Published online Jan 27, 2024. doi: 10.4240/wjgs.v16.i1.205
Peer-review started: August 28, 2023
First decision: November 24, 2023
Revised: December 9, 2023
Accepted: January 9, 2024
Article in press: January 9, 2024
Published online: January 27, 2024
Processing time: 149 Days and 21.7 Hours
Primary liver cancer is a malignant tumor with a high recurrence rate that significantly affects patient prognosis. Postoperative adjuvant external radiation therapy (RT) has been shown to effectively prevent recurrence after liver cancer resection. However, there are multiple RT techniques available, and the differential effects of these techniques in preventing postoperative liver cancer re
To assess the advantages and disadvantages of various adjuvant external RT methods after liver resection based on overall survival (OS) and disease-free survival (DFS) and to determine the optimal strategy.
This study involved network meta-analyses and followed the PRISMA guidelines. The data of qualified studies published before July 10, 2023, were collected from PubMed, Embase, the Web of Science, and the Cochrane Library. We included relevant studies on postoperative external beam RT after liver resection that had OS and DFS as the primary endpoints. The magnitudes of the effects were determined using risk ratios with 95% confidential intervals. The results were analyzed using R software and STATA software.
A total of 12 studies, including 1265 patients with hepatocellular carcinoma (HCC) after liver resection, were included in this study. There was no significant heterogeneity in the direct paired comparisons, and there were no significant differences in the inclusion or exclusion criteria, intervention measures, or outcome indicators, meeting the assumptions of heterogeneity and transitivity. OS analysis revealed that patients who underwent stereotactic body radiotherapy (SBRT) after resection had longer OS than those who underwent intensity modulated radiotherapy (IMRT) or 3-dimensional conformal RT (3D-CRT). DFS analysis revealed that patients who underwent 3D-CRT after resection had the longest DFS. Patients who underwent IMRT after resection had longer OS than those who underwent 3D-CRT and longer DFS than those who underwent SBRT.
HCC patients who undergo liver cancer resection must consider distinct advantages and disadvantages when choosing between SBRT and 3D-CRT. IMRT, a RT technique that is associated with longer OS than 3D-CRT and longer DFS than SBRT, may be a preferred option.
Core Tip: The core focus of this study is the comparative analysis of various adjuvant external beam radiation therapy (RT) methods following hepatectomy based on the network meta-analysis. The key aspects emphasized in the study include the efficacy of different RT methods in terms of disease-free survival and overall survival for postoperative liver cancer patients. The research aims to provide valuable insights for future investigations and clinical applications, potentially influencing the direction of liver cancer treatment.
- Citation: Yang GY, He ZW, Tang YC, Yuan F, Cao MB, Ren YP, Li YX, Su XR, Yao ZC, Deng MH. Unraveling the efficacy network: A network meta-analysis of adjuvant external beam radiation therapy methods after hepatectomy. World J Gastrointest Surg 2024; 16(1): 205-214
- URL: https://www.wjgnet.com/1948-9366/full/v16/i1/205.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i1.205
Primary liver cancer is the second leading cause of cancer-related death, and its recurrence has always been an important factor affecting the prognosis of liver cancer patients. Liver resection surgery is currently the preferred treatment for liver cancer, but approximately 70% of patients experience recurrence within 5 years after surgery, severely impacting patient survival time[1,2]. Additionally, a second surgery increases the treatment risk and is influenced by factors such as patient health status, liver function, and liver reserve that further burden patients both physically and mentally and hinder subsequent treatment. Therefore, preventing postoperative recurrence of liver cancer has become an important research topic.
Postoperative adjuvant therapy, including interventional therapy, chemotherapy, and targeted therapy, has been proven effective in preventing liver cancer recurrence after liver resection[3,4]. As an emerging treatment modality relying on technological advances, external beam radiotherapy (EBRT), including stereotactic body radiotherapy (SBRT), intensity-modulated radiotherapy (IMRT)/image-guided radiotherapy (IGRT), volumetric modulated arc therapy (VMAT), and three-dimensional conformal radiation therapy (3D-CRT), features precision and safety and offers significantly better efficacy and safety than traditional radiotherapy. EBRT has recently been considered to achieve curative effects in several early-stage liver cancers and has also been applied as adjuvant therapy after liver resection surgery. However, there are multiple EBRT methods available, and they have not yet become commonly used approaches for postoperative adjuvant treatment of liver cancer. As a result, there is a lack of clarity on which EBRT method is better for preventing recurrence after liver cancer resection, and related research is relatively insufficient. In this study, we aim to use network meta-analysis methods based on the results of current clinical trials to evaluate the advantages and disadvantages of EBRT methods for preventing recurrence after liver resection surgery, thereby providing a reference for future clinical experiments and the selection of clinical treatments.
This network meta-analysis has been registered in the PROSPERO international database (https://www.crd.york.ac.uk/prospero/). The PROSPERO Registration Number is CRD42023448817. The entire analysis process was conducted in accordance with the PRISMA guidelines, as shown in Supplementary Table 1[5].
As of July 10, 2023, the participants in this study completed the retrieval of all relevant and language-unrestricted literature from PubMed, Embase, the Web of Science, and the Cochrane Library. The search terms used were as follows: "liver cancer", "hepatocellular carcinoma", "radiotherapy", "radiation", "external beam radiotherapy", "EBRT", "radical surgery", "hepatectomy", "postoperative", "recurrence", "stereotactic body radiotherapy", "SBRT", "intensity-modulated radiotherapy", "IMRT", "image-guided radiotherapy", "IGRT", "3-dimensional conformal radiation therapy", "3D-CRT", "volumetric modulated arc therapy", "VMAT", and "proton beam therapy". The participants in this study also cross-checked and supplemented the literature included in this analysis based on relevant published meta-analyses and references cited in reviews to ensure that the retrieved literature met the inclusion criteria and that the search scope was sufficient.
Study population: Adult hepatocellular carcinoma (HCC) patients diagnosed with primary liver cancer who underwent surgical resection and had negative surgical margins according to postoperative pathology.
Interventions: Only radiation therapy (RT) methods classified as EBRT techniques were considered interventions.
Outcome measures: Two-year disease-free survival (DFS) and 2-year overall survival (OS).
Study design: Randomized controlled trials (RCTs), prospective studies, and retrospective studies that included propensity score matching or controls; the inclusion criteria included the following: (1) RCTs focusing on adjuvant radiotherapy for HCC patients after liver cancer resection; (2) studies reporting the following outcome measures were included: 2-year OS and DFS, with hazard ratio and 95% confidential intervals (95%CI) data; and (3) studies involving patients who underwent liver cancer resection and received only EBRT or no other treatment. The exclusion criteria were as follows: (1) Clinical studies without the specified outcome measures of this study; (2) studies that did not compare adjuvant radiotherapy with no adjuvant radiotherapy or single-arm studies; (3) studies including misdiagnosed or underage patients; (4) studies including HCC patients with positive surgical margins or unclear surgical margins; (5) studies including HCC patients receiving other liver cancer-related treatments in addition to EBRT; and (6) literature published in the form of conference abstracts, research proposals, case reports, or reviews, not representing clinical studies.
The literature search and data extraction for the included studies were conducted by three research participants (Yang GY, He ZW and Tang YC) based on the aforementioned search strategy. Any disagreements were resolved by the fourth research participant (Yao ZC). The following data were extracted from each included study: (1) Study characteristics, publication year, author names, study design, study population, and number of participants; (2) specific radiotherapy methods; and (3) outcome measures, including 2-year DFS and OS. Two researchers assessed the quality of the included RCTs using the Cochrane Collaboration's risk of bias tool. The quality of the non-RCT studies was assessed using the Newcastle-Ottawa Scale, with a score of 0-3 indicating low quality, 3-6 indicating moderate quality, and ≥ 7 indicating high quality. Any disagreements were resolved by the third research participant. An overview of the inclusion and exclusion criteria for the included studies can be found in Supplementary Table 2.
The software packages "rjags4-14" and "gemt1.0-1" in R software version 4.3.1 (https://www.r-project.org/) and Stata SE version 15.1 were used for conducting network meta-analyses of different intervention measures. The Bayesian network framework was employed to assess the consistency of direct and indirect comparisons, and the Markov chain Monte Carlo model was used to detect model heterogeneity (I2). An I2 value less than 50 indicated acceptable heterogeneity and satisfied the assumption of consistency. The convergence of the model was evaluated using region and density plots and Brooks–Gelman–Rubin diagnostic plots. The model was considered to have good convergence when the iteration number reached 20000 and the bandwidth approached 0. The "rank" method was used to generate a ranking plot of the probability of treatment efficacy. The advantages and disadvantages of intervention measures were inferred by calculating the area under the cumulative ranking curve (SUCRA).
A flowchart of the studies included in this research is presented in Supplementary Figure 1. A total of 735 articles were initially extracted through a preliminary search based on the search strategy. After removal of 149 duplicate articles, the remaining 104 articles were screened based on their titles and abstracts. Subsequently, 92 articles were excluded for the following reasons: not related to the objective (n = 18); not compare postoperative adjuvant radiotherapy with no adjuvant radiotherapy (n = 46); published as conference abstracts, study protocols, case reports, or reviews (n = 17); and not yet completed (n = 11). Finally, 12 clinical studies were included in this network meta-analysis. The included studies were conducted from 2014 to 2023, and all the research centers were located in China. A total of 1265 patients were included in this study. The NMT approach was used to evaluate the effects of three different external radiotherapy techniques, namely, SBRT[6,7], IMRT[8-12], and 3D-CRT[13-17], on preventing postoperative recurrence of liver cancer, with sample sizes ranging from 9 to 82 patients. The baseline characteristics of the included studies are shown in Supplementary Table 3.
The specific scoring criteria for the included RCTs in this study can be found in Supplementary Figure 2. Any disagreements were resolved by the third research participant. Due to the current realities of clinical practice, conducting double-blind RCTs with both patients and health care providers is almost impractical. Some randomized trials were open-label, and the proportion of non-RCT studies among the included studies was relatively high. The blinding of participants and the potential risk of bias resulting from the lack of blinding could affect the outcomes. We believe that as long as the sequence of participant enrollment is randomized, the bias risk of participants may be considered relatively low.
A total of 12 studies were included in this research, and the trace and density plots can be found in Supplemen
The evaluators of this study assessed the inclusion and exclusion criteria of the included studies to ensure that the patients involved met the criteria. Additionally, it was ensured that the same radiotherapy methods used in the included studies did not significantly affect treatment variables, such as the number of radiotherapy sessions and radiation dose. In most of the studies, the total radiation dose for patients receiving posthepatectomy adjuvant radiotherapy was above 40 Gy; in more than half of the studies, the 2-year OS rate was above 84.4%, and the 2-year DFS rate was above 71.1%, indicating good generalizability of the included studies. Moreover, all the research centers included in the studies were located in China. No significant differences in patient samples related to etiology were found in the included studies, indicating high geographical consistency and compliance with the assumption of generalizability.
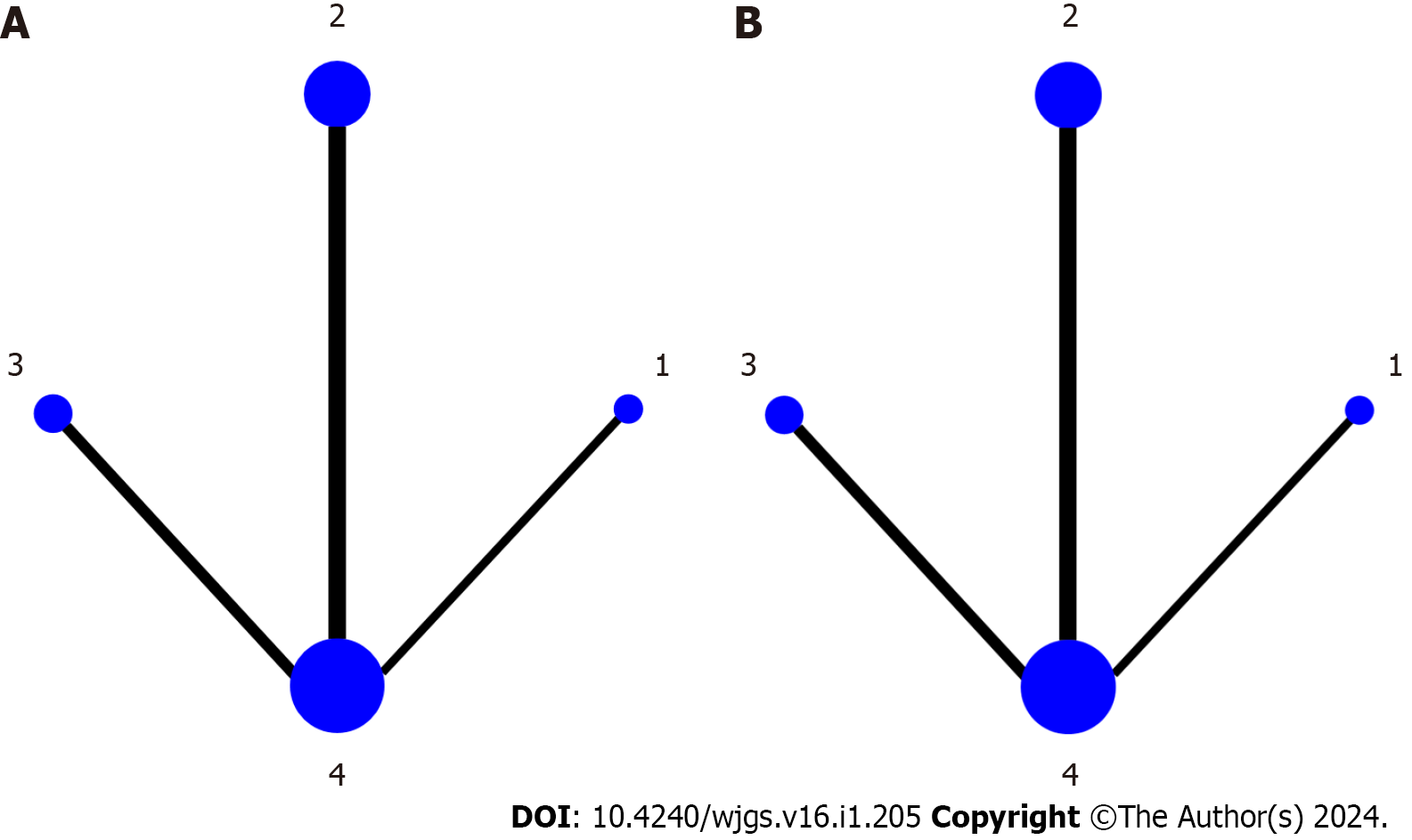
A total of 12 included studies involved 1265 enrolled patients and included three types of posthepatectomy adjuvant external radiotherapy methods. The network geometry plots directly comparing no adjuvant radiotherapy with posthepatectomy adjuvant radiotherapy using SBRT, IMRT, and 3D-CRT are shown in Figure 2. For all direct pairwise comparisons, there was no statistically significant heterogeneity among the included studies. Among the studies related to OS and DFS, two studies compared posthepatectomy SBRT with no adjuvant radiotherapy, five studies compared posthepatectomy IMRT with no adjuvant radiotherapy, and three studies compared posthepatectomy 3D-CRT with no adjuvant.
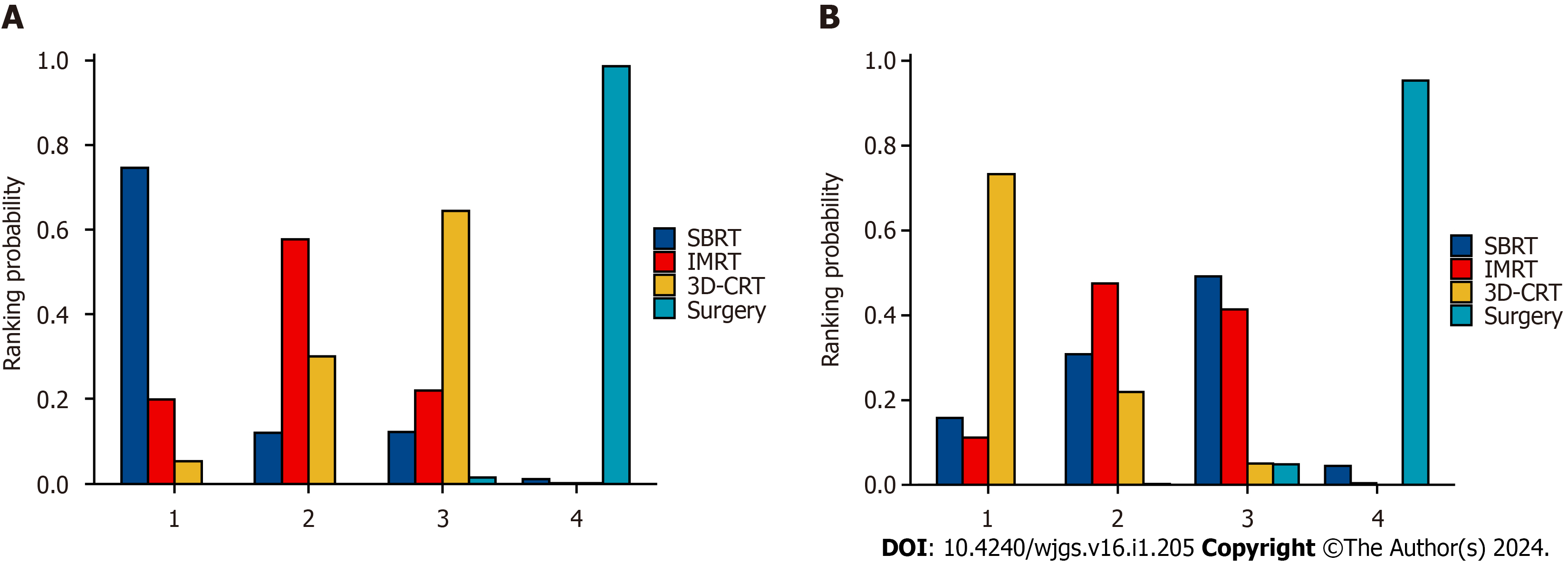
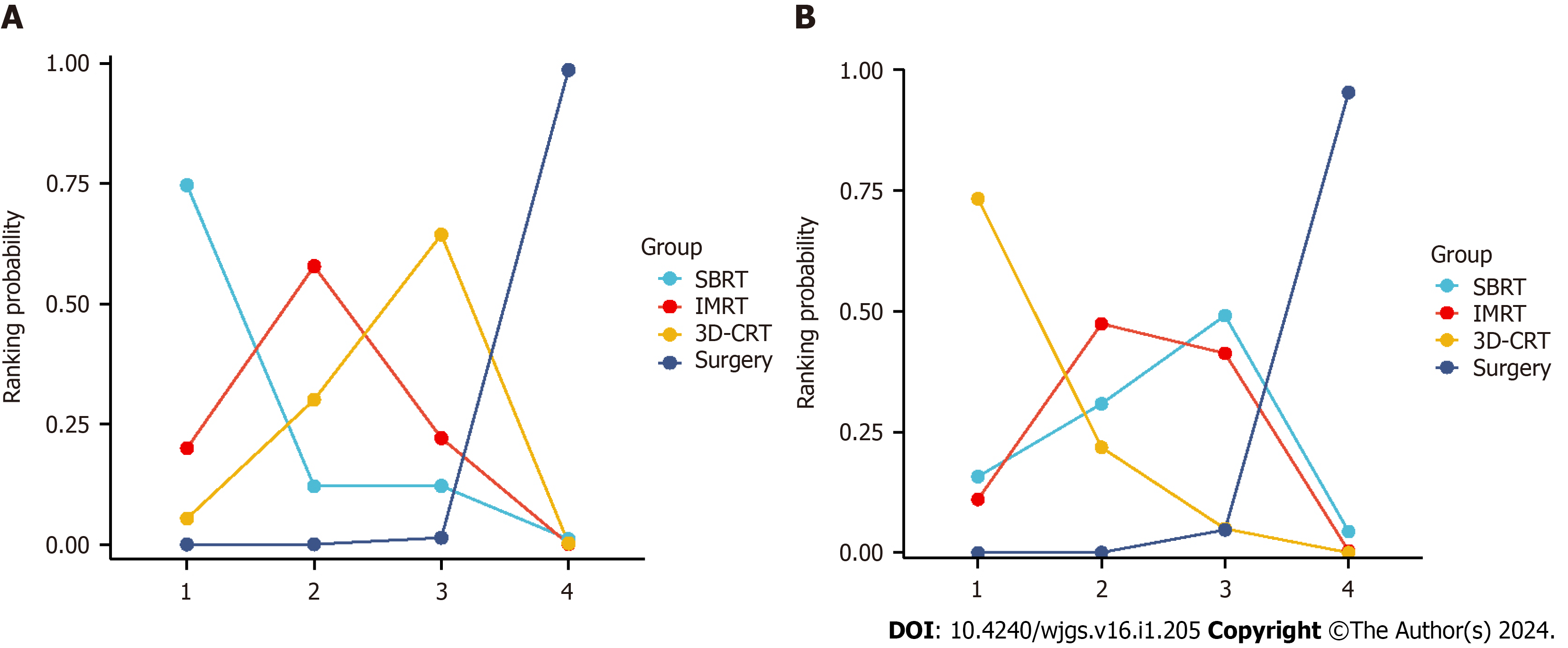
A comparison of OS and DFS after liver cancer resection and adjuvant radiotherapy in patients with HCC, along with 95%CI, can be found in Tables 1 and 2. The probability histograms for the rankings of OS and DFS according to different adjuvant radiotherapy methods after liver resection are shown in Figure 3. The area under the SUCRA calculations are shown in Figure 4. In terms of OS, patients who underwent SBRT after surgery had longer OS than those who underwent IMRT or 3D-CRT. Patients who underwent IMRT after surgery had a better OS than those who underwent 3D-CRT. Regarding DFS, patients who underwent 3D-CRT after surgery experienced a better preventive effect against HCC recurrence than did patients who underwent SBRT or IMRT. IMRT was more effective than SBRT at prolonging patient DFS. Among the three techniques, SBRT resulted in the longest OS, while 3D-CRT resulted in the longest DFS.
| Log odds ratio (95%CI) | ||||
| SBRT | IMRT | 3D-CRT | Surgery | |
| SBRT | - | -0.8913 (-4.685, 1.333) | -1.241 (-4.957, 0.9864) | -2.38 (-6.083, -0.2884) |
| IMRT | 0.8913 (-1.333, 4.685) | - | -0.3267 (-1.379, 0.7683) | -1.475 (-2.325, -0.6613) |
| 3D-CRT | 1.241 (-0.9864, 4.957) | 0.3267 (-0.7683, 1.379) | - | -1.14 (-1.874, -0.5102) |
| Surgery | 2.38 (0.2884, 6.083) | 1.475 (0.6613, 2.325) | 1.14 (0.5102, 1.874) | - |
| Log odds ratio (95%CI) | ||||
| SBRT | IMRT | 3D-CRT | Surgery | |
| SBRT | - | 0.07723 (-1.135, 1.230) | 0.5162 (-0.6510, 1.697) | -0.8220 (-1.878, 0.1440) |
| IMRT | -0.07723 (-1.230, 1.135) | - | 0.4326 (-0.3994, 1.323) | -0.9057 (-1.545, -0.3127) |
| 3D-CRT | -0.5162 (-4.697, 0.651) | -0.4326 (-1.323, 0.3994) | - | -1.339 (-1.994, -0.7873) |
| Surgery | 0.8220 (-0.1440, 1.878) | 0.9057 (0.3127, 1.545) | 1.339 (0.7873, 1.994) | - |
Based on the high recurrence rates in clinical practice, we explored adjuvant treatment options after liver cancer resection. Compared to invasive treatments such as repeat surgery or ablation, transcatheter arterial chemoembolization, and expensive systemic treatments such as targeted therapy and immunotherapy, as well as chemotherapy with important side effects, external radiotherapy seems to have unique advantages and can serve as a new option for adjuvant therapy. Given the limited specific research on adjuvant radiotherapy methods after liver cancer resection and the lack of a definitive consensus in clinical practice, we chose to evaluate which external RT method was most effective at preventing recurrence after liver cancer surgery through NMT. In this study, we conducted NMT on 1265 patients from 12 clinical studies to assess which external RT method after liver cancer surgery could effectively prevent recurrence. We also selected OS and DFS, which are commonly used to evaluate the efficacy of liver cancer treatments, as the primary outcome measures.
Unlike previous comparisons between RT and ablation and between RT and interventional therapy, we focused on RT itself. Although we collected almost all clinical studies on adjuvant radiotherapy after liver cancer resection, only 12 studies met the inclusion criteria. Among them, there were only 2 studies on SBRT after liver resection and no clinical studies on VMAT, proton radiotherapy, or IGRT after liver resection. Additionally, no clinical studies comparing different EBRT methods were found. This posed a challenge for our study because it made it difficult for us to construct a perfect network meta-analysis structure for EBRT.
However, we believe this finding reflects the current clinical practice. In recent years, SBRT has been considered an alternative to ablation for achieving local tumor control, but it is rarely used as adjuvant radiotherapy for irregular or extensive liver cancer resection margins. Although IMRT is the most commonly used external radiotherapy method in our country, IMRT did not have overall advantages in terms of OS or DFS compared to the other two methods in this study; moreover, it demonstrated noninferiority in prolonging OS and preventing recurrence in HCC patients. This finding indicates that IMRT may be a good option for extending OS while effectively preventing recurrence. Although 3D-CRT has the greatest advantage in terms of improving DFS, the results of the related analysis on OS in this study were not optimistic for 3D-CRT patients. Similar findings were reported by Jiang et al[18], who reported that for patients with unresectable but localized HCC, IMRT was superior to 3D-CRT in terms of treatment response and potential survival. Hou et al[19] also reported that IMRT was more effective than 3D-CRT in HCC patients with portal or inferior vena cava tumor thrombus without increasing radiation-related toxic reactions. Chen et al[20] reported that IMRT had a better conformal index for the planned target volume than did 3D-CRT, with a lower hot spot (V = 110%). These findings suggest that IMRT, and even IGRT based on advanced guidance techniques, may achieve better treatment outcomes than 3D-CRT, including better prolongation of patient survival. Although new adjuvant 3D-CRT regimens have emerged with the advancement of associated technologies, their effectiveness has not been definitively confirmed.
Previous studies comparing the advantages and disadvantages of adjuvant radiotherapy methods after liver cancer surgery have been extremely limited. This article attempts to fill this research gap and provides some guidance and assistance in selecting the appropriate radiotherapy method after liver cancer resection. This approach is attended by a certain degree of novelty. The search strategy of this study was extensive and included all relevant research, without restrictions on language, country, or year. The use of 2-year OS and 2-year DFS as primary outcome measures and the relatively long follow-up period added to the representativeness of the study.
Although every effort was made to include all relevant studies, the limited number of reports on clinical trials, especially RCTs, increased the potential risk of bias in the research findings. The lack of published clinical trials comparing different radiotherapy methods also affected the assessment of the consistency of the studies. We minimized bias through methodological approaches such as study identification, data selection, statistical analysis, and heterogeneity analysis, enhancing the accuracy of the study.
As surgical resection is the preferred treatment for liver cancer, the role of RT in the field of liver cancer treatment is still being explored, and its position remains undetermined. Therefore, the use of RT in the clinical treatment of liver cancer is still considered rare and insufficiently effective by some medical practitioners. Currently, when there have been no breakthroughs in surgical approaches or techniques, it is important to find ways to "add value" to liver cancer resection surgery. Numerous basic research studies have demonstrated that RT can influence the epigenetic regulation of liver cancer, apoptosis programming, and even the tumor immune microenvironment[21]. Therefore, postoperative adjuvant RT is not simply a "wider surgical excision" but may also prolong DFS and OS by reshaping the tumor microenvironment, inducing genetic mutations in pericancerous liver cells, and regulating the expression of cellular products through various pathways and mechanisms. Exploring the mechanisms of RT can also provide theoretical feasibility for combining RT with chemotherapy, targeted therapy, and immunotherapy, thus guiding further experimental investigations and clinical practices. Due to the limited number of clinical trials on various combined therapies, including RT, for postoperative liver cancer, we were unable to conduct an in-depth study on the relevant questions. However, we look forward to exploring these questions in the future. Answers to issues such as the synergistic or antagonistic effects of RT with certain drugs, the pathways involved, and the impact on patients may provide new directions and insights for liver cancer treatment.
After liver cancer resection in HCC patients, SBRT resulted in the longest OS, while 3D-CRT provided the longest DFS. IMRT, a RT technique associated with longer OS than 3D-CRT and longer DFS than SBRT, may be a good choice of postoperative RT.
Patients with primary liver cancer face a high recurrence rate, impacting prognosis significantly. Research has demonstrated the effectiveness of postoperative adjuvant external radiation therapy (RT) in preventing liver cancer recurrence after resection. However, the varying effects of different RT techniques on postoperative liver cancer recurrence necessitate further exploration and investigation.
This study aims to uncover and compare the efficacy of different adjuvant external beam RT (EBRT) methods after hepatectomy. Ultimately, the goal is to guide future experimental investigations, clinical practices, and potentially identify new directions for liver cancer treatment.
This study conducted a network meta-analysis to evaluate different adjuvant external RT methods following liver resection, focusing on overall survival (OS) and disease-free survival (DFS) to identify the optimal approach.
In adherence to PRISMA guidelines, this study utilized network meta-analyses to collect data from qualified studies published before July 10, 2023, from various reputable databases. Specifically, relevant studies pertaining to postoperative EBRT following liver resection with OS and DFS as primary endpoints were included for analysis. The effects were evaluated using risk ratios and 95% confidential intervals, with data analysis performed via R and STATA software for comprehensive assessment.
Inclusive of 1265 patients with hepatocellular carcinoma (HCC) post-liver resection, 12 studies formed the basis of this study. The absence of significant heterogeneity in direct paired comparisons, and consistency in inclusion/exclusion criteria, intervention measures, and outcome indicators, corroborated the assumptions of heterogeneity and transitivity. Findings from the analysis of OS indicated that patients who received stereotactic body radiotherapy (SBRT) after resection exhibited longer OS compared to those who underwent intensity modulated radiotherapy (IMRT) or 3-dimensional conformal RT (3D-CRT). Moreover, the analysis of DFS revealed that patients treated with 3D-CRT after resection had the longest DFS, while those undergoing IMRT post-resection demonstrated longer OS compared to 3D-CRT and longer DFS compared to SBRT.
When considering RT options after liver cancer resection for HCC patients, IMRT stands out as a preferred choice due to its association with longer OS than 3D-CRT and longer DFS than SBRT.
The role of RT in liver cancer treatment is currently uncertain, with some practitioners deeming its use rare and insufficiently effective alongside surgical resection. However, basic research shows that RT can influence epigenetic regulation, apoptosis programming, and the tumor immune microenvironment, potentially extending disease-free and OS. Further understanding of RT mechanisms can pave the way for combining it with chemotherapy, targeted therapy, and immunotherapy, offering new directions for clinical practices.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Goja S, India S-Editor: Lin C L-Editor: A P-Editor: Zheng XM
| 1. | Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Harding JJ, Merle P, Rosmorduc O, Wyrwicz L, Schott E, Choo SP, Kelley RK, Sieghart W, Assenat E, Zaucha R, Furuse J, Abou-Alfa GK, El-Khoueiry AB, Melero I, Begic D, Chen G, Neely J, Wisniewski T, Tschaika M, Sangro B. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 739] [Article Influence: 184.8] [Reference Citation Analysis (0)] |
| 2. | Tang ZY. Hepatocellular carcinoma surgery--review of the past and prospects for the 21st century. J Surg Oncol. 2005;91:95-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Liu S, Guo L, Li H, Zhang B, Sun J, Zhou C, Zhou J, Fan J, Ye Q. Postoperative Adjuvant Trans-Arterial Chemoembolization for Patients with Hepatocellular Carcinoma and Portal Vein Tumor Thrombus. Ann Surg Oncol. 2018;25:2098-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Zhang W, Zhang B, Chen XP. Adjuvant treatment strategy after curative resection for hepatocellular carcinoma. Front Med. 2021;15:155-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 5. | Ellis FH Jr, Gibb SP. Esophageal reconstruction for complex benign esophageal disease. J Thorac Cardiovasc Surg. 1990;99:192-7; discussion 197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4165] [Cited by in RCA: 5279] [Article Influence: 527.9] [Reference Citation Analysis (1)] |
| 6. | Liu L, Shui Y, Yu Q, Guo Y, Zhang L, Zhou X, Yu R, Lou J, Wei S, Wei Q. Narrow-Margin Hepatectomy Resulted in Higher Recurrence and Lower Overall Survival for R0 Resection Hepatocellular Carcinoma. Front Oncol. 2020;10:610636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Shi C, Li Y, Geng L, Shen W, Sui C, Dai B, Lu J, Pan M, Yang J. Adjuvant stereotactic body radiotherapy after marginal resection for hepatocellular carcinoma with microvascular invasion: A randomised controlled trial. Eur J Cancer. 2022;166:176-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 8. | Sun J, Yang L, Shi J, Liu C, Zhang X, Chai Z, Lau WY, Meng Y, Cheng SQ. Postoperative adjuvant IMRT for patients with HCC and portal vein tumor thrombus: An open-label randomized controlled trial. Radiother Oncol. 2019;140:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Wei X, Jiang Y, Feng S, Lu C, Huo L, Zhou B, Meng Y, Lau WY, Zheng Y, Cheng S. Neoadjuvant intensity modulated radiotherapy for a single and small (≤5 cm) hepatitis B virus-related hepatocellular carcinoma predicted to have high risks of microvascular invasion: a randomized clinical trial. Int J Surg. 2023;109:3052-3060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 10. | Wang WH, Wang Z, Wu JX, Zhang T, Rong WQ, Wang LM, Jin J, Wang SL, Song YW, Liu YP, Ren H, Fang H, Wang WQ, Liu XF, Yu ZH, Li YX. Survival benefit with IMRT following narrow-margin hepatectomy in patients with hepatocellular carcinoma close to major vessels. Liver Int. 2015;35:2603-2610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Long L, Chen B, Wang H, Zhao Y, Wu F, Wang L, Rong W, Wu J, Li Y, Wang W. Survival benefit of radiotherapy following narrow-margin hepatectomy in patients with hepatocellular carcinoma: A propensity score-matched analysis based on phase II study. Radiother Oncol. 2023;180:109462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 12. | Wang L, Wang W, Rong W, Li Z, Wu F, Liu Y, Zheng Y, Zhang K, Siqin T, Liu M, Chen B, Wu J. Postoperative adjuvant treatment strategy for hepatocellular carcinoma with microvascular invasion: a non-randomized interventional clinical study. BMC Cancer. 2020;20:614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Wei X, Jiang Y, Zhang X, Feng S, Zhou B, Ye X, Xing H, Xu Y, Shi J, Guo W, Zhou D, Zhang H, Sun H, Huang C, Lu C, Zheng Y, Meng Y, Huang B, Cong W, Lau WY, Cheng S. Neoadjuvant Three-Dimensional Conformal Radiotherapy for Resectable Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Randomized, Open-Label, Multicenter Controlled Study. J Clin Oncol. 2019;37:2141-2151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 14. | Rong W, Yu W, Wang L, Wu F, Zhang K, Chen B, Miao C, Liu L, An S, Tao C, Wang W, Wu J. Adjuvant radiotherapy in central hepatocellular carcinoma after narrow-margin hepatectomy: A 10-year real-world evidence. Chin J Cancer Res. 2020;32:645-653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (2)] |
| 15. | Bai T, Chen J, Xie ZB, Wu FX, Wang SD, Liu JJ, Li LQ. The efficacy and safety of postoperative adjuvant transarterial embolization and radiotherapy in hepatocellular carcinoma patients with portal vein tumor thrombus. Onco Targets Ther. 2016;9:3841-3848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Yu W, Wang W, Rong W, Wang L, Xu Q, Wu F, Liu L, Wu J. Adjuvant radiotherapy in centrally located hepatocellular carcinomas after hepatectomy with narrow margin (<1 cm): a prospective randomized study. J Am Coll Surg. 2014;218:381-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Wang L, Wang W, Yao X, Rong W, Wu F, Chen B, Liu M, Lin S, Liu Y, Wu J. Postoperative adjuvant radiotherapy is associated with improved survival in hepatocellular carcinoma with microvascular invasion. Oncotarget. 2017;8:79971-79981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Jiang T, Zeng ZC, Yang P, Hu Y. Exploration of Superior Modality: Safety and Efficacy of Hypofractioned Image-Guided Intensity Modulated Radiation Therapy in Patients with Unresectable but Confined Intrahepatic Hepatocellular Carcinoma. Can J Gastroenterol Hepatol. 2017;2017:6267981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Hou JZ, Zeng ZC, Wang BL, Yang P, Zhang JY, Mo HF. High dose radiotherapy with image-guided hypo-IMRT for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombi is more feasible and efficacious than conventional 3D-CRT. Jpn J Clin Oncol. 2016;46:357-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Chen D, Wang R, Meng X, Liu T, Yan H, Feng R, Liu S, Jiang S, Xu X, Zhu K, Dou X. A comparison of liver protection among 3-D conformal radiotherapy, intensity-modulated radiotherapy and RapidArc for hepatocellular carcinoma. Radiat Oncol. 2014;9:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Yang G, Yan H, Tang Y, Yuan F, Cao M, Ren Y, Li Y, He Z, Su X, Yao Z, Deng M. Advancements in understanding mechanisms of hepatocellular carcinoma radiosensitivity: A comprehensive review. Chin J Cancer Res. 2023;35:266-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |