Published online Dec 27, 2024. doi: 10.4240/wjgs.v16.i12.3780
Revised: September 24, 2024
Accepted: October 22, 2024
Published online: December 27, 2024
Processing time: 91 Days and 1.7 Hours
The Kasai procedure (KPE) is an important treatment for biliary atresia (BA), the most common cause of neonatal obstructive jaundice.
To investigate the efficacy of robotic-assisted Kasai portoenterostomy (RAKPE) in patients with BA.
Clinical data of 10 patients with BA who underwent RAKPE at the Seventh Medi
RAKPE was successfully completed in all nine patients, with an average total operative time of 352.2 minutes (including intraoperative cholangiography). Milk feeding resumed on an average 9.89 days postoperatively, and the average time of drainage tube removal was 18.11 days. All patients were followed up for 6 mon
RAKPE is technically feasible, safe, and effective for treating BA. Once the technique is mastered, RAKPE may achieve CJ outcomes comparable to those of OKPE.
Core Tip: We presented the results of some patients with biliary atresia treated with Robotic-assisted Kasai portoenterostomy and discussed the technical details. The conclusion is satisfactory, and the surgical outcome is largely consistent with that of Open Kasai portoenterostomy. Robot-assisted Kasai portoenterostomy can overcome the obstacles of traditional laparoscopic surgery and is likely to be more widely applied in the future.
- Citation: Xing GD, Wang XQ, Duan L, Liu G, Wang Z, Xiao YH, Xia Q, Xie HW, Shen Z, Yu ZZ, Huang LM. Robotic-assisted Kasai portoenterostomy for child biliary atresia. World J Gastrointest Surg 2024; 16(12): 3780-3785
- URL: https://www.wjgnet.com/1948-9366/full/v16/i12/3780.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i12.3780
The Kasai procedure (KPE) is a key treatment for biliary atresia (BA), the most common cause of neonatal obstructive jaundice. The clearance of jaundice (CJ) rate 3 months post- surgery is the primary index for evaluating the effect of KPE. Laparoscopic Kasai portoenterostomy (LKPE) is technically challenging and remains controversial. In its early stages, LKPE was not recommended by the International Pediatric Endosurgery Group in a 2007 study due to its low CJ rate[1]. However, a later study demonstrated that LKPE was comparable to Open Kasai portoenterostomy (OKPE) in terms of CJ rate. The smaller anatomical area of the porta hepatis and overcoming the learning curve were potential factors believed to contribute to the improved CJ rate[2].
Robotic-assisted Kasai portoenterostomy (RAKPE), which allows fiber block dissection at an optimal angle, may facilitate extensive dissection at the porta hepatis, transection of shallow fibrous masses, and CJ. Herein, we present the clinical outcomes of 10 patients with BA treated with RAKPE and discuss the technical details.
We conducted a retrospective single-center analysis of 10 patients with BA who underwent RAKPE at the Seventh Medical Center of the People's Liberation Army General Hospital between December 2018 and December 2021. One patient underwent OKPE due to intraoperative bleeding, leaving nine patients in the study group. Fifty-two patients who underwent OKPE during the same period were selected as the control group. The selection process for the surgical approach involved fully informing the parents of the child patient about the advantages and disadvantages of both surgical options, with the final decision being made by the parents themselves. Preoperative and postoperative bio
| Variables | RAKPE group (n = 9) | OKPE group (n = 52) | P value |
| Sex | 0.550 | ||
| Male | 3 | 30 | |
| Female | 6 | 22 | |
| Age at operation (days) | 66.11 (15.037) | 63.98 (15.727) | 0.707 |
| Height (cm) | 57 (3.391) | 57 (2.584) | 1.000 |
| Weight (kg) | 5.167 (0.6144) | 4.896 (0.9199) | 0.280 |
| Preoperative TBIL (μmol/L) | 169 (38.7879) | 184.1 (44.0634) | 0.339 |
| Preoperative DBIL(μmol/L) | 124.889 (33.0471) | 126.738 (31.91) | 0.874 |
| ALT (U/L) | 158.78 (113.796) | 149.23 (110.796) | 0.813 |
| Variables | RAKPE group (n = 9) | OKPE group (n = 52) | P value |
| Operative time (minutes) | 352.22 (18.047) | 180.1 (46.752) | 0.000 |
| intraoperative bleeding (mL) | 22.22 (12.528) | 13.02 (13.908) | 0.069 |
| Time to milk feeding (days) | 9.89 (4.014) | 7.9 (2.098) | 0.182 |
| Time of drainage tube removal (days) | 18.11 (3.951) | 13.46 (3.878) | 0.008 |
| Postoperative hospital stay (days) | 23.35 (10.972) | 22 (10.701) | 0.986 |
| CJ at 3 months (%) | 88.9 (8/9) | 71.1 (37/52) | 0.423 |
This study was approved by the Ethics Committee of the Seventh Medical Center of the People's Liberation Army General Hospital. The legal guardians of the patients were fully informed and signed the informed consent form.
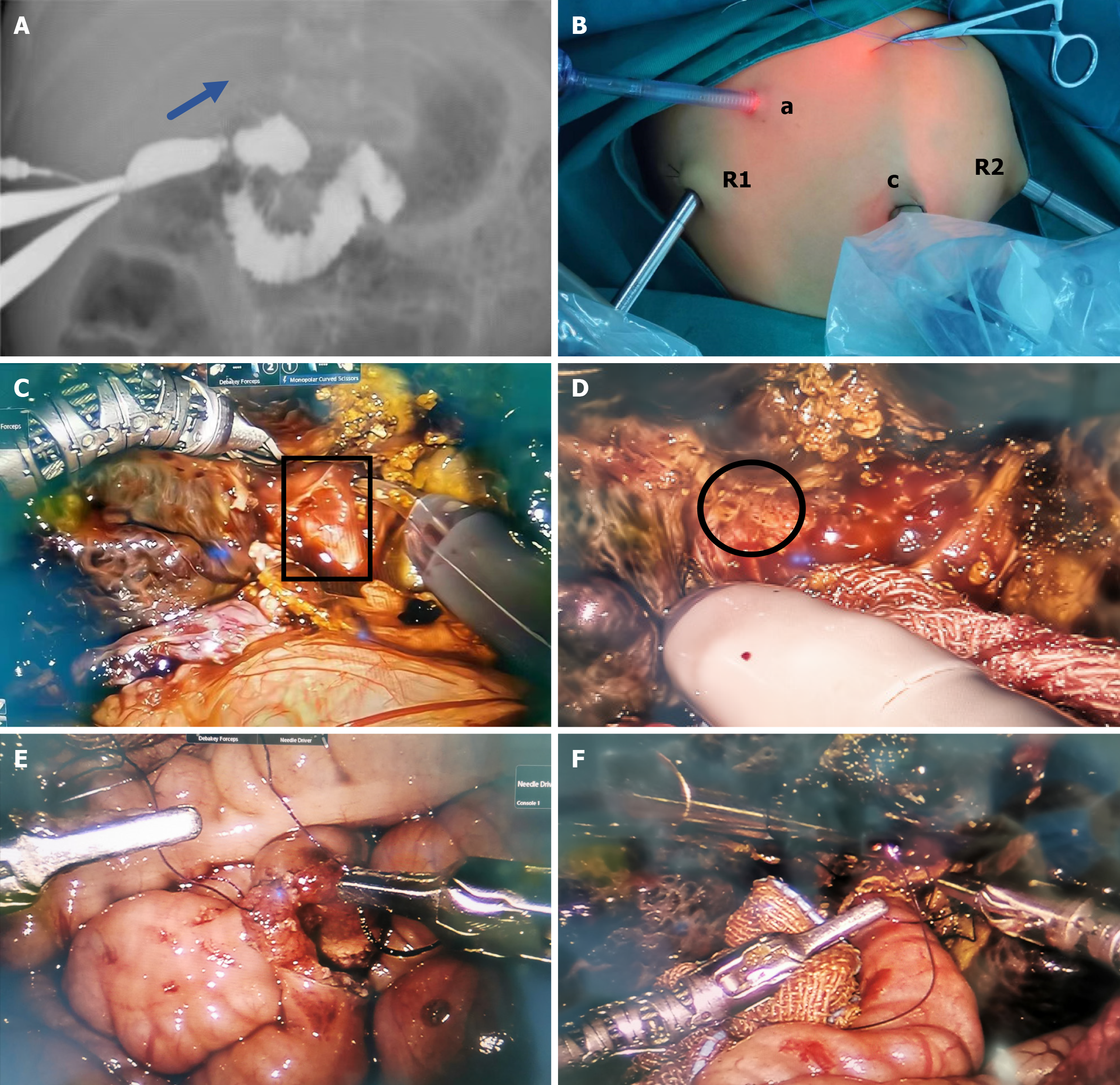
Intraoperative cholangiography: Following general anesthesia, each patient was placed in the supine position with indwelling gastric tubes. A 8.5-mm trocar was introduced through a small incision in the umbilical ring, and a lapa
RAKPE: First, each patient was placed in the supine position. The three-armed Da Vinci robotic system was positioned directly at the head of the bed. The trocar positions are shown in Figure 1B. An 8.5-mm trocar was used to establish carbon dioxide pneumoperitoneum at a pressure of 6-8 mmHg (1 mmHg = 0.133 kPa) and served as the camera port (C). The robotic instruments were 8 mm in diameter. Two 8-mm operation ports (R1 and R2) were placed in the right upper middle and left lower abdomen, respectively. Another 5-mm accessory port (A) was introduced at the small incision of the body surface projection of the gallbladder with the camera and instruments placed. Second, we dissociated the gallbladder, dissected the hepatoduodenal ligament and the right branch of the hepatic artery, ligated the middle hepatic artery, and exposed the portal vein. Small branches (arrows) of the portal vein (triangles) were dissected using coa
The patients were followed up at 1 month, 3 months, 6 months, 9 months, and 1 year after discharge, and once every 6 months after that. The indicators included routine blood tests, liver function tests, and liver ultrasonography. Patients were readmitted to the hospital at any time for further anti-infective treatment when they suffered from fever and jaundice aggravation or if cholangitis was highly suspected.
Ten patients with BA underwent RAKPE, of whom one patient was converted to OKPE. The criterion for transitioning from RAKPE to OKPE was the presence of uncontrollable bleeding factors. The operation was successfully completed in nine patients, with an average total operative time of 352.2 minutes (including intraoperative cholangiography). Milk feeding was resumed on an average of 9.89 days post-surgery, and drainage tubes were removed on an average 18.11 days after surgery. The average postoperative hospital stay was 23.35 days. All patients were followed up for 6 months to 2 years. The liver function indicators and bilirubin levels of 8 of the 9 patients returned to normal within 3 months after surgery. Three patients developed recurrent cholangitis after discharge, and their white blood cell counts, liver function indicators, and bilirubin levels increased to varying degrees, requiring hospitalization for intravenous antibiotic treat
Since the completion of the first LKPE for BA in 2002[3], few relevant studies have been published. Over the past 20 years, LKPE has been tested and opposed by some scholars[4-6]. It is generally believed that the early CJ rate achieved with LKPE is not comparable with that of OKPE. Porta hepatis dissection is relatively complex, and excessive electrocoagulation for local bleeding during laparoscopy can cause varying degrees of thermal injury to the micro-bile ducts[7]. Moreover, the limited scissor angle when excising the fibrous mass in the hepatic portal region raises questions about whether LKPE can achieve satisfactory bile drainage[8], contributing to prolonged learning curve[9]. Furthermore, the learning curve for LKPE in BA is longer due to associated morbidity. However, no clear evidence supports that LKPE results in minimal intra-abdominal adhesions during subsequent liver transplantation[5,6].
Recent studies have demonstrated that satisfactory bile drainage can be achieved without excessive dissection at the porta hepatis, with the CJ rate consistent with that achieved through extensive dissection at the porta hepatis, a pre
Among the 10 RAKPE cases in this study, one was converted to OKPE due to intraoperative bleeding caused by the operator’s inexperience, and the remaining nine patients completed the surgery. The RAKPE group had a longer ope
The robotic-assisted technique provides new perspectives to overcome the limitations of traditional laparoscopic sur
As a new technology, using simulators for training can improve surgeons’ proficiency and reduce surgery time, shortening the learning curve. In this study, the requirements for the robotic operating space were high since the patients who underwent RAKPE weighed approximately 5 kg and were approximately 2 months old. We overcame this difficulty by retracting the umbilical trocar and adding a conventional 5 mm operating instrument to guarantee a smooth operation. During the operation, suspending specific organs with silk threads can also enhance the surgical space.
Because the intestinal anastomosis and the anastomosis between the porta hepatis and the jejunum were performed with robotic assistance, there is a concern of anastomotic leak. Therefore, we removed the drainage tube in the RAKPE group later than in the OKPE group; however, the postoperative hospital stay was not significantly different. The main postoperative complication of both surgical methods is cholangitis, and the treatment plan involves strengthening anti-infection measures, drawing blood cultures for drug sensitivity testing, and using antibiotics rationally. The short-term outcomes of the patients in the RAKPE group (Table 2) revealed that the CJ rates were comparable with those of the OKPE group. Thus, we believe that RAKPE can replace OKPE. Given the shorter learning curve for RAKPE and its superior ability to navigate the anatomy of the porta hepatis, the outcomes of RAKPE are expected to be better than those of conventional LKPE.
Our results demonstrate that RAKPE is a technically feasible, safe, and effective surgical treatment for BA. After crossing the learning curve, it achieved CJ rates similar to those of OKPE. However, the mid/Long-term effects and long-term survival rates of the two techniques should be investigated in prospective, large-sample studies.
| 1. | Dutta S, Woo R, Albanese CT. Minimal access portoenterostomy: advantages and disadvantages of standard laparoscopic and robotic techniques. J Laparoendosc Adv Surg Tech A. 2007;17:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Meehan JJ, Elliott S, Sandler A. The robotic approach to complex hepatobiliary anomalies in children: preliminary report. J Pediatr Surg. 2007;42:2110-2114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Esteves E, Clemente Neto E, Ottaiano Neto M, Devanir J Jr, Esteves Pereira R. Laparoscopic Kasai portoenterostomy for biliary atresia. Pediatr Surg Int. 2002;18:737-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Wong KK, Chung PH, Chan KL, Fan ST, Tam PK. Should open Kasai portoenterostomy be performed for biliary atresia in the era of laparoscopy? Pediatr Surg Int. 2008;24:931-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Hussain MH, Alizai N, Patel B. Outcomes of laparoscopic Kasai portoenterostomy for biliary atresia: A systematic review. J Pediatr Surg. 2017;52:264-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Joob B, Wiwanitkit V. Laparoscopic versus conventional Kasai portoenterostomy. J Laparoendosc Adv Surg Tech A. 2013;23:177. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Ji Y, Yang K, Zhang X, Jin S, Jiang X, Chen S, Xu Z. The short-term outcome of modified laparoscopic Kasai portoenterostomy for biliary atresia. Surg Endosc. 2021;35:1429-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Ji Y, Zhang X, Chen S, Li Y, Yang K, Zhou J, Xu Z. Medium-term outcomes after laparoscopic revision of laparoscopic Kasai portoenterostomy in patients with biliary atresia. Orphanet J Rare Dis. 2021;16:193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Chan KW, Lee KH, Tsui SY, Wong YS, Pang KY, Mou JW, Tam YH. Laparoscopic versus open Kasai portoenterostomy in infant with biliary atresia: a retrospective review on the 5-year native liver survival. Pediatr Surg Int. 2012;28:1109-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Zhang M, Cao G, Li X, Zhang X, Li Y, Chi S, Rong L, Tang ST. Robotic-assisted Kasai portoenterostomy for biliary atresia. Surg Endosc. 2023;37:3540-3547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |