Published online Jan 27, 2024. doi: 10.4240/wjgs.v16.i1.155
Peer-review started: November 30, 2023
First decision: December 18, 2023
Revised: December 21, 2023
Accepted: January 5, 2024
Article in press: January 5, 2024
Published online: January 27, 2024
Processing time: 55 Days and 21.4 Hours
Neutrophil-lymphocyte ratio (NLR), fibrosis index based on four factors (Fib4), aspartate aminotransferase-to-platelet ratio index (APRI) can be used for prog
To screen the factors that affect the prognosis of hepatocellular carcinoma and establish a nomogram model that predicts postoperative liver failure after hepatic resection in patients with hepatocellular carcinoma.
In total, 220 patients with hepatocellular carcinoma treated in our hospital from January 2022 to January 2023 were selected. They were divided into 154 parti
Binary logistic regression showed that Child-Pugh grading, Surgical site, NLR, Fib4, and APRI were all risk factors for liver failure after hepatic resection in patients with hepatocellular carcinoma. The modeling cohort built a column-line graph model, and the area under the ROC curve was 0.986 [95% confidence in
NLR, Fib4, and APRI independently influence posthepatectomy liver failure in patients with hepatocellular carcinoma. The column-line graph prediction model exhibited strong prognostic capability, with substantial concordance between predicted and actual events.
Core Tip: Postoperative liver failure in hepatocellular carcinoma is a serious complication that seriously affects the survival and quality of life of patients. Our work showed that neutrophil-lymphocyte ratio, fibrosis index based on four factors, and aspartate aminotransferase-to-platelet ratio index independently influenced the occurrence of liver failure following hepatectomy in patients with hepatocellular carcinoma. The column-line graph prediction model constructed in this study for the occurrence of liver failure after hepatectomy in patients with hepatocellular carcinoma showed good predictive ability, and the consistency between the predicted and actual events was high. This model has broad potential as a tool to prevent liver failure after hepatectomy in patients with hepatocellular carcinoma.
- Citation: Kuang TZ, Xiao M, Liu YF. Predictive value of NLR, Fib4, and APRI in the occurrence of liver failure after hepatectomy in patients with hepatocellular carcinoma. World J Gastrointest Surg 2024; 16(1): 155-165
- URL: https://www.wjgnet.com/1948-9366/full/v16/i1/155.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i1.155
Hepatocellular carcinoma is a common malignant tumor, and its incidence and mortality rates are increasing worldwide[1]. Hepatic resection is a primary treatment for hepatocellular carcinoma; however, postoperative liver failure is a serious complication that severely affects patient survival and quality of life[2,3]. Therefore, an accurate assessment of the risk of developing postoperative liver failure is essential to guide clinical treatment and improve patient prognosis. In recent years, inflammation and fibrosis indices have received extensive attention for prognostic assessment of patients with hepatocellular carcinoma. Neutrophil-lymphocyte ratio (NLR) is the ratio of neutrophils to lymphocytes in the blood and is usually associated with inflammatory responses, infections, tumors, and other diseases[4]. The fibrosis index based on four factors (Fib4) is used to assess the degree of liver fibrosis and is often used to evaluate patients with chronic liver disease; the higher the value, the more severe the degree of liver fibrosis[5]. The aspartate aminotransferase-to-platelet ratio index (APRI) is the ratio of aspartate transaminase (AST) to platelets (PLT) in the blood, and changes in this ratio can offer insights into the liver’s state and extent of inflammation[6]. These three indices are commonly used indicators of inflammation and fibrosis and have been shown to be strongly associated with the prognosis of patients with hepatocellular carcinoma. However, few studies have investigated the predictive value of these indicators in the development of postoperative liver failure and their changes. Therefore, this study aimed to investigate the changes in the levels of NLR, Fib4, and APRI in patients with hepatocellular carcinoma after hepatic resection and to establish a corresponding pre
A total of 220 patients with hepatocellular carcinoma who received treatment in our hospital from January 2022 to January 2023 were selected as the study objects, and were divided into a modeling cohort of 154 patients and a model validation cohort of 66 patients according to a ratio of 7:3. The model validation cohort was divided into liver failure group (n = 21) and non-liver failure group (n = 45). The study has been approved by the hospital ethics committee.
Inclusion criteria: (1) Meeting the diagnostic criteria for hepatocellular carcinoma[7]; (2) the age is above 18 years old; (3) the condition is stable and non-life threatening; (4) all patients received hepatectomy; and (5) complete clinical data.
Exclusion criteria: (1) There are other types of liver cancer; (2) complicated with heart, kidney, lung and other im
Observation of grouping and prognosis: At the same time, according to whether the patients had liver failure after hepatectomy, the modeling group was divided into liver failure group (n = 53) and no liver failure group (n = 101). The validation cohort was divided into liver failure group (n = 21) and non-liver failure group (n = 45). The outcome was observed, and postoperative liver failure was taken as the end event. Criteria for hepatic failure: Increased international normalized ratios and associated hyperbilirubinemia on or after the 5th d after surgery.
Index observation and method: General data of patients with and without liver failure were collected through electronic medical records of our hospital: Age, gender, body mass index (BMI), smoking history, drinking history, hepatitis B, tumor diameter, cirrhosis, tumor number, Child-Pugh grade of liver function, surgical site, alpha-fetoprotein, and post
Child-Pugh grading: including the assessment of general condition, ascites, bilirubin, albumin, prothrombin time, etc., with 1-3 points scored and a total of 15 points, of which 5-6 points are graded as grade A, indicating the presence of a small surgical risk; 7-9 points are graded as grade B, indicating the presence of a moderate surgical risk; and ≥ 10 points are graded as grade C, indicating the presence of a large surgical risk.
To analyze the changes of NLR, Fib4 and APRI levels in patients with hepatocellular carcinoma after hepatectomy, as well as their predictive value for the occurrence of postoperative liver failure, and to establish and validate a roadmap prediction model. The formula for calculating NLR: NLR = Neutrophil count/lymphocyte count; the formula for calcu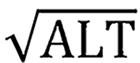 ); APRI calculation formula: APRI = (AST/upper limit normal)/PLT count × 100%.
); APRI calculation formula: APRI = (AST/upper limit normal)/PLT count × 100%.
SPSS 26.0 software and R software were used to analyze the data collected in this collection, and all the collected measures were tested for normality by the Shapiro-Wilk method, with P > 0.05 for normally distributed data expressed as (mean ± SD) and t-test, and with P < 0.05 for non-normally distributed data described as median (quartiles) and Mann-Whitney U test. Collected count data were expressed as (%), χ2 or Fisher exact test was used for data that were unordered, and Mann-Whitney U test was used for data that were ordered. Univariate and multivariate logistic regression was used to analyze the factors affecting the development of liver failure after hepatectomy in patients with hepatocellular carcinoma, to develop a predictive model for the column-line diagram, and the discriminative power of the validation set and the calibration plot were used to assess the accuracy of the column-line diagram. The area under the patient ope
A total of 220 patients with hepatocellular carcinoma were included, including 154 in the modeling cohort and 66 in the model validation cohort. The mean age of the patients was (53.11 ± 2.58) years, with 124 males (56.36%) and 96 females (43.64%). There were 86 males (55.84%) and 68 females (44.16%) in the modeling cohort. There were 38 males (57.58%) and 28 females (42.42%) in the validation cohort. The baseline data of the modeling cohort and the validation cohort were shown in Table 1. Except for differences in Child-Pugh grade, surgical site and Fib4, there were no statistically significant differences in other general data between the two groups (P > 0.05, Table 1).
| Index | Total cases (n = 220) | Modeling queue (n = 154) | Validation queue (n = 66) | χ2 | P value | |
| Gender | Male | 124 (56.36) | 86 (55.84) | 38 (57.58) | 3.333 | 0.068 |
| Female | 96 (43.64) | 68 (44.16) | 28 (42.42) | |||
| Age (yr) | 53.11 ± 2.58 | 53.03 ± 3.98 | 52.98 ± 3.54 | 0.088 | 0.930 | |
| BMI (kg/m2) | 21.26 ± 2.07 | 21.36 ± 2.36 | 21.28 ± 2.11 | 0.238 | 0.812 | |
| Smoking history | No | 106 (48.18) | 74 (48.05) | 32 (48.48) | 0.222 | 0.638 |
| Yes | 114 (51.82) | 80 (51.95) | 34 (51.52) | |||
| Drinking history | No | 114 (51.82) | 79 (51.30) | 35 (53.03) | 0.346 | 0.556 |
| Yes | 106 (48.18) | 75 (48.70) | 31 (46.97) | |||
| Hepatitis B | No | 124 (56.36) | 86 (55.84) | 38 (57.58) | 3.333 | 0.068 |
| Yes | 96 (43.64) | 68 (44.16) | 28 (42.42) | |||
| Tumor diameter | < 5 cm | 121 (55.00) | 85 (55.19) | 36 (54.55) | 1.757 | 0.185 |
| ≥ 5 cm | 99 (45.00) | 69 (44.81) | 30 (45.45) | |||
| Liver cirrhosis | No | 168 (76.36) | 125 (81.47) | 43 (65.15) | ||
| Yes | 52 (23.64) | 29 (18.83) | 23 (34.85) | 0.346 | 0.556 | |
| Number of tumors | < 2 | 114 (51.82) | 79 (51.30) | 35 (53.03) | ||
| ≥ 2 | 106 (48.18) | 75 (48.70) | 31 (46.97) | |||
| Child-Pugh classification | Grade A | 111 (50.45) | 90 (58.44) | 21 (31.82) | 26.615 | 0.000 |
| Grade B | 54 (24.55) | 31 (20.13) | 23 (34.85) | |||
| Grade C | 55 (25.00) | 33 (21.43) | 22 (33.33) | |||
| Surgical site | Left half liver | 109 (49.55) | 87 (56.49) | 22 (33.33) | 7.312 | 0.007 |
| Right half liver | 67 (30.45) | 33 (21.43) | 34 (51.52) | 19.842 | 0.000 | |
| Bilateral hemiliver | 44 (20.00) | 34 (22.08) | 10 (15.15) | |||
| AFP | < 400 μg/L | 121 (55.00) | 85 (55.19) | 36 (54.55) | 1.757 | 0.185 |
| ≥ 400 μg/L | 99 (45.00) | 69 (44.81) | 30 (45.45) | |||
| NLR | 5.35 ± 3.23 | 5.36 ± 3.25 | 4.49 ± 2.55 | 1.934 | 0.054 | |
| Fib4 | 9.76 ± 2.53 | 9.72 ± 2.36 | 7.72 ± 3.42 | 4.999 | 0.000 | |
| APRI | 0.58 ± 0.21 | 0.54 ± 0.22 | 0.53 ± 0.23 | 0.305 | 0.761 | |
There were no significant differences in gender, age, BMI, tumor diameter, resection range and tumor number in the modeling cohort (all P > 0.05). There were significant differences in Child-Pugh grade, surgical site, NLR, Fib4 and APRI between the two groups, and the levels of NLR, Fib4, and APRI indexes in the liver failure group were significantly higher than those in the non-liver failure group (all P < 0.05, Table 2).
| Index | Liver failure group (n = 53) | No liver failure group (n = 101) | χ2 | P value | |
| Gender | Male | 29 (54.72) | 57 (56.44) | 0.042 | 0.838 |
| Female | 24 (45.28) | 44 (43.56) | |||
| Age (yr) | 52.95 ± 4.20 | 53.11 ± 3.76 | 0.241 | 0.810 | |
| BMI (kg/m2) | 21.44 ± 2.06 | 21.08 ± 2.23 | 0.977 | 0.330 | |
| Smoking history | No | 23 (43.40) | 51 (50.50) | 0.520 | 0.471 |
| Yes | 30 (56.60) | 50 (49.50) | |||
| Drinking history | No | 26 (49.06) | 53 (52.48) | 0.033 | 0.857 |
| Yes | 27 (50.94) | 48 (47.52) | |||
| Hepatitis B | No | 29 (54.72) | 57 (56.44) | 0.042 | 0.838 |
| Yes | 24 (45.28) | 44 (43.56) | |||
| Tumor diameter | < 5 cm | 26 (49.06) | 59 (58.42) | 1.231 | 0.267 |
| ≥ 5 cm | 27 (50.94) | 42 (41.58) | |||
| Liver cirrhosis | No | 40 (75.47) | 85 (84.16) | 0.768 | 0.381 |
| Yes | 13 (24.53) | 16 (15.84) | |||
| Number of tumors | < 2 | 26 (49.06) | 53 (52.48) | 0.033 | 0.857 |
| ≥ 2 | 27 (50.94) | 48 (47.52) | |||
| Child-Pugh classification | Grade A | 4 (7.55) | 86 (85.15) | 102.766 | 0.000 |
| Grade B | 16 (30.19) | 15 (14.85) | |||
| Grade C | 33 (62.26) | 0 (0.00) | |||
| Surgical site | Left half liver | 4 (7.55) | 83 (82.18) | 88.726 | 0.000 |
| Right half liver | 18 (33.96) | 15 (14.85) | |||
| Bilateral hemiliver | 31 (58.49) | 3 (2.97) | |||
| AFP | < 400 μg/L | 26 (49.06) | 59 (58.42) | 1.231 | 0.267 |
| ≥ 400 μg/L | 27 (50.94) | 42 (41.58) | |||
| NLR | 8.31 ± 2.52 | 3.22 ± 1.57 | 15.406 | 0.000 | |
| Fib4 | 12.41 ± 4.59 | 6.45 ± 1.73 | 11.600 | 0.000 | |
| APRI | 0.79 ± 0.25 | 0.32 ± 0.19 | 13.044 | 0.000 | |
Binary logistic regression analysis with liver failure = 1 and no liver failure = 0 as dependent variables and factors with significant differences in the above univariate analyses as covariates showed that Child-Pugh classification, BCLC stage, NLR, Fib4, and APRI were all risk factors for the development of liver failure after hepatic resection in patients with hepatocellular carcinoma.
The logarithm of the odds of liver failure was modeled using the following equation: Log (P) = 2.023 × Child-Pugh grading + 1.269 × surgical site + 0.505 × NLR + 0.569 × Fib4 + 5.254 × APRI - 16.266 (Table 3).
| B | SE | Wals | P value | OR | OR, 95%CI | |
| Child-Pugh classification | 2.023 | 0.942 | 4.610 | 0.032 | 7.559 | 1.193-47.901 |
| Surgical site | 1.269 | 0.638 | 3.960 | 0.047 | 3.557 | 1.019-12.416 |
| NLR | 0.505 | 0.211 | 5.761 | 0.016 | 1.658 | 1.097-2.505 |
| Fib4 | 0.569 | 0.283 | 4.033 | 0.045 | 1.766 | 1.014-3.076 |
| APRI | 5.254 | 2.425 | 4.694 | 0.030 | 191.283 | 1.650-22174.958 |
| Constant | -16.266 | 3.824 | 18.094 | 0.000 | 0.000 | - |
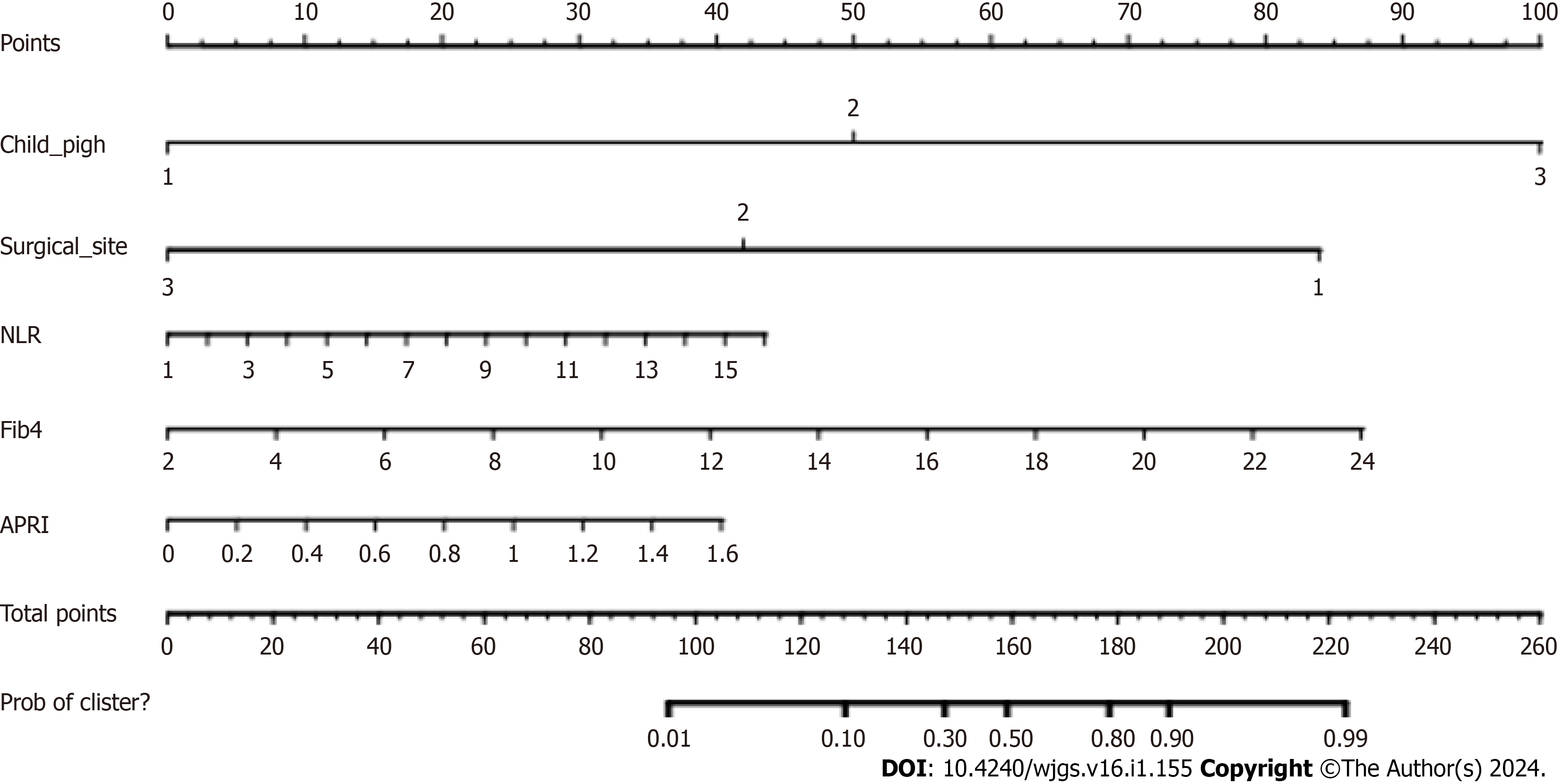
Five independent risk factors (Child-Pugh grade, surgical site, NLR, Fib4, and APRI) were obtained by R software to build a prediction model, and a nomogram model was established (Figure 1). After calibration of the generated nomo
Based on the clinical data of patients in the validation cohort (n = 66) (Table 4), the ROC curve was used for external validation of the ATC risk nomogram, and the results showed that the lower product of the ROC curve was 0.692 (95%CI: 0.548-0.837) (Figure 3A). The slope of the calibration curve of the generated nomogram was close to 1 (Figure 3B), and the result of Hosmer-Lemeshow test was χ2 = 1.784, P = 0.987. The decision curve shows that the model has a higher net benefit (Figure 3C), suggesting that the nomogram model has better calibration ability in the validation group.
| Index | Liver failure group (n = 21) | No liver failure group (n = 45) | χ2 | P value | |
| Gender | Male | 11 (52.38) | 27 (60.00) | 0.891 | 0.345 |
| Female | 10 (47.62) | 18 (40.00) | |||
| Age (yr) | 52.84 ± 2.54 | 52.76 ± 2.19 | 0.131 | 0.896 | |
| BMI (kg/m2) | 21.33 ± 2.08 | 21.29 ± 2.25 | 0.069 | 0.945 | |
| Smoking history | No | 12 (57.14) | 20 (44.44) | 0.015 | 0.904 |
| Yes | 9 (42.86) | 25 (55.56) | |||
| Drinking history | No | 11 (52.38) | 24 (53.33) | 0.187 | 0.665 |
| Yes | 10 (47.62) | 21 (46.67) | |||
| Hepatitis B | No | 11 (52.38) | 27 (60.00) | 0.891 | 0.345 |
| Yes | 10 (47.62) | 18 (40.00) | |||
| Tumor diameter | < 5 cm | 15 (71.43) | 21 (46.67) | 1.939 | 0.164 |
| ≥ 5 cm | 6 (28.57) | 24 (53.33) | |||
| Liver cirrhosis | No | 13 (61.90) | 30 (66.67) | 4.785 | 0.029 |
| Yes | 8 (38.10) | 15 (33.33) | |||
| Number of tumors | < 2 | 11 (52.38) | 24 (53.33) | 0.187 | 0.665 |
| ≥ 2 | 10 (47.62) | 21 (46.67) | |||
| Child-Pugh classification | Grade A | 3 (14.29) | 18 (40.00) | 4.381 | 0.112 |
| Grade B | 9 (42.86) | 14 (31.11) | |||
| Grade C | 9 (42.86) | 13 (28.89) | |||
| Surgical site | Left half liver | 5 (23.81) | 17 (37.78) | 25.414 | 0.000 |
| Right half liver | 6 (28.57) | 28 (62.22) | |||
| Bilateral hemiliver | 10 (47.62) | 0 (0.00) | |||
| AFP | < 400 μg/L | 15 (71.43) | 21 (46.67) | 1.939 | 0.164 |
| ≥ 400 μg/L | 6 (28.57) | 24 (53.33) | |||
| NLR | 6.97 ± 3.41 | 3.33 ± 0.09 | 7.220 | 0.000 | |
| Fib4 | 10.54 ± 4.12 | 6.41 ± 1.99 | 5.516 | 0.000 | |
| APRI | 0.75 ± 0.21 | 0.43 ± 0.15 | 7.080 | 0.000 | |
In recent years, the safety of hepatic resection has been greatly improved and the perioperative morbidity and mortality rates have been reduced by about 15% with the continuous development of surgical techniques, the widespread use of relevant instruments, and the continuous advancement of intensive care techniques[8]. Liver failure after hepatic re
In this study, patients were divided into modeling cohort and validation cohort, and clinical data of patients were compared. In the modeling cohort, 53 patients (21.90%) with liver failure and 101 patients (78.10%) without liver failure were found, indicating that the prognosis of patients after comprehensive hepatectomy was better. At the same time, by comparing the clinical data of the two groups of subjects in the modeling cohort, it was found that there were significant differences in Child-Pugh grade, surgical site, NLR, Fib4, and APRI between the two groups. Among them, the levels of NLR, Fib4, and APRI indexes in the liver failure group were significantly higher than those in the non-liver failure group, while there were no significant differences in other indexes. Binary logistic regression analysis with liver failure = 1 and no liver failure = 0 as dependent variables and the factors with significant differences in the above univariate analyses as covariates showed that Child-Pugh grading, BCLC staging, NLR, Fib4, and APRI were risk factors for liver failure after hepatic resection in patients with hepatocellular carcinoma. Child-Pugh grading was mainly based on the following five indicators to assess liver function: Total bilirubin level, serum albumin level, prothrombin time, and the presence or absence of ascites and encephalopathy. Higher Child-Pugh grades (B and C) mean poorer liver function and more damage to liver cells, which can result in the liver not being able to perform its functions properly, including synthesizing proteins, detoxifying and metabolizing medications, making the liver unable to efficiently deal with toxins and wastes produced by the body and increasing the risk of liver failure[10]. At the same time, hepatectomy can result in partial removal or damage to the liver, which can affect the function of the liver. The liver has a very important physiological function in the human body, including metabolism, detoxification, synthesis of important proteins, etc., so even partial resection may have a certain impact on the body. In addition, after hepatectomy, the remaining liver tissue needs to undertake more functions to maintain normal physiological activities, and if the surgical site is removed too far, the remaining liver tissue may not be able to meet the needs of the body, resulting in an increased risk of liver dysfunction and even liver failure[11]. Neutrophil count reflects the pro-inflammatory state of the body and lymphocyte count reflects the immune state of the body, NLR is the ratio of these two values, a high NLR value implies an increased inflammatory response and immune dysfunction which further promotes liver injury, thus greatly increasing the risk of liver failure after surgery[12]. In addition, patients with liver failure have a dysregulated immune system, which is characterized by systemic inflammation and immune paralysis, leading to bacterial infections[13]. As a result, neutrophils and lympho
Recently, there has been increasing evidence of the utility of non-invasive liver fibrosis-related markers, such as the Fib4 index[14] with APRI[15]. A study observed the value of Fib4 in the prognosis of patients with hepatic failure, and it was noted in the study that patients with hepatic failure tend to have underlying chronic liver disease and cirrhosis, and that Fib4 can, to a certain extent, reflect the level of liver fibrosis in them[16]. Fib4 is an important indicator for non-invasive and objective evaluation of liver fibrosis and cirrhosis, and its role in liver fibrosis and cirrhosis is even greater, and the degree of fibrosis is positively correlated with the Fib4 value[17]. The same result was also obtained in the study by Zhang et al[18] and is similar to the results of the study in this paper. In addition, APRI also affects the occurrence of postoperative liver failure in hepatocellular carcinoma patients undergoing hepatic resection to some extent. Yugawa et al[19] showed that APRI was the best independent predictor of liver failure after severe hepatic resection in patients with hepatocellular carcinoma, which was similar to the findings of the present study. Although the Child-Pugh score has long been the most commonly used tool for evaluating liver function in the clinic, the Child-Pugh score relies mainly on the observation and judgment of the patient’s symptoms and signs, resulting in possible subjective differences, and can only provide a general risk assessment and cannot accurately predict the occurrence of liver failure. There is a significant correlation between APRI and the degree of fibrosis and hepatic impairment in hepatic histopathology. Among the APRIs, the PLT count is an important factor representing hepatic fibrosis, and a low PLT level has been associated with advanced hepatic fibrosis and liver cirrhosis[20]. In addition, serum AST has a high sensitivity to reflect the presence of liver fibrosis or cirrhosis. Therefore, APRI is more accurate in predicting liver failure after hepatectomy.
In order to clarify the predictive value of NLR, Fib4, and APRI in the occurrence of liver failure after hepatic resection in patients with hepatocellular carcinoma, the present study used the modeling cohort to establish a column-line diagram model, the area under the ROC curve of the column-line diagram prediction model was larger, and the predictive efficacy was better, and the subjects in the validation cohort predicted the probability of the occurrence of liver failure in the validation group by the column-line diagram of , suggesting that has a certain predictive value. In addition, the factors of the model are all patients’ medical record data, which are easier to obtain and have higher clinical adaptability. In addition, as can be seen from the validation cohort calibration curve graph, the deviation between the actual outcome curve and the calibration curve is small, indicating that the consistency between the predicted events and the actual events is high. As can be seen from the validation cohort decision analysis curve, the decision analysis curve is located in the upper right corner usually indicates that the model has a high true positive rate and a low false positive rate, which means that this model has a certain degree of accuracy and reliability.
In summary, NLR, Fib4, and APRI are all independent influences on the occurrence of liver failure after hepatectomy in patients with hepatocellular carcinoma. The column-line graph prediction model constructed in this study for the occurrence of liver failure after hepatectomy in patients with hepatocellular carcinoma showed good predictive ability, and the consistency between the predicted events and the actual events was high. The model has a broad potential as a tool to prevent the occurrence of liver failure after hepatectomy in patients with hepatocellular carcinoma. As this study is a retrospective analysis with limited clinical data of subjects, the selection of indicators may not be comprehensive enough. At the same time, there are initial differences in the modeling and verification of groups of patients, which may lead to differences in research results. Therefore, a large sample size and multi-indicator analysis can be conducted in the future to establish a more comprehensive prediction model.
Hepatectomy is a common surgical procedure for hepatocellular carcinoma, but liver failure can occur after surgery, which is a serious complication that can be life-threatening to some extent. Therefore, predicting the occurrence of liver failure is very important for postoperative management and patient care. Neutrophil-lymphocyte ratio (NLR), fibrosis index based on four factors (Fib4), aspartate aminotransferase-to-platelet ratio index (APRI) are indicators derived from a simple blood test that reflect liver function and degree of fibrosis. By analyzing the relationship between these indicators and the occurrence of liver failure, we can evaluate their potential value in predicting liver failure and provide a basis for clinical practice.
Hepatectomy is an important treatment for hepatocellular carcinoma, but the occurrence of postoperative liver failure may bring serious complications and risks to patients. Abnormal expressions of NLR, Fib4, and APRI are common in patients with liver failure. However, there are few studies on the predictive value and changes of these indicators in the occurrence of postoperative liver failure.
To analyze the expression differences of NLR, Fib4, and APRI in hepatocellular carcinoma patients with liver failure after hepatectomy and their predictive value in postoperative liver failure, and establish and verify their nomogram prediction models.
A total of 220 patients with hepatocellular carcinoma who received treatment in our hospital from January 2022 to January 2023 were retrospectively selected as research objects, and were divided into a modeling cohort of 154 patients and a model validation cohort of 66 patients according to a ratio of 7:3. At the same time, according to whether the pa
Child-Pugh grade, surgical site, NLR, Fib4, and APRI were all risk factors for liver failure in patients with hepatocellular carcinoma after hepatectomy. In addition, in this study, the deviation between the actual result curve and the calibration curve of the nomogram generated by the modeling queue and the verification queue is small, and the consistency between the predicted event and the actual event is high. The validity of the nomogram prediction model is further confirmed in the decision analysis curve of modeling queue and verifying queue prediction model.
NLR, Fib4, and APRI were all independent factors influencing the occurrence of liver failure in hepatocellular carcinoma patients after hepatectomy, and the further constructed nomogram prediction model of liver failure in hepatocellular carcinoma patients after hepatectomy showed good prediction ability, with high consistency between predicted events and actual events. This model has broad potential as a tool to prevent liver failure in patients with hepatocellular car
This study is a retrospective analysis with limited clinical data of subjects, and the selection of indicators may not be comprehensive enough. At the same time, there are initial differences in the modeling and validation of groups of patients, which may lead to differences in study results. Therefore, more clinical indicators need to be added for further comprehensive evaluation and a more comprehensive prediction model needs to be established.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Nabarawi M, Egypt S-Editor: Chen YL L-Editor: A P-Editor: Xu ZH
| 1. | Chidambaranathan-Reghupaty S, Fisher PB, Sarkar D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv Cancer Res. 2021;149:1-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 554] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 2. | Sparrelid E, Olthof PB, Dasari BVM, Erdmann JI, Santol J, Starlinger P, Gilg S. Current evidence on posthepatectomy liver failure: comprehensive review. BJS Open. 2022;6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 51] [Reference Citation Analysis (0)] |
| 3. | Zhang C, Chen ZL, Ma YF, Song DY. [Multifactorial Logistic Regression Analysis and Predictive Model Construction of Progression-Free Survival in Patients with Hepatocellular Carcinoma after Laparoscopic Ultrasonic Partial Hepatectomy]. Weichangbingxue He Ganbingxue Zazhi. 2023;32:90-94. [DOI] [Full Text] |
| 4. | Cao XF, Wu MH. [Predictive value of neutrophil-lymphocyte ratio on poor prognosis of patients with acute cerebral infarction]. Hebei Yixue. 2020;26:1072-1075. [DOI] [Full Text] |
| 5. | Lee J, Vali Y, Boursier J, Spijker R, Anstee QM, Bossuyt PM, Zafarmand MH. Prognostic accuracy of FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: A systematic review. Liver Int. 2021;41:261-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 234] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 6. | Lu WQ, Xue MY, Feng WK, He ZC, Hu WB, Wang X, Wu JX. [Evaluation of the value of blood ammonia combined with PTA and APRI in the adjunctive diagnosis of hepatomegaly cirrhosis complicated by hepatic encephalopathy]. Jilin Yixue. 2022;43:1243-1246. [DOI] [Full Text] |
| 7. | Cong WM, Bu H, Chen J, Dong H, Zhu YY, Feng LH. [Guidelines for standardized pathological diagnosis of primary liver cancer (2015 edition)]. Jiefangjun Yixue Zazhi. 2015;40:865-872. [DOI] [Full Text] |
| 8. | Qiu J, Mo XS, Teng YJ, Chen SX, Tang WZ. [Establishment and evaluation of a column-line diagram risk prediction model for serious complications after hepatectomy in patients with hepatocellular carcinoma]. Zhongguo Putong Waike Zazhi. 2021;30:24-31. [DOI] [Full Text] |
| 9. | Ocak İ, Topaloğlu S, Acarli K. Posthepatectomy liver failure. Turk J Med Sci. 2020;50:1491-1503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Kang N, Qi LC, Yuan Y, Liu L, Bai Y, Zheng JM, Cui ZJ, Zhang J, Wang CK, Wang YZ. [Study on the predictive value of PTAR combined with Child-Pugh and MELD scores on the occurrence of slow plus acute liver failure in cirrhotic patients]. Weichangbingxue He Ganbingxue Zazhi. 2020;29:1171-1178. [DOI] [Full Text] |
| 11. | Zhang L, LI YM, Cong S. [Predictors of risk of liver failure in patients with alveolar hepatic echinococcosis after hepatectomy]. Ganzang. 2021;26:4. [DOI] [Full Text] |
| 12. | Zhu XW, Wang WB, Yuan FB, Wu X. [Exploring the value of NLR combined with serum IL-6 Level in predicting the recent prognosis of patients with slow plus acute hepatitis B liver failure]. Shiyong Ganzangbing Zazhi. 2023;26:67-70. [DOI] [Full Text] |
| 13. | Li Y, Kong Y, Shi K, Huang Y, Zhang Q, Zhu B, Zeng H, Wang X. CD200R Combined Neutrophil-Lymphocyte Ratio Predict 90-Day Mortality in HBV-Related Acute-On-Chronic Liver Failure. Front Med (Lausanne). 2021;8:762296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Toyoda H, Johnson PJ. The ALBI score: From liver function in patients with HCC to a general measure of liver function. JHEP Rep. 2022;4:100557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 82] [Reference Citation Analysis (1)] |
| 15. | Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1609] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 16. | Yugawa K, Maeda T, Nagata S, Sakai A, Edagawa M, Omine T, Kometani T, Yamaguchi S, Konishi K, Hashimoto K. A novel combined prognostic nutritional index and aspartate aminotransferase-to-platelet ratio index-based score can predict the survival of patients with hepatocellular carcinoma who undergo hepatic resection. Surg Today. 2022;52:1096-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Graupera I, Thiele M, Serra-Burriel M, Caballeria L, Roulot D, Wong GL, Fabrellas N, Guha IN, Arslanow A, Expósito C, Hernández R, Aithal GP, Galle PR, Pera G, Wong VW, Lammert F, Ginès P, Castera L, Krag A; Investigators of the LiverScreen Consortium. Low Accuracy of FIB-4 and NAFLD Fibrosis Scores for Screening for Liver Fibrosis in the Population. Clin Gastroenterol Hepatol. 2022;20:2567-2576.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 153] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 18. | Zhang ZQ, Yang B, Zou H, Xiong L, Miao XY, Wen Y, Zhou JJ. ALBI/ST ratio vs FIB-4 and APRI as a predictor of posthepatectomy liver failure in hepatocellular carcinoma patients. Medicine (Baltimore). 2019;98:e15168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Yugawa K, Maeda T, Nagata S, Shiraishi J, Sakai A, Yamaguchi S, Konishi K, Hashimoto K. Impact of aspartate aminotransferase-to-platelet ratio index based score to assess posthepatectomy liver failure in patients with hepatocellular carcninoma. World J Surg Oncol. 2022;20:248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 20. | Yan J. [The value of applying plasma coagulation factors and platelet parameters testing in the diagnosis of severe liver cirrhosis]. Jianyan Yixue Yu linchuang. 2020;17:151-153, 157. [DOI] [Full Text] |