Published online Dec 27, 2024. doi: 10.4240/wjgs.v16.i12.3772
Revised: September 10, 2024
Accepted: September 12, 2024
Published online: December 27, 2024
Processing time: 99 Days and 1.3 Hours
The global incidence of esophageal cancer (EC) remains high. Despite advan
To analyze the survival prognosis and influencing factors of esophageal squamous cell carcinoma (ESCC).
A retrospective analysis was conducted on the clinical data of 115 patients with pT3N0M0 ESCC who underwent radical surgery alone from January 1, 2013, to December 31, 2019. The Kaplan–Meier method was used to evaluate the 1-year, 3-year, and 5-year survival rates and median survival time of the patients. The Cox proportional hazards regression model was used to assess the hazard ratios (HRs) and 95% confidence intervals (95%CIs) of risk factors.
The 1-year, 3-year, and 5-year overall survival (OS) rates for the 115 EC patients analyzed were 85.22%, 50.43%, and 37.48%, respectively. The median OS was 37.00 (95%CI: 24.93-49.07) months, and the median disease-free survival was 21.00 (95%CI: 14.71-27.29) months. Both univariate and multivariate Cox regression analyses revealed that high body mass index (BMI; HR = 1.137, 95%CI: 1.054-1.226), positive perineural invasion (PNI; HR = 13.381, 95%CI: 4.899-36.547), and smoking (HR = 2.415, 95%CI: 1.388-4.203) were independent risk factors for a poor prognosis. In contrast, compared to the upper thoracic location of the tumor, middle thoracic (HR = 0.441, 95%CI: 0.240-0.810) and lower thoracic (HR = 0.328, 95%CI: 0.144-0.750) locations were protective factors.
BMI, tumor location, PNI, and smoking are associated with the prognosis of ESCC patients. This study highlights the prognostic risk factors for T3N0M0 ESCC patients and offers personalized insights for clinical treatment.
Core Tip: Esophageal squamous cell carcinoma (ESCC) has a relatively high incidence globally, imposing a significant societal burden. This study conducted a survival analysis on 115 patients with T3N0M0 stage ESCC and found that high body mass index, tumor location, positive perineural invasion and smoking were associated with the prognosis of ESCC patients. In the future, prospective multi-center hospital studies need to be carried out to confirm the risk factors affecting prognosis, with a view to providing individualized and standardized treatment measures after surgery.
- Citation: Ren ZT, Kang M, Zhu LY, Li P. Long-term survival and risk factors in esophageal squamous cell carcinoma: A Kaplan-Meier and cox regression study. World J Gastrointest Surg 2024; 16(12): 3772-3779
- URL: https://www.wjgnet.com/1948-9366/full/v16/i12/3772.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i12.3772
Esophageal cancer (EC) is a prevalent malignant tumor of the digestive tract. According to the International Agency for Research on Cancer, in 2020, there were 600000 new cases of EC globally, resulting in 540000 deaths[1]. China has a high incidence of EC, with 224000 new cases and 187500 deaths in 2022, accounting for over 40% of global new cases[2]. In China, esophageal squamous cell carcinoma (ESCC) is the primary type of EC, comprising over 90% of cases, which differs from Western countries where esophageal adenocarcinoma (EA) is more common[3]. Despite efforts to prevent and control the disease, the incidence and mortality rates of EC in China have declined but remain significant[4].
Compared to other types of EC, ESCC has a lower long-term survival rate. Radical esophagectomy is the main treatment, but the prognosis is still unsatisfactory[5]. Adjuvant therapy after surgery can enhance treatment outcomes and is the main method of treatment[6]. However, there is controversy regarding the necessity of adjuvant therapy in patients at the T3N0M0 stage who have not experienced lymph node or distant organ metastasis. A retrospective study based on the National Cancer Database in the United States found that adjuvant therapy did not improve the long-term prognosis of patients with pT24aN0M0 EA after radical resection[7]. In contrast, a Japanese study (JCOG9204) included a total of 242 patients with locally advanced ESCC and randomly divided them into a group receiving surgery combined with adjuvant chemotherapy and a group receiving surgery only. The results showed that the 5-year disease-free survival (DFS) rate of the postoperative adjuvant chemotherapy group was significantly higher than that of the surgery-only group (55% vs 45%, P = 0.037). Notably, there was no statistically significant difference in the 5-year overall survival (OS) rate between the two groups (61% vs 52%, P = 0.130). However, further subgroup analysis revealed that the benefit of postoperative adjuvant chemotherapy might be more significant in patients with positive pathological lymph nodes, while no significant benefit was observed in patients with negative pathological lymph nodes[8]. Therefore, for patients with lymph node-negative ESCC, individualized conditions and the impact of comprehensive risk factors on prognosis should be considered when determining the need for postoperative adjuvant therapy[8].
This study aimed to analyze the factors influencing survival and prognosis in postoperative patients with ESCC through follow-up surveys, providing a scientific basis for postoperative prevention and treatment of the disease.
A retrospective analysis was conducted on the clinical data of 115 patients with pT3N0M0 ESCC who underwent simple radical surgery in our hospital from January 1, 2013, to December 31, 2019. Trained investigators obtained information from medical records, admission and discharge records, and the first pages of pathological diagnosis reports. The information included baseline characteristics such as sex, age at diagnosis, body mass index (BMI), smoking, drinking, marital status, and ethnicity, as well as tumor-related information such as pathological type, anatomical location, degree of differentiation, and pathological stage. According to the International Classification of Diseases for Oncology, Third edition[9], the classification of tumor anatomical locations was coded as C15.0 to C15.9. C15.0, C15.1, and C15.2 were defined as the cervical, thoracic, and abdominal esophageal segments, respectively.
The inclusion criteria for patients were as follows: (1) Patients diagnosed with advanced ESCC through tissue biopsy pathology obtained by gastroscopy, as well as barium meal and computed tomography (CT) examinations[10]; (2) Patients at TNM stage T3N0M0 who received simple radical surgery for the first time; (3) Patients with radiologically assessable cancer lesions; and (4) Patients expected to survive for more than 3 months.
The exclusion criteria for patients were as follows: (1) Patients with solid malignant tumors or hematological tumors in other parts of the body; (2) Patients with autoimmune diseases; (3) Patients who could not cooperate with long-term follow-up; and (4) Patients with missing key information.
All patients received regular telephone follow-ups and outpatient reexaminations after being discharged, which included esophageal radiography, chest ultrasound, and CT scans. The follow-up period ended on December 31, 2021.
Within the first 2 years, patients were followed up every 3 months, and in the third year, the follow-up occurred every 6 months. DFS and OS calculations were performed for all patients. DFS was defined as the time from surgery until disease progression or death caused by any reason, including the end of the follow-up period. On the other hand, OS was defined as the time from surgery until death caused by any reason or the end of the follow-up period. The survival time was analyzed statistically and measured in months.
The sample size was estimated based on the sample size calculation formula, which is n = μα2pq/δ2. In this formula, p represents the 5-year mortality rate of EC patients, q = 1-p, α = 0.05, μα = 1.96, and the allowable error is 4%. According to previous literature, the 5-year mortality rate of cancer after lung cancer surgery is 53%. Based on this, the calculated sample size is 100 cases. Considering an additional 10% for loss of follow-up, the final calculated sample size is 110 cases. In this study, 115 cases were selected as the sample size, which meets the requirements.
The data was analyzed and entered into SPSS 25.0. Measurement data are presented as mean ± SD, and the comparison between groups was tested using a two-tailed t-test. The comparison of enumeration data between groups was conducted using a χ2 test. Multivariate Cox regression analysis was used to identify factors that influence the prognosis of patients with advanced ESCC. The Kaplan-Meier method was used to plot the OS curves for these patients based on the influencing factors, and the log-rank test was used to examine the differences in survival rates. A P value of < 0.05 was considered statistically significant.
A total of 115 ESCC patients were included in this study, and none were lost to follow-up, resulting in a follow-up rate of 100%. As of December 31, 2021, 38 patients (33.04%) were alive, and 77 patients (66.96%) had died. The clinical characteristics of the study subjects are presented in Table 1.
| Variables | Group | Death (n = 77) | Survival (n = 38) | χ2/t | P value |
| Gender, n (%) | 1.019 | 0.313 | |||
| Male | 63 (81.8) | 28 (73.7) | |||
| Females | 14 (18.2) | 10 (26.3) | |||
| Age (year), n (%) | 0.059 | 0.808 | |||
| ≤ 60 | 24 (31.2) | 11 (28.9) | |||
| > 60 | 53 (68.8) | 27 (71.7) | |||
| BMI (kg/m2) | 22.09 ± 2.94 | 20.81 ± 2.68 | 2.251 | 0.026 | |
| Tumor location, n (%) | 5.493 | 0.064 | |||
| Upper segments | 14 (18.2) | 3 (7.9) | |||
| Middle segments | 53 (68.8) | 24 (63.2) | |||
| Lower segments | 10 (13.0) | 11 (28.9) | |||
| Degree of differentiation, n (%) | 4.540 | 0.103 | |||
| Poorly differentiated | 21 (27.3) | 6 (15.8) | |||
| Moderately differentiated | 54 (70.1) | 28 (73.7) | |||
| Well-differentiated | 2 (2.6) | 4 (10.5) | |||
| Perineural invasion, n (%) | 1.255 | 0.263 | |||
| No | 72 (93.5) | 38 (100.0) | |||
| Yes | 5 (6.5) | 0 (0.0) | |||
| Tumor length (mm) | 40.18 ± 15.09 | 39.21 ± 11.49 | 0.350 | 0.727 | |
| Tumor width (mm) | 25.66 ± 11.26 | 24.82 ± 8.37 | 0.411 | 0.682 | |
| Retrieved lymph nodes number | 14.23 ± 10.05 | 14.76 ± 7.97 | 0.284 | 0.777 | |
| Smoking, n (%) | 5.216 | 0.022 | |||
| No | 60 (77.9) | 36 (94.7) | |||
| Yes | 17 (22.1) | 2 (5.3) | |||
| Drinking, n (%) | 1.612 | 0.105 | |||
| No | 68 (88.3) | 37 (97.4) | |||
| Yes | 9 (11.7) | 1 (2.6) | |||
The 1-year, 3-year, and 5-year survival rates for all patients were 85.22%, 50.43%, and 37.48%, respectively. The median OS was 37.00 (95%CI: 24.93-49.07) months, and the median DFS was 21.00 (95%CI: 14.71-27.29) months.
To investigate the influencing factors on prognosis in patients with advanced ESCC, both univariate and multivariate Cox regression models were established. Categorical variables included sex (coded as 0 for male and 1 for female), age (coded as 0 for age ≤ 60 years and 1 for age > 60 years), tumor location (coded as 0 for the upper segment, 1 for the middle segment, and 2 for the lower segment), degree of differentiation (coded as 0 for well-differentiated, 1 for poorly differentiated, and 2 for moderately differentiated), perineural invasion (PNI) status (coded as 0 for no and 1 for yes), smoking (coded as 0 for no and 1 for yes), alcohol consumption (coded as 0 for no and 1 for yes), BMI, tumor length, and tumor width (continuous variables entered as their original values) as independent variables. The OS of patients with T3N0M0 stage ESCC served as the dependent variable (coded as 0 for alive and 1 for dead).
The results of the univariate Cox regression analysis revealed that BMI, tumor location, PNI, and smoking were significantly associated with the prognosis of ESCC patients (P < 0.05). The multivariate Cox regression analysis further indicated that high BMI levels, PNI, and smoking were risk factors affecting the prognosis of ESCC patients (P < 0.05). Compared to tumors located in the upper thoracic segment, those in the middle and lower segments were found to be protective factors (P < 0.05), as shown in Table 2.
| Factors | Univariate Cox | Multivariate Cox | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Gender | 1.197 (0.896-1.599) | 0.225 | - | - |
| Age | 0.862 (0.532-1.398) | 0.548 | - | - |
| BMI | 1.111 (1.032-1.197) | 0.005 | 1.137 (1.054-1.226) | 0.001 |
| Tumor location | ||||
| Upper segments | 1 | - | 1 | - |
| Middle segments | 0.521 (0.287-0.944) | 0.032 | 0.441 (0.240-0.810) | 0.008 |
| Lower segments | 0.399 (0.177-0.901) | 0.027 | 0.328 (0.144-0.750) | 0.008 |
| Degree of differentiation | ||||
| Well-differentiated | 1 | - | - | - |
| Poorly differentiated | 3.045 (0.712-13.015) | 0.133 | - | - |
| Moderately differentiated | 2.265 (0.552-9.302) | 0.257 | - | - |
| Perineural invasion | 7.947 (3.079-20.513) | < 0.001 | 13.381 (4.899-36.547) | < 0.001 |
| Tumor length | 1.011 (0.994-1.029) | 0.196 | - | - |
| Tumor width | 1.014 (0.991-1.037) | 0.240 | - | - |
| Retrieved lymph nodes number | 1.008 (0.983-1.033) | 0.551 | - | - |
| Smoking | 1.947 (1.135-3.339) | 0.016 | 2.415 (1.388-4.203) | 0.002 |
| Drinking | 1.510 (0.753-3.030) | 0.246 | - | - |
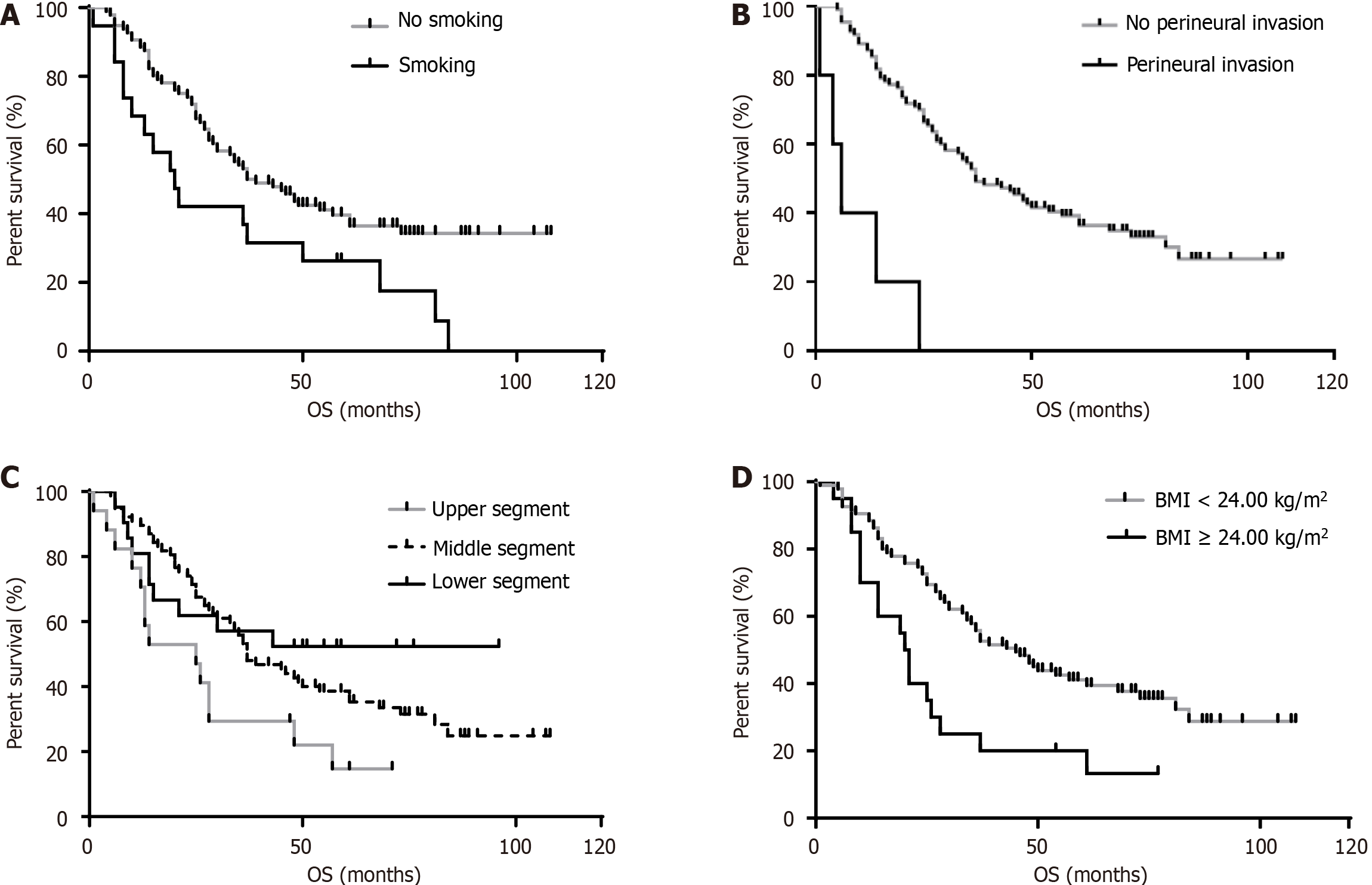
The survival curves were further plotted to compare the differences between groups in terms of statistically significant influencing factors. The continuous variable of BMI was categorized using a boundary of 24.0 kg/m2. The results indicated that the group with BMI < 24.0 kg/m2 had a higher survival rate than the group with BMI ≥ 24.0 kg/m2. Additionally, the non-smoking group had a higher survival rate compared to the smoking group, as did the group without PNI compared to the group with PNI. Furthermore, those with tumors in the lower and middle segments of the thorax had a higher survival rate than those with upper-segment tumors. The corresponding P values were 0.003, 0.013, 0.040, and < 0.001, respectively (Figure 1).
In recent years, as society has developed and progressed, the adoption of healthy lifestyles and the implementation of comprehensive cancer prevention and control measures have led to a gradual decline in the incidence of EC in China[11]. However, given China's large population and the increasingly prominent issue of aging, it is crucial to remain vigilant about the health threat of EC to the population.
In this study, the 3-year and 5-year survival rates of 115 hospitalized patients with stage T3N0M0 ESCC were found to be 50.43% and 37.48%, respectively. These survival rates were similar to those reported in a multicenter study conducted by He et al[12], which involved 18 hospitals in 6 regions of China and reported rates of 49.98% and 39.07%. Additionally, the 5-year survival rates were higher than the estimated 20.9% reported by the National Cancer Center of China[13]. The most recent data on EC survival rates in the United States reveals a 5-year survival rate of 21%[14], with white patients experiencing better survival rates compared to black patients. These findings highlight significant differences in the 5-year survival rates of EC patients across different regions.
It is important to note that in this study, the included patients had squamous cell carcinoma and were in stage T3N0M0, which differs somewhat from previous research subjects, leading to variations in the data. In this study, the results showed that high BMI levels, the presence of nerve invasion, and smoking were risk factors that affected the prognosis of ESCC patients. Additionally, tumors located in the middle and lower segments were found to be protective factors compared to those located in the upper thoracic segment.
BMI is a reliable indicator for assessing nutritional and metabolic status, and previous studies have shown that it can potentially be used to evaluate the prognosis of EC patients[15]. However, the exact correlation between BMI and survival outcomes in EC patients is still inconclusive. A meta-analysis by Pan et al[16], which included 4823 EC patients, found that a high BMI was associated with better DFS and OS in EC patients compared to normal BMI. However, among patients with ESCC, a high BMI was correlated with a poorer prognosis, suggesting that the prognostic role of BMI differs significantly between squamous cell carcinoma and adenocarcinoma. This indicates that the association between BMI and EC prognosis is influenced by the pathological types of the tumor. Additionally, research by He et al[12] revealed that EC patients with high or normal BMI had higher 5-year OS rates than those with low BMI. However, several studies have also indicated no significant association between BMI levels and EC prognosis[17,18].
In our current study on ECSS, we found that patients with high BMI had a worse prognosis compared to those with low BMI, which is consistent with the findings of Pan et al[16]. This suggests that BMI holds some value in predicting ECSS prognosis. Research has shown that an increase in BMI may cause an accumulation of adipose tissue, leading to hypoxia. This not only induces compensatory angiogenesis but also promotes the expression of transcription factor hypoxia-inducible factor alpha, thereby upregulating the expression of related molecules and pro-inflammatory factors in the fibrosis pathway. These processes can ultimately affect patient prognosis[19]. In addition, there is evidence suggesting that BMI may influence EC, particularly in relation to smoking status[20].
Smoking has been identified as a risk factor that negatively impacts the prognosis of ESCC, as confirmed by several epidemiological studies[21,22]. Studies have also explored the connection between smoking and EC prognosis. For instance, a prospective cohort study involving 45 newly diagnosed patients with EA investigated the potential impact of smoking and alcohol consumption on EA progression. The findings indicated that the risk of EA increased with both the number of years smoked and the duration of smoking, but no significant association was observed with alcohol consumption[23]. Another systematic review that analyzed 12 studies examined the relationship between smoking prior to diagnosis and the risk of death from EC. The review found that, for patients who smoked before diagnosis, the risk of death from ESCC increased by 1.19 times, but no significant association was found for EA[24].
The present study also confirmed the detrimental effects of smoking on the prognosis of ESCC. One possible mechanism is that tobacco contains various carcinogens that can cause DNA damage in esophageal epithelial cells. For example, it can lead to loss of heterozygosity and promoter methylation of P16, resulting in the transformation of normal esophageal mucosal epithelium into intraepithelial neoplasia and adenocarcinoma[25]. Nevertheless, while numerous studies provide compelling evidence of the link between alcohol consumption, ESCC risk, and prognosis[26,27], this particular study did not find any association. This may be attributed to the study's relatively small sample size.
In this study, we also observed a correlation between tumor location and prognosis in ESCC, with tumors in the lower thoracic segment of the esophagus associated with a better prognosis. Notably, a retrospective study by Cheng et al[28] on 14394 EC patients revealed that the 5-year OS rate for tumors located in the upper third of the esophagus (15.0%) was inferior to that of cancers in the middle third (18.4%) or lower third (19.1%). Additional evidence also suggests that patient survival increases with the distal location of the esophagus[29]. Overall, the poorer prognoses associated with proximal tumors may be due to their higher propensity for lymphatic spread along both sides of the recurrent laryngeal nerve[30]. Similarly, our study highlights the importance of other factors affecting prognosis, such as PNI. The impact of PNI on prognosis has been well-documented in the literature. For instance, Ma et al[31] evaluated the impact of PNI on the prognosis of 349 patients with ESCC. The researchers found that PNI was an independent factor affecting DFS and OS in ESCC. Overall, patients with positive PNI tended to have poorer OS compared to those with negative PNI. Similar findings were also observed in two other studies on the correlation between PNI and prognosis in Chinese patients with ESCC[32,33]. In this study, patients with positive PNI also exhibited poorer OS, which was consistent with previous research results.
The limitations of this study should be taken into consideration. Firstly, this was a hospital-based, single-center study with a relatively small sample size. Therefore, caution should be exercised when extrapolating the results. Secondly, since this was a retrospective study, the collected information on variables was limited, which may have limited the explanatory power of the constructed Cox model. Lastly, specifying concrete postoperative treatment options for patients proved to be challenging, and no information was collected regarding the patients' family history. As a result, the influence of these factors on prognosis remains uninvestigated. Furthermore, it is important to note that the study's results are limited in their applicability since only patients with T3N0M0 ESCC were included. To confirm these findings, future studies should be conducted prospectively.
This study investigated and analyzed the postoperative survival rate and its influencing factors among 115 patients with T3N0M0 stage ESCC. The study found that the 5-year survival rate of ESCC patients was relatively high. Additionally, the prognosis of ESCC patients was affected by factors such as BMI level, tumor location, PNI, and smoking. In the future, prospective multi-center hospital studies should be carried out to confirm the risk factors affecting prognosis, with the aim of providing individualized and standardized treatment measures after surgery.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64583] [Article Influence: 16145.8] [Reference Citation Analysis (176)] |
| 2. | Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. 2024;4:47-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 707] [Article Influence: 707.0] [Reference Citation Analysis (0)] |
| 3. | Zhang X, Yang L, Liu S, Cao LL, Wang N, Li HC, Ji JF. [Interpretation on the report of global cancer statistics 2022]. Zhonghua Zhong Liu Za Zhi. 2024;46:710-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 4. | Gu BL, Ma LX, Shi LL, Liang GF, Qi YJ, Gao SG. [Advances and Prospects in Epidemiological Research on Esophageal Cancer]. Shiguan Jibing. 2024;6:148-151. [DOI] [Full Text] |
| 5. | Xia LL, Wang J, Fan XL, Chen YS. [Curative effect,survival status and prognostic factors of patients with superficial esophageal carcinoma]. Wuhan Daxue Xuebao (Yixueban). 2023;44:1204-1208. [DOI] [Full Text] |
| 6. | Pape M, Vissers PAJ, Beerepoot LV, van Berge Henegouwen MI, Lagarde SM, Mook S, Moehler M, van Laarhoven HWM, Verhoeven RHA. A population-based study in resected esophageal or gastroesophageal junction cancer aligned with CheckMate 577. Ther Adv Med Oncol. 2022;14:17588359221075495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 7. | Rucker AJ, Raman V, Jawitz OK, Voigt SL, Harpole DH, D'Amico TA, Tong BC. The Impact of Adjuvant Therapy on Survival After Esophagectomy for Node-negative Esophageal Adenocarcinoma. Ann Surg. 2022;275:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, Ikeda K, Kanda T, Tsujinaka T, Nakamura K, Fukuda H. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 1051] [Article Influence: 75.1] [Reference Citation Analysis (0)] |
| 9. | Wang QQ, Mao JF. [Dynamic Coding Changes of Bone Marrow Proliferative Diseases in ICD-0-3 in International Disease Classification]. Zhongguo Bingan. 2018;19:32-34. [DOI] [Full Text] |
| 10. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2430] [Article Influence: 486.0] [Reference Citation Analysis (3)] |
| 11. | Chen W, Zheng R, Zhang S, Zeng H, Xia C, Zuo T, Yang Z, Zou X, He J. Cancer incidence and mortality in China, 2013. Cancer Lett. 2017;401:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 348] [Article Influence: 43.5] [Reference Citation Analysis (1)] |
| 12. | He Y, Liang D, Du L, Guo T, Liu Y, Sun X, Wang N, Zhang M, Wei K, Shan B, Chen W. Clinical characteristics and survival of 5283 esophageal cancer patients: A multicenter study from eighteen hospitals across six regions in China. Cancer Commun (Lond). 2020;40:531-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 13. | Zeng H, Zheng R, Guo Y, Zhang S, Zou X, Wang N, Zhang L, Tang J, Chen J, Wei K, Huang S, Wang J, Yu L, Zhao D, Song G, Chen J, Shen Y, Yang X, Gu X, Jin F, Li Q, Li Y, Ge H, Zhu F, Dong J, Guo G, Wu M, Du L, Sun X, He Y, Coleman MP, Baade P, Chen W, Yu XQ. Cancer survival in China, 2003-2005: a population-based study. Int J Cancer. 2015;136:1921-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 499] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 14. | Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 9929] [Article Influence: 4964.5] [Reference Citation Analysis (2)] |
| 15. | Sun P, Zhang F, Chen C, An X, Li YH, Wang FH, Zhu ZH. Comparison of the prognostic values of various nutritional parameters in patients with esophageal squamous cell carcinoma from Southern China. J Thorac Dis. 2013;5:484-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 16. | Pan W, Sun Z, Xiang Y, Fang W. The correlation between high body mass index and survival in patients with esophageal cancer after curative esophagectomy: evidence from retrospective studies. Asia Pac J Clin Nutr. 2015;24:480-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 17. | Deng HY, Alai G, Li G, Luo J, Zhuo ZG, Lin YD. High BMI has no impact on the survival of Chinese patients with lower thoracic esophageal adenocarcinoma treated with curative esophagectomy: a propensity score-matched study. Dis Esophagus. 2019;32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Hasegawa T, Kubo N, Ohira M, Sakurai K, Toyokawa T, Yamashita Y, Yamazoe S, Kimura K, Nagahara H, Amano R, Shibutani M, Tanaka H, Muguruma K, Ohtani H, Yashiro M, Maeda K, Hirakawa K. Impact of body mass index on surgical outcomes after esophagectomy for patients with esophageal squamous cell carcinoma. J Gastrointest Surg. 2015;19:226-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Sun K, Tordjman J, Clément K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 689] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 20. | Sun P, Zhang F, Chen C, Ren C, Bi XW, Yang H, An X, Wang FH, Jiang WQ. Prognostic impact of body mass index stratified by smoking status in patients with esophageal squamous cell carcinoma. Onco Targets Ther. 2016;9:6389-6397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Lindblad M, Rodríguez LA, Lagergren J. Body mass, tobacco and alcohol and risk of esophageal, gastric cardia, and gastric non-cardia adenocarcinoma among men and women in a nested case-control study. Cancer Causes Control. 2005;16:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 198] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 22. | Spreafico A, Coate L, Zhai R, Xu W, Chen ZF, Chen Z, Patel D, Tse B, Brown MC, Heist RS, Dodbiba L, Teichman J, Kulke M, Su L, Eng L, Knox J, Wong R, Darling GE, Christiani DC, Liu G. Early adulthood body mass index, cumulative smoking, and esophageal adenocarcinoma survival. Cancer Epidemiol. 2017;47:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Hardikar S, Onstad L, Blount PL, Odze RD, Reid BJ, Vaughan TL. The role of tobacco, alcohol, and obesity in neoplastic progression to esophageal adenocarcinoma: a prospective study of Barrett's esophagus. PLoS One. 2013;8:e52192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Fahey PP, Mallitt KA, Astell-Burt T, Stone G, Whiteman DC. Impact of pre-diagnosis behavior on risk of death from esophageal cancer: a systematic review and meta-analysis. Cancer Causes Control. 2015;26:1365-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 25. | Cook MB, Kamangar F, Whiteman DC, Freedman ND, Gammon MD, Bernstein L, Brown LM, Risch HA, Ye W, Sharp L, Pandeya N, Webb PM, Wu AH, Ward MH, Giffen C, Casson AG, Abnet CC, Murray LJ, Corley DA, Nyrén O, Vaughan TL, Chow WH. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: a pooled analysis from the international BEACON consortium. J Natl Cancer Inst. 2010;102:1344-1353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 26. | Thrift AP, Nagle CM, Fahey PP, Russell A, Smithers BM, Watson DI, Whiteman DC; Australian Cancer Study Clinical Follow-Up Study. The influence of prediagnostic demographic and lifestyle factors on esophageal squamous cell carcinoma survival. Int J Cancer. 2012;131:E759-E768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Huang Q, Luo K, Yang H, Wen J, Zhang S, Li J, Ela Bella A, Liu Q, Yang F, Zheng Y, Hu R, Chen J, Fu J. Impact of alcohol consumption on survival in patients with esophageal carcinoma: a large cohort with long-term follow-up. Cancer Sci. 2014;105:1638-1646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Cheng YF, Chen HS, Wu SC, Chen HC, Hung WH, Lin CH, Wang BY. Esophageal squamous cell carcinoma and prognosis in Taiwan. Cancer Med. 2018;7:4193-4201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Rice TW, Lerut TE, Orringer MB, Chen LQ, Hofstetter WL, Smithers BM, Rusch VW, van Lanschot J, Chen KN, Davies AR, D'Journo XB, Kesler KA, Luketich JD, Ferguson MK, Räsänen JV, van Hillegersberg R, Fang W, Durand L, Allum WH, Cecconello I, Cerfolio RJ, Pera M, Griffin SM, Burger R, Liu JF, Allen MS, Law S, Watson TJ, Darling GE, Scott WJ, Duranceau A, Denlinger CE, Schipper PH, Ishwaran H, Apperson-Hansen C, DiPaola LM, Semple ME, Blackstone EH. Worldwide Esophageal Cancer Collaboration: neoadjuvant pathologic staging data. Dis Esophagus. 2016;29:715-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 30. | Law S, Kwong DL, Kwok KF, Wong KH, Chu KM, Sham JS, Wong J. Improvement in treatment results and long-term survival of patients with esophageal cancer: impact of chemoradiation and change in treatment strategy. Ann Surg. 2003;238:339-47; discussion 347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 126] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 31. | Ma Y, Chen J, Yao X, Li Z, Li W, Wang H, Zhu J. Patterns and prognostic predictive value of perineural invasion in esophageal squamous cell carcinoma. BMC Cancer. 2022;22:1287. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 32. | Sheng L, Ji Y, Du X. Perineural invasion correlates with postoperative distant metastasis and poor overall survival in patients with PT1-3N0M0 esophageal squamous cell carcinoma. Onco Targets Ther. 2015;8:3153-3157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Tsai CY, Yeh CJ, Chao YK, Chang HK, Tseng CK, Liu YH. Perineural invasion through the sheath in posttherapy esophagectomy specimens predicts poor survival in patients with esophageal squamous cell carcinoma. Eur J Surg Oncol. 2017;43:1970-1976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |