Published online Dec 27, 2024. doi: 10.4240/wjgs.v16.i12.3703
Revised: September 20, 2024
Accepted: October 25, 2024
Published online: December 27, 2024
Processing time: 192 Days and 3 Hours
Near-infrared fluorescence imaging via using intravenous indocyanine green (ICG) has a wide range of applications in multiple surgical scenarios. In lapa
To investigate the novel imaging strategy MCFI in LC.
This was a single-center retrospective study conducted at Peking Union Medical College Hospital, Beijing, China. Patients who underwent LC from June 2022 to March 2023 by the same surgical team were enrolled. Demographic features, clinical and surgical information were collected. The clarity, visual comfort, and effectiveness of different imaging strategies were subjectively evaluated by surgeons.
A total of 155 patients were included, 60 patients were in the non-ICG group in which only bright light illuminance without ICG was applied, 60 patients were in the SCFI group, and 35 patients were in the MCFI group. No statistically significant differences were found in demographics or clinical history. Post-surgical complications were minimal in all 3 groups with no significant differences observed. MCFI improved the clarity of imaging and visual comfort. Clarity of imaging and visual comfort were improved with MCFI.
MCFI improves biliary visualization and reduces liver fluorescence contamination, which supports its routine use in LC. MCFI may also be a better choice than SCFI in other clinical settings.
Core Tip: Near-infrared fluorescence imaging using intravenous indocyanine green facilitates intraoperative identification of biliary anatomy. However, the conventional single color strategy has limited distinguishable differences and the scale range of the fluorescence intensity; and the green fluorescence could cause visual fatigue on a reddish surgical background. Therefore, we aim to develop a novel multi-color fluorescence imaging strategy to correlate the fluorescence intensity. To help alleviate visual fatigue and observe structures with extreme low fluorescence intensity, we utilized a color range of blue to purple, and performed primary clinical trials in a large Chinese gallbladder disease center.
- Citation: Li JY, Ping L, Lin BZ, Wang ZH, Fang CH, Hua SR, Han XL. Efficacy of multi-color near-infrared fluorescence with indocyanine green: A new imaging strategy and its early experience in laparoscopic cholecystectomy. World J Gastrointest Surg 2024; 16(12): 3703-3709
- URL: https://www.wjgnet.com/1948-9366/full/v16/i12/3703.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i12.3703
In recent years, the utilization of near-infrared fluorescence (NIRF) combined with indocyanine green (ICG) has significantly increased in both open and laparoscopic surgeries across various clinical settings[1-4]. When exposed to near-infrared light, ICG binds to plasma proteins and emits light at a peak wavelength of approximately 830 nm. Near-infrared light exhibits minimal auto-fluorescence and possesses the ability to penetrate tissues up to a depth of 1 centimeter. After administration, ICG is exclusively eliminated by the liver, with biliary excretion lasting from several minutes to 24 hours[5]. The most prevalent clinical applications of NIRF ICG include evaluating anastomotic leakage, assessing flap perfusion, and identifying structures and organs[6]. Because it is metabolized by the liver, ICG is suitable to intraoperatively visualize biliary anatomy in a safe, convenient, efficient, and cost-effective manner during the laparoscopic cholecystectomy (LC)[5]. Consequently, it obviates the need for intraoperative ultrasound or cholangiography to guide dissection and identify the desired structures[1,7,8].
Since the advent of NIRF, the imaging strategies have seen improvements in both camera sensitivity and intraoperative display, including three dimensional imaging and novel dyes with improved target-to-background ratios[9]. However, the usual single-color fluorescence imaging (SCFI) strategy in which the signal intensity is directly correlated with the brightness of the single color (usually green) on the screen remains largely unchanged. Traditional fluorescence imaging relies solely on brightness to differentiate anatomical structures with different signal intensity. However, in actual clinical scenarios, various anatomical structures with a wide range of colors and brightness would easily distract the surgeon’s attention, diminishing the surgeon’s ability to distinguish brightness within a single-color setting at the same time. The common bile duct (CBD), in reality, would often appears as a far lighter shade of green than the liver. Clinically, this appearance may cause the bile duct to remain indistinct because the surgeon’s attention is completely occupied by the heavily fluorescence-polluted liver.
Based on these observations, we aimed to develop a new imaging strategy that can improve the ability of surgeons to recognize a wider range of fluorescence intensities. To that end, we have designed a multi-color fluorescence imaging (MCFI) strategy in which the signal intensity is correlated with not only the brightness but also different colors as well. We hereby aimed to test whether the MCFI strategy could adequately visualize the biliary system, reduce surgical time, and increase the visual comfort of surgeons compared with the SCFI and non-ICG strategies. This early experience would signify that MCFI can augment real-time intraoperative localization of the extrahepatic biliary tree, thereby offering a safe, accurate, and efficient strategy to guide surgical interventions.
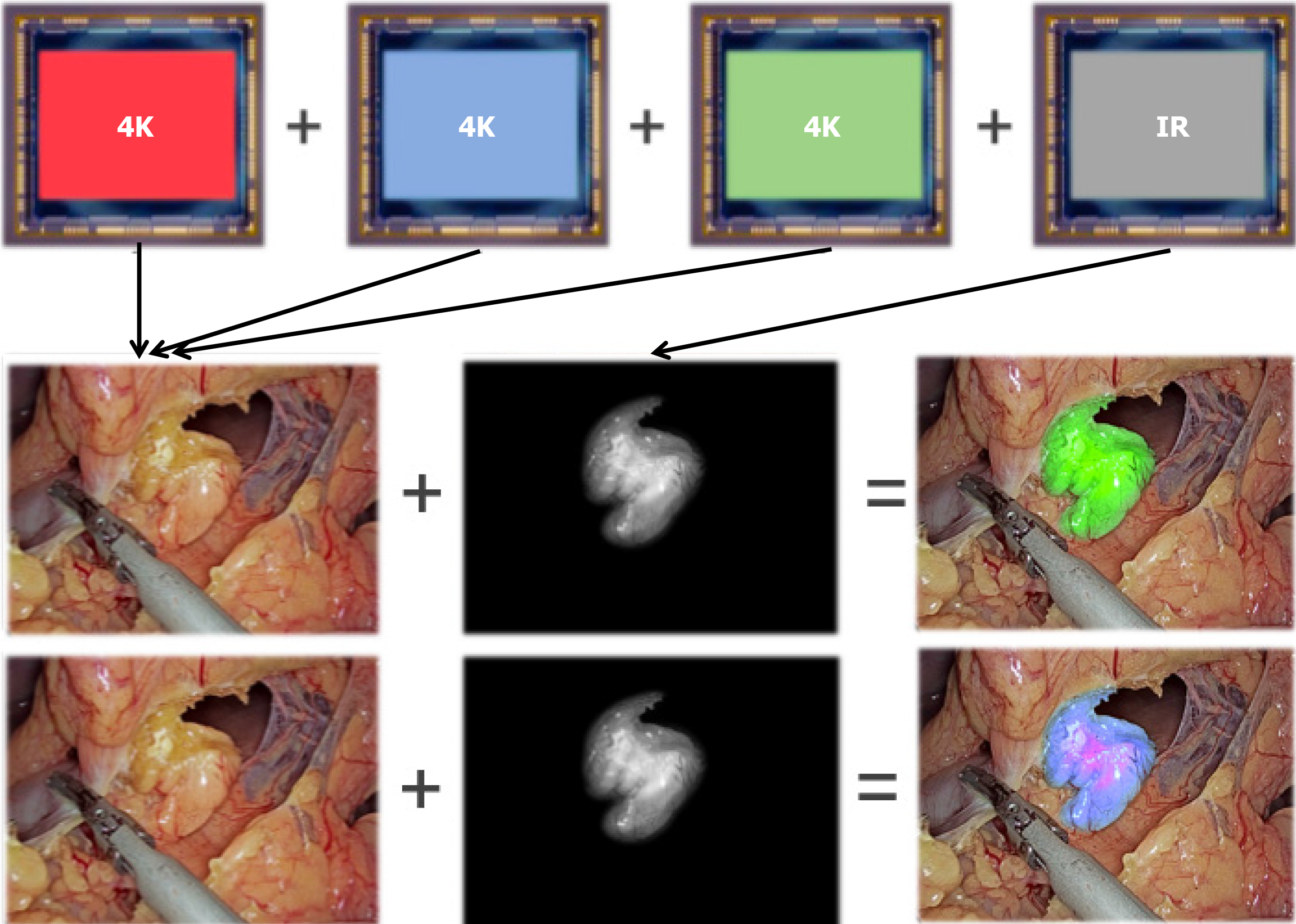
In our endoscopic fluorescence imaging system, we use the MCFI to visualize ICG fluorescence via the addition of a laser excitation at 805 nm and filter sets. The surgical videos were recorded with a resolution of 1980 × 1020 pixels. The surgeons then assessed the visualization of various anatomical structures, including the common hepatic duct, cystic duct, CBD, and aberrant ducts. They also compared the MCFI with SCFI and non-ICG in terms of assisting in the identification of biliary anatomy. The surgeons evaluated the clarity of the imaging, the extent to which the new strategy helped in completing the surgery compared to SCFI, and the visual comfort of MCFI compared to SCFI. The assessment was based on a Likert scale of 0 to 4, with higher scores indicating better performance (0: Unsatisfactory, detracts from visualization; 1: No additional value; 2: Satisfactory, some added value, marginal aid to identification of anatomy; 3: Good, appreciable added value, sufficient identification of anatomy; 4: Excellent, exemplary added value, identification of anatomy is very clear).
A dose of 0.5 mg of ICG was administered intravenously 20 minutes prior to surgery. After injection, only the raw fluorescence intensity data without color information were collected by the fluorescence camera. In our MCFI strategy, the displayed pseudocolored is restored by a staining algorithm that correlates the intensity of the fluorescence with multiple colors. Specifically, MCFI represents the brightness of fluorescence with a blue (weak)-to-purple (strong) color range (Figure 1). Compared with the traditional SCFI strategy, the MCFI strategy features 2 major novel aspects. First, reprogramming signal intensities into different colors on the display strengthens the surgeon’s ability to recognize a wider range. Second, the use of a blue-purple color scale instead of a green scale could reduce visual discomfort and fatigue, providing optimal imaging effects in body tissues, which are mainly red and yellow.
This work was a single-center retrospective study conducted at Peking Union Medical College Hospital, Beijing, China. Patients who underwent LC between June 2022 and March 2023 by the same surgical team were chronologically enrolled in this study. We excluded patients who had allergies or experienced adverse reactions to ICG, iodine, or dyes containing iodine. Patients were divided into non-ICG, SCFI, or MCFI groups according to the mode of display used in their surgeries. Demographic information, including age, sex, ethnicity, body mass index, preoperative diagnosis, and com
Mann-Whitney U tests were conducted to quantitatively and qualitatively assess any significant differences among the three imaging strategies. A P value of < 0.05 was considered statistically significant. All tests were performed using GraphPad Prism software version 9.4.1.
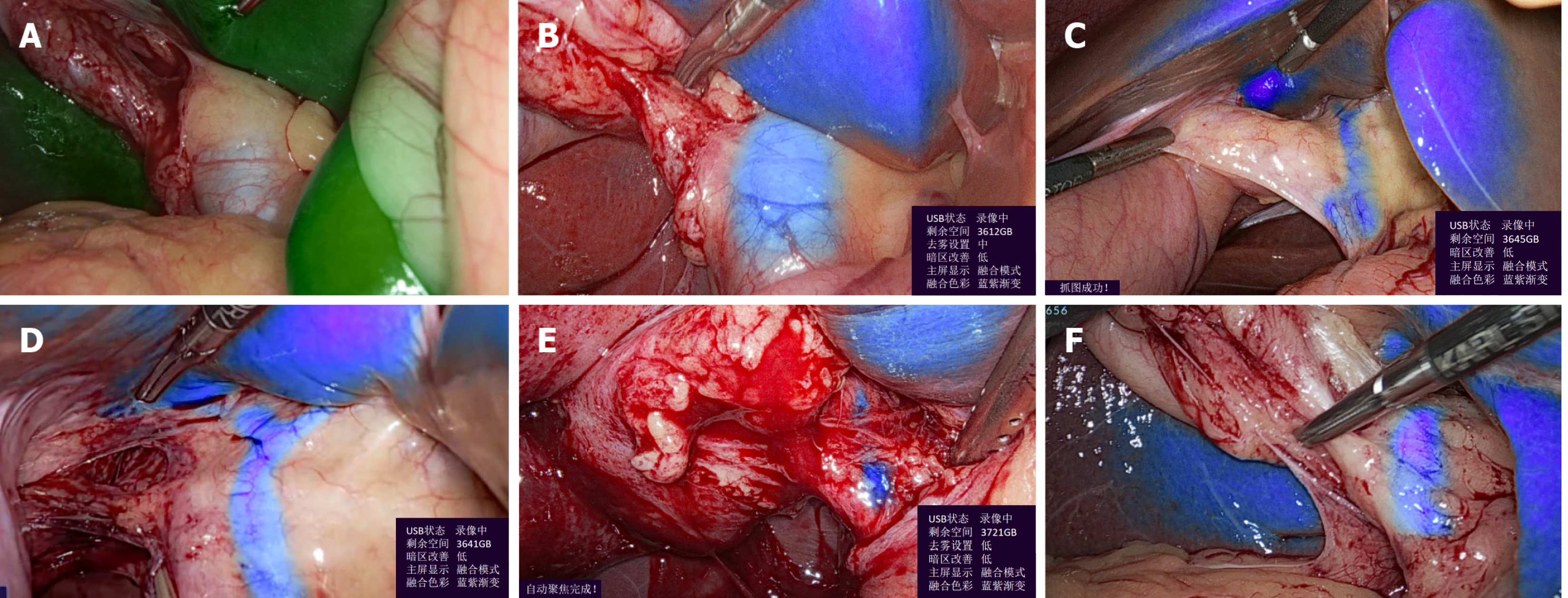
The new MCFI strategy helps to visualize the CBD when SCFI-ICG cannot be used (Figure 2A and B), and helps to predict the shape of the left and right hepatic ducts (Figure 2C and D). Thus, it reduces the risk of bile ducts injury, especially in cases of heavy adhesion and inflammatory edema (Figure 2E). The appearance of the surgical instruments did not disturb the imaging of the hepatic ducts (Figure 2F).
Between March 2022 and March 2023, a total of 155 patients were enrolled in the study. Of these, 35 patients were in the MCFI group, 60 patients were in the SCFI group, and 60 patients were in the non-ICG group. A majority of the patients (60.64%, 94/155) of the patients were treated for cholelithiasis, whereas the remaining patients underwent surgery for cholecystitis, choledocholithiasis, cholangitis, gallstone pancreatitis, or gallbladder polyps (Table 1).
| Patient characteristic | MCFI (n = 35) | SCFI (n = 60) | Non-ICG (n = 60) |
| Age (years), median (range) | 51.87 (31-70) | 53.76 (23-85) | 58.70 (27-81) |
| Sex ratio (M:F) | 1.059 | 0.935 | 1.400 |
| BMI (kg/m2), median | 22.32 | 22.74 | 25.2 |
| Preoperative diagnosis | |||
| Cholelithiasis | 12 (34.29) | 43 (67.3) | 39 (65.0) |
| Acute cholecystitis | 14 (40.00) | 3 (3.3) | 3 (5.0) |
| Chronic cholecystitisa,b | 5 (14.3) | 11 (20.4) | 8 (13.3) |
| Gallbladder polyps | 3 (8.6) | 2 (2.2) | 7 (11.7) |
| Cholecystic adenomyosis | 1 (2.9) | 1 (1.1) | 3 (5.0) |
| Post-surgical complications | |||
| None | 32 (91.43) | 57 (95.00) | 53 (88.3) |
| Pain | 3 (8.57) | 3 (5.00) | 7 (11.7) |
| Operative time/minutes, medianb | 85.00 | 83.42 | 108.0 |
No significant differences in demographics or clinical history were observed among the three groups. Post-surgical complications were minimal, with only 7 (11.7%) in the group, 3 (8.57%) in the MCFI group, and 3 (5.00%) patients in the SCFI group, experiencing pain (P = 0.614). The mean operative time, from the start of surgery to clamping of the cystic duct, was 85.00, 83.42, and 108.0 minutes for the three groups, respectively (P = 0.001). We encountered and successfully completed two challenging surgeries in the MCFI group (one with a short biliary duct, and another with severe inflammatory adhesion) (Video 1 and 2).
The subjective evaluation of the MCFI strategy compared with that of the single-color and without fluorescence involved five experienced surgeons. The average score for clarity of imaging was 3.49 (appreciable to exemplary added value) on a scale of 0 to 4, and MCFI had added value in identifying the biliary anatomy. Compared with SCFI, this new imaging strategy could help surgeons to complete the surgery compared to SCFI with an average score of 2.17 (sa
Although LC performed without non-ICG demonstrated comparable efficacy in most cases, the MCFI strategy has been proven to be more effective and preferable by surgeons in emergency or complex situations. This was particularly notable in cases involving thick and edematous anterior bile duct tissue as well as fatty liver, where the non-ICG display failed to predict the misshaping of the duct system, which could lead to bile duct injury. When the gallbladder is dissected from its bed, excessively deep cuts can damage the liver, whereas a shallow cut may compromise the gallbladder. As the amount of tissue increases, the thickness of the lymph and adipose tissue thickness increases, and the fluorescence weakens. The multi-color method delineates the boundary between the liver surface and the gallbladder bed. The new MCFI strategy helps to visualize the when SCFI-ICG cannot be used, which is especially useful in cases involving fatty liver and inflammatory edema (Figure 2E and Video 1). It also helps to predict the shape of the left and right hepatic ducts, thus reducing the risk of bile duct injury (Figure 2C and D, Video 2).
Our study focuses on how the new MCFI strategy helps the biliary surgical team to improve the surgical efficacy in patients undergoing LC. During LC surgery, the mainstream imaging methods include the biliary radiography method and the NIRF imaging. After the imaging agent is injected, the conventional biliary radiography method could con
Our MCFI strategy is a major advancement, highlighting the ICG concentration in the blue to purple range. To the best of our knowledge, this is the first study to report MCFI’s clinical application. The findings of this study indicate that the use of MCFI enhances the contrast between the background or liver fluorescence, thereby improving the visualization of the extrahepatic biliary tree. This imaging strategy has the potential to decrease the time needed to adequately visualize tissues from dosing to surgery and offers better performance in challenging cases. Furthermore, we believe that this technique is beneficial for delineating complications, including iatrogenic injury and bile leakage, during surgeries conducted by inexperienced surgeons[12].
At our center, this imaging strategy is beginning to be applied in partial hepatectomy, the partial nephrectomy, the Beger procedure, the evaluation of the blood supply of the parathyroid gland in endoscopic thyroidectomy, and the evaluation of the anastomotic blood supply in Dixon. More data are needed to prove its performance in demonstrating structures in low fluorescence intensity in various surgical settings. Most surgeries were laparoscopic cholecystectomies. Importantly, this study is retrospective and aims to promote the adoption of this new imaging strategy. While most MCFI procedures are comparable in terms of surgical difficulty, the decision to adopt fluorescence is ultimately based on the surgeon’s preference due to the limited availability of MCFI-supported machines at our center. However, in emergency situations and difficult surgical settings, most surgeons prefer the MCFI strategy. These findings are in accordance with our results, which showed that MCFI has been more widely applied to acute cholecystitis than the single-green fluorescence. Additionally, the MCFI strategy proved particularly beneficial in cases with anatomical difficulties in the gallbladder triangle; it improved visualization and shortened surgical time. Moreover, this new imaging strategy enables the identification of the left and right hepatic ducts, which is especially useful in cases involving fatty liver and inflammatory edema.
Nevertheless, this study is subject to limitations. First, its retrospective nature introduces patient bias and includes unmatched cases in the three groups. Notably, the SCFI group included more patients than the MCFI group. Second, because the number of available MCFI machines was limited, surgeons tended to use MCFI in difficult cases that may be associated with longer surgical time and an increased risk of complications. This study represents one of an initial experience in applying the MCFI strategy in LC, and more carefully designed prospective controlled studies to assess its effectiveness in specific patient groups, especially those at high risk. Finally, this study solely verifies the feasibility and safety of the novel MCFI strategy. Most of our retrospective clinical data were retrieved from electronic health records; and some of surgical videos were not available, thus data analyses of specific quantitative metrics to compare the imaging strategies could not be conducted. Non-inferiority and superiority studies are currently being conducted with a rigorous head-to-head design involving long-term large samples to further evaluate the effectiveness of this imaging strategy.
In the present study, we developed a novel MCFI imaging strategy to increase the performance of fluorescence imaging in laparoscopic surgeries and preliminarily explored its effectiveness in treating LC. Compared with the SCFI strategy, the MCFI strategy delineates biliary anatomy better in cases of low fluorescence intensity and decreases visual fatigue in surgeons. Future prospective studies with MCFI could further elucidate its efficacy, and we believe MCFI could become a routine and practical procedure in LC. In addition, MCFI may be a better choice than SCFI in additional clinical surgical settings.
| 1. | Ishizawa T, Bandai Y, Ijichi M, Kaneko J, Hasegawa K, Kokudo N. Fluorescent cholangiography illuminating the biliary tree during laparoscopic cholecystectomy. Br J Surg. 2010;97:1369-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 250] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 2. | Liberale G, Bourgeois P, Larsimont D, Moreau M, Donckier V, Ishizawa T. Indocyanine green fluorescence-guided surgery after IV injection in metastatic colorectal cancer: A systematic review. Eur J Surg Oncol. 2017;43:1656-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Skubleny D, Dang JT, Skulsky S, Switzer N, Tian C, Shi X, de Gara C, Birch DW, Karmali S. Diagnostic evaluation of sentinel lymph node biopsy using indocyanine green and infrared or fluorescent imaging in gastric cancer: a systematic review and meta-analysis. Surg Endosc. 2018;32:2620-2631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Purich K, Dang JT, Poonja A, Sun WYL, Bigam D, Birch D, Karmali S. Intraoperative fluorescence imaging with indocyanine green in hepatic resection for malignancy: a systematic review and meta-analysis of diagnostic test accuracy studies. Surg Endosc. 2020;34:2891-2903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Ishizawa T, Tamura S, Masuda K, Aoki T, Hasegawa K, Imamura H, Beck Y, Kokudo N. Intraoperative fluorescent cholangiography using indocyanine green: a biliary road map for safe surgery. J Am Coll Surg. 2009;208:e1-e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 182] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 6. | Pollmann L, Juratli M, Roushansarai N, Pascher A, Hölzen JP. Quantification of Indocyanine Green Fluorescence Imaging in General, Visceral and Transplant Surgery. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Dip F, Roy M, Lo Menzo E, Simpfendorfer C, Szomstein S, Rosenthal RJ. Routine use of fluorescent incisionless cholangiography as a new imaging modality during laparoscopic cholecystectomy. Surg Endosc. 2015;29:1621-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Matsui A, Tanaka E, Choi HS, Winer JH, Kianzad V, Gioux S, Laurence RG, Frangioni JV. Real-time intra-operative near-infrared fluorescence identification of the extrahepatic bile ducts using clinically available contrast agents. Surgery. 2010;148:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Lim ZY, Mohan S, Balasubramaniam S, Ahmed S, Siew CCH, Shelat VG. Indocyanine green dye and its application in gastrointestinal surgery: The future is bright green. World J Gastrointest Surg. 2023;15:1841-1857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Donnellan E, Coulter J, Mathew C, Choynowski M, Flanagan L, Bucholc M, Johnston A, Sugrue M. A meta-analysis of the use of intraoperative cholangiography; time to revisit our approach to cholecystectomy? Surg Open Sci. 2021;3:8-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Verbeek FP, Schaafsma BE, Tummers QR, van der Vorst JR, van der Made WJ, Baeten CI, Bonsing BA, Frangioni JV, van de Velde CJ, Vahrmeijer AL, Swijnenburg RJ. Optimization of near-infrared fluorescence cholangiography for open and laparoscopic surgery. Surg Endosc. 2014;28:1076-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Humm GL, Peckham-Cooper A, Chang J, Fernandes R, Gomez NF, Mohan H, Nally D, Thaventhiran AJ, Zakeri R, Gupte A, Crosbie J, Wood C, Dawas K, Stoyanov D, Lovat LB. Surgical experience and identification of errors in laparoscopic cholecystectomy. Br J Surg. 2023;110:1535-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |