Published online Sep 27, 2023. doi: 10.4240/wjgs.v15.i9.2089
Peer-review started: May 30, 2023
First decision: July 4, 2023
Revised: July 12, 2023
Accepted: August 1, 2023
Article in press: August 1, 2023
Published online: September 27, 2023
Processing time: 115 Days and 4.8 Hours
In the translational therapy of giant hepatocellular carcinoma (HCC), hepatic arterial infusion chemotherapy (HAIC) combined with anti-PD-1 immunotherapy and tyrosine kinase inhibitors (TKI) after laparoscopic portal vein ligation (PVL) is extremely rare. This is a dual conversion therapy that combines surgery and oncology. Here, we report two cases of successful surgical completion after dual conversion therapy.
We report that a 54-year-old man and a 69-year-old woman were diagnosed with primary HCC combined with hepatitis B cirrhosis (case 2 also combined with fatty liver) on physical examination. Due to the insufficient residual liver volume assessed before surgery, laparoscopic right PVL was performed, followed by HAIC combined with anti-PD-1 immunotherapy and TKI. Finally, surgical resection was successfully completed, and pathology confirmed that the tumor was mostly necrotic (90%) in one case, and no live tumor tissue was found in the other case.
In the process of surgical transformation, our treatment plan takes into account the control and transformation of oncology at the same time, which is expected to provide more opportunities for radical hepatectomy and improve the prognosis of patients with large liver cancer.
Core Tip: There have been many clinical studies on translational therapy for giant hepatocellular carcinoma, but simply seeking surgical transformation always carries the risk of tumor progression. There's a combination of control and transformation in oncology either immunotherapy, targeted therapy, or a combination of both. In addition, we added hepatic arterial infusion chemotherapy to seek better prognosis. In addition to the success of surgical transformation in the 2 patients reported by us, the postoperative pathology also suggested good oncology control and transformation. This treatment regimen is promising to provide more opportunities for radical hepatectomy and better prognosis for patients with large liver cancer.
- Citation: Gao Q, Zhu GZ, Han CY, Ye XP, Huang HS, Mo ST, Peng T. Dual transformation therapy for giant hepatocellular carcinoma: Two case reports and review of literature. World J Gastrointest Surg 2023; 15(9): 2089-2097
- URL: https://www.wjgnet.com/1948-9366/full/v15/i9/2089.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i9.2089
The incidence and mortality rate of hepatocellular carcinoma (HCC) are comparable, making it one of the most common malignant tumors worldwide. According to global cancer statistics data in 2020, there were 600000 new cases of HCC worldwide, with over half of the cases occurring in China[1,2]. Surgical resection remains the first choice for HCC treatment, but the recurrence rate after surgery can be as high as 70%[3]. Some HCC patients may not be suitable for extensive liver resection due to insufficient future liver remnant (FLR) volume[4]. According to the results of the BRIDGE study, 64% of liver cancer patients in China are diagnosed at CNLC-Stage II and III [Barcelona Clinic Liver Cancer (BCLC) Stage B and C], with a median survival period of approximately 2 years[5,6]. Most patients in the advanced stages are not suitable for surgical resection and should receive mainly local and systemic treatments. In recent years, non-surgical treatments for liver cancer have made significant progress. Drug therapy, especially the combination of anti-angiogenic drugs and immune therapy, can achieve an objective response rate of about 30% and a median survival period of around 20 mo in the treatment of advanced or unresectable liver cancer[7-9].
The reasons for unresectability of liver cancer can be divided into two levels. One level is unresectability from a surgical perspective, including patients who cannot tolerate surgical trauma due to their overall condition, cannot tolerate liver dysfunction, or have insufficient FLR volume, which is considered unresectable from a surgical perspective. The other level is technically resectable, but the postoperative efficacy is not better than non-surgical treatment, which is considered unresectable from an oncological or biological perspective. The goal of conversion therapy is to eliminate these two reasons and achieve conversion from unresectable to resectable liver cancer[10]. The main methods of surgical conversion therapy for insufficient FLR volume include associating liver partition and portal vein ligation for staged hepatectomy (ALPPS), portal vein ligation (PVL), and portal vein embolization (PVE), but there is still a risk of tumor progression while the residual liver volume is growing. In our previous clinical study of 30 cases of conversion resection with insufficient FLR volume in HCC using PVL combined with apatinib (anti-VEGFR2) and camrelizumab (anti-PD1), the median preoperative estimated FLR/standard liver volume (SLV) was 32.9% (19.1%-39.9%). According to mRECIST, 4 cases (13.3%) achieved complete response (CR) and 8 cases (26.7%) achieved partial response (PR). Twenty-three cases (76.7%) met the criteria for second-stage surgery, and 20 of them (66.7%) completed second-stage liver tumor resection. Double conversion therapy had better clinical outcomes than simple surgical conversion. However, the control of tumors after PVL in our systemic therapy (objective response rate of 40%, disease control rate of 76.7%) was comparable to that of targeted immunotherapy. Considering the promising results of local combined systemic therapy in tumor control, we added hepatic arterial infusion chemotherapy (HAIC) on the basis of PVL combined with targeted immunotherapy.
Here, we report two cases of successful treatment of large liver cancer patients at our center. After PVL, the patients underwent HAIC combined with targeted immunotherapy, and finally successfully completed right hepatectomy.
Case 1: A 54-year-old Chinese male was admitted to the Department of Hepatobiliary Surgery due to upper abdominal discomfort for 2 wk.
Case 2: A 69-year-old Chinese woman was admitted to the hepatobiliary surgery clinic for 20 d due to physical examination.
Case 1: The patient reported right upper abdominal discomfort without obvious inducement more than half a month ago, presenting intermittent dull pain, each lasting 1-2 min.
Case 2: The patient reported a solid space occupying lesion in the liver during abdominal computed tomography (CT) examination 20 d ago.
Case 1: Hepatitis B cirrhosis for more than 30 years without systematic treatment.
Case 2: Hepatitis B cirrhosis for more than 30 years without systematic treatment; He had a history of fatty liver 3 years ago and was treated regularly with drugs.
The patient denied any family history of malignant tumours.
There were no positive signs in either.
Case 1: Laboratory tests indicated that AFP was 876.62 ng/mL and PIVKA-II was 12943.43 mAU/mL. Liver function, blood routine, prothrombin time, and international normalized ratio were all within normal ranges. AFP and PIVKA-II were 6.16 ng/mL and 114.89 mAU/mL before right hemihepatectomy.
Case 2: Laboratory tests showed no abnormalities.
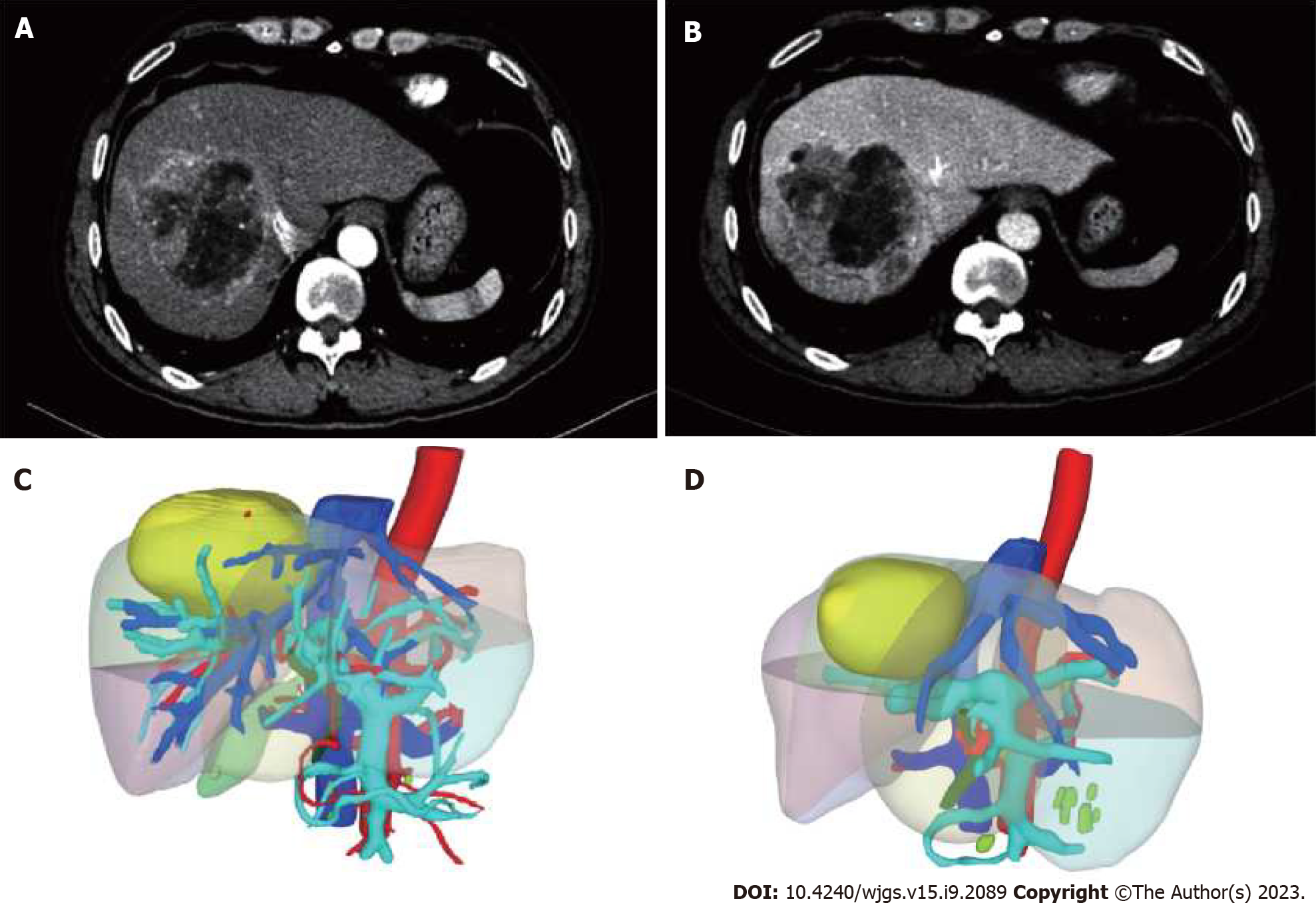
Case 1: The outer edge of the liver was not smooth, and the proportion of each lobe was disordered. Irregular masses with slightly low density were seen in S5, 6, and 8 segments of the liver, with the size of about 6.7 cm × 6.5 cm × 7.5 cm, complete capsule and small difficult vessels were seen, and the density was not uniform. After enhancement, three stages of scanning were performed: The lesion enhancement was obvious in the arterial stage, but decreased in the portal and equilibrium stages, and the density was lower than that of liver parenchyma. Diagnosis: S5, 6, 8 segments of liver occupying, massive liver cancer was considered. Cirrhosis of the liver (Figure 1A and B). The preoperative SLV was estimated to be 1370.52 mL, and the FLR was estimated to be 418.39 mL using a three-dimensional CT reconstruction system. FLR/SLV was 30.5% (Figure 1C).
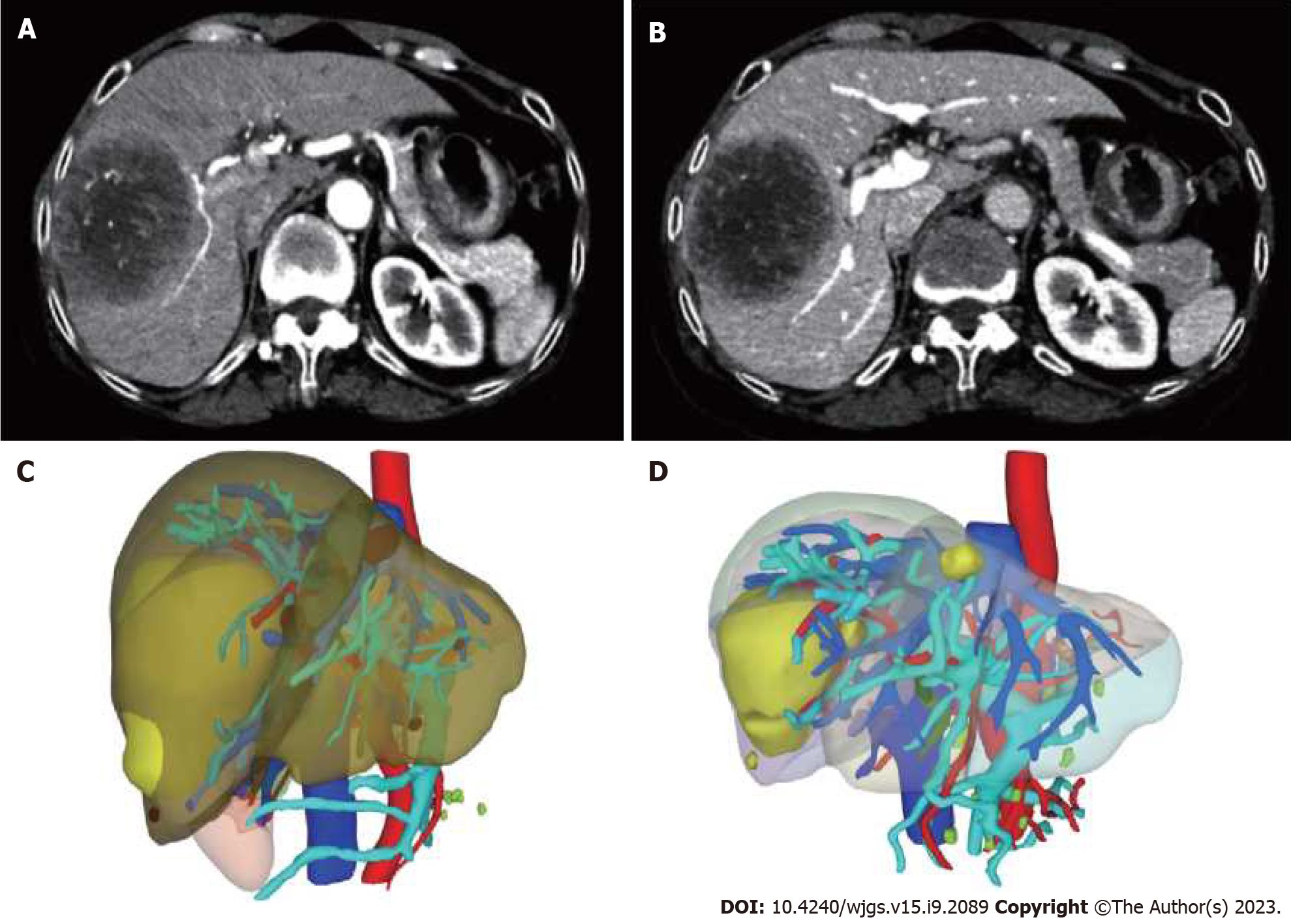
Case 2: A round mixed low-density mass with a size of 8.0 cm × 7.5 cm × 6.2 cm was seen in the right lobe of liver (S7/8 segment), with a clear margin. The third stage of enhancement scan showed that the edge of the disease showed continuous enhancement, mainly marginal enhancement, with a density slightly lower than that of open parenchyma, and patchy non-enhancement lesions were still seen in the equilibrium stage. The remaining hepatic parenchyma density was normal and no abnormal enhancement was observed. Diagnosis: right lobe of liver mass, primary liver cancer (mixed type?) was considered (Figure 2A and B). The preoperative SLV was estimated to be 913.5 mL, and the FLR was estimated to be 310.59 mL using a three-dimensional CT reconstruction system. FLR/SLV was 34% (Figure 2C).
Combined with the patient’s medical history, the final diagnosis was HCC (CNLC Ib stage, BCLC A grade, Child-Pugh A grade); hepatitis B cirrhosis.
Combined with the patient’s medical history, the final diagnosis was HCC (CNLC Ib stage, BCLC A grade, Child-Pugh A grade); hepatitis B cirrhosis; fatty liver.
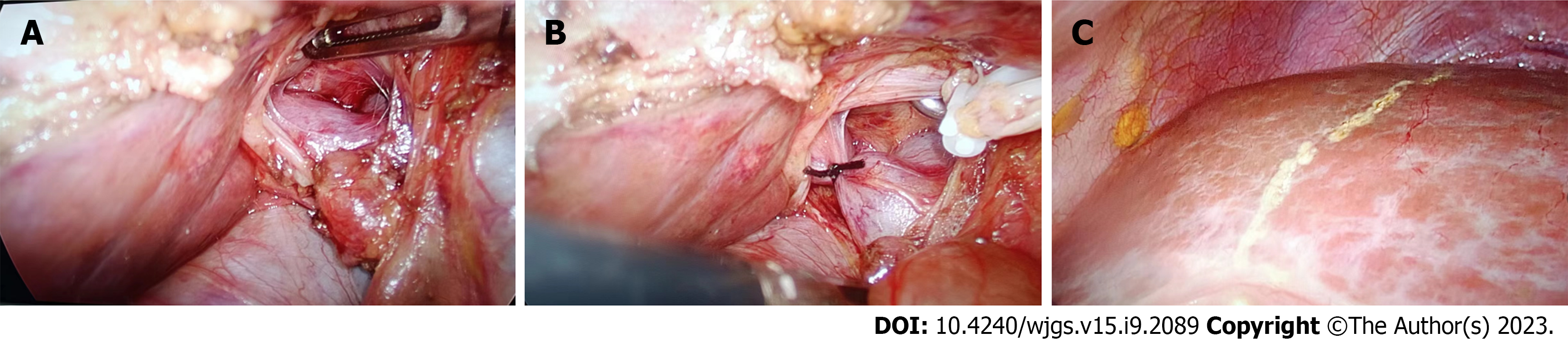
Both patients in this series underwent complete laparoscopic right PVL surgery. The surgical steps were as follows: The patient was placed in a supine position, and pneumoperitoneum was established with trocar placement. Intraoperative ultrasound was used to confirm the location and boundaries of the tumor. The right portal vein branch near the first porta hepatis was dissected within the Glisson's sheath, and then titanium clips or No. 7 sutures were used for ligation. An electrocautery hook was used to mark the ischemic line on the liver surface (Figure 3). Case 1 and case 2 completed PVL on July 12 and 29, 2022, respectively.
Case 1: The patient underwent a total of 4 cycles of HAIC combined with targeted immunotherapy, using the FOLFOX regimen for 46 h. The microcatheter is pushed into the hepatic artery and the drug is transfused through the hepatic artery. Oxaliplatin, 130 mg/m2 before 2 h on day 1, calcium folinate, 400 mg/m2 from 2-3 h on day 1, fluorouracil 400 mg/m2 at 3 h on day 1, 2400 mg/m2 over 24 h. On the following dates: July 15, 2022; August 8, 2022; September 6, 2022; and October 13, 2022. Bevacizumab was administered intravenously at a dose of 200 mg on July 18, 2022; August 11, 2022; September 9, 2022; and October 16, 2022. In addition, the patient received oral lenvatinib starting from July 18, 2022, until prior to surgery. After discontinuation of the medication for four weeks, the FLR to SLV ratio was reevaluated and found to be 60.5% (Figure 1D). Right hemihepatectomy was performed on November 25, 2022.
Case 2: On August 12 and September 16, 2022, the patient received HAIC treatment using the same protocol as before. On September 19, Carfilzomib was administered intravenously at a dose of 200mg. After a four-week drug holiday, the FLR/SLV was re-evaluated and found to be 47% (as shown in Figure 2D). On October 26, right hepatectomy was performed to remove the right half of the liver.
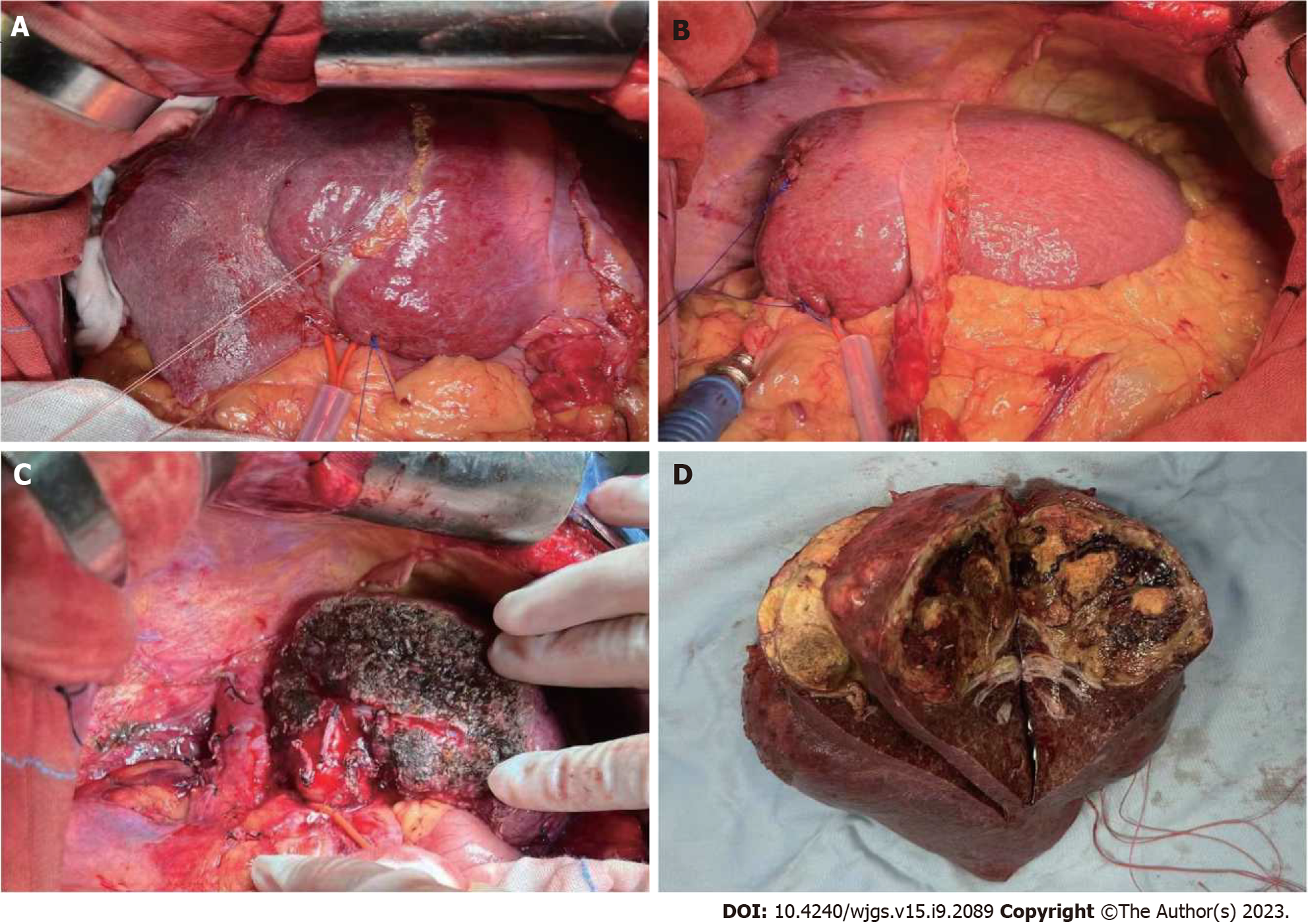
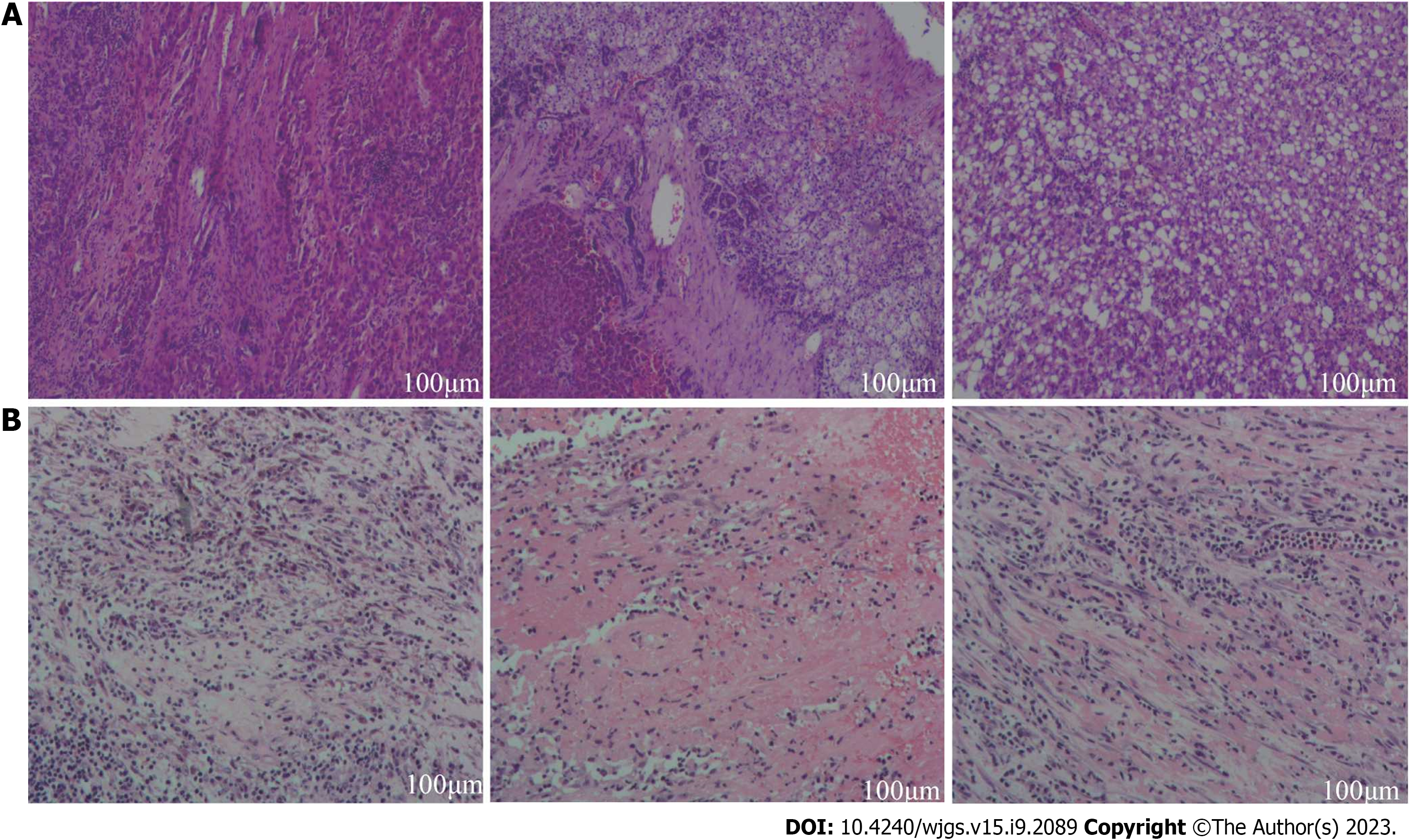
Case 1: During the initial surgical exploration, left liver hypertrophy was easily observed (Figure 4). After separation of adhesions, right hepatic pedicle was dissected intrafascially. Then, the liver parenchyma was continuously separated along the previously marked ischemic line using an ultrasonic knife. The right hepatic vein was transected with a stapling closure device until complete removal of the diseased liver. There were no major complications during the postoperative recovery period. Histopathological examination of the tumor confirmed that the majority of the tumor was necrotic (90% necrosis), with some residual viable tumor tissue at the periphery. The tumor was classified as HCC grade II, with a nodular pattern, with approximately 40% of tumor cells showing clear cell changes and approximately 40% of tumor cells showing macrovesicular fatty changes. Fibrous tissue proliferation with foam cell and multinucleated giant cell reactions were observed, along with deposition of hemosiderin and infiltration of lymphocytes. No satellite nodules or neural bundle invasion were observed, and the microvascular invasion was graded as MO. There was no tumor involvement in the liver capsule or surgical margins. The surrounding liver tissue showed chronic hepatitis changes, with a CS grade of C3S3 (Figure 5A).
Case 2: The postoperative pathological examination revealed extensive necrosis with no residual viable tumor tissue. Fibrous tissue proliferation with abundant lymphocyte and plasma cell infiltration, hemosiderin deposition, and foam cell reaction were observed around the necrotic tissue, consistent with changes after interventional treatment. Microvascular invasion and nerve bundle invasion could not be assessed due to the absence of viable tumor components. There was no tumor involvement in the liver capsule or surgical margins. The surrounding liver tissue showed chronic hepatitis with a grade of G2S2 (Figure 5B).
Cases 1 and 2: As of July 2023, the patient is still alive and has no recurrence.
Currently, international centers have similar criteria for determining the required liver reserve function for safe liver resection, which includes normal liver function (Child-Pugh Class A) with a retention rate of indocyanine green at 15 min (ICG-R15) of 20% to 30%; patients with chronic liver disease or liver parenchymal damage (including cirrhosis, severe fatty liver, and liver damage related to chemical drug treatment) require a FLR to SLV ratio of > 40%; for patients with impaired liver function, a higher FLR is required (e.g., ICG-R15 of 10% to 20%, and patients with chronic liver disease and cirrhosis require FLR/SLV > 50%)[10,11]. Insufficient FLR is an important criterion for unresectable liver cancer, and the goal of conversion therapy is to achieve a sufficient FLR.
In 1990, Makuuchi et al[12] reported the first series of PVE in 14 patients with hilar cholangiocarcinoma, demonstrating the safety and feasibility of this technique in reducing postoperative liver failure. PVE has been used in clinical practice for a long time, with a conversion success rate of 60% to 80% and a complication rate of 10% to 20%. The time required for residual liver hypertrophy after PVE is relatively long (usually 4 to 6 wk, during which time tumor progression may occur). In addition, more than 20% of patients may lose the opportunity for surgery due to tumor progression or insufficient volume of residual liver hypertrophy[13-15]. This has prompted exploration of alternative techniques. There is no significant difference in the induction of FLR hypertrophy between PVE and PVL (relative increase in FLR volume: PVE 43.2%, PVL 38.5%). The induction of FLR hypertrophy with PVL also takes 4 to 8 wk. However, the resection rate with PVL is higher than with PVE, resulting in significantly fewer patients who have to cancel surgery due to insufficient FLR hypertrophy. There is no statistically significant difference in the incidence of postoperative complications and mortality between the two techniques[16,17].
In 2012, ALPPS was first described as a surgical technique for increasing FLR in patients with liver tumors[18]. It is a more radical method of portal vein occlusion and elimination of collateral vessels from FLR to the diseased segment, resulting in faster and more extensive FLR enhancement. ALPPS can induce FLR hypertrophy of up to 47% to 192% within 1-2 wk, which is much higher than PVE. Due to the short interval between the two-stage surgeries, it can minimize the risk of tumor progression, with a tumor resection rate of 95% to 100%[19]. The main limitation of this technique is its high morbidity and mortality rates. Compared to PVE, the 90-d mortality rate remains at 8%-9%[20], and both mortality and morbidity rates are increased[21]. These findings were initially attributed to increased invasiveness of the surgery and subsequent bile leakage, with bile leakage occurring in 20% of the cases reported by Schnitzbauer et al[18].
When the tumor volume is too large, involves critical ducts, or cannot achieve an R0 resection, tumor reduction through anti-tumor treatment can be achieved before surgery to further improve long-term outcomes. Treatment options include local therapy, systemic therapy, and combination therapy[11].
In clinical practice, there is no consensus on which systemic treatment regimen to choose for potentially resectable liver cancer patients. Based on current clinical research data on first-line systemic treatment for liver cancer, lenvatinib has a higher objective response rate (ORR) than sorafenib[22]. Combination targeted therapy and immunotherapy (targeted immunotherapy combination)[7,8,23,24], represented by lenvatinib combined with pembrolizumab, nivolumab combined with ramucirumab, and bevacizumab, and atezolizumab combined with cetuximab, has an ORR > 20% for treating unresectable liver cancer, with stronger potential for conversion. Zhu et al[25] reported a conversion resection rate of 23.8% in 101 cases of initially unresectable liver cancer patients treated with PD-1 inhibitors combined with tyrosine kinase inhibitors (TKIs)[25]. Zhu et al[26] reported a conversion resection rate of 15.9% in 63 cases of initially unresectable liver cancer patients treated with PD-1 inhibitors combined with TKIs. At the 2022 Annual Meeting of the American Association for the Study of Liver Diseases, we reported the effectiveness and safety of hepatic portal vein ligation (PVL) combined with apatinib (anti-VEGFR2) + camrelizumab (anti-PD1) (also known as double A combination) in treating insufficient FLR volume HCC. According to mRECIST, among them, 4 cases (13.3%) achieved CR, 8 cases (26.7%) achieved PR, 11 cases (36.7%) had stable disease (SD), and 7 cases (23.3%) had progressive disease (PD), with an ORR of 40% and a disease control rate (DCR) of 76.7%. This is a dual conversion regimen that considers both surgical and oncological aspects. The results of this study suggest that PVL combined with apatinib and camrelizumab may become a potential treatment option for insufficient FLR volume HCC.
In Asia, particularly in Japan and South Korea, HAIC has been used to improve the prognosis of advanced HCC and has been incorporated into treatment guidelines[27]. In a randomized phase III study (9810) announced at ESMO 2020, the surgical conversion rate of unresectable HCC with HAIC (oxaliplatin, fluorouracil, and folinic acid) was 23.8% in the HAIC group compared to 11.5% in the transarterial chemoembolization group (P < 0.004). Furthermore, the study also found that the ORR of advanced HCC treated with HAIC plus targeted therapy and immune therapy according to mRECIST criteria was 67.6%[28]. The results of clinical studies by Luo et al[29] and Zhang et al[30] at their respective centers, using HAIC in combination with targeted immunotherapy for advanced liver cancer, were encouraging, with objective tumor response rates of 57.2% and 96%, respectively, and good safety profiles. These studies demonstrate that combined systemic and local treatment can lead to better tumor response, providing new options for future HAIC-based conversion therapies.
In the two cases reported in our study, the main feature was the increase in FLR volume after PVL combined with systemic and local treatment. FLR increased by 30% and 13% in case 1 and case 2, respectively, with time intervals of 17 wk and 12 wk, respectively. Postoperative pathology confirmed that the majority of the tumor in case 1 had necrotized (90% necrosis), and the tumor in case 2 had completely necrotized, with both cases showing good recovery during follow-up. Dual conversion in terms of surgical and oncological outcomes was achieved, indicating that PVL combined with HAIC and targeted immunotherapy is a safe and effective dual conversion treatment approach.
Our treatment approach aims to achieve surgical conversion while also addressing tumor control and conversion, offering potential opportunities for curative liver resection in patients with advanced HCC and improving their prognosis. However, research in this area is limited at present. Further studies are needed to confirm the safety, feasibility, and efficacy of our approach.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Akbulut S, Turkey; Martín Del Olmo JC, Spain S-Editor: Yan JP L-Editor: A P-Editor: Cai YX
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64637] [Article Influence: 16159.3] [Reference Citation Analysis (176)] |
| 2. | Cao M, Li H, Sun D, Chen W. Cancer burden of major cancers in China: A need for sustainable actions. Cancer Commun (Lond). 2020;40:205-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 3. | General Office of National Health Commission. Diagnosis and Treatment Guidelines for Primary liver Cancer (2022 edition). Linchuang Gandanbing Zazhi. 2022;38:288-303. [DOI] [Full Text] |
| 4. | Chan A, Zhang WY, Chok K, Dai J, Ji R, Kwan C, Man N, Poon R, Lo CM. ALPPS Versus Portal Vein Embolization for Hepatitis-related Hepatocellular Carcinoma: A Changing Paradigm in Modulation of Future Liver Remnant Before Major Hepatectomy. Ann Surg. 2021;273:957-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 5. | Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, Kudo M, Johnson P, Wagner S, Orsini LS, Sherman M. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155-2166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 569] [Cited by in RCA: 944] [Article Influence: 94.4] [Reference Citation Analysis (0)] |
| 6. | Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, Zhou W, Bie P, Liu L, Wen T, Han G, Wang M, Liu R, Lu L, Ren Z, Chen M, Zeng Z, Liang P, Liang C, Yan F, Wang W, Ji Y, Yun J, Cai D, Chen Y, Cheng W, Cheng S, Dai C, Guo W, Hua B, Huang X, Jia W, Li Y, Liang J, Liu T, Lv G, Mao Y, Peng T, Ren W, Shi H, Shi G, Tao K, Wang X, Xiang B, Xing B, Xu J, Yang J, Yang Y, Ye S, Yin Z, Zhang B, Zhang L, Zhang S, Zhang T, Zhao Y, Zheng H, Zhu J, Zhu K, Shi Y, Xiao Y, Dai Z, Teng G, Cai J, Cai X, Li Q, Shen F, Qin S, Dong J, Fan J. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer. 2020;9:682-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 575] [Article Influence: 115.0] [Reference Citation Analysis (1)] |
| 7. | Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Mody K, Llovet JM. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol. 2020;38:2960-2970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 661] [Cited by in RCA: 876] [Article Influence: 175.2] [Reference Citation Analysis (0)] |
| 8. | Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, Shao G, Xu L, Yin T, Liu J, Ren Z, Xiong J, Mao X, Zhang L, Yang J, Li L, Chen X, Wang Z, Gu K, Pan Z, Ma K, Zhou X, Yu Z, Li E, Yin G, Zhang X, Wang S, Wang Q. Camrelizumab in Combination with Apatinib in Patients with Advanced Hepatocellular Carcinoma (RESCUE): A Nonrandomized, Open-label, Phase II Trial. Clin Cancer Res. 2021;27:1003-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 399] [Article Influence: 99.8] [Reference Citation Analysis (0)] |
| 9. | Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, Tovoli F, Knox JJ, Ruth He A, El-Rayes BF, Acosta-Rivera M, Lim HY, Neely J, Shen Y, Wisniewski T, Anderson J, Hsu C. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020;6:e204564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 908] [Cited by in RCA: 968] [Article Influence: 193.6] [Reference Citation Analysis (0)] |
| 10. | Sun HC, Xie Q, Jia WD, Zhao M, Liu XF, Bi XY, Li G, Bai XL, Ji Y, Xu L, Wang Z, Zhu XD. Chinese Expert Consensus on transformation Therapy for liver Cancer (2021 edition). Zhongguo Shiyong Waike Zazhi. 2021;41:618-632. [DOI] [Full Text] |
| 11. | Zhao HT, Bi XY, Zhao H, Sun YK, Zhou JG, Chen B, Zhang W. Chinese expert consensus on neoadjuvant and translational therapy for hepatocellular carcinoma. Ganai Dianza Zazhi. 2022;9:23-28. [DOI] [Full Text] |
| 12. | Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P, Yamazaki S, Hasegawa H, Ozaki H. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521-527. [PubMed] |
| 13. | Aloia TA. Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy: Portal Vein Embolization Should Remain the Gold Standard. JAMA Surg. 2015;150:927-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Piron L, Deshayes E, Escal L, Souche R, Herrero A, Pierredon-Foulongne MA, Assenat E, le Lam N, Quenet F, Guiu B. [Portal vein embolization: Present and future]. Bull Cancer. 2017;104:407-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | European Association for the Study of the Liver; European Organisation for research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4520] [Article Influence: 347.7] [Reference Citation Analysis (2)] |
| 16. | Jaeck D, Oussoultzoglou E, Rosso E, Greget M, Weber JC, Bachellier P. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004;240:1037-49; discussion 1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 365] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 17. | Isfordink CJ, Samim M, Braat MNGJA, Almalki AM, Hagendoorn J, Borel Rinkes IHM, Molenaar IQ. Portal vein ligation versus portal vein embolization for induction of hypertrophy of the future liver remnant: A systematic review and meta-analysis. Surg Oncol. 2017;26:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R, Kroemer A, Loss M, Rümmele P, Scherer MN, Padberg W, Königsrainer A, Lang H, Obed A, Schlitt HJ. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 933] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 19. | Popescu GA, Alexandrescu ST, Grigorie RT, Stoica L, Apavaloaie CA, Hrehoreţ D. GOOD TO KNOW: The ALPPS Procedure - Embracing a New Technique. Chirurgia (Bucur). 2017;112:332-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Sandström P, Røsok BI, Sparrelid E, Larsen PN, Larsson AL, Lindell G, Schultz NA, Bjørnbeth BA, Isaksson B, Rizell M, Björnsson B. ALPPS Improves Resectability Compared With Conventional Two-stage Hepatectomy in Patients With Advanced Colorectal Liver Metastasis: Results From a Scandinavian Multicenter Randomized Controlled Trial (LIGRO Trial). Ann Surg. 2018;267:833-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 228] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 21. | Eshmuminov D, Raptis DA, Linecker M, Wirsching A, Lesurtel M, Clavien PA. Meta-analysis of associating liver partition with portal vein ligation and portal vein occlusion for two-stage hepatectomy. Br J Surg. 2016;103:1768-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 22. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3826] [Article Influence: 546.6] [Reference Citation Analysis (1)] |
| 23. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4696] [Article Influence: 939.2] [Reference Citation Analysis (2)] |
| 24. | Ren Z, Fan J, Xu J, Bai Y, Xu A, Cang S, Du C, Liu B, Li Q, Lu Y, Chen Y, Shao G, Guo Y, Chen Z, Yang Y, Chen M, Wang Y, Zhou H. LBA2 Sintilimab plus bevacizumab biosimilar vs sorafenib as first-line treatment for advanced hepatocellular carcinoma (ORIENT-32)2. Ann Oncol. 2020;31 Suppl 6:S1287. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Zhu XD, Huang C, Shen YH, Xu B, Ge NL, Ji Y, Qu XD, Chen L, Chen Y, Li ML, Zhu JJ, Tang ZY, Zhou J, Fan J, Sun HC. Hepatectomy After Conversion Therapy Using Tyrosine Kinase Inhibitors Plus Anti-PD-1 Antibody Therapy for Patients with Unresectable Hepatocellular Carcinoma. Ann Surg Oncol. 2023;30:2782-2790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 26. | Zhu XD, Huang C, Shen YH, Ji Y, Ge NL, Qu XD, Chen L, Shi WK, Li ML, Zhu JJ, Tan CJ, Tang ZY, Zhou J, Fan J, Sun HC. Downstaging and Resection of Initially Unresectable Hepatocellular Carcinoma with Tyrosine Kinase Inhibitor and Anti-PD-1 Antibody Combinations. Liver Cancer. 2021;10:320-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 162] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 27. | Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, Nagano H, Hatano E, Izumi N, Kaneko S, Kudo M, Iijima H, Genda T, Tateishi R, Torimura T, Igaki H, Kobayashi S, Sakurai H, Murakami T, Watadani T, Matsuyama Y. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49:1109-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 396] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 28. | Shi M, Li Q, He M, Guo R. 981O Hepatic arterial infusion chemotherapy (HAIC) with oxaliplatin, fluorouracil, and leucovorin (FOLFOX) vs transarterial chemoembolization (TACE) for unresectable hepatocellular carcinoma (HCC): A randomised phase III trial. Ann Oncol. 2020;31:S688. |
| 29. | Luo L, Xiao Y, Zhu G, Huang A, Song S, Wang T, Ge X, Xie J, Deng W, Hu Z, Wen W, Mei H, Wan R, Shan R. Hepatic arterial infusion chemotherapy combined with PD-1 inhibitors and tyrosine kinase inhibitors for unresectable hepatocellular carcinoma: A tertiary medical center experience. Front Oncol. 2022;12:1004652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 30. | Zhang J, Zhang X, Mu H, Yu G, Xing W, Wang L, Zhang T. Surgical Conversion for Initially Unresectable Locally Advanced Hepatocellular Carcinoma Using a Triple Combination of Angiogenesis Inhibitors, Anti-PD-1 Antibodies, and Hepatic Arterial Infusion Chemotherapy: A Retrospective Study. Front Oncol. 2021;11:729764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |