Published online Jan 27, 2024. doi: 10.4240/wjgs.v16.i1.124
Peer-review started: November 21, 2023
First decision: December 5, 2023
Revised: December 16, 2023
Accepted: December 28, 2023
Article in press: December 28, 2023
Published online: January 27, 2024
Processing time: 64 Days and 22.2 Hours
The incidence of colorectal cancer (CRC) is increasing annually. Laparoscopic radical resection of CRC is a minimally invasive procedure preferred in clinical practice.
To investigate the clinical effect of laparoscopic radical resection of CRC on the basis of propensity score matching (PSM).
The clinical data of 100 patients who received inpatient treatment for CRC at Changde Hospital, Xiangya School of Medicine, Central South University (The First People’s Hospital of Changde City) were analyzed retrospectively. The control group included patients who underwent open surgery (n = 43), and those who underwent laparoscopic surgery formed the observation group (n = 57). The baseline information of both groups was equipoised using 1 × 1 PSM. Differences in the perioperative parameters, inflammatory response, immune function, degree of pain, and physical status between the groups were analyzed.
Thirty patients from both groups were successfully matched. After PSM, baseline data showed no statistically significant differences between the groups: (1) Perioperative parameters: The observation group had a longer surgery time, less intra
Laparoscopic radical resection of CRC has significant benefits, such as reducing postoperative pain and postoperative inflammatory response, avoiding excessive immune inhibition, and contributing to postoperative recovery.
Core Tip: Clinical data from 100 patients who underwent radical resection for colorectal cancer were retrospectively analyzed to compare the clinical effects of open and laparoscopic surgeries in terms of perioperative parameters, inflammatory response, immune function, degree of pain, and physical status.
- Citation: Liu Y, Wang XX, Li YL, He WT, Li H, Chen H. Clinical effect of laparoscopic radical resection of colorectal cancer based on propensity score matching. World J Gastrointest Surg 2024; 16(1): 124-133
- URL: https://www.wjgnet.com/1948-9366/full/v16/i1/124.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i1.124
Colorectal cancer (CRC) is a common cancer of the digestive system with high incidence and mortality[1]. There would be approximately 1.93 million new cases of CRC and 940000 deaths worldwide in 2020, representing 10% and 9.4% of global cancer morbidity and mortality, respectively[2]. However, early symptoms of CRC remain unclear. Growing tumors can cause abdominal pain, changes in stool characteristics, bloody stools, and other symptoms. At this time, the disease often progresses to the middle and late stages, and its prognosis is poor[3]. Currently, the treatment for CRC is largely based on surgery. Early surgical resection, blocking tumor progression, and avoiding post-diffusion metastasis are key to improving the prognosis of patients with CRC.
Traditional open surgery can effectively remove lesions but has the disadvantages of much trauma, slow recovery of postoperative function, and many complications[4]. Laparoscopic surgery has recently become increasingly popular. It can achieve complete resection of lesions and promote rapid recovery of patients after surgery, while reducing surgical side injuries[5]. However, laparoscopic surgery is complicated, and the anatomy of the colon and rectum increases its difficulty[6]. The application of laparoscopic radical resection of CRC remains controversial at the present clinical stage. Therefore, the clinical data of 100 patients with CRC were retrospectively analyzed in this study, and propensity score matching (PSM) was used to balance confounding variables between the observation group and the control group to control confounding bias and reduce the bias[7]. The aim of this study was to explore the clinical effects of laparoscopic radical resection for CRC, and to provide a reference for the selection of clinical surgical modalities.
The clinical data of 100 patients who received inpatient treatment for CRC between January 2022 and March 2023 at Changde Hospital, Xiangya School of Medicine, Central South University (The First People’s Hospital of Changde City) were analyzed retrospectively. Inclusion criteria were: (1) First diagnosed as CRC by histopathological examination; (2) Age ≥ 18 years old; (3) Tumor-node-metastasis (TNM) stage of the tumor was I-III; and (4) Received radical surgical resection, open surgery or laparoscopic surgery. Exclusion criteria were as follows: (1) Previous history of abdominal surgery; (2) Perforation, bleeding, acute intestinal obstruction, and other acute surgeries; (3) Combined with other malignant tumors or malignant tumor history; (4) Combined with major organ dysfunction; (5) Pregnant and lactating women; and (6) Missing the data required for this study.
Open surgery: Lithotomy position after general anesthesia. The size and position of the incision was confirmed based on the size and position of the lesion. First, a normal abdominal examination was performed to determine the location of the tumor and its proximal tissues and organs. The upper and lower regular and corresponding mesenteric vessels of the tumor were first ligated, and the intestinal canal was freed. The tumor was removed and intestinal tubes at each end of the tumor and its corresponding mesentery were fitted. Lymph node dissection, intestinal anastomosis, abdominal cavity irrigation, lining drainage, and abdominal cavity closure were completed.
Laparoscopic surgery: Lithotomy position after general anesthesia. Laparoscopic access was established by opening 3–5 small holes (5–10 mm) in the abdominal wall and introducing the laparoscopic and surgical instruments. A 5 cm incision was made in the abdominal wall, based on the location of the lesion, to remove the tumor tissue. A CO2 pneumothorax was established, and the intraperitoneal condition was investigated. The mesenteric arterial and peripheral connective tissues were isolated. The tumor, appropriate intestinal tubes at each end of the tumor, and corresponding mesentery were removed, and the lymph nodes were dissected. Colorectal anastomosis was performed, bowel ducts were rationalized, the abdominal cavity was irrigated, internal drainage was performed, instruments were withdrawn, and the abdominal cavity was closed.
Data were collected from patients through the hospital information system, including baseline data such as age, sex, body mass index (BMI), tumor diameter, lesion location, and the American Society of Anesthesiologists (ASA) grade.
(1) Perioperative parameters such as surgery duration, intraoperative blood loss, number of lymph node dissections, first ambulation time, bowel sound recovery time, first anal exhaust time, gastric tube indwelling time, and complication rate were compared between the groups; (2) Inflammatory response: Five milliliters of venous blood was collected after fasting preoperatively and 24 h postoperatively. After centrifugation, the levels of interleukin-6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor-α (TNF-α) were determined using an enzyme-linked immunosorbent assay; (3) Immune function: Blood samples were collected as described above. CD4-positive T-lymphocytes (CD4+) and CD8-positive T-lymphocytes (CD8+) counts were quantified using a flow cytometer and companion kit (BD FACSCalibur; Becton, Dickinson And Company., United States); (4) Degree of pain: The visual analog scale (VAS) was used to evaluate the degree of pain preoperatively and 24 h and 72 h postoperatively. The VAS score is 0–10, with a higher score indicating more intense pain; and (5) Physical status: The Karnofsky performance score (KPS) was used to evaluate the physical status preoperatively, and 1 month and 3 mo postoperatively. The KPS can be divided into 11 grades from disease-free (100 points) to death (0 points), with higher scores indicating better conditions.
R software (R 4.1.3; Bell Laboratories., Auckland, New Zealand) was used for the PSM. The nearest neighbor matching method and the caliper matching method were used. When the caliper value was set to 0.2, age, tumor diameter, lesion location, and ASA were matched at a ratio of 1 × 1 between groups, and the standardized mean difference (SMD) was applied to evaluate the matching effect. SMD < 0.1 can was considered as a good matching effect. SPSS software (version 26.0; IBM Corp., Armonk, NY, United States) was used for data processing and analysis. Quantitative data according to the Gaussian distribution was described as mean ± standard (mean ± SD), the paired sample t-test was applied to compare within groups and the independent sample t-test to compare among groups. Quantitative continuous data that did not conform to the Gaussian distribution are shown as median (M) and interquartile range [M (P25-P75)], and the Mann–Whitney U test was applied for comparison. Categorical data were expressed as numbers and percentages, n (%), and the chi-square test was applied for comparison. Statistical significance was set at P < 0.05.
Among the 100 patients in the study, 43 who underwent open surgery were included in the control group, and 57 who underwent laparoscopic surgery were included in the observation group. There were statistically significant differences in age, tumor diameter, lesion location, and ASA between the groups (Table 1).
| Data | Control group (n = 43) | Observation group (n = 57) | t/χ2/Z | P value |
| Age (yr, mean ± SD) | 56.44 ± 7.48 | 52.37 ± 11.71 | 2.116 | 0.037 |
| Sex, n (%) | 0.220 | 0.887 | ||
| Male | 24 (55.81) | 31 (54.39) | ||
| Female | 19 (44.19) | 26 (45.61) | ||
| BMI (kg/m2, mean ± SD) | 22.06 ± 1.50 | 22.41 ± 1.61 | 1.102 | 0.273 |
| Underlying disease, n (%) | ||||
| Hypertension | 11 (25.58) | 19 (33.33) | 0.701 | 0.402 |
| Diabetes | 14 (35.56) | 10 (17.54) | 3.029 | 0.082 |
| CHD | 8 (18.60) | 12 (21.05) | 0.092 | 0.762 |
| Tumor diameter (cm, mean ± SD) | 3.93 ± 0.48 | 3.70 ± 0.52 | 2.188 | 0.031 |
| TNM stage, n (%) | 1.142 | 0.254 | ||
| I | 20 (46.51) | 32 (56.14) | ||
| II | 17 (39.53) | 21 (36.84) | ||
| III | 6 (13.95) | 4 (7.02) | ||
| Histological type, n (%) | 0.256 | 0.968 | ||
| Adenocarcinoma | 17 (39.53) | 25 (43.86) | ||
| Mucinous adenocarcinoma | 12 (27.91) | 14 (24.56) | ||
| Squamous cell carcinoma | 9 (20.93) | 11 (19.30) | ||
| Other | 5 (11.63) | 7 (12.28) | ||
| Tumor location, n (%) | 8.501 | 0.037 | ||
| Rectum | 20 (46.51) | 24 (42.11) | ||
| Descending colon | 13 (30.23) | 7 (12.28) | ||
| Ascending colon | 6 (13.95) | 11 (19.30) | ||
| Sigmoid flexure | 4 (9.30) | 15 (26.32) | ||
| ASA grade, n (%) | 2.026 | 0.043 | ||
| I | 18 (41.86) | 14 (24.56) | ||
| II | 16 (37.21) | 22 (38.60) | ||
| III | 6 (13.95) | 15 (26.32) | ||
| IV | 3 (6.98) | 6 (10.53) |
Sixty patients were successfully matched after 1:1 PSM. The SMD for age, tumor diameter, lesion location, and ASA classification were 0.014, 0.090, 0.092, and 0.035, respectively, which can be considered a good matching effect. After PSM, there were no significant differences between the groups in terms of age, sex, BMI, underlying disease, tumor diameter, TNM stage, histological type, lesion location, or ASA classification (Table 2).
| Data | Control group (n = 30) | Observation group (n = 30) | t/χ2/Z | P value |
| Age (year, mean ± SD) | 54.97 ± 7.54 | 54.83 ± 11.52 | 0.053 | 0.958 |
| Sex, n (%) | 0.067 | 0.795 | ||
| Male | 17 (56.67) | 16 (53.33) | ||
| Female | 13 (43.33) | 14 (46.67) | ||
| BMI (kg/m2, mean ± SD) | 22.11 ± 1.54 | 22.01 ± 1.62 | 0.237 | 0.814 |
| Underlying disease, n (%) | ||||
| Hypertension | 7 (23.33) | 10 (33.33) | 0.739 | 0.390 |
| Diabetes | 10 (33.33) | 5 (16.67) | 2.222 | 0.136 |
| CHD | 7 (23.33) | 4 (13.33) | 0.445 | 0.505 |
| Tumor diameter (cm, mean ± SD) | 3.86 ± 0.48 | 3.81 ± 0.56 | 0.347 | 0.730 |
| TNM stage, n (%) | 0.701 | 0.483 | ||
| I | 15 (50.00) | 17 (56.67) | ||
| II | 12 (40.00) | 12 (40.00) | ||
| III | 3 (10.00) | 1 (3.33) | ||
| Histological type, n (%) | 0.842 | 0839 | ||
| Adenocarcinoma | 11 (36.67) | 13 (43.33) | ||
| Mucinous adenocarcinoma | 10 (33.33) | 7 (23.33) | ||
| Squamous cell carcinoma | 6 (20.00) | 6 (20.00) | ||
| Other | 3 (10.00) | 4 (13.33) | ||
| Tumor location, n (%) | 4.149 | 0.246 | ||
| Rectum | 12 (40.00) | 17 (56.67) | ||
| Descending colon | 9 (30.00) | 3 (10.00) | ||
| Ascending colon | 6 (20.00) | 6 (20.00) | ||
| Sigmoid flexure | 3 (10.00) | 4 (13.33) | ||
| ASA grade, n (%) | 0.008 | 0.994 | ||
| I | 12 (40.00) | 10 (33.33) | ||
| II | 10 (33.33) | 15 (50.00) | ||
| III | 5 (16.67) | 2 (6.67) | ||
| IV | 3 (10.00) | 3 (10.00) |
There were no significant differences between the groups in the number of lymph node dissections, bowel sound recovery time, or rate of complications (P > 0.05). The observation group had a longer surgery time, lesser intraoperative blood loss, earlier first ambulation time, shorter first anal exhaust time, and shorter gastric tube indwelling time than the control group (Table 3).
| Parameters | Control group (n = 30) | Observation group (n = 30) | t | P value |
| Surgery time (min, mean ± SD) | 157.70 ± 14.14 | 203.13 ± 20.07 | 10.138 | < 0.001 |
| Intraoperative blood loss (mL, mean ± SD) | 172.07 ± 26.94 | 131.93 ± 21.84 | 6.338 | < 0.001 |
| Number of lymph nodes dissected (piece, mean ± SD) | 17.73 ± 2.48 | 17.17 ± 3.08 | 0.786 | 0.435 |
| First ambulation time (h, mean ± SD) | 47.60 ± 5.37 | 38.73 ± 6.76 | 5.626 | < 0.001 |
| Bowel sounds recovery time (h, mean ± SD) | 67.80 ± 8.06 | 65.97 ± 6.61 | 0.963 | 0.339 |
| First anal exhaust time (h, mean ± SD) | 78.33 ± 16.01 | 67.73 ± 18.20 | 2.396 | 0.020 |
| Gastric tube indwelling time, d, M (P25-P75) | 4.00 (4.00, 5.00) | 3.50 (3.00, 4.00) | 4.621 | < 0.001 |
| Rate of complications, n (%) | 5 (16.67) | 3 (10.00) | 0.144 | 0.704 |
There were no differences between groups in the levels of IL-6, CRP, and TNF-α preoperatively (P > 0.05). At 24 h after surgery, the IL-6, CRP, and TNF-α levels of both groups were higher than preoperatively, and those in the observation group were lower than the control group (Table 4).
| Group | IL-6 (ng/L) | CRP (mg/L) | TNF-α (ng/L) | |||
| Preoperative | 24 h after surgery | Preoperative | 24 h after surgery | Preoperative | 24 h after surgery | |
| Control group (n = 30) | 8.49 ± 1.23 | 16.68 ± 4.22a | 4.96 ± 1.22 | 21.24 ± 4.32a | 24.81 ± 3.36 | 49.37 ± 7.58a |
| Observation group (n = 30) | 9.06 ± 1.68 | 13.78 ± 2.34a | 5.34 ± 1.41 | 18.96 ± 3.56a | 23.92 ± 4.07 | 43.62 ± 5.68a |
| t | 1.501 | 3.294 | 1.099 | 2.235 | 0.920 | 3.326 |
| P value | 0.139 | 0.002 | 0.276 | 0.029 | 0.361 | 0.002 |
CD4+ counts and CD4+/CD8+ in both groups were lower postoperatively and CD8+ counts were higher 24 h after surgery. The observation group had higher CD4+ counts and CD4+/CD8+ and lower CD8+ counts than the control group at 24 h after surgery (Table 5).
| Group | CD4+ (%) | CD8+ (%) | CD4+/CD8+ | |||
| Preoperative | 24 h after surgery | Preoperative | 24 h after surgery | Preoperative | 24 h after surgery | |
| Control group (n = 30) | 44.80 ± 6.32 | 32.17 ± 4.78a | 27.00 ± 3.46 | 33.40 ± 3.41a | 1.69 ± 0.36 | 0.97 ± 0.19a |
| Observation group (n = 30) | 44.23 ± 5.74 | 36.13 ± 4.97a | 26.10 ± 4.67 | 31.53 ± 2.99a | 1.76 ± 0.45 | 1.15 ± 0.18a |
| t | 0.364 | 3.150 | 0.848 | 2.254 | 0.625 | 3.736 |
| P value | 0.718 | 0.003 | 0.400 | 0.028 | 0.535 | < 0.001 |
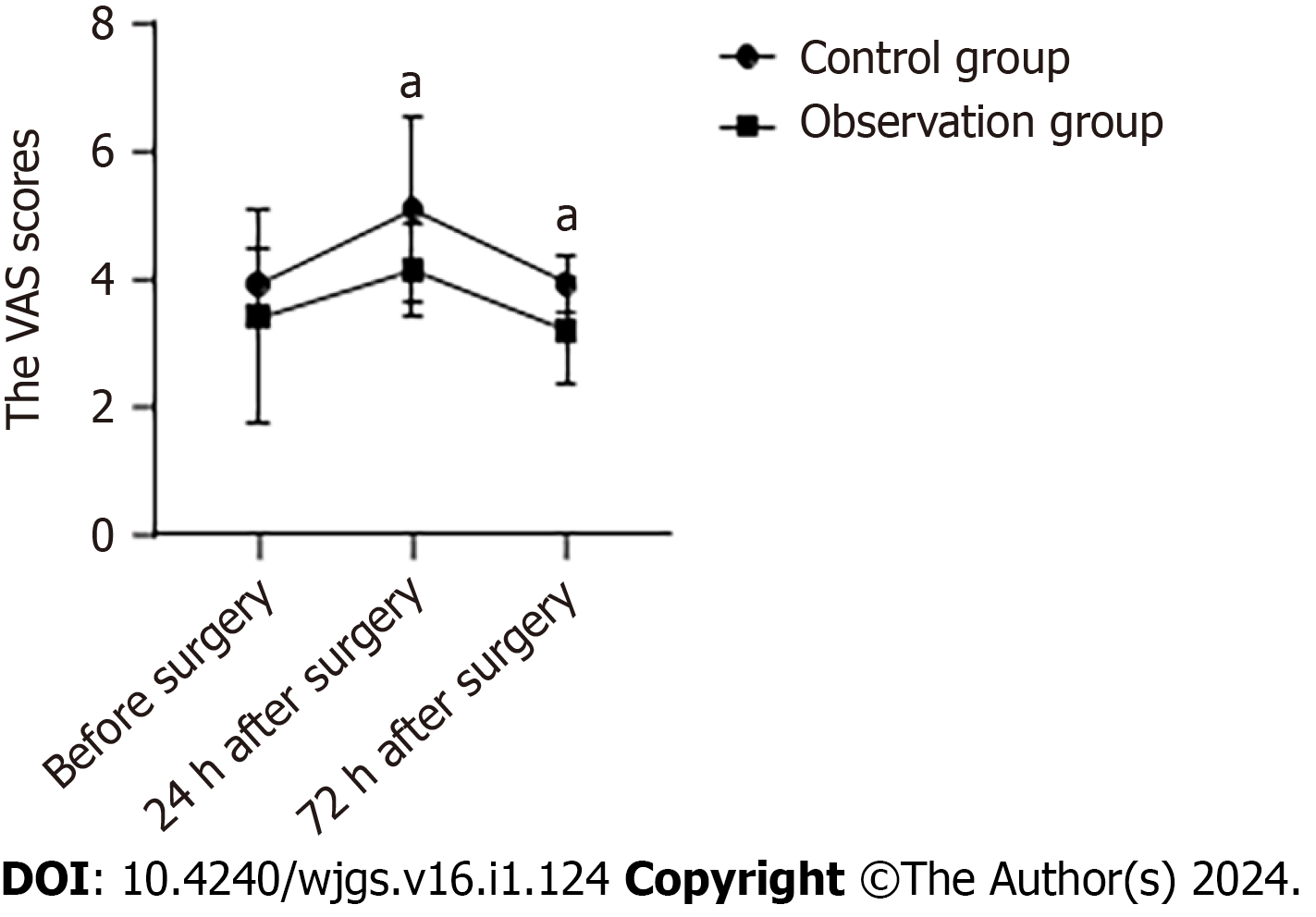
Before surgery, the average VAS score of the control group was (3.90 ± 0.55) and the observation group was (3.40 ± 1.67), with no significant differences between groups (P > 0.05). At 24 h after surgery, the average VAS score of the control group was (5.07 ± 1.44) and the observation group was (4.13 ± 0.73). At 72 h after surgery, the average VAS score of the control group was (3.93 ± 0.45) and the observation group was (3.20 ± 0.85). The VAS scores in the observation group were significantly lower than those in the control group at 24 h and 72 h after surgery (Figure 1).
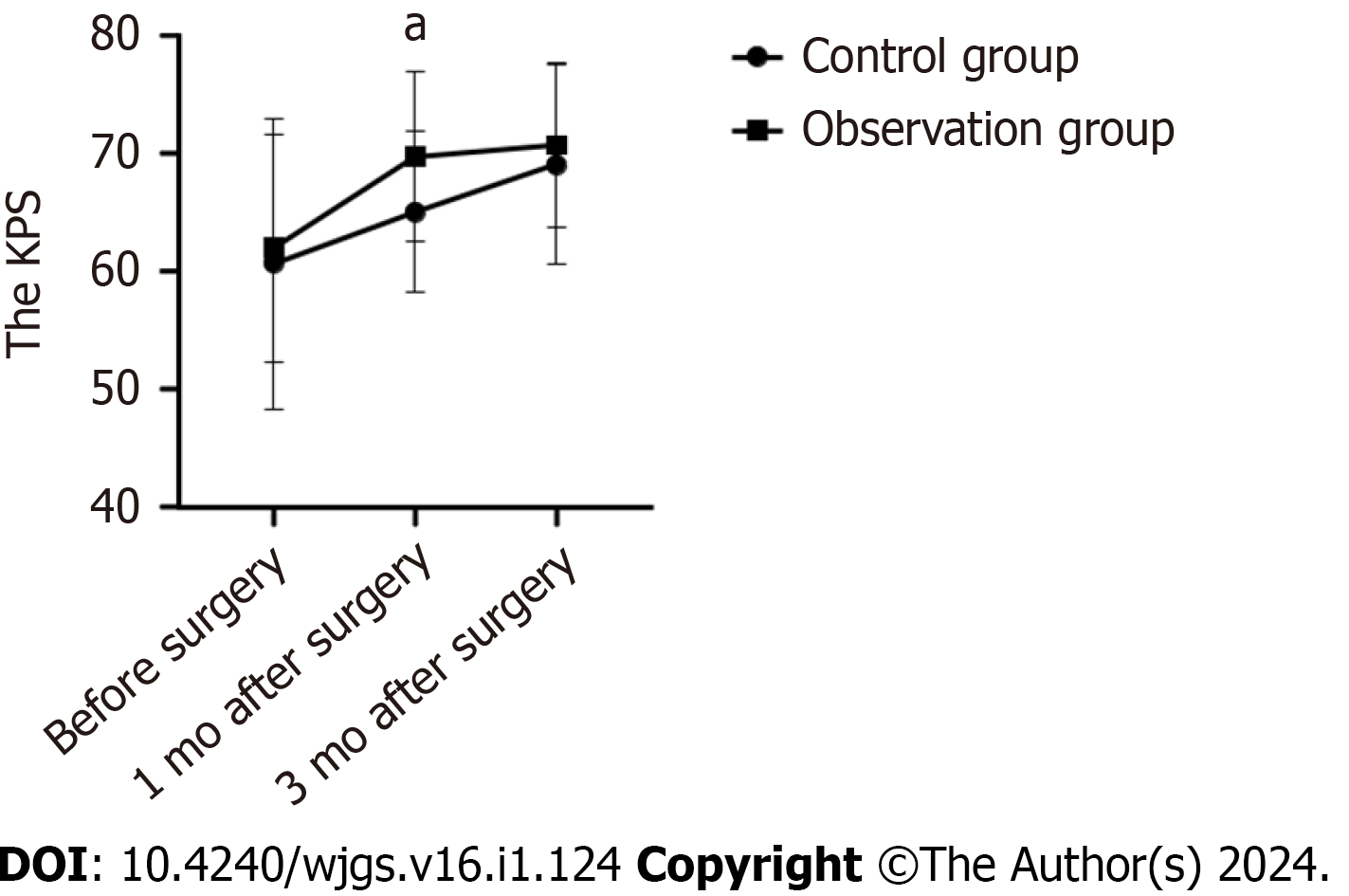
Before surgery, the average KPS of the control group was (60.67 ± 12.30) and the observation group was (62.00 ± 9.61). The average KPS of the control group was (65.00 ± 6.82) and the observation group was (69.67 ± 7.18) one month after surgery. The average KPS of the control group and the observation group was (69.00 ± 8.45) and (70.67 ± 6.915) respectively, three months after surgery. Preoperatively and three months after surgery, there were no significant differences in KPS scores among the groups (P > 0.05). The observation group had a higher KPS score than the control group one month after surgery (Figure 2).
The etiology of CRC is complex and is linked to diet, digestive tract diseases, lifestyle, genetics, and other factors. The long-term interaction of these factors affects the intestinal peristaltic ability and increases the contact time between carcinogens and the intestine, thus continuously stimulating the intestinal mucosal cells, causing them to proliferate out of control and eventually form tumor tissues[6]. With an improvement in living conditions, changes in dietary structure and mode of life have caused a significant increase in the morbidity of CRC, and the age of onset has gradually become lesser[8]. Currently, CRC is generally treated based on the principle of clearing the tumor and lymph nodes, and inhibiting the transfer and invasion of cancer cells[9].
Surgery is the only curative treatment for CRC[10]. Open radical resection for CRC has a long history of clinical application. An abdominal opening can be used to observe the abdominal cavity and locate the intestinal segment of the lesion, and resection of the tumor and the affected intestinal segment can be completed under direct vision to achieve complete removal of the tumor[11]. However, open surgery, with its long incisions and extensive lymph node dissection, is prone to a strong stress response. In addition, the risk of infection increases with a long exposure time of the abdominal cavity, which affects the recovery of the body after surgery[12]. Recently, laparoscopic surgery has become increasingly popular for treating CRC. The magnification of laparoscopic images broadens the surgical domain and helps surgeons more clearly identify important structures, such as blood vessels, nerves, and ureters, facilitating delicate surgical manipulation. Laparoscopic surgery results in a smaller wound, which avoids prolonged exposure of the abdominal cavity to air and reduces the damage to the body caused by invasive surgery to a certain extent[13,14]. However, la
We collected clinical data of 43 patients who underwent open radical resection and compared them with those of 57 patients who underwent laparoscopic radical resection for CRC. After 1:1 PSM, 60 patients were matched successfully. By comparing perioperative parameters, we found that open radical resection and laparoscopic radical resection for CRC had similar clinical effects, including the number of lymph nodes removed, bowel sound recovery time, and incidence of complications. Laparoscopic radical resection of CRC results in a longer surgery time, less intraoperative blood loss, earlier time to get out of bed and first anal exit, and shorter time to remove the stomach tube. Considering that the visual field of laparoscopic surgery has a planar structure, the surgeon needs to use an instrument to sense the location of the lesion, which enhances the difficulty of the procedure to a certain extent, thus prolonging the surgery time. VAS scores 24 and 72 h postoperatively were significantly lower in patients who underwent laparoscopic radical response for CRC, and they also had a higher KPS one month after surgery. At three months after surgery, there were no significant differences in the KPS scores between the groups. These results confirmed that laparoscopic surgery can reduce early postoperative pain and contribute to early physical recovery.
Invasive surgery can easily induce a stress response, mainly manifested as excessive expression of inflammatory factors[19]. On the one hand, the production of large amounts of inflammatory cells can increase the incidence of postoperative infection; on the other hand, it can directly affect the surgical outcome[20]. IL-6 and TNF-α are typical pro-inflammatory factors, which are important mediators that trigger and initiate inflammatory responses. CRP levels can be markedly elevated post-trauma. The results of our study showed that patients receiving laparoscopic radical resection of CRC had lower levels of IL-6, CRP, and TNF-α at 24 h after surgery. This indicates that laparoscopic surgery may reduce the early postoperative inflammatory response compared to open surgery. This is consistent with the results reported by Chen et al[10]. At the same time, surgical trauma can also cause the temporary inhibition of immune function[21]. CD4+ T cells are helper cells and induce T cells with anti-tumor effects, CD8+ T cells are inhibitory T cells that inhibit the immune reaction, and CD4+/CD8+ is an important marker reflecting the body's immune regulation efficacy[22]. The results of our study showed that patients who underwent laparoscopic radical resection for CRC had higher CD4+ counts and CD4+/CD8+ ratios and lower CD8+ counts than patients who underwent open surgery. This suggests that laparoscopic surgery can avoid excessive immunosuppression compared with open surgery. Strong postoperative inflammatory responses and immunosuppression can lead to delayed healing, which is detrimental to the postoperative recovery.
Although PSM was used to eliminate the influence of some confounding factors and increase the reliability of the study results, there are still some limitations: (1) This study has a retrospective design with a low level of evidence; (2) The number of cases included in the study was small, and the research data were all from the same institution; (3) Based on a single-center retrospective study, in addition to demographic and pathological characteristics, there are still some confounding factors regarding the treatment differences, such as chemoradiotherapy regimen and tumor metastasis; and (4) Lack of long-term observation data. Future studies with large sample sizes and high-quality randomized controlled trials are still needed to confirm this.
Our results indicated that laparoscopic radical resection of CRC has significant benefits such as reducing postoperative pain and postoperative inflammatory response, avoiding excessive immune inhibition, and contributing to postoperative recovery.
Currently, there is some debate about the merits of laparoscopic surgery of colorectal cancer (CRC).
The advantages of laparoscopic surgery for CRC require further validation through additional studies and data.
Exploring the advantages of laparoscopic radical resection vs open surgery for CRC.
Data from 43 patients with CRC who underwent open surgery and 53 who underwent laparoscopic surgery were compared retrospectively, and differences between the groups were analyzed using 1:1 propensity score matching equilibrium treatment.
Compared with open surgery, laparoscopic radical resection of CRC showed better early inflammatory, immune, and pain indicators, and better physical status one month after surgery.
Laparoscopic radical resection of CRC can reduce postoperative pain and postoperative inflammatory responses, prevent excessive immune inhibition, and contribute to postoperative recovery.
To analyze the early clinical effects of laparoscopic radical resection for CRC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Schulman AR, United States; Tortora G, Italy S-Editor: Li L L-Editor: A P-Editor: Li L
| 1. | Lorente-Herce JM, Parra-Membrives P, Martínez-Baena D, Cañete-Gómez J, Segura-Sampedro JJ. Influence of surgical site infection on oncological prognosis after curative resection for colorectal cancer: An observational single-institution study. Cir Cir. 2021;89:574-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64542] [Article Influence: 16135.5] [Reference Citation Analysis (176)] |
| 3. | Zorzi M, Battagello J, Selby K, Capodaglio G, Baracco S, Rizzato S, Chinellato E, Guzzinati S, Rugge M. Non-compliance with colonoscopy after a positive faecal immunochemical test doubles the risk of dying from colorectal cancer. Gut. 2022;71:561-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 4. | Ni X, Jia D, Chen Y, Wang L, Suo J. Is the Enhanced Recovery After Surgery (ERAS) Program Effective and Safe in Laparoscopic Colorectal Cancer Surgery? A Meta-Analysis of Randomized Controlled Trials. J Gastrointest Surg. 2019;23:1502-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 5. | Tan SJ, Jiang Y, Xi QL, Meng QY, Zhuang QL, Han YS, Wu GH. [Meta-analysis of laparoscopic vs open surgery for palliative resection of the primary tumor in stage IV colorectal cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23:589-596. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Zhang Q, Chen M, Wang Z, Qi C, Cao Y, Zhang J, Peng Z, Wang X, Lu M, Shen L, Li J. Efficacy and Safety Comparison of Regorafenib and Fruquintinib in Metastatic Colorectal Cancer-An Observational Cohort Study in the Real World. Clin Colorectal Cancer. 2022;21:e152-e161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 7. | Hu TWY, Huang Y, Li N, Nie D, Li Z. Comparison of laparoscopic vs open radical hysterectomy in patients with early-stage cervical cancer: a multicenter study in China. Int J Gynecol Cancer. 2020;30:1143-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Osagiede O, Spaulding AC, Cochuyt JJ, Naessens J, Merchea A, Colibaseanu DT. Trends in the Use of Laparoscopy and Robotics for Colorectal Cancer in Florida. J Laparoendosc Adv Surg Tech A. 2019;29:926-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | de Neree Tot Babberich MPM, van Groningen JT, Dekker E, Wiggers T, Wouters MWJM, Bemelman WA, Tanis PJ; Dutch Surgical Colorectal Audit. Laparoscopic conversion in colorectal cancer surgery; is there any improvement over time at a population level? Surg Endosc. 2018;32:3234-3246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Chen Y, Xi D, Zhang Q. Laparoscopic Radical Resection vs Routine Surgery for Colorectal Cancer. Comput Math Methods Med. 2022;2022:4899555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Park SJ, Lee KY, Lee SH. Laparoscopic Surgery for Colorectal Cancer in Korea: Nationwide Data from 2013 to 2018. Cancer Res Treat. 2020;52:938-944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Zhou S, Wang X, Zhao C, Liu Q, Zhou H, Zheng Z, Zhou Z, Liang J. Laparoscopic vs open colorectal cancer surgery in elderly patients: short- and long-term outcomes and predictors for overall and disease-free survival. BMC Surg. 2019;19:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Vallance AE, Keller DS, Hill J, Braun M, Kuryba A, van der Meulen J, Walker K, Chand M. Role of Emergency Laparoscopic Colectomy for Colorectal Cancer: A Population-based Study in England. Ann Surg. 2019;270:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Keller DS, de Paula TR, Qiu J, Kiran RP. The Trends in Adoption, Outcomes, and Costs of Laparoscopic Surgery for Colorectal Cancer in the Elderly Population. J Gastrointest Surg. 2021;25:766-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Hiyoshi Y, Miyamoto Y, Eto K, Nagai Y, Iwatsuki M, Iwagami S, Baba Y, Yoshida N, Baba H. Laparoscopic surgery for colorectal cancer with persistent descending mesocolon. World J Surg Oncol. 2019;17:190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41-55. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15213] [Cited by in RCA: 15340] [Article Influence: 365.2] [Reference Citation Analysis (0)] |
| 17. | Matsuo K, Nusbaum DJ, Machida H, Huang Y, Khetan V, Matsuzaki S, Klar M, Grubbs BH, Roman LD, Wright JD. Populational trends and outcomes of postoperative radiotherapy for high-risk early-stage cervical cancer with lymph node metastasis: concurrent chemo-radiotherapy vs radiotherapy alone. Am J Obstet Gynecol. 2020;222:484.e1-484.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Guo C, Tang X, Meng Y, Zhang Y, Zhang X, Guo J, Lei X, Qiu J, Hua K. Effect of the surgical approach on survival outcomes in patients undergoing radical hysterectomy for cervical cancer: A real-world multicenter study of a large Chinese cohort from 2006 to 2017. Cancer Med. 2020;9:5908-5921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | He LH, Yang B, Su XQ, Zhou Y, Zhang Z. Comparison of clinical efficacy and postoperative inflammatory response between laparoscopic and open radical resection of colorectal cancer. World J Clin Cases. 2022;10:4042-4049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (2)] |
| 20. | Wang H, Zhang L, Sun M, Kang L, Wei X. Perioperative treatment compliance, anxiety and depression of elderly patients with ophthalmic surgery and the influential factors. Ann Palliat Med. 2021;10:2115-2122. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Erus S, Öztürk AB, Albayrak Ö, İncir S, Kapdağlı MH, Cesur EE, Yavuz Ö, Tanju S, Dilege Ş. Immune profiling after minimally invasive lobectomy. Interact Cardiovasc Thorac Surg. 2021;32:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Song H, Song J, Liang Y, Fu W, Xu Y, Zheng J, Xu W. [Comparison of immune response after laparoscopic and open surgery for colorectal carcinoma: a meta-analysis]. Zhonghua Wei Chang Wai Ke Za Zhi. 2014;17:799-804. [PubMed] |