Published online Dec 27, 2024. doi: 10.4240/wjgs.v16.i12.3754
Revised: October 11, 2024
Accepted: October 18, 2024
Published online: December 27, 2024
Processing time: 93 Days and 1.7 Hours
Traditional surgical procedures are highly invasive and risky for children with pancreatic and biliary diseases. Endoscopic retrograde cholangiopancreatography (ERCP) has been used mostly in adults because it is a safe and effective surgical procedure. Its application in children will contribute to the treatment and prog
To analyze the efficacy and safety of ERCP for the treatment of pediatric pancreatobiliary diseases.
A retrospective study was performed using the medical records of 101 pediatric patients who received treatment for pancreatobiliary diseases at Children’s Hospital Capital Institute of Pediatrics from April 2022 to April 2024. The patients were divided into an observation group (n = 52, treated with ERCP) and a control group (n = 49, treated with traditional surgical methods). Diagnostic and therapeutic outcomes of ERCP were statistically analyzed. Treatment efficacy, time to resume eating, and hospital stay duration were compared between the two groups. Indicators of liver function were monitored preoperatively and one week postoperatively. Dynamic changes in C-reactive protein (CRP) and serum amylase levels were assessed preoperatively and at 6 and 24 hours postoperatively. Postoperative complications were also compared. Logistic multivariate regression was used to analyze the independent effect of ERCP on outcomes.
For the observation group, 36 and 16 patients were diagnosed with biliary and pancreatic diseases, respectively. Compared with the control group, the observa
ERCP effectively enhances the treatment efficacy of pediatric pancreatobiliary diseases, with a reduced inflammatory response, faster postoperative recovery, and fewer complications. ERCP is a safe and effective diagnostic and therapeutic method for pediatric pancreatobiliary diseases.
Core Tip: Pancreatic and biliary diseases are serious illnesses. Children with pancreatic and biliary diseases have traditionally received surgery; however, these procedures are traumatic and risky. Thus far, endoscopic retrograde pancreaticobiliary angiography has been used in adults as a safe and effective surgical procedure; however, there are few studies analyzing its efficacy in children. The purpose of this study was to evaluate the effect and safety of endoscopic retrograde pancreaticobiliary angiography for the treatment of pancreatic and biliary diseases in children.
- Citation: Wang XQ, Kong CH, Ye M, Diao M. Analysis of the efficacy and safety of endoscopic retrograde cholangiopancreatography for the treatment of pediatric pancreatobiliary diseases. World J Gastrointest Surg 2024; 16(12): 3754-3763
- URL: https://www.wjgnet.com/1948-9366/full/v16/i12/3754.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i12.3754
Pediatric biliary and pancreatic diseases are predominantly congenital and encompass acute and chronic pancreatitis. Because of the dynamic nature of children’s growth and development, the clinical presentations vary significantly with age[1]. Traditionally, pediatric patients with pancreatobiliary diseases are treated surgically, which is high risk and associated with considerable trauma. Therefore, identifying minimally invasive and safer treatment methods is of paramount importance for the diagnosis and treatment of pediatric pancreatobiliary diseases[2].
Since the initial use of endoscopic retrograde cholangiopancreatography (ERCP) by McCune et al[3] in 1968, this technique remains indispensable for diagnosing and treating biliary and pancreatic diseases. In 1976, Waye[4] introduced ERCP to pediatric patients; however, its application was limited by high operational risks and equipment constraints[5]. With the advent of pediatric-specific duodenoscopes and related devices, ERCP use has increased in pediatric cases and demonstrated significant diagnostic and therapeutic value[6,7]. Despite these advancements, however, the anatomical complexity of pediatric pancreatobiliary diseases poses greater challenges compared with that in adults. The lack of pediatric-specific endoscopic equipment necessitates the use of adult devices, which may increase the occurrence of postoperative complications[8,9]. Furthermore, there is a scarcity of studies comparing the safety and efficacy of ERCP with traditional surgical methods in the pediatric population. Thus, a comprehensive analysis of ERCP in pediatric pancreatobiliary diseases is warranted.
In this study, we analyzed the efficacy and safety of ERCP for the treatment of pediatric pancreatobiliary diseases and provided data for selecting the appropriate treatment for these conditions.
A retrospective analysis was performed on 101 pediatric patients with pancreatobiliary diseases treated at Children’s Hospital Capital Institute of Pediatrics from April 2022 to April 2024. Based on the treatment method, patients were categorized into an observation group (n = 52) that underwent ERCP, and a control group (n = 49) receiving traditional surgical intervention. The inclusion criteria were as follows: (1) Children who met the diagnostic criteria for pancreatobiliary diseases; (2) Children aged below 12 years; and (3) Patients with complete medical records. The exclusion criteria were as follows: (1) Patients with other malignant tumors; (2) Patients with severe hepatic or renal dysfunction; (3) Patients with contraindications to surgery; and (4) Patients with immunodeficiency disorders.
All patients underwent complete blood count, liver and kidney function tests, coagulation profiles, abdominal ultra
Patients with control group underwent conventional surgical treatment specific to their condition. Two pediatric surgeons with over 10 years of experience in pancreatobiliary diseases performed the surgeries, which included laparoscopic and classic open surgeries. Postoperative care included intensive monitoring, oxygen therapy, fasting, gastric decompression, prophylactic antibiotics, and regular checks of blood counts and biochemical indices. Healing of the surgical incision was monitored and the diet was gradually transitioned from liquid to regular food.
Observation group patients were treated with ERCP. The procedure was performed using Olympus JF240 and TJF240 endoscopes with distal end outer diameters of 12.6 mm and 13.5 mm, respectively. They were equipped with specialized ERCP accessories from cook medical, which included various guidewires, duodenal papillotomy knives, dilators, and pancreatobiliary stents. Because of the difficulty in patient cooperation, poor tolerance, and the delicate nature of the pediatric gastrointestinal tract, all procedures were done under general anesthesia with endotracheal intubation by a dedicated anesthesiologist. Experienced endoscopists conducted the procedures.
During ERCP, the pancreatic or bile ducts were cannulated, and X-rays were taken to confirm the pancreatobiliary pathology. Appropriate ERCP techniques were selected based on the specific condition. The most common procedures for chronic pancreatitis, pancreatic duct stenosis, and pancreatic divisum included ERCP with endoscopic retrograde pancreatic drainage (ERPD) and ERCP with endoscopic sphincterotomy (EST) plus ERPD. For bile duct stones, the procedures included ERCP with EST and balloon stone extraction, ERPD with endoscopic retrograde biliary drainage, and ERCP with balloon stone extraction plus endoscopic nasobiliary drainage.
Post-ERCP, the patients were kept fasting and provided with symptomatic supportive treatments, such as inhibition of digestive secretion and broad-spectrum antibiotics, based on intraoperative findings. Regular monitoring of blood counts and serum amylase levels was done along with periodic monitoring of blood biochemistry and electrolyte levels. The clinical manifestations of the patients were closely observed.
Diagnostic and therapeutic outcomes of ERCP: The results of the diagnostic and therapeutic procedures performed by ERCP were statistically analyzed.
Comparison of treatment efficacy: The treatment efficacy between the two groups was compared and classified as follows: Markedly effective: Laboratory indices returned to normal and symptoms completely disappeared. Effective: Significant improvement in laboratory indices and symptoms. Ineffective: No significant relief of symptoms postoperatively.
Time to resume eating and hospital stay duration: The time required for the patient to resume eating and the duration of hospital stay were compared between the two groups.
Liver function indicators: Using a biochemical analyzer (BECKMANAU680), liver function indicators, including total bilirubin (TBil), aspartate aminotransferase (AST), and alanine aminotransferase (ALT), were monitored preoperatively and one week postoperatively in both groups.
Dynamic monitoring of inflammatory markers: Preoperative and postoperative (6 and 24 hours) C-reactive protein (CRP) and serum amylase levels were dynamically monitored in both groups.
Postoperative complications: The incidence of postoperative complications, including hyperamylasemia, stress-induced hyperglycemia, transient acute pancreatitis, and bleeding, was compared between the two groups.
Impact of ERCP on outcomes: Logistic multivariate regression was used to analyze the independent impact of ERCP on outcomes.
Data analysis was performed using SPSS 23.0 software and graphical representations were created using GraphPad Prism 8.0. The sample size of this study was calculated using the formula: n = Z × [P × (1-P)]/E, where n is the sample size; Z is the statistic, Z = 1.96 when the confidence level is 95%; Z = 1.64 when the confidence level is 90%; E is the error value. The “statistics power calculator” was used for power analysis to determine whether the sample size was sufficient. All continuous variables in this study were normal. The categorical data are expressed as n (%) and the χ2 test was used for comparison. Continuous data are expressed as the mean ± SD. Between-group comparisons were conducted using the independent samples t-test, whereas within-group comparisons before and after intervention were conducted using the paired samples t-test. A P value less than 0.05 was considered statistically significant.
The results of a power analysis revealed that the power threshold of the sample size could reach 0.9. No significant differences were observed between the two groups in terms of gender, age, and preoperative body mass index (P > 0.05; Table 1)[10].
| Factor | Observation group (n = 52) | Control group (n = 49) | χ2 | P value |
| Gender | 0.013 | 0.909 | ||
| Male | 27 (51.92) | 26 (53.06) | ||
| Female | 25 (48.08) | 23 (46.94) | ||
| Age (years) | 0.058 | 0.809 | ||
| ≤ 7 | 32 (61.54) | 29 (59.18) | ||
| > 7 | 20 (38.46) | 20 (40.82) | ||
| BMI (kg/m2) | 0.016 | 0.899 | ||
| ≤ 16 | 28 (53.85) | 27 (55.10) | ||
| > 16 | 24 (46.15) | 22 (44.90) | ||
| JSGPM-PBM[10] | 0.050 | 0.997 | ||
| A | 16 (30.77) | 15 (30.61) | ||
| B | 15 (28.85) | 15 (30.61) | ||
| C | 13 (25.00) | 12 (24.49) | ||
| D | 8 (15.38) | 7 (14.29) |
Thirty-six cases were diagnosed with biliary diseases, including 20 cases of simple common bile duct stones, 8 cases of bile duct stricture/dilatation, 2 cases of bile duct dilatation and gallbladder stones, 2 cases of bile duct stones and abnormal bile-pancreatic junction, 2 cases of bile duct stones combined with bile duct stricture, and 2 cases of suspected bile duct stones. Sixteen cases were diagnosed with pancreatic diseases, including 6 cases of chronic pancreatitis, 3 cases of pancreatic pseudocysts, 2 cases after pancreatic duct stenting, 3 cases of pancreatic divisum, and 2 cases of pancreatic duct stricture (Table 2 and Table 3).
| Preoperative imaging diagnosis | Postoperative ERCP diagnosis | |||||
| Simple common bile duct stones | Bile duct stricture/dilatation | Bile duct dilatation and gallstones | Choledocholithiasis and abnormal biliopancreatic junction | Bile duct stones combined with bile duct stricture | Suspected bile duct stones | |
| Bile duct stones | 17 | 1 | 1 | 0 | 1 | 0 |
| Abnormal bile duct signs | 0 | 0 | 0 | 0 | 0 | 1 |
| Bile duct stricture/dilatation | 0 | 5 | 0 | 1 | 0 | 0 |
| Bile duct dilatation and gallstones | 3 | 2 | 1 | 0 | 1 | 1 |
| Biliary pancreatitis | 0 | 0 | 0 | 1 | 0 | 0 |
| Preoperative imaging diagnosis | Postoperative ERCP diagnosis | ||||
| Chronic pancreatitis | Pancreatic pseudocyst | Pancreatic schizophrenia | Pancreatic duct stent placement | Pancreatic duct stenosis | |
| Chronic recurrent pancreatitis | 5 | 0 | 0 | 0 | 2 |
| Pancreatic cyst | 1 | 3 | 0 | 0 | 0 |
| Acute pancreatitis | 0 | 0 | 3 | 0 | 0 |
| After pancreatic duct stent placement | 0 | 0 | 0 | 2 | 0 |
The number of children showing a marked effect, effective, and ineffective in the observation group was 30, 20, and 2, respectively, whereas the number of children with a marked effect, effective, and ineffective in the control group was 20, 18, and 11, respectively. The total treatment efficacy of the observation group was markedly higher compared with that of the control group (96.15% vs 77.55%, P < 0.05; Table 4).
| Efficacy | Observation group (n = 52) | Control group (n = 49) | χ2 | P value |
| Marked effect | 30 (57.69) | 20 (40.86) | - | - |
| Effective | 20 (38.46) | 18 (36.73) | - | - |
| Ineffective | 2 (3.85) | 11 (22.45) | - | - |
| The total treatment efficacy | 50 (96.15) | 38 (77.55) | 7.785 | 0.005 |
The time to resume eating and hospitalization time of the children in the observation group were shorter compared with those in the control group (P < 0.05; Table 5).
| Project | Observation group (n = 52) | Control group (n = 49) | t value | P value |
| Time to resume eating (day) | 2.12 ± 0.38 | 4.86 ± 0.61 | 27.26 | < 0.001 |
| Hospitalization time (day) | 8.19 ± 1.09 | 15.29 ± 1.1 | 32.57 | < 0.001 |
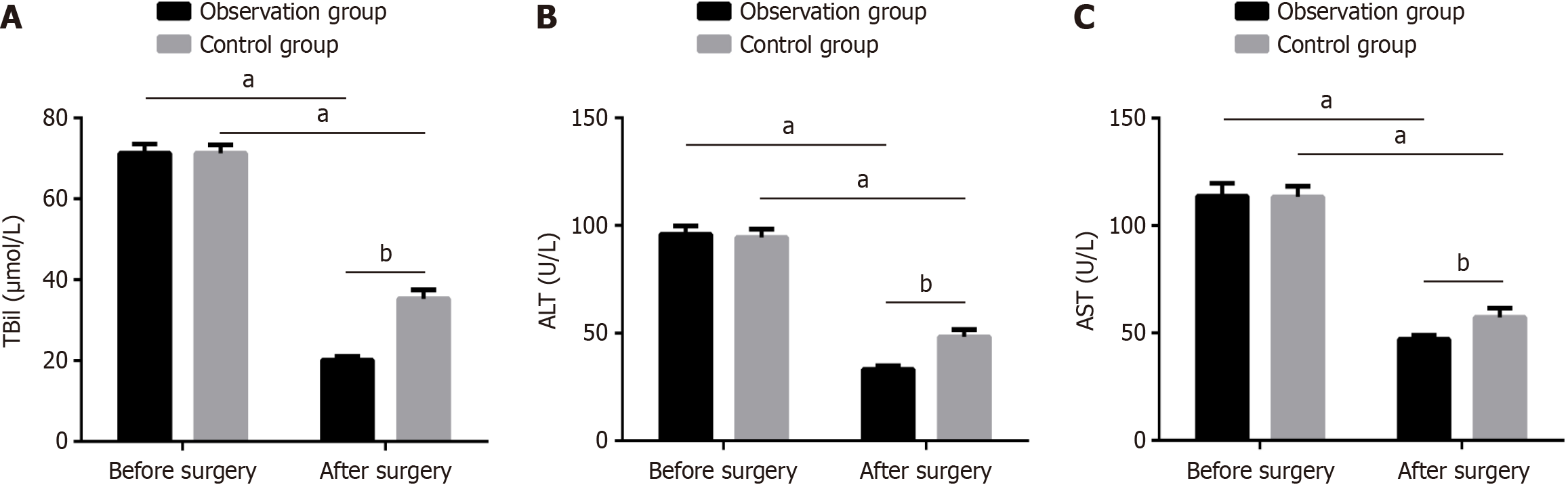
Before surgery, two groups were observed with insignificant differences in liver function indicators (TBil, ALT, AST; P > 0.05). After treatment, TBil, ALT, and AST for both groups were improved; however, such improvement of the observation group was more apparent compared with the control group (P < 0.05; Figure 1).
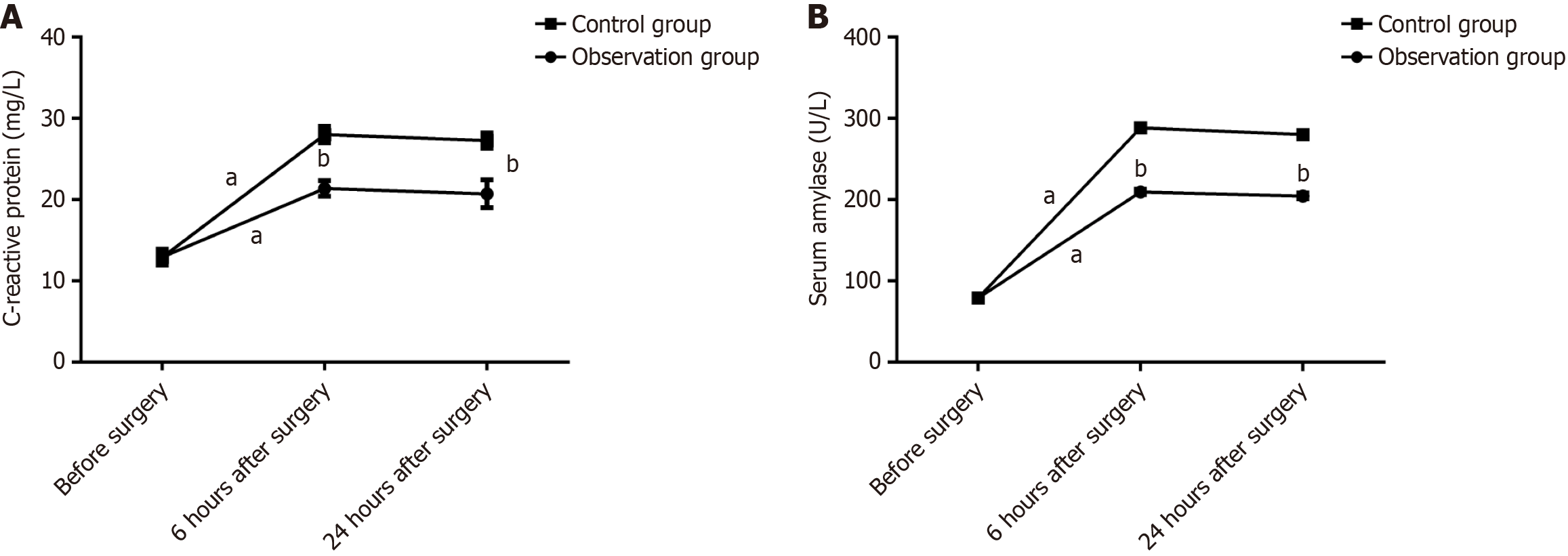
No significant difference was observed in the levels of CRP and serum amylase between the two groups before surgery (P > 0.05). The CRP and serum amylase levels in both groups of children at 6 and 24 hours after surgery were significantly higher compared with those before surgery (P < 0.05); however, the levels of CRP and serum amylase in the observation group were markedly lower compared with those in the control group (P < 0.05; Figure 2).
For the observation group, the number of children with hyperamylasemia, stress hyperglycemia, transient acute pancreatitis, and bleeding was 12, 2, 2 and 0, respectively, whereas the complication rate was 30.77%. For the control group, the number of children with hyperamylasemia, stress hyperglycemia, transient acute pancreatitis, and bleeding was 17, 4, 3, and 1, respectively, whereas the complication rate was 51.07%. The complication rate was notably lower in the observation group compared with the control group (P < 0.05; Table 6).
| Complication | Observation group (n = 52) | Control group (n = 49) | χ2 | P value |
| Hyperamylasemia | 12 (23.07) | 17 (34.69) | - | - |
| Stress-induced hyperglycemia | 2 (3.85) | 4 (8.16) | - | - |
| Transient acute pancreatitis | 2 (3.85) | 3 (6.12) | - | - |
| Bleeding | 0 | 1 (2.04) | - | - |
| Overall incidence | 16 (30.77) | 25 (51.01) | 4.290 | 0.038 |
Logistic regression was used to analyze the independent effect of ERCP on the results. The results indicated that there were differences between the two groups in terms of time to resume eating, length of hospital stay, postoperative liver function indicators (TBil, ALT, AST), CRP, serum amylase, and complication rate. The e variables with statistically significant differences were assigned values (Table 7) and included in the multivariate logistic regression analysis. The results indicated that TBil, ALT, AST, CRP, serum amylase are independent factors affecting treatment effectiveness (Table 8).
| Factor | Assignment |
| Recovery feeding time | Continuous variables |
| Length of hospital stay | Continuous variables |
| TBil | Continuous variables |
| ALT | Continuous variables |
| AST | Continuous variables |
| C-reactive protein | Continuous variables |
| Serum amylase | Continuous variables |
| Complication | Complications occurred = 1, no complications occurred = 0 |
| Factor | B | SE | Wals | P value | OR | 95%CI | |
| Lower limit | Upper limit | ||||||
| Recovery feeding time | -0.145 | 0.888 | 0.027 | 0.870 | 0.865 | 0.152 | 1.927 |
| Length of hospital stay | -0.706 | 0.711 | 0.985 | 0.321 | 0.494 | 0.123 | 1.989 |
| TBil | -2.496 | 1.066 | 5.487 | 0.019 | 0.082 | 0.010 | 0.665 |
| ALT | -0.679 | 0.325 | 4.349 | 0.037 | 0.507 | 0.268 | 0.960 |
| AST | -0.616 | 0.296 | 4.314 | 0.038 | 0.540 | 0.302 | 0.966 |
| C-reactive protein | -1.248 | 0.586 | 4.532 | 0.033 | 0.287 | 0.091 | 0.906 |
| Serum amylase | 0.837 | 0.361 | 5.380 | 0.020 | 2.308 | 1.138 | 4.680 |
| Complication | -2.648 | 1.855 | 2.037 | 0.153 | 0.071 | 0.002 | 2.686 |
ERCP is a routine technique for the diagnosis and treatment of pancreaticobiliary diseases in adults. With advancements in non-invasive cross-sectional imaging technologies, including MRCP, MRCP has largely replaced ERCP for the diagnosis of pancreaticobiliary diseases. Nonetheless, ERCP remains the preferred therapeutic method for these cases. A systematic analysis of the pediatric ERCP literature in China revealed that ERCP is increasingly recognized as a therapeutic technique for pediatric patients, with therapeutic ERCP accounting for 77% of the total procedures with the proportion continuing to rise annually[11]. Currently, ERCP is not only a diagnostic technique, but also a therapeutic tool.
Although the indications for pediatric ERCP are similar to those in adults, there are differences. Compared with adults, congenital anomalies of the pancreaticobiliary anatomy are more common in children[12]. Research data from the United States indicate that recurrent pancreatitis, pancreatic duct stones, and congenital anatomical anomalies, such as pancreaticobiliary maljunction, pancreatic divisum, and annular pancreas, are common indications for ERCP in pediatric patients, which differs from those in adults[13]. In addition, the incidence and detection rate of common bile duct stones and choledochal cysts are higher in children compared with that in adults. In the present study, the most common condition treated with ERCP was bile duct stones, followed by chronic pancreatitis, pancreatic divisum, and pancreatic duct strictures. Other studies have indicated that common bile duct stones are the most common indication for ERCP in pediatric patients with biliary diseases, whereas chronic pancreatitis is a frequent indication among children with pancreatic diseases[14]. Pancreatic divisum and pancreatic duct strictures are common congenital anatomical anomalies of the pancreas in children. With the development of ERCP in pediatric patients, they may now be effectively treated. Further analysis of the therapeutic efficacy of ERCP in pediatric pancreaticobiliary diseases in the present study revealed that the overall treatment efficacy in the observation group was significantly higher compared with that in the control group. In addition, the time to resume eating in the observation group was significantly shorter compared with that in the control group. This suggests that ERCP for pediatric pancreaticobiliary diseases not only improves treatment efficacy, but also contributes to postoperative recovery[15]. The likely reason for these outcomes is that the pancreaticobiliary diseases involved in this study were primarily common bile duct stones. As a diagnostic and therapeutic method that does not require incision of the common bile duct, ERCP can display the morphology of the bile duct and stones when combined with contrast agents, accurately identifying their location, number, and size. This ensures the complete removal of stones, reduces stone residue, minimizes physiological impairment, and enhances postoperative recovery[16,17].
To further assess postoperative recovery, we compared liver function indicators in both groups before and after surgery. TBil is an important clinical indicator for diagnosing liver and biliary abnormalities, whereas ALT is used for assessing liver damage. Increased levels indicate possible liver injury[18,19]. Following ERCP treatment, both indicators were significantly decreased, indicating effective improvement in liver function. This was likely the result of the noninvasive nature of ERCP, which avoids incising the common bile duct and maximizes its integrity[20]. However, ERCP is an invasive procedure that induces an inflammatory response and elevates serum amylase levels[21]. Therefore, we compared the changes in CRP and serum amylase levels postoperatively between the two groups. The results indicated that both CRP and serum amylase levels significantly increased postoperatively in both groups; however, the increases were more pronounced in the control group, suggesting that although ERCP induces some inflammatory response, it is less than that caused by traditional surgery.
ERCP is an invasive diagnostic and therapeutic technique. It may also result in complications, such as hyperamylasemia, postoperative pancreatitis, gastrointestinal bleeding, and perforation. Post-ERCP pancreatitis is the most common adverse event, with a reported incidence ranging from 3% to 15%, and approximately 5% of the cases progressing to severe disease[22]. The pathophysiology of post-ERCP pancreatitis is not well understood, although physical, chemical, and microbial factors may contribute to early enzyme activation and pancreatic injury. In most cases, post-ERCP pancreatitis is mild and has a good prognosis; however, some patients may develop organ failure or local complications resulting in severe acute pancreatitis that worsens rapidly with a poor prognosis[23]. In the present study, the most common complication in the observation group was hyperamylasemia, which typically resolved within 48 to 72 hours postoperatively, and a 5.5% incidence of post-ERCP pancreatitis. A retrospective study in Turkey analyzing pediatric ERCP data over ten years reported a complication rate of 15.23%, with post-ERCP pancreatitis incidence at 11.42%. This indicates that ERCP is safe and reliable for pediatric patients[24]. Nevertheless, strict adherence to ERCP indications is essential to minimize unnecessary procedures.
ERCP is an effective and safe method for the diagnosis and treatment of pediatric pancreaticobiliary diseases. Studies suggest that ERCP will be the preferred method for certain types of pediatric pancreaticobiliary diseases in the future; however, careful consideration of indications and contraindications is necessary for the management of these conditions. With advancements in operator skills, improved equipment, and appropriate anesthesia methods, ERCP will increasingly play an important role in the diagnosis and treatment of pediatric pancreaticobiliary diseases because of its minimally invasive nature, efficacy, safety, repeatability, and good prognosis. Nonetheless, this study has certain limitations. First, it is a retrospective analysis and there may be certain biases in the data collection. Second, because of the relatively small sample size and the lack of related studies, large-sample, multicenter randomized controlled trials are needed to corroborate our findings.
| 1. | Keil R, Drábek J, Lochmannová J, Šťovíček J, Koptová P, Wasserbauer M, Frýbová B, Šnajdauf J, Matouš J, Kotalová R, Rygl M, Hlava Š. ERCP in infants, children, and adolescents-Different roles of the methods in different age groups. PLoS One. 2019;14:e0210805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Felux J, Sturm E, Busch A, Zerabruck E, Graepler F, Stüker D, Manger A, Kirschner HJ, Blumenstock G, Malek NP, Goetz M. ERCP in infants, children and adolescents is feasible and safe: results from a tertiary care center. United European Gastroenterol J. 2017;5:1024-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | McCune WS, Shorb PE, Moscovitz H. Endoscopic cannulation of the ampulla of vater: a preliminary report. Ann Surg. 1968;167:752-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 374] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Waye JD. Endoscopic retrograde cholangiopancreatography in the infant. Am J Gastroenterol. 1976;65:461-463. [PubMed] |
| 5. | Lou Q, Sun J, Zhang X, Shen H. Successful Therapeutic ERCP in a 99-Day-Old Child With Common Bile Duct Stones: A Case Report and Discussions on the Particularities of the ERCP in Children. Front Pediatr. 2020;8:435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Abu-El-Haija M, Kumar S, Quiros JA, Balakrishnan K, Barth B, Bitton S, Eisses JF, Foglio EJ, Fox V, Francis D, Freeman AJ, Gonska T, Grover AS, Husain SZ, Kumar R, Lapsia S, Lin T, Liu QY, Maqbool A, Sellers ZM, Szabo F, Uc A, Werlin SL, Morinville VD. Management of Acute Pancreatitis in the Pediatric Population: A Clinical Report From the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition Pancreas Committee. J Pediatr Gastroenterol Nutr. 2018;66:159-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 163] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 7. | Asenov Y, Akın M, Cantez S, Gün Soysal F, Tekant Y. Endoscopic retrograde cholangiopancreatography in children: Retrospective series with a long-term follow-up and literature review. Turk J Gastroenterol. 2019;30:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Trocchia C, Khalaf R, Amankwah E, Ruan W, Fishman DS, Barth BA, Liu QY, Giefer M, Kim KM, Martinez M, Dall'oglio L, Torroni F, De Angelis P, Faraci S, Bitton S, Werlin SL, Dua K, Gugig R, Huang C, Mamula P, Quiros JA, Zheng Y, Piester T, Grover A, Fox VL, Wilsey M, Troendle DM. Pediatric ERCP in the Setting of Acute Pancreatitis: A Secondary Analysis of an International Multicenter Cohort Study. J Pediatr Gastroenterol Nutr. 2023;76:817-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Åvitsland TL, Aabakken L. Endoscopic retrograde cholangiopancreatography in infants and children. Endosc Int Open. 2021;9:E292-E296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Zhu L, Xiong J, Lv Z, Liu J, Huang X, Xu W. Type C Pancreaticobiliary Maljunction Is Associated With Perforated Choledochal Cyst in Children. Front Pediatr. 2020;8:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Zhang J, Gao LC, Guo S, Mei TL, Zhou J, Wang GL, Yu FH, Fang YL, Xu BP. Endoscopic retrograde cholangiopancreatography in the treatment of pancreaticopleural fistula in children. World J Gastroenterol. 2020;26:5718-5730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Pan G, Yang K, Gong B, Deng Z. Analysis of the Efficacy and Safety of Endoscopic Retrograde Cholangiopancreatography in Children With Symptomatic Pancreas Divisum. Front Pediatr. 2021;9:761331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Rashid R, Arfin MS, Karim ASMB, Alam MB, Mahmud S. Endoscopic Retrograde Cholangiopancreatography in Bangladeshi Children: Experiences and Challenges in a Developing Country. Pediatr Gastroenterol Hepatol Nutr. 2022;25:332-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Gómez SR, Trujillo Ceballos AA, Montaño Rozo GA Sr, Solano Mariño J, Carias Domínguez AM, Vera Chamorro JF. Endoscopic Retrograde Cholangiopancreatography in Children: Nine Years' Experience at Santa Fe Foundation in Bogota, Colombia. Cureus. 2023;15:e41835. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Gong SC, An S, Shin IS, Jung PY. Usefulness of Endoscopic Retrograde Cholangiopancreatography in the Diagnosis and Treatment of Traumatic Pancreatic Injury in Children. Diagnostics (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 16. | Kandırıcı A, Gürbüz E. Our endoscopic retrograde cholangiopancreatography (ERCP) experience in pediatric patients in a training and research hospital. Hippokratia. 2022;26:152-156. [PubMed] |
| 17. | Perera KDR, Nawarathne NMM, Samarawickrama VT, Deraniyagala MP, Luxman WGE, Fernandopulle ANR. Endoscopic Retrograde Cholangiopancreatography in Children: Feasibility, Success, and Safety with Standard Adult Endoscopes and Accessories. Pediatr Gastroenterol Hepatol Nutr. 2022;25:406-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Liu W, Liu Q, Wang W, Wang P, Chen J, Hong T, Zhang N, Li B, Qu Q, He X. Differential diagnostic roles of the serum CA19-9, total bilirubin (TBIL) and the ratio of CA19-9 to TBIL for benign and malignant. J Cancer. 2018;9:1804-1812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Sehatpour F, Salehi A, Molavi Vardanjani H, Poustchi H, Gandomkar A, Malekzadeh R. Upper Normal Limit of Serum Alanine Aminotransferase and Its Association with Metabolic Risk Factors in Pars Cohort Study. Middle East J Dig Dis. 2020;12:19-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Zhang XL, Sun JH, Wu Y, Xie M, Li CC, Lv D, Yu W, Cui PL. Therapeutic outcomes of early and delayed endoscopic retrograde cholangiopancreatography and percutaneous transhepatic cholangial drainage in patients with obstructive severe acute biliary pancreatitis. J Clin Transl Res. 2023;9:160-167. [PubMed] |
| 21. | Lv ZH, Kou DQ, Guo SB. Three-hour post-ERCP amylase level: a useful indicator for early prediction of post-ERCP pancreatitis. BMC Gastroenterol. 2020;20:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Morales SJ, Sampath K, Gardner TB. A Review of Prevention of Post-ERCP Pancreatitis. Gastroenterol Hepatol (N Y). 2018;14:286-292. [PubMed] |
| 23. | Shih HY, Hsu WH, Kuo CH. Postendoscopic retrograde cholangiopancreatography pancreatitis. Kaohsiung J Med Sci. 2019;35:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Dişçi E, Peksöz R, Yıldız M, Yıldırgan Mİ, Albayrak Y, Fakirullahoğlu M, Fırıncı B, Atamanalp SS. Endoscopic Retrograde Cholangiopancreatography in Pediatric Patients. J Laparoendosc Adv Surg Tech A. 2022;32:320-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |