 0.84 ± 0.19 | 0.518 | 0.388 | -0.109 |
| Body mass index (kg/m2) | 22.52 ± 4.25 | 23.47 ± 4.96 | 22.07 ± 4.98 | 23.44 ± 7.21 | 0.855 | 0.784 | 0.036 |
| Age (years) | 59 ± 16 | 62 ± 18 | 58 ± 23 | 73 ± 13 | 0.406 | 0.232 | 0.140 |
| Group-group | P value | ||||||
| Mean bowel sound rate | Duration of bowel sounds | Amplitude of bowel sounds | Average frequency of bowel sounds | Interval time between bowel sounds | Gastrin | Acute Physiology and Chronic Health Evaluation II | |
| III-II | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 0.265 | 1.000 |
| III-I | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 0.034a | 1.000 |
| II-I | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 0.354 |
| III-non-AGI | 0.066 | 0.043a | 0.060 | 0.080 | 0.218 | 0.370 | 1.000 |
| II-non-AGI | 0.002b | 0.018a | 0.017a | 0.004b | 0.002b | 1.000 | 0.018a |
| I-non-AGI | < 0.001b | < 0.001b | < 0.001b | < 0.001b | < 0.001b | 0.172 | 0.515 |
Correlation analysis showed that several factors were negatively correlated with AGI grade, including: (1) Mean bowel sound rate (r = -0.621, P < 0.001), duration (r = -0.572, P < 0.001), amplitude (r = -0.568, P < 0.001), average frequency (r =
Collinearity diagnosis: (1) The mean bowel sound rate, duration, amplitude, average frequency, and interval time; (2) Cit level; (3) Platelet count; (4) Total protein level; (5) Blood gas pH; (6) HCO3- level; (7) IL-6 level; (8) SOFA score; and (9) APACHE II score were significantly correlated with AGI. To further diagnose collinearity among the variables, we used the variance inflation factor (VIF). There is multiple collinearity when VIF > 5 and eliminating the collinearity variables is required. The VIF of: (1) Mean bowel sound rate (VIF = 4.451), amplitude (VIF = 2.984), and interval time (VIF = 2.819); (2) Cit level (VIF = 1.297); (3) Gastrin level (VIF = 1.326); (4) SOFA score (VIF = 4.934); (5) APACHE II score (VIF = 3.902); (6) Platelet count (VIF = 1.905); (7) Total protein level (VIF = 1.258); (8) PH (VIF = 1.231); and (9) HCO3- level (VIF = 1.289) were all lower than 5, and each index had no multiple collinearity; hence, they could be used for the discriminant analysis.
Establishment of discriminant model: The results revealed the existence of two discriminant functions for analysis. Model 1 showed Wilks’ lambda = 0.175, χ² = 77.651, P < 0.001, and model 2 showed Wilks’ lambda = 0.454, χ² = 35.125, P = 0.019. Both models demonstrated statistical significance, underscoring the capability of the included variables to effectively classify cases and enhance the accuracy of the model.
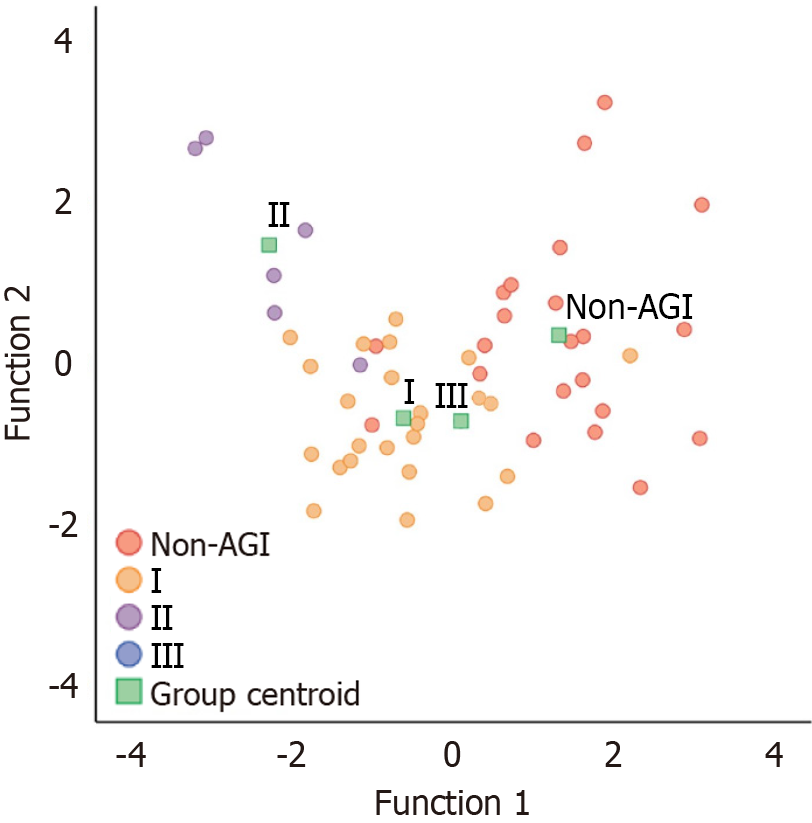
Table 10 presents the coefficients for the constructed canonical discriminant functions. We substituted various observation indicators of AGI to be evaluated into the following two discriminant functions, the AGI class to which the AGI being assessed belongs can be determined by identifying the group centroid closest to the obtained (Y1, Y2), as illustrated in Figure 4.
| Variables | Function | |
| 1 | 2 | |
| Acute Physiology and Chronic Health Evaluation II | -0.039 | 0.197 |
| Sequential Organ Failure Assessment | 0.053 | -0.386 |
| Gastrin | 0.019 | 0.032 |
| Citrulline | 0.132 | 0.076 |
| Interval time | 0.297 | 0.250 |
| Amplitude | -8.676 | 7.212 |
| Mean bowel sound rate | 0.473 | -0.202 |
| Total protein | 0.018 | 0.001 |
| Platelets | 0.004 | -0.005 |
| Potential of hydrogen | 5.856 | -0.090 |
| Bicarbonate | 0.005 | -0.065 |
| Constant | -50.170 | -2.538 |
Y1 = C + a1X1 + a2X2 + a3X3 +···+ anXn
Y2 = C + b1X1 + b2X2 + b3X3 +···+ bnXn
C is constant, a1, a2, a3...an and b1, b2, b3...bn are discriminant coefficients, X1, X2, X3...Xn are observation indexes or variables.
The Fisher linear discriminant function coefficients for different AGI grades are detailed in Table 11.
| Variables | Non-acute gastrointestinal injury | I | II | III |
| Acute Physiology and Chronic Health Evaluation II | 5.865 | 5.746 | 6.215 | 5.383 |
| Sequential Organ Failure Assessment | -0.983 | -0.694 | -1.595 | -0.361 |
| Gastrin | 0.174 | 0.098 | 0.152 | 0.382 |
| Citrulline | 4.486 | 4.156 | 4.093 | 4.133 |
| Interval time | 6.162 | 5.274 | 5.449 | 7.783 |
| Amplitude | -853.653 | -844.614 | -813.995 | -839.793 |
| Mean bowel sound rate | 28.872 | 28.205 | 26.892 | 27.004 |
| Total protein | 0.604 | 0.574 | 0.532 | 0.325 |
| Platelets | 0.235 | 0.232 | 0.214 | 0.229 |
| Potential of hydrogen | 2436.999 | 2426.586 | 2414.747 | 2398.098 |
| Bicarbonate | -0.939 | -0.880 | -1.032 | -0.936 |
| Constant | -9230.872 | -9136.797 | -9048.069 | -8942.058 |
Non-AGI group: F1 = C01 + C11X1 + C21X2 + C31X3 +···+Cm1Xm
AGI I group: F2 = C02 + C12X1 + C22X2 + C32X3 +···+Cm2Xm
AGI II group: F3 = C03 + C13X1 + C23X2 + C33X3 +···+Cm3Xm
AGI III group: F4 = C04 + C14X1 + C24X2 + C34X3 +···+Cm4Xm
Cjk is the discriminant coefficients (j = 0, 1, 2,···m, k = 1, 2, 3, 4), X1, X2, X3,···Xm are the variable values.
The indices of the unevaluated AGI were substituted into the above four discriminant functions, and the four values obtained were compared. Higher values of these indices indicated the AGI grade of the unevaluated AGI. Compared to the canonical discriminant function, the two discriminant results were the same, whereas the Fisher linear discriminant function was more convenient to use.
Discriminant effect evaluation: In the retrospective assessment of misjudgment probability, we resubstituted the research objects to establish the discriminant function into the function for discrimination. The model correctly classified 92.5% of the original grouped cases with a misjudgment probability of 0.075. This finding, which is below 0.1, indicates its application value.
The leave-one-out cross-validation procedure sequentially eliminated a research object, using the remaining research object data to establish a discriminant function, and subsequently used the discriminant function to identify the deleted research object, repeating the above steps 75 times. The above model correctly classified 69.8% of the cross-validated grouped cases.
We employed discriminant analysis as a statistical method to develop a diagnostic discriminant model for AGI. This model was constructed using intestinal sound characteristic data, biomarkers, and related clinical information. The misjudgment probability of the model was 0.075, and its accuracy was high, with good application value. In the leave-one-out cross-validation, the accuracy of the model was 69.8%, possibly related to the small sample size. Gastrointestinal dysfunction is a common coexisting disease in critically ill patients. Previously available assessment tools include the Lausanne Intestinal Failure Estimation (2008)[14], the Gastrointestinal Failure score[15], and the European Society for Clinical Nutrition and Metabolism endorses recommendations for IF[16]. These recommendations primarily depend on the assessment of clinical symptoms based on the subjective judgment of the observer, rather than objective laboratory parameters and specific biomarkers. However, these tools have not been formally verified; therefore, there is still a lack of consistent and effective methods to assess the severity of gastrointestinal dysfunction. The AGI definition and grading diagnostic criteria proposed by ESICM in 2012 based on available medical evidence and expert opinions are now widely used in ICU[5]. In a recent prospective multicenter study based on the theory of AGI classification, structural components of symptoms were employed to quantitatively evaluate gastrointestinal dysfunction while increasing the prognostic value of existing scoring systems. However, lack of consistent and effective methods to assess the severity of gastrointestinal dysfunction remains. While the approach seems to be a reliable clinical tool to evaluate the severity and short-term prognosis of critically ill patients[17], its effectiveness and reproducibility still need to be confirmed by further prospective studies. In our study, prospective observation and data collection were conducted among patients admitted to the ICU, accurately reflecting the situation in the ICU. The model was constructed using objective data parameters, such as bowel sound, biomarkers, disease severity score, and biochemical indicators, to avoid subjective factors and comprehensively and objectively evaluate AGI and its severity. To the best of our knowledge, this is the first such attempt in this field of research.
Our results showed that the characteristic index of bowel sound is negatively correlated with AGI grade. The higher the AGI grade, the more severe the gastrointestinal injury and the lower the monitoring value of the bowel sound characteristic index, indicating a decline in gastrointestinal motility function. Among these indices, the mean bowel sound rate, amplitude, and interval time played a pivotal role in the model, with a larger coefficient and a substantial contribution to discrimination. However, in the AGI II group, the duration and amplitude of bowel sounds were longer and higher, respectively, whereas the interval time in the AGI III–IV group was longer, and the differences in characteristic indexes of intestinal sounds between the groups were not ideal, which may be associated with the complex condition of ICU patients and the various treatment methods and procedures used that interfere with the evaluation of gastrointestinal function to some extent. Furthermore, abdominal symptoms and signs are not always related only to the gastrointestinal tract. In the ICU, more than 50% of patients receive sedatives and analgesics[18]. Opioids act on opioid receptors, which can inhibit the excitability of intestinal neurons, disrupt nerve transmission, and cause an imbalance in the release of neurotransmitters, thus damaging the normal movement of the gastrointestinal tract[19]. Mechanically ventilated patients receiving sedative therapy may experience varying degrees of slow gastric emptying and proximal food retention[20]. Catecholamines, widely used in ICU patients, have a direct dose-dependent inhibitory effect on small intestinal motility[21]. An autopsy case study showed that the number of interstitial cells of Cajal (ICCs) in critically ill patients was significantly lower than that in individuals in the control group [0.45/high power field (HPF) vs 7.25/HPF], and ICCs were almost depleted, suggesting damage to the colon and ICCs in critically ill patients, leading to gastrointestinal motility disorders[22]. In summary, in critically ill patients, gastrointestinal motility is very sensitive to any stress response, such as hemodynamic instability, multiple organ failure, abdominal surgery, trauma, inflammation, hypoxia, malnutrition, water and electrolyte imbalance, abnormal glucose levels, and the use of multiple drugs, etc., which can cause the gastrointestinal tract to lose the balance between sympathetic and parasympathetic nerve signals[23]. Due to the complexity of gastrointestinal function assessment, few studies have used bowel sounds, abdominal pain and other signs as assessment criteria for gastrointestinal dysfunction. Nevertheless, in our study, digital bowel sound monitoring technology was used. Compared with the time-consuming and labor-intensive traditional stethoscope method of obtaining bowel sound information, digital bowel sound monitoring technology can achieve non-invasive, portable, remote and real-time continuous collection of bowel sounds. Computer technology can be used to effectively identify bowel sounds while reducing noise and eliminating interference, and output bowel sound information comprehensively and objectively[6]. A study in patients with irritable bowel syndrome and those with non-ulcerative dyspepsia showed the value of abdominal computer auscultation in the objective classification of patients with functional bowel disease[24]. In patients with intestinal obstruction, the sound duration of colorectal obstruction was notably longer and the dominant frequency was higher than those in patients with small intestinal obstruction. These acoustic characteristics can help determine the possible location of the obstruction[25]. Kim et al[26] designed a non-invasive intestinal motility estimation algorithm based on the back-propagation neural network model of bowel sounds design that can continuously monitor and evaluate intestinal motility. Computer-assisted acoustic gastrointestinal monitoring not only distinguishes healthy controls from postoperative patients but also further distinguishes patients with intestinal obstruction from those without intestinal obstruction[27]. Various types and severities of gastrointestinal diseases have different intestinal sound characteristics, which is also proven by including intestinal sound characteristic data in the AGI discriminant model in our study. These findings suggest that continuous digital bowel sound monitoring technology has great potential for application and may be a powerful tool for diagnosing AGI.
Although gastrointestinal dysfunction in ICU patients is common and complex, its assessment relies predominantly on clinical symptoms and subjective judgment. There has been no clear distinction between gastrointestinal dysfunction at different severities. Therefore, there has been a widespread interest in identifying biomarkers that can objectively assess the pathological development of AGI to replace or improve clinical assessments. Cit and I-FABP have certain clinical application prospects. Plasma Cit is a reliable marker for quantitatively evaluating intestinal function, and a reduced Cit level indicates a substantial reduction in intestinal cell mass and function in different human disease conditions. This reduction indicates intestinal cell mass loss and dysfunction[9,10]. Low plasma Cit levels are substantially prevalent among critically ill patients and are associated with a poor prognosis. A Cit level < 10 μmol/L is considered to be an effective threshold for evaluating intestinal dysfunction in critically ill patients[28]. I-FABP is an effective biomarker that reflects the injury of ischemic intestinal cells and can be employed for early diagnosis of intestinal ischemia[11,29]. The results of a previous multicenter study demonstrated that, compared with conventional biochemical markers, serum I-FABP level exhibited superior sensitivity, positive predictive value, and negative predictive value for diagnosing small intestinal ischemia and could effectively identify patients with acute abdomen at risk of small intestinal ischemia[30]. However, to the best of our knowledge, no studies are available on the use of biomarkers to evaluate AGI grading. In our study, we observed that Cit level is an independent risk factor for AGI, possesses diagnostic value for AGI, correlates with AGI grading, and can be used to construct an AGI grading prediction model. However, the study of I-FABP results thus far cannot fully support its application in AGI grading, which is consistent with the results of a previous study[31]. In this study, the I-FABP level gradually increased in the non-AGI, AGI I, and AGI II groups, while the I-FABP level in the AGI III group decreased significantly, approaching that of the non-AGI group. This may be due to the small sample size of the AGI III group, which has a certain bias and cannot accurately represent the true level of I-FABP at the severity level of AGI III. A recent prospective cohort study showed that serum I-FABP and Cit concentrations were not ideal predictors of gastrointestinal dysfunction[32]. Critically ill patients with complex medical conditions, often combined with systemic inflammatory response syndrome and acute renal failure, have increased extraintestinal Cit synthesis and renal accumulation, resulting in pseudo-elevated blood Cit concentrations that mask the reduced synthesis of Cit in the intestine[33], which may partly account for the lack of significant differences in Cit between groups. Most of the current studies are based on the present descriptive AGI grading diagnostic criteria, and the diagnosis of AGI grading itself may be affected by a certain degree of subjective factors. In addition, it is important to note that Cit and I-FABP levels may be elevated in many diseases associated with gastrointestinal, renal, or liver disorders, and critically ill patients may have these diseases simultaneously. Therefore, we cannot completely deny the association between Cit and I-FABP and AGI, and multicenter studies with larger sample sizes are needed to further explore and clarify its clinical value in AGI.
Our model included variables such as APACHE II and SOFA scores. Critically ill patients frequently experience multiple organ dysfunction, and the evaluation of gastrointestinal function is affected by other organ dysfunction; therefore, it is reasonable to use the SOFA and APACHE II scores as AGI predictive model variables. These patients are often unable to eat, their neuroendocrine function is affected, and their gastrointestinal hormone levels are altered. Additionally, critically ill patients are frequently hypermetabolic, and gastrointestinal dysfunction often affects the implementation of nutrition. Furthermore, various coexisting diseases affect the immune, secretory, and dynamic functions of the gastrointestinal tract to varying degrees, resulting in vomiting, diarrhea, gastric retention, malnutrition, and electrolytic acid-base imbalances. Therefore, gastrin, total protein, platelet count, pH, and HCO3- included in the model may be effective indicators for evaluating AGI, and their effectiveness and accuracy need to be further studied and verified.
This study has some limitations. First, it was conducted at a single center, the sample size was small, we used retrospective misclassification probability assessment and leave-one-out cross-validation to internally validate the model. No additional sample size was added for external validation, and the universality of the model was not fully evaluated. All patients involved were given active treatment and medication. Some critically ill patients died before the gastrointestinal injury progressed to AGI IV, and some patients opted to discontinue treatment; therefore, only patients with AGI I–III were included in our study. Second, the mechanism of gastrointestinal function injury is complex; hence, the selected indicators may still be one-sided and cannot be used for comprehensive evaluation of AGI, and their diagnostic value is limited. In addition, digital bowel sound monitoring technology may require further optimization and improvement, and collaborations between clinical professionals, engineers, and software developers in the future may help make new progress in gastrointestinal diagnosis. Due to these limitations, the current value of the model is limited and needs further verification. However, the method of using objective indicators to build a discriminant model requires more research. Objective indicators based on a larger sample size and optimize the model must be researched. More statistical methods such as discriminant analysis and the establishment of predictive models may be used, such as the AGI diagnostic model, which is based on statistical analysis and may be useful as a clinical diagnostic tool for gastrointestinal dysfunction.
Using objective indices such as intestinal sound characteristic data, biomarkers, disease severity scores, and blood biochemistry parameters, a discriminant model for AGI diagnosis was developed based on discriminant analysis. This model may be an effective method for clinical AGI grading. However, its accuracy and application value require further study.
We are grateful to the clinical and research staff in the ICU of The First Affiliated Hospital of Xi'an Jiaotong University for support during this study, and to Zeng LX for statistical advice.
| 1. | Reintam A, Parm P, Kitus R, Kern H, Starkopf J. Gastrointestinal symptoms in intensive care patients. Acta Anaesthesiol Scand. 2009;53:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Reintam Blaser A, Poeze M, Malbrain ML, Björck M, Oudemans-van Straaten HM, Starkopf J; Gastro-Intestinal Failure Trial Group. Gastrointestinal symptoms during the first week of intensive care are associated with poor outcome: a prospective multicentre study. Intensive Care Med. 2013;39:899-909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 3. | Mittal R, Coopersmith CM. Redefining the gut as the motor of critical illness. Trends Mol Med. 2014;20:214-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 241] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 4. | Nieuwenhuijzen GA, Goris RJ. The gut: the 'motor' of multiple organ dysfunction syndrome? Curr Opin Clin Nutr Metab Care. 1999;2:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Reintam Blaser A, Malbrain ML, Starkopf J, Fruhwald S, Jakob SM, De Waele J, Braun JP, Poeze M, Spies C. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems. Intensive Care Med. 2012;38:384-394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 364] [Article Influence: 28.0] [Reference Citation Analysis (1)] |
| 6. | Zhao K, Jiang H, Wang Z, Chen P, Zhu B, Duan X. Long-Term Bowel Sound Monitoring and Segmentation by Wearable Devices and Convolutional Neural Networks. IEEE Trans Biomed Circuits Syst. 2020;14:985-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Zhou P, Lu M, Chen P, Wang D, Jin Z, Zhang L. Feasibility and basic acoustic characteristics of intelligent long-term bowel sound analysis in term neonates. Front Pediatr. 2022;10:1000395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Blaser A, Padar M, Tang J, Dutton J, Forbes A. Citrulline and intestinal fatty acid-binding protein as biomarkers for gastrointestinal dysfunction in the critically ill. Anaesthesiol Intensive Ther. 2019;51:230-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Shen LJ, Guan YY, Wu XP, Wang Q, Wang L, Xiao T, Wu HR, Wang JG. Serum citrulline as a diagnostic marker of sepsis-induced intestinal dysfunction. Clin Res Hepatol Gastroenterol. 2015;39:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr. 2008;27:328-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 303] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 11. | Kanda T, Fujii H, Tani T, Murakami H, Suda T, Sakai Y, Ono T, Hatakeyama K. Intestinal fatty acid-binding protein is a useful diagnostic marker for mesenteric infarction in humans. Gastroenterology. 1996;110:339-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 183] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Kanda T, Fujii H, Fujita M, Sakai Y, Ono T, Hatakeyama K. Intestinal fatty acid binding protein is available for diagnosis of intestinal ischaemia: immunochemical analysis of two patients with ischaemic intestinal diseases. Gut. 1995;36:788-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Schellekens DH, Grootjans J, Dello SA, van Bijnen AA, van Dam RM, Dejong CH, Derikx JP, Buurman WA. Plasma intestinal fatty acid-binding protein levels correlate with morphologic epithelial intestinal damage in a human translational ischemia-reperfusion model. J Clin Gastroenterol. 2014;48:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Berger MM, Oddo M, Lavanchy J, Longchamp C, Delodder F, Schaller MD. Gastrointestinal failure score in critically ill patients. Crit Care. 2008;12:436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Reintam A, Parm P, Kitus R, Starkopf J, Kern H. Gastrointestinal failure score in critically ill patients: a prospective observational study. Crit Care. 2008;12:R90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Pironi L, Arends J, Baxter J, Bozzetti F, Peláez RB, Cuerda C, Forbes A, Gabe S, Gillanders L, Holst M, Jeppesen PB, Joly F, Kelly D, Klek S, Irtun Ø, Olde Damink SW, Panisic M, Rasmussen HH, Staun M, Szczepanek K, Van Gossum A, Wanten G, Schneider SM, Shaffer J; Home Artificial Nutrition & Chronic Intestinal Failure; Acute Intestinal Failure Special Interest Groups of ESPEN. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin Nutr. 2015;34:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 414] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 17. | Reintam Blaser A, Padar M, Mändul M, Elke G, Engel C, Fischer K, Giabicani M, Gold T, Hess B, Hiesmayr M, Jakob SM, Loudet CI, Meesters DM, Mongkolpun W, Paugam-Burtz C, Poeze M, Preiser JC, Renberg M, Rooijackers O, Tamme K, Wernerman J, Starkopf J. Development of the Gastrointestinal Dysfunction Score (GIDS) for critically ill patients - A prospective multicenter observational study (iSOFA study). Clin Nutr. 2021;40:4932-4940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (1)] |
| 18. | Richards-Belle A, Canter RR, Power GS, Robinson EJ, Reschreiter H, Wunsch H, Harvey SE. National survey and point prevalence study of sedation practice in UK critical care. Crit Care. 2016;20:355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Wood JD, Galligan JJ. Function of opioids in the enteric nervous system. Neurogastroenterol Motil. 2004;16 Suppl 2:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 249] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 20. | Nguyen NQ, Chapman MJ, Fraser RJ, Bryant LK, Burgstad C, Ching K, Bellon M, Holloway RH. The effects of sedation on gastric emptying and intra-gastric meal distribution in critical illness. Intensive Care Med. 2008;34:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Fruhwald S, Scheidl S, Toller W, Petnehazy T, Holzer P, Metzler H, Hammer HF. Low potential of dobutamine and dopexamine to block intestinal peristalsis as compared with other catecholamines. Crit Care Med. 2000;28:2893-2897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Shimizu K, Ogura H, Matsumoto N, Ikeda M, Yamamoto H, Mori M, Morii E, Shimazu T. Interstitial cells of Cajal are diminished in critically ill patients: Autopsy cases. Nutrition. 2020;70:110591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Fruhwald S, Holzer P, Metzler H. Intestinal motility disturbances in intensive care patients pathogenesis and clinical impact. Intensive Care Med. 2007;33:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Craine BL, Silpa ML, O'Toole CJ. Two-dimensional positional mapping of gastrointestinal sounds in control and functional bowel syndrome patients. Dig Dis Sci. 2002;47:1290-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Ching SS, Tan YK. Spectral analysis of bowel sounds in intestinal obstruction using an electronic stethoscope. World J Gastroenterol. 2012;18:4585-4592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 26. | Kim KS, Seo JH, Song CG. Non-invasive algorithm for bowel motility estimation using a back-propagation neural network model of bowel sounds. Biomed Eng Online. 2011;10:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Spiegel BM, Kaneshiro M, Russell MM, Lin A, Patel A, Tashjian VC, Zegarski V, Singh D, Cohen SE, Reid MW, Whitman CB, Talley J, Martinez BM, Kaiser W. Validation of an acoustic gastrointestinal surveillance biosensor for postoperative ileus. J Gastrointest Surg. 2014;18:1795-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Piton G, Belon F, Cypriani B, Regnard J, Puyraveau M, Manzon C, Navellou JC, Capellier G. Enterocyte damage in critically ill patients is associated with shock condition and 28-day mortality. Crit Care Med. 2013;41:2169-2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 29. | Thuijls G, van Wijck K, Grootjans J, Derikx JP, van Bijnen AA, Heineman E, Dejong CH, Buurman WA, Poeze M. Early diagnosis of intestinal ischemia using urinary and plasma fatty acid binding proteins. Ann Surg. 2011;253:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 30. | Kanda T, Tsukahara A, Ueki K, Sakai Y, Tani T, Nishimura A, Yamazaki T, Tamiya Y, Tada T, Hirota M, Hasegawa J, Funaoka H, Fujii H, Hatakeyama K. Diagnosis of ischemic small bowel disease by measurement of serum intestinal fatty acid-binding protein in patients with acute abdomen: a multicenter, observer-blinded validation study. J Gastroenterol. 2011;46:492-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Wang J, Yu L, Xia Y, Gao Y, Yu W, Sun H, Sun Y. [Diagnostic value of citrulline and intestinal fatty acid binding protein on acute gastrointestinal injury in critical patients: a prospective study of 530 patients]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2017;29:999-1003. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 32. | Onuk S, Ozer NT, Ozel M, Sipahioglu H, Kahriman G, Baskol G, Temel S, Gundogan K, Akin A. Gastric ultrasound, citrulline, and intestinal fatty acid-binding protein as markers of gastrointestinal dysfunction in critically ill patients: A pilot prospective cohort study. JPEN J Parenter Enteral Nutr. 2023;47:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 33. | Piton G, Manzon C, Cypriani B, Carbonnel F, Capellier G. Acute intestinal failure in critically ill patients: is plasma citrulline the right marker? Intensive Care Med. 2011;37:911-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |