Published online Sep 27, 2023. doi: 10.4240/wjgs.v15.i9.2021
Peer-review started: June 23, 2023
First decision: July 4, 2023
Revised: July 10, 2023
Accepted: July 27, 2023
Article in press: July 27, 2023
Published online: September 27, 2023
Processing time: 91 Days and 0.4 Hours
Cold ischemia-reperfusion of the liver is an inevitable occurrence in liver trans
To analyze the data of children who underwent LDLT to determine the risk factors for intraoperative myocardial injury.
We retrospectively analyzed the inpatient records of pediatric patients who underwent LDLT in Tianjin First Central Hospital from January 1, 2020, to January 31, 2022. Recipient-related data and donor-related data were collected. The patients were divided into a myocardial injury group and a nonmyocardial injury group according to the value of the serum cardiac troponin I at the end of surgery for analysis. Univariate analysis and multivariate logistic regression were used to evaluate the risk factors for myocardial injury during LDLT in pediatric patients.
A total of 302 patients met the inclusion criteria. The myocardial injury group had 142 individuals (47%), and the nonmyocardial injury group included 160 patients (53%). Age, height, and weight were significantly lower in the myocardial injury group (P < 0.001). The pediatric end-stage liver disease (PELD) score, total bilirubin, and international standardized ratio were significantly higher in the myocardial injury group (P < 0.001). The mean arterial pressure, lactate, hemoglobin before reperfusion, duration of the anhepatic phase, cold ischemic time, incidence of postreperfusion syndrome (PRS), and fresh frozen plasma transfusion were significantly different between the two groups (P < 0.05). The postoperative intensive care unit stay and peak total bilirubin values in the first 5 d after LDLT were significantly higher in the myocardial injury group (P < 0.05). The pediatric patients with biliary atresia in the nonmyocardial injury group who underwent LDLT had a considerably higher one-year survival rate than those in the myocardial injury group (P = 0.015). Multivariate logistic regression revealed the following independent risk factors for myocardial injury: a high PELD score [odds ratio (OR) = 1.065, 95% confidence interval (CI): 1.013-1.121; P = 0.014], a long duration of the anhepatic phase (OR = 1.021, 95%CI: 1.003-1.040; P = 0.025), and the occurrence of intraoperative PRS (OR = 1.966, 95%CI: 1.111-3.480; P = 0.020).
A high PELD score, a long anhepatic phase duration, and the occurrence of intraoperative PRS were independent risk factors for myocardial injury during LDLT in pediatric patients with biliary atresia.
Core Tip: This is a retrospective study to investigate data for children who underwent living donor liver transplantation to determine risk factors for intraoperative myocardial injury. Myocardial injury during liver transplantation is associated with postoperative adverse outcomes in pediatric patients; it can increase the incidence of postoperative mortality. Our findings demonstrated that a high pediatric end-stage liver disease score, a long anhepatic phase duration, and the occurrence of intraoperative postreperfusion syndrome are independent risk factors for myocardial injury.
- Citation: Wu YL, Li TY, Gong XY, Che L, Sheng MW, Yu WL, Weng YQ. Risk factors for myocardial injury during living donor liver transplantation in pediatric patients with biliary atresia. World J Gastrointest Surg 2023; 15(9): 2021-2031
- URL: https://www.wjgnet.com/1948-9366/full/v15/i9/2021.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i9.2021
Cold ischemia-reperfusion of the liver is an inevitable pathophysiological occurrence in liver transplantation. This process not only causes liver damage but also causes damage to distant organs such as the heart, brain, and kidneys[1]. Huang et al[2] reported that the incidence of perioperative myocardial injuries in adult patients who underwent liver transplantation was 40.4%, while the 30-d mortality rate of patients who developed a myocardial injury was 11.4%, which was significantly higher than that of patients without a myocardial injury. In the research of Sheng et al[3], the serum cardiac troponin I (cTnI) level was detected in 123 children who underwent living donor liver transplantation (LDLT), and the results showed that the rate of myocardial injury (cTnI ≥ 0.07 ng/mL) at 30 min in the neohepatic stage was as high as 52%, and cardiovascular adverse events such as ventricular extrasystole and myocardial ischemia could occur during the operation[3]. After noncardiac surgery, myocardial injury is an independent predictor for 30-d mortality[4]. Studies have shown that a myocardial injury is an important cause of postoperative death, and it can lead to an increase in the incidence of postoperative mortality during liver transplantation[5,6]. For a long time, research on myocardial injury has mainly focused on adults, but there is relatively little information on the incidence of myocardial injury in children who have undergone LDLT. Therefore, in this study, our main objective was to further identify the independent risk factors for myocardial injury in pediatric LDLT to provide clinical direction for the prevention of a myocardial injury during LDLT in children.
The Institutional Review Committee of Tianjin First Central Hospital (approval No. 2022DZX02) authorized this retrospective observational study. This study included 302 biliary atresia (BA) pediatric patients (< 18 years old) who underwent LDLT in Tianjin First Central Hospital from January 1, 2020, to January 31, 2022.
The children arrived without having any anesthetic premedications and were monitored using ECG, pulse oximetry, and noninvasive monitoring. Anesthesia was induced with midazolam (0.15 mg/kg), propofol (2-3 mg/kg), fentanyl (2-5 μg/kg), and rocuronium (0.6-1.0 mg/kg). After intubation, mechanical air flow was performed with a fraction of inspired oxygen (FiO2) of 50%-60%, a tidal extent of 8-10 mL/kg, a respiratory rate of 20-28/min, an inspiration-to-expiration ratio of (1.0:1.5)-2.0, and an end-tidal CO2 partial pressure of 30-35 mmHg. Anesthesia was maintained with sevoflurane (1.5%-2.5%), intravenous infusion of propofol (9-15 mg/kg/h), intermittent intravenous fentanyl (1-3 μg/kg), and intravenous infusion of atracurium besylate (1-2 μg/kg/min). Right internal jugular vein puncture was performed under ultrasound guidance to monitor central venous pressure (CVP). A radial artery puncture was performed to monitor invasive arterial pressure. Based on hemodynamic parameters and CVP, albumin and acetate Ringer’s solution were used for fluid therapy. Red blood cells (RBCs) were given to maintain a hemoglobin level of 80-100 g/L. The patients’ coagulation functions were assessed using a Sonoclot analyzer (Sienco, Inc., Arvada, CO, United States). When there was an obvious coagulation disorder, fresh frozen plasma (FFP) was infused. The patients were monitored, and fluctuations in systolic blood pressure and heart rate during surgery were maintained within 20% of the baseline values. We administered anesthetics, cardioactive drugs, and fluids when hemodynamic changes occurred.
For the donor, a left lobectomy was performed, and piggyback liver transplantation was performed for the recipient. Histidine-tryptophan-ketoglutarate (HTK) solution was used as a perfusion solution to perfuse the transplanted liver. The specific components of the HTK solution include NaCl, KCl, MgCl2·6H2O, histidine·HCI·H2O, histidine, tryptophan, mannitol, CaCl2·2H2O, and 2-ketoglutarate-hydrogen-potassium. After occlusion of the inferior vena cava (IVC), the left hepatic vein of the transplanted liver was anastomosed with the recipient hepatic vein, and the IVC was opened after the anastomosis was completed. The donor and recipient portal veins were anastomosed. The opening of the portal vein indicates the start of reperfusion of the liver graft. Venovenous bypass was not performed during the operation. The left hepatic artery of the donor was anastomosed with the hepatic artery of the recipient. The bile duct was connected to the recipient’s jejunum (Roux-en-Y cholangiojejunostomy) after arterial reperfusion. The vascular morphology and blood flow velocity were examined by ultrasound after hepatic artery opening and abdominal closure, respectively.
The third-generation enhanced AccuTnI assay (Beckman Coulter, Brea, CA, United States) was used to analyze the serum cTnI levels in this study. Myocardial injury was described as cTnI ≥ 0.07 ng/mL, which used to be the lowest value mea
The preoperative recipient variables included age, sex, height, weight, left ventricular ejection fraction (LVEF), QTC interval, pediatric end-stage liver disease (PELD) score, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TB), international standardized ratio (INR), creatinine (Cr) and hemoglobin. The graft weight, cold ischemia time, hemodynamic parameters, blood gas parameters, central body temperature before reperfusion, anhepatic phase duration, incidence of postreperfusion syndrome (PRS), operation time, anesthesia duration, blood loss, urine volume, blood transfusion volume, and FFP transfusion volume were recorded during the operation. The postoperative observation indexes included the duration of mechanical ventilation, days in the intensive care unit (ICU), days of hospitalization, peak values of ALT, AST, and TB in the first 5 d after the operation, incidence of acute kidney injury (AKI), and 1-year survival rate. AKI was assessed using the kidney disease improving global outcomes (KDIGO) criteria[7].
All continuous variable data were tested for normality by the Shapiro-Wilk test and Q-Q plots. Measures that conformed to the normal distribution are shown using the mean ± SD, and independent samples t tests were applied. The nonnormally distributed continuous variables were expressed using the median (interquartile range), and Mann-Whitney U tests were used. Categorical variables are shown by the number of cases and percentages, using the Pearson chi-square test. Potentially relevant variables with P values < 0.10 in the univariate analysis were further examined by stepwise binary logistic regression to find the independent risk factors connected to myocardial injury. The data are reported in the form of odds ratios (ORs) and corresponding 95% confidence intervals (CIs). The survival status of the patients was determined using the Kaplan-Meier method. The log-rank test was used to compare the survival status of the two groups. SPSS software version 20.0 (SPSS, Inc., Chicago, IL, United States) was used for statistical analysis. All variables with P values < 0.05 were considered statistically significant.
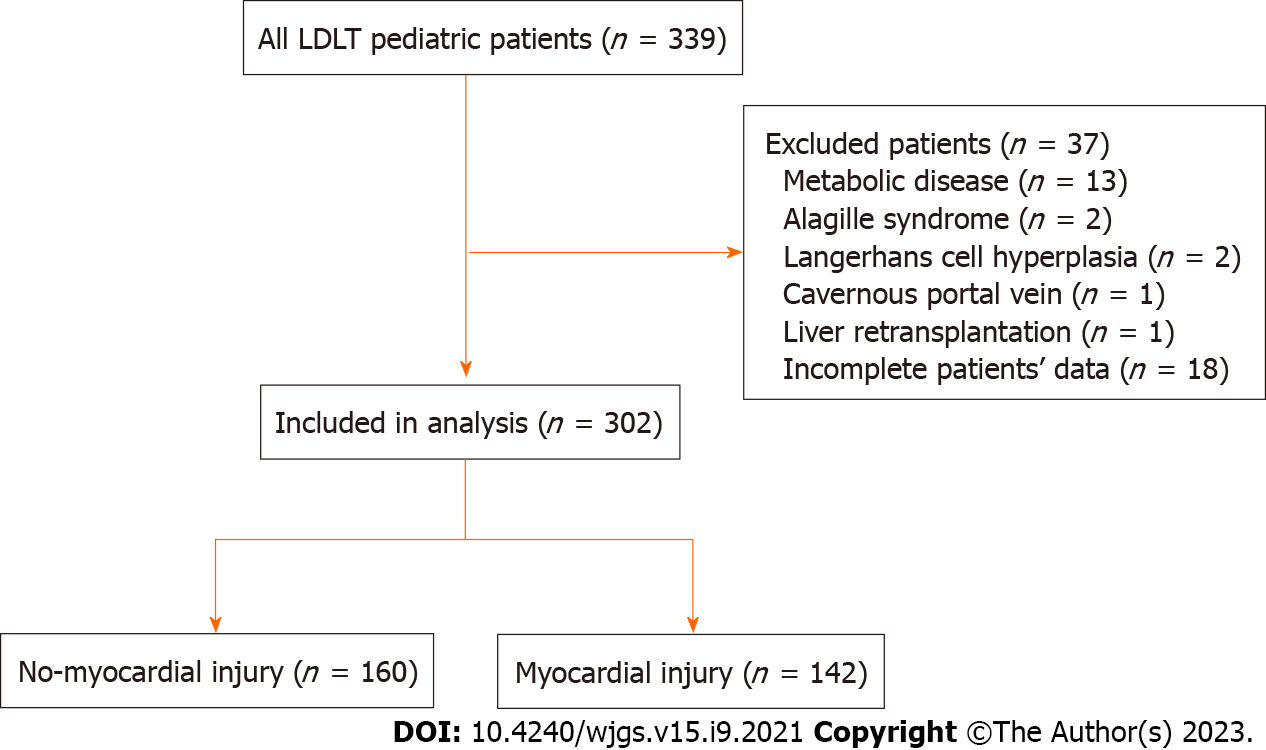
During the study period, 339 pediatric patients underwent LDLT. Metabolic disease occurred in 13 patients, Alagille syndrome in 2 patients, Langerhans cell hyperplasia in 2 patients, the cavernous portal vein in 1 patient, and liver retransplantation in 1 patient, and 18 patients had incomplete data and were excluded. Three hundred two patients with biliary atresia were included in this retrospective study; among them, 142 patients had myocardial injuries, and the incidence rate was 47% (Figure 1).
The demographic data of the recipients are shown in Table 1. The data in Table 1 show that the age [7.0 (6.0-11.0) vs 9.5 (6.0-16.8) mo; P < 0.001], height [65 (62-70) vs 70 (65-82) cm; P < 0.001], and weight [7.0 (6.0-8.0) vs 8.0 (6.5-11.0) kg; P < 0.001] of pediatric patients with myocardial injuries were significantly lower than those in the nonmyocardial injury group. The PELD score [22 (15-28) vs 14 (4-21); P < 0.001], TB level [262 (157-349) vs 166 (40-278) µmol/L; P < 0.001], and INR [1.52 (1.23-2.04) vs 1.29 (1.09-1.63); P < 0.001] in the myocardial injury group were significantly higher than those in the nonmyocardial injury group. The comparison of the preoperative data between the two groups showed that there was no significant difference in male sex, LVEF, QTc, preoperative liver function indexes (ALT, AST), Cr, and hemoglobin (Table 1).
| Variable | Non-myocardial injury (n = 160) | Myocardial injury (n = 142) | P value |
| Age (mo) | 9.5 (6.0-16.8) | 7.0 (6.0-11.0) | < 0.001 |
| Male gender (%) | 78 (48.8) | 72 (50.7) | 0.735 |
| Height (cm) | 70 (65-82) | 65 (62-70) | < 0.001 |
| Weight (kg) | 8.0 (6.5-11.0) | 7.0 (6.0-8.0) | < 0.001 |
| LVEF (%) | 64 (62-67) | 65 (62-67) | 0.167 |
| QTc(ms) | 404 (387-427) | 405 (384-424) | 0.596 |
| PELD score | 14 (4-21) | 22 (15-28) | < 0.001 |
| ALT (U/L) | 106 (61-168) | 103 (70-172) | 0.494 |
| AST (U/L) | 177 (103-319) | 196 (129-295) | 0.186 |
| TB (µmol/L) | 166 (40-278) | 262 (157-349) | < 0.001 |
| INR | 1.29 (1.09-1.63) | 1.52 (1.23-2.04) | < 0.001 |
| Cr (µmol/L) | 14.0 (12.0-16.0) | 13.5 (10.0-16.0) | 0.528 |
| Hemoglobin (g/L) | 93 ± 17 | 93 ± 16 | 0.671 |
The data in Table 2 show that the MAP before reperfusion [56 (51-62) vs 60 (54-67) mmHg; P = 0.001], lactate [2.7 (2.2-3.8) vs 2.5 (1.8-3.2) mmol/L; P = 0.002], hemoglobin before reperfusion [81 (72-91) vs 84 (75-93) g/L; P =0.038], duration of the anhepatic phase [62 (50-76) vs 56 (47-67) min; P = 0.019], cold ischemic time [90 (71-111) vs 80 (64-106) min; P = 0.023], incidence of PRS (53.5% vs 33.8%; P = 0.001), and FFP transfusion volume [0 (0-200) vs 0 (0-100) mL; P = 0.009] were significantly different between the two groups. There was no statistically significant difference in other intraoperative indexes (Table 2).
| Variable | Non-myocardial injury (n = 160) | Myocardial injury (n = 142) | P value |
| Vital signs immediately before reperfusion | |||
| HR (bpm/min) | 118 ± 13 | 117±13 | 0.515 |
| MAP (mmHg) | 60 (54-67) | 56 (51-62) | 0.001 |
| CVP (mmHg) | 5 (4-7) | 5 (3-7) | 0.454 |
| Temperature (°C) | 36.5 (35.9-37.0) | 36.5 (35.8-37.0) | 0.652 |
| Laboratory findings before reperfusion | |||
| pH | 7.39 ± 0.07 | 7.39 ± 0.07 | 0.952 |
| PCO2 (mmHg) | 34 (30-39) | 33 (30-38) | 0.148 |
| PO2 (mmHg) | 257 (176-309) | 242 (170-312) | 0.472 |
| Lactate (mmol/L) | 2.5 (1.8-3.2) | 2.7 (2.2-3.8) | 0.002 |
| Base excess (mmol/L) | -4.2 (-6.5--1.7) | -4.8 (-6.8--3.1) | 0.102 |
| Ca (mmol/L) | 1.08 (1.01-1.17) | 1.09 (1.00-1.15) | 0.648 |
| K (mmol/L) | 3.8±0.6 | 3.8±0.5 | 0.448 |
| Hemoglobin (g/L) | 84 (75-93) | 81 (72-91) | 0.038 |
| Duration of anhepatic phase (min) | 56 (47-67) | 62 (50-76) | 0.019 |
| Donor-related data | |||
| Cold ischemic time (min) | 80 (64-106) | 90 (71-111) | 0.023 |
| Graft weight (g) | 244 (217-277) | 243 (208-278) | 0.654 |
| PRS (%) | 54 (33.8) | 76 (53.5) | 0.001 |
| The data at the end of the surgery | |||
| Duration of surgery (min) | 542 (495-600) | 540 (484-593) | 0.814 |
| Duration of anesthesia (min) | 616 (555-651) | 610 (559-650) | 0.984 |
| Blood loss (mL) | 300 (200-400) | 350 (200-400) | 0.378 |
| Urine output (mL) | 400 (300-600) | 400 (248-600) | 0.909 |
| RBCs transfusion (units) | 2.0 (1.5-3.0) | 2.0 (2.0-3.0) | 0.078 |
| FFP transfusion (mL) | 0 (0-100) | 0 (0-200) | 0.009 |
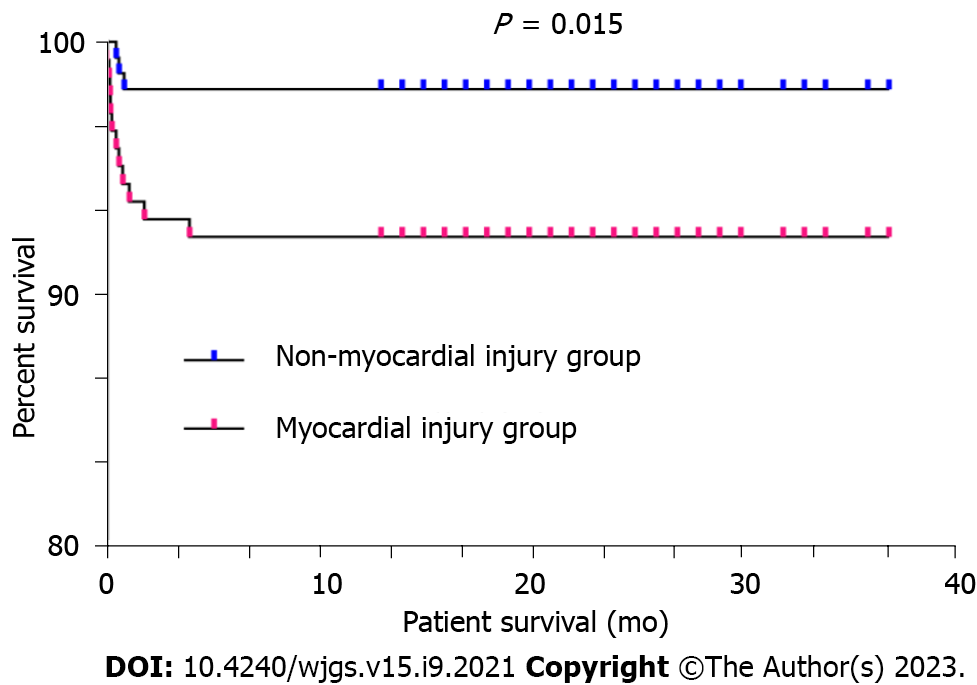
The postoperative data revealed no statistically significant differences between the two groups in terms of the duration of mechanical ventilation, number of hospitalization days, incidence of AKI, and peak ALT and AST values in the first five days following LDLT. However, the number of postoperative ICU stay days [3 (2.0-3.5) vs 2 (2.0-3.0) d, P = 0.041] and peak values of total bilirubin in the first 5 d after LDLT [88 (65-126) vs 80 (52-114) µmol/L, P = 0.034] in the myocardial injury group were significantly higher than those in the nonmyocardial injury group (Table 3). The pediatric patients with biliary atresia in the nonmyocardial injury group who underwent LDLT had a considerably higher one-year survival rate than those in the myocardial injury group (98.1% vs 92.3%, P = 0.015) (Figure 2).
| Variable | Non-myocardial injury (n = 160) | Myocardial injury (n =142) | P value |
| Ventilation time (min) | 220 (129-351) | 196 (146-321) | 0.785 |
| ICU stay (d) | 2 (2.0-3.0) | 3 (2.0-3.5) | 0.041 |
| hospital stay (d) | 20 (16-29) | 21 (17-26) | 0.732 |
| AKI (%) | 35 (21.9) | 35 (24.6) | 0.569 |
| Liver function test during the first five days after LDLT | |||
| Peak ALT (U/L) | 551 (386-972) | 619 (416-1151) | 0.108 |
| Peak AST (U/L) | 666 (472-1186) | 748 (495-1386) | 0.103 |
| Peak TB (µmol/L) | 80 (52-114) | 88 (65-126) | 0.034 |
| One-year survival rate (%) | 157 (98.1) | 131 (92.3) | 0.015 |
The results of the univariate analysis showed that low height, low weight, a high PELD score, a high total bilirubin level, a high INR, a low MAP before reperfusion, high lactate and low hemoglobin levels before reperfusion, a long anhepatic phase duration, a long cold ischemic time, the occurrence of PRS, and massive FFP transfusion were identified as risk factors for myocardial injury in pediatric patients with biliary atresia (Table 4). The multivariate logistic regression analysis showed that a high PELD score (OR = 1.065, 95%CI: 1.013-1.121; P = 0.014), a long anhepatic phase duration (OR = 1.021, 95%CI: 1.003-1.040; P = 0.025), and the occurrence of PRS (OR = 1.966, 95%CI: 1.111-3.480; P = 0.020) were independent risk factors for myocardial injury (Table 4).
| Factor | Univariate analysis OR (95%CI) | P value | Multivariate analysis OR (95%CI) | P value |
| Preoperative recipient-related data | ||||
| Age | 0.989 (0.974-1.003) | 0.125 | ||
| Male gender | 1.081 (0.688-1.699) | 0.735 | ||
| Height | 0.969 (0.951-0.988) | 0.002 | 1.011 (0.964-1.060) | 0.659 |
| Weight | 0.889 (0.827-0.955) | 0.001 | 0.953 (0.800-1.136) | 0.593 |
| LVEF | 1.044 (0.980-1.112) | 0.178 | ||
| QTc | 0.999 (0.990-1.008) | 0.774 | ||
| PELD | 1.074 (1.049-1.100) | < 0.001 | 1.065 (1.013-1.121) | 0.014 |
| ALT | 1.001 (0.999-1.002) | 0.540 | ||
| AST | 1.001 (1.000-1.002) | 0.218 | ||
| Total Bilirubin | 1.004 (1.002-1.006) | < 0.001 | 1.001 (0.999-1.003) | 0.353 |
| INR | 2.167 (1.435-3.272) | < 0.001 | 0.957 (0.554-1.653) | 0.875 |
| Creatinine | 0.99 (0.951-1.031) | 0.632 | ||
| Hemoglobin | 1.031 (0.897-1.184) | 0.670 | ||
| Vital signs immediately before reperfusion | ||||
| HR | 0.994 (0.977-1.012) | 0.513 | ||
| MAP | 0.971 (0.949-0.993) | 0.010 | 0.997 (0.971-1.023) | 0.819 |
| CVP | 0.974 (0.896-1.057) | 0.524 | ||
| Temperature | 1.088 (0.819-1.444) | 0.560 | ||
| Laboratory findings before reperfusion | ||||
| pH | 0.902 (0.032-25.155) | 0.952 | ||
| PCO2 | 0.971 (0.935-1.009) | 0.129 | ||
| PO2 | 0.999 (0.996-1.002) | 0.478 | ||
| Lactate | 1.267 (1.092-1.469) | 0.002 | 1.158 (0.990-1.355) | 0.067 |
| Base excess | 0.946 (0.881-1.015) | 0.122 | ||
| Ca | 0.374 (0.066-2.117) | 0.266 | ||
| K | 0.857 (0.575-1.277) | 0.447 | ||
| Hemoglobin | 0.836 (0.713-0.981) | 0.029 | 0.956 (0.828-1.105) | 0.544 |
| Duration of anhepatic phase | 1.019 (1.004-1.035) | 0.016 | 1.021 (1.003-1.040) | 0.025 |
| Donor-related data | ||||
| Cold ischemic time | 1.006 (1.001-1.012) | 0.016 | 1.003 (0.997-1.010) | 0.334 |
| Graft weight | 0.999 (0.996-1.003) | 0.755 | ||
| PRS | 2.260 (1.420-3.598) | 0.001 | 1.966 (1.111-3.480) | 0.020 |
| The data at the end of the surgery | ||||
| Duration of surgery | 1 (0.998-1.002) | 0.854 | ||
| Duration of anesthesia | 1.001 (0.999-1.003) | 0.481 | ||
| Blood loss | 1.001 (1.000-1.002) | 0.128 | ||
| RBCs transfusion | 1.151 (0.970-1.366) | 0.107 | ||
| FFP transfusion | 1.002 (1.000-1.004) | 0.017 | 1.001 (0.999-1.003) | 0.441 |
Under normal circumstances, cTnI cannot pass through an intact cell membrane into the blood circulation, but after myocardial cell damage, cTnI will be released into the blood. CTnI is a commonly used marker that, when elevated, is indicative of myocardial injury[8]. There is reliable evidence showing that the measurement of troponin levels is not only the gold standard for the diagnosis of cardiac injury[9] but also an effective screening and risk stratification tool[10,11]. van Waes et al[4] conducted an observational cohort study and defined myocardial injury as a troponin I level > 0.06 ng/mL, which was the lowest value measurable with a 10% coefficient of variation above the 99th percentile (0.04 ng/mL) of the assay used. However, there are very few studies in which researchers provide reference cutoff values for elevated cTnI levels in pediatric patients. Bailey et al[12] found that TnI was exceptionally high at birth, abruptly decreased shortly after birth, and reached adult concentrations 3 mo after birth, and the manufacturer assay's 99th percentile URL was reasonable for children older than 3 mo. Sheng’s study also showed that a cTnI level ≥ 0.07 ng/mL could be used as a specific marker of myocardial injury in pediatric living donor liver recipients[3]. Therefore, in our study, we defined myocardial injury as a cTnI level ≥ 0.07 ng/mL.
The PELD scoring system is used to evaluate the severity and prognosis of end-stage liver disease in children. Oh et al[13] found that a PELD score > 25 was an independent risk factor for graft function loss after LDLT in children. Sheng et al[14] conducted a retrospective analysis of LDLT in 112 children with biliary atresia and found that there was a positive correlation between the preoperative PELD score and the increase in intraoperative cTnI; that was, the higher the PELD, the higher the serum cTnI level at 30 min in the neohepatic stage. It has been suggested that the main cause of a myocardial injury associated with liver transplantation is the inflammatory response[15]. As the largest group of macrophages in the body, Kupffer cells can release many inflammatory factors during ischemic cold preservation and reperfusion injury, which becomes the most noteworthy factor that contributes to the occurrence of a myocardial injury[16,17]. The higher the preoperative PELD score of children with biliary atresia, the more sensitive the liver is to ischemia-reperfusion, the more likely Kupffer cells are activated and release inflammatory factors, and the more likely a cardiomyocyte injury will occur during the perioperative period.
PRS is characterized by a decrease of more than 30% in the mean arterial pressure (MAP) that occurs in the first 5 min after liver graft reperfusion and lasts at least 1 min[18]. PRS is one of the most common perioperative complications of liver transplantation and has a reported incidence between 34.7% and 50%[19-22], which can lead to slower heart rate, lower blood pressure, arrhythmia, increased mortality, and a serious effect on the patients’ quality of life after the operation[18,23]. At present, the mechanism of PRS has not been fully clarified. Prolonged cold ischemia time of the graft can lead to mitochondrial dysfunction, cell metabolic disorder, the release of reactive oxygen species and inflammatory factors, and aggravate hepatic ischemia-reperfusion injury[24]. Sahmeddini et al[25] showed that recipient age > 60 years, high end-stage liver disease model score and preoperative serum sodium < 130 mmol/L were independent risk factors for PRS in orthotopic liver transplantation. The literature showed that in LDLT, male sex, reduced left ventricular end-diastolic diameter and increased graft volume were risk factors for PRS, while increased serum calcium concentration and decreased pulmonary artery pressure before reperfusion were protective factors for PRS[26].
The anhepatic phase was defined as the time from the physical removal of the liver from the recipient to the recirculation of the graft[27]. The inferior vena cava needs to be blocked before anastomosing the left hepatic vein of the graft with the recipient hepatic vein. If the inferior vena cava is blocked for a long time, it will lead to a decrease in cardiac blood volume, a decrease in stroke volume, and a compensatory increase in heart rate. The above factors can lead to a decrease in the myocardial oxygen supply, an increase in oxygen consumption, an imbalance of cardiac oxygen supply and demand, and finally lead to myocardial injury. During the anhepatic period, the blood vessels of the donor’s liver had not yet been anastomosed because of the resection of the diseased liver. At this time, the body is unable to metabolize acid substances, thereby making it extremely prone to metabolic acidosis and causing decreased blood pressure and unstable circulation. Because the coagulation factor of the human body depends on liver synthesis, a long anhepatic period can lead to a decrease in the coagulation factor level. A recent scientific study found that coagulation factor XI was a liver protein that could prevent diastolic dysfunction, maintain the ejection fraction, and protect the heart from injury[28]. Thus, it can be seen that the decrease in the coagulation factor levels not only affects the blood coagulation function but can also diminish heart function.
This study’s sample size is very large, and thus far, there has been no retrospective study with a large sample size that has been conducted to evaluate the risk factors for intraoperative myocardial injury in children who are subjected to LDLT, which may be the advantage of this study. However, the limitation of this study is that the follow-up period was short, and the effect of myocardial injury on the long-term prognosis and quality of life of children who underwent liver transplantation has not been observed. In addition, different centers have different anesthetic management strategies, and the results of a single-center study may be biased, thus requiring multicenter large sample data for analysis in the future.
In summary, our study suggests that a high PELD score, a long anhepatic phase duration, and the occurrence of intraoperative PRS are independent risk factors for the development of a myocardial injury. For anesthesiologists, the findings may help predict the likelihood of a myocardial injury in pediatric patients who undergo LDLT. Effective anesthetic management strategies that have been designed to prevent the occurrence of a myocardial injury during LDLT will help to improve the prognosis and postoperative survival rate of the patients.
Myocardial injury during liver transplantation is associated with postoperative adverse outcomes in pediatric patients; it can increase the incidence of postoperative mortality.
For a long time, research on myocardial injury has mainly focused on adults, but there is relatively little information on myocardial injury in children who have undergone living donor liver transplantation (LDLT).
To analyze the data of children who underwent LDLT to determine the risk factors for intraoperative myocardial injury.
We retrospectively analyzed the inpatient records of pediatric patients who underwent LDLT in Tianjin First Central Hospital from January 1, 2020, to January 31, 2022. Recipient-related data and donor-related data were collected. The patients were divided into a myocardial injury group and a nonmyocardial injury group according to the value of the serum cardiac troponin I at the end of surgery for analysis. Univariate analysis and multivariate logistic regression were used to evaluate the risk factors for myocardial injury during LDLT in pediatric patients.
A total of 302 patients met the inclusion criteria. The myocardial injury group had 142 individuals (47%), and the nonmyocardial injury group included 160 patients (53%). The pediatric patients with biliary atresia in the nonmyocardial injury group who underwent LDLT had a considerably higher one-year survival rate than those in the myocardial injury group (98.1% vs 92.3%, P = 0.015). Multivariate logistic regression revealed the following independent risk factors for myocardial injury: a high pediatric end-stage liver disease (PELD) score [odds ratio (OR) = 1.065, 95% confidence interval (CI): 1.013-1.121; P = 0.014], a long duration of the anhepatic phase (OR = 1.021, 95%CI: 1.003-1.040; P = 0.025), and the occurrence of intraoperative postreperfusion syndrome (PRS) (OR = 1.966, 95%CI: 1.111-3.480; P = 0.020).
A high PELD score, a long anhepatic phase duration, and the occurrence of intraoperative PRS were independent risk factors for myocardial injury during LDLT in pediatric patients with biliary atresia.
This study’s sample size is very large, and thus far, there has been no retrospective study with a large sample size that has been conducted to evaluate the risk factors for intraoperative myocardial injury in children who are subjected to LDLT, which may be the advantage of this study. But different centers have different anesthetic management strategies, and the results of a single-center study may be biased, thus requiring multicenter large sample data for analysis in the future.
The authors would like to thank Professor Weng YQ for his contribution to this writing support and selfless help. In particular, the authors would like to thank the editors and reviewers for the recognition of our research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anesthesiology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tchilikidi KY, Russia; Tsoulfas G, Greece S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Daugaard TR, Pommergaard HC, Rostved AA, Rasmussen A. Postoperative complications as a predictor for survival after liver transplantation - proposition of a prognostic score. HPB (Oxford). 2018;20:815-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Huang S, Apinyachon W, Agopian VG, Wray CL, Busuttil RW, Steadman RH, Xia VW. Myocardial injury in patients with hemodynamic derangements during and/or after liver transplantation. Clin Transplant. 2016;30:1552-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Sheng M, Lin Y, Weng Y, Xu R, Sun Y, Yu W, Du H. Predictive Value of Intraoperative Troponin I Elevation in Pediatric Living Donor Liver Transplant Recipients With Biliary Atresia. Transplantation. 2017;101:2385-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | van Waes JA, Nathoe HM, de Graaff JC, Kemperman H, de Borst GJ, Peelen LM, van Klei WA; Cardiac Health After Surgery (CHASE) Investigators. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. 2013;127:2264-2271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 236] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 5. | Sun K, Wang Y, Yan M, Xia VW. Pretransplant left ventricular hypertrophy in association with postoperative myocardial injury in liver transplantation. Clin Transplant. 2020;34:e13847. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Vilchez-Monge AL, Garutti I, Jimeno C, Zaballos M, Jimenez C, Olmedilla L, Piñeiro P, Duque P, Salcedo M, Asencio JM, Lopez-Baena JA, Maruszewski P, Bañares R, Perez-Peña JM. Intraoperative Troponin Elevation in Liver Transplantation Is Independently Associated With Mortality: A Prospective Observational Study. Liver Transpl. 2020;26:681-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 925] [Cited by in RCA: 1222] [Article Influence: 111.1] [Reference Citation Analysis (0)] |
| 8. | Chaulin A. Cardiac Troponins: Contemporary Biological Data and New Methods of Determination. Vasc Health Risk Manag. 2021;17:299-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 9. | Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; ESC Scientific Document Group. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40:237-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1021] [Cited by in RCA: 1047] [Article Influence: 149.6] [Reference Citation Analysis (1)] |
| 10. | de Lemos JA, deFilippi CR. Prevalence and significance of detectable troponins as measured by highly sensitive assays in the general population. Coron Artery Dis. 2013;24:705-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Brown JL, Hirsh DA, Mahle WT. Use of troponin as a screen for chest pain in the pediatric emergency department. Pediatr Cardiol. 2012;33:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Bailey D, Colantonio D, Kyriakopoulou L, Cohen AH, Chan MK, Armbruster D, Adeli K. Marked biological variance in endocrine and biochemical markers in childhood: establishment of pediatric reference intervals using healthy community children from the CALIPER cohort. Clin Chem. 2013;59:1393-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 13. | Oh SH, Kim KM, Kim DY, Lee YJ, Rhee KW, Jang JY, Chang SH, Lee SY, Kim JS, Choi BH, Park SJ, Yoon CH, Ko GY, Sung KB, Hwang GS, Choi KT, Yu E, Song GW, Ha TY, Moon DB, Ahn CS, Kim KH, Hwang S, Park KM, Lee SG. Long-term outcomes of pediatric living donor liver transplantation at a single institution. Pediatr Transplant. 2010;14:870-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Sheng MW, Du HY, Weng YQ, Xu RB, Sun Y, Yu WL. Prognostic value of serum cardiac troponin I in acute lung injury after living-donor liver transplantation for children with biliary atresia. Zhonghua Qigaung Yizhi Zazhi. 2016;37:581-585. [DOI] [Full Text] |
| 15. | Chen JB, Chen GY, Wei SD, Sun JJ, Liu GB, Xie ZT, Tang GF. Inflammatory factor in donor liver and its effect on recipient myocardial injury after liver transplantation. Eur Rev Med Pharmacol Sci. 2017;21:4362-4368. [PubMed] |
| 16. | Tsuchihashi S, Ke B, Kaldas F, Flynn E, Busuttil RW, Briscoe DM, Kupiec-Weglinski JW. Vascular endothelial growth factor antagonist modulates leukocyte trafficking and protects mouse livers against ischemia/reperfusion injury. Am J Pathol. 2006;168:695-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Buras JA, Reenstra WR. Endothelial-neutrophil interactions during ischemia and reperfusion injury: basic mechanisms of hyperbaric oxygen. Neurol Res. 2007;29:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Jeong SM. Postreperfusion syndrome during liver transplantation. Korean J Anesthesiol. 2015;68:527-539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Chung IS, Kim HY, Shin YH, Ko JS, Gwak MS, Sim WS, Kim GS, Lee SK. Incidence and predictors of post-reperfusion syndrome in living donor liver transplantation. Clin Transplant. 2012;26:539-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (2)] |
| 20. | Zhang L, Tian M, Xue F, Zhu Z. Diagnosis, Incidence, Predictors and Management of Postreperfusion Syndrome in Pediatric Deceased Donor Liver Transplantation: A Single-Center Study. Ann Transplant. 2018;23:334-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Hilmi I, Horton CN, Planinsic RM, Sakai T, Nicolau-Raducu R, Damian D, Gligor S, Marcos A. The impact of postreperfusion syndrome on short-term patient and liver allograft outcome in patients undergoing orthotopic liver transplantation. Liver Transpl. 2008;14:504-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 185] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 22. | Li T, Wu Y, Gong X, Che L, Sheng M, Jia L, Li H, Yu W, Weng Y. Risk factors for postreperfusion syndrome during living donor liver transplantation in paediatric patients with biliary atresia: a retrospective analysis. BMJ Paediatr Open. 2023;7. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Khosravi MB, Sattari H, Ghaffaripour S, Lahssaee M, Salahi H, Sahmeddini MA, Bahador A, Nikeghbalian S, Parsa S, Shokrizadeh S, Malek-Hosseini SA. Post-reperfusion Syndrome and Outcome Variables after Orthotopic Liver Transplantation. Int J Organ Transplant Med. 2010;1:115-120. [PubMed] |
| 24. | Ye L, He S, Mao X, Zhang Y, Cai Y, Li S. Effect of Hepatic Macrophage Polarization and Apoptosis on Liver Ischemia and Reperfusion Injury During Liver Transplantation. Front Immunol. 2020;11:1193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 25. | Sahmeddini MA, Tehran SG, Khosravi MB, Eghbal MH, Asmarian N, Khalili F, Vatankhah P, Izadi S. Risk factors of the post-reperfusion syndrome during orthotopic liver transplantation: a clinical observational study. BMC Anesthesiol. 2022;22:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Umehara K, Karashima Y, Yoshizumi T, Yamaura K. Factors Associated With Postreperfusion Syndrome in Living Donor Liver Transplantation: A Retrospective Study. Anesth Analg. 2022;135:354-361. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Ijtsma AJ, van der Hilst CS, de Boer MT, de Jong KP, Peeters PM, Porte RJ, Slooff MJ. The clinical relevance of the anhepatic phase during liver transplantation. Liver Transpl. 2009;15:1050-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Cao Y, Wang Y, Zhou Z, Pan C, Jiang L, Meng Y, Charugundla S, Li T, Allayee H, Seldin MM, Lusis AJ. Liver-heart cross-talk mediated by coagulation factor XI protects against heart failure. Science. 2022;377:1399-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |