Published online Jul 27, 2023. doi: 10.4240/wjgs.v15.i7.1542
Peer-review started: March 28, 2023
First decision: April 14, 2023
Revised: April 28, 2023
Accepted: May 11, 2023
Article in press: May 11, 2023
Published online: July 27, 2023
Processing time: 119 Days and 7.2 Hours
Intraductal papillary neoplasm of the bile duct (IPNB) and intraductal papillary mucinous neoplasm (IPMN) of the pancreas have similar pathological manifestations. However, they often develop separately and it is rare for both to occur together. Patients presenting with heterochronic IPMN after IPNB are prone to be misdiagnosed with tumor recurrence.
A 67-year-old male patient was admitted 8.5 years after IPNB carcinoma and 4 years after the discovery of a pancreatic tumor. A left hepatic bile duct tumor with distal bile duct dilatation was found 8.5 years ago by the computed tomography; therefore, a left hepatectomy was performed. The postoperative pathological diagnosis was malignant IPNB with negative cutting edge and pathological stage T1N0M0. Magnetic resonance imaging 4 years ago showed cystic lesions in the pancreatic head with pancreatic duct dilatation, and carcinoembryonic antigen continued to increase. Positron emission tomography showed a maximum standard uptake value of 11.8 in the soft tissue mass in the pancreatic head, and a malignant tumor was considered. Radical pancreatoduodenectomy was performed. Postoperative pathological diagnosis was pancreatic head IPMN with negative cutting edge, pancreaticobiliary type, stage T3N0M0. He was discharged 15 d after the operation. Follow-up for 6 mo showed no tumor recurrence, and quality of life was good.
IPNB and IPMN are precancerous lesions with similar pathological characteristics and require active surgery and long-term follow-up.
Core Tip: We report a rare case of heterochronous onset of malignant intraductal papillary neoplasm of the bile duct (IPNB) and malignant intraductal papillary mucinous neoplasm of the pancreas (IPMN). The time difference between the onset of the two diseases was 4.5 years. Left hepatectomy and radical pancreaticoduodenectomy were performed with excellent results. This case suggests that IPNB and IPMN are precancerous lesions of low-grade malignancy that require aggressive surgery and long-term postoperative follow-up.
- Citation: Xiao G, Xia T, Mou YP, Zhou YC. Reoperation for heterochronic intraductal papillary mucinous neoplasm of the pancreas after bile duct neoplasm resection: A case report. World J Gastrointest Surg 2023; 15(7): 1542-1548
- URL: https://www.wjgnet.com/1948-9366/full/v15/i7/1542.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i7.1542
Intraductal papillary mucinous neoplasm of the pancreas (IPMN) is a mucus-producing tumor involving the main or branch pancreatic ducts that lacks the characteristic ovarian-like interstitium of mucinous cystic neoplasm. IPMN is characterized by intraductal papillary growth with excessive mucus secretion, resulting in cystic dilatation of the pancreas’ primary and/or branch ducts[1]. Intraductal papillary neoplasm of the bile duct (IPNB) is a rare disease entity with a previously reported prevalence of 4% to 15% among bile duct tumors[2]. It was classified as a distinct pathological type by the World Health Organization in 2010[3]. IPNB is a slow-growing biliary tumor with intraductal papillae but can eventually progress to bile duct cancer. IPNB has a similar pathological presentation to IPMN, with excessive mucin secretion but with different histological subtypes and growth patterns. Cases of concurrent IPMN and IPNB are rare[4], with only 11 reported in the literature to our knowledge[5-15] (Table 1), and the heterochronic onset of IPNB with IPMN is even rarer.
| Ref. | Sex | Age | IPNB | IPMN | Heterochronic occurrence | Pancreaticobiliary maljunction | ||
| Location | Pathology | Location | Pathology | |||||
| Joo et al[5], 2000 | Male | 60 | Left IHD | LGD | Branch duct | LGD | No | No |
| Ishida et al[6], 2002 | Male | 67 | B1 | Without dysplasia | Branch duct | Without dysplasia | No | No |
| Yamaguchi et al[7], 2005 | Male | 69 | Left IHD | IC | Branch duct | MIC | No | No |
| Zalinski et al[8], 2007 | Female | 65 | Bilateral IHD | IC | Main duct | HGD | No | No |
| Park et al[9], 2010 | Male | 67 | Left IHD | LGD | Mixed duct | LGD | No | No |
| Valente et al[10], 2012 | Male | 76 | Right IHD | IC | Branch duct | LGD | No | No |
| Xu et al[11], 2012 | Female | 68 | Left IHD | LGD | Branch duct | Without dysplasia | No | No |
| Moon et al[12], 2014 | Female | 66 | Left IHD | HGD | Main duct | HGD | No | No |
| Bansal et al[13], 2016 | Male | 70 | Right IHD | IC | Main duct | IC | No | No |
| Kitahama et al[14], 2021 | Male | 52 | CBD | MIC | Main duct | LGD | No | No |
| Aslam et al[15], 2020 | Male | 73 | Left IHD | LGD | Main duct | HGD | No | No |
| Our case | Male | 67 | Left IHD | IC | Main duct | IC | Yes | Yes |
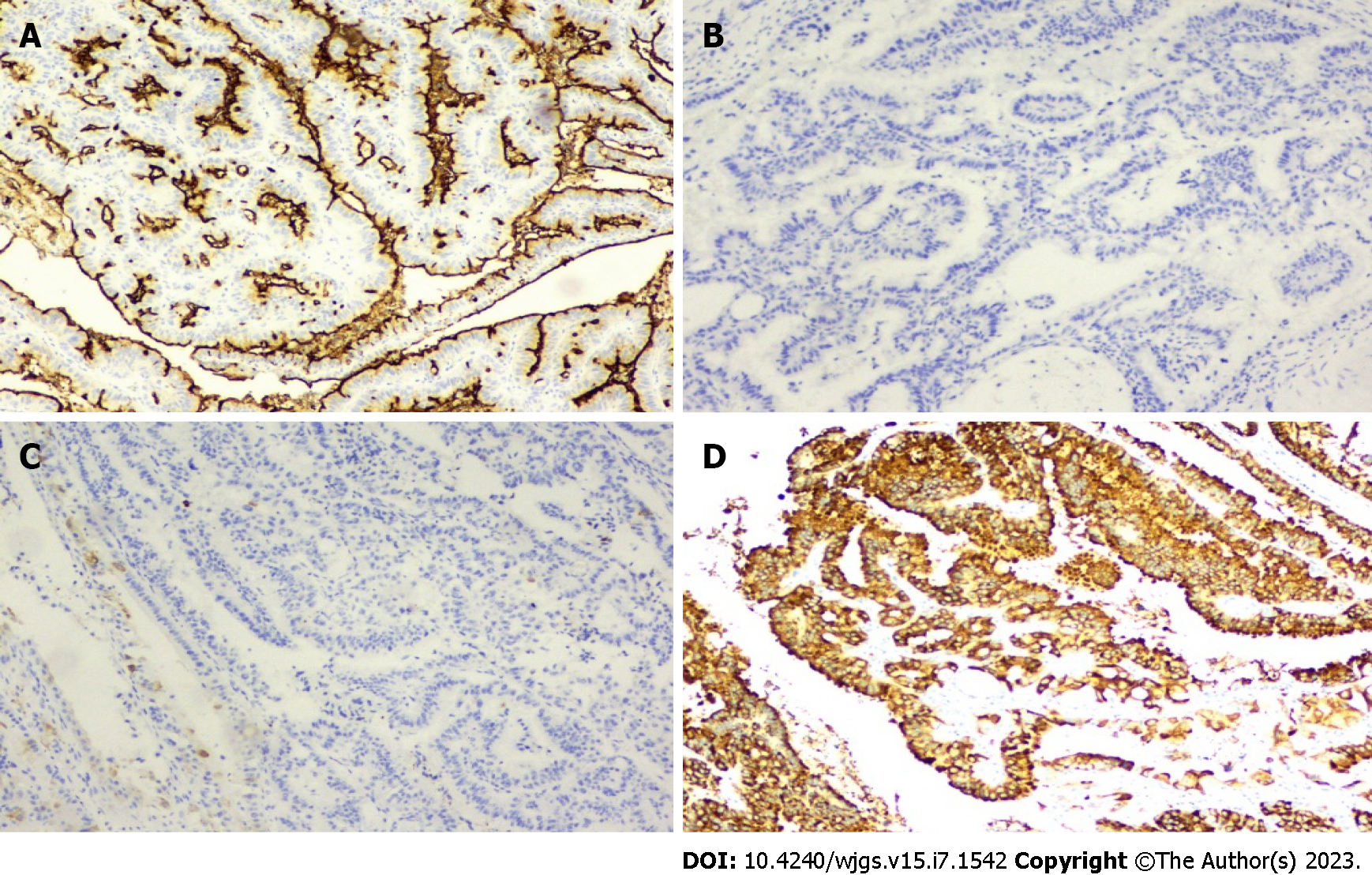
We report a case of heterochronic onset of IPNB with IPMN at an interval of 4.5 years, and this is the first case reported in the literature. We performed aggressive reoperation on this patient, which had been diagnosed as recurrent tumor metastasis several times in different hospitals, and confirmed as heterochronous IPMN (Figure 1).
A 67-year-old Chinese man presented to our Department of Gastrointestinal and Pancreatic Surgery 8.5 years after diagnosis of IPNB and 4 years after diagnosis of pancreatic tumor.
The patient had a 10-year history of primary hypertension and a 15-year history of asthma.
The patient had no past illness.
The patient had no family history.
The patient’s vital signs on physical examination were stable, with a body temperature of 37.0 °C on admission. No jaundice or superficial lymphadenopathy was observed. No apparent abnormalities were observed upon pulmonary and cardiac examination. An old surgical scar about 30 cm in length was visible under the right costal margin, and a nodule about 1 cm in size was palpable, with no abdominal pressure or rebound pain.
Serum tumor markers showed a significant increase in carcinoembryonic antigen of 20.7 mg/L, but carbohydrate antigen 19-9 was normal. Blood biochemistry showed that transaminases, total bilirubin, serum amylase, and blood glucose were within normal ranges.
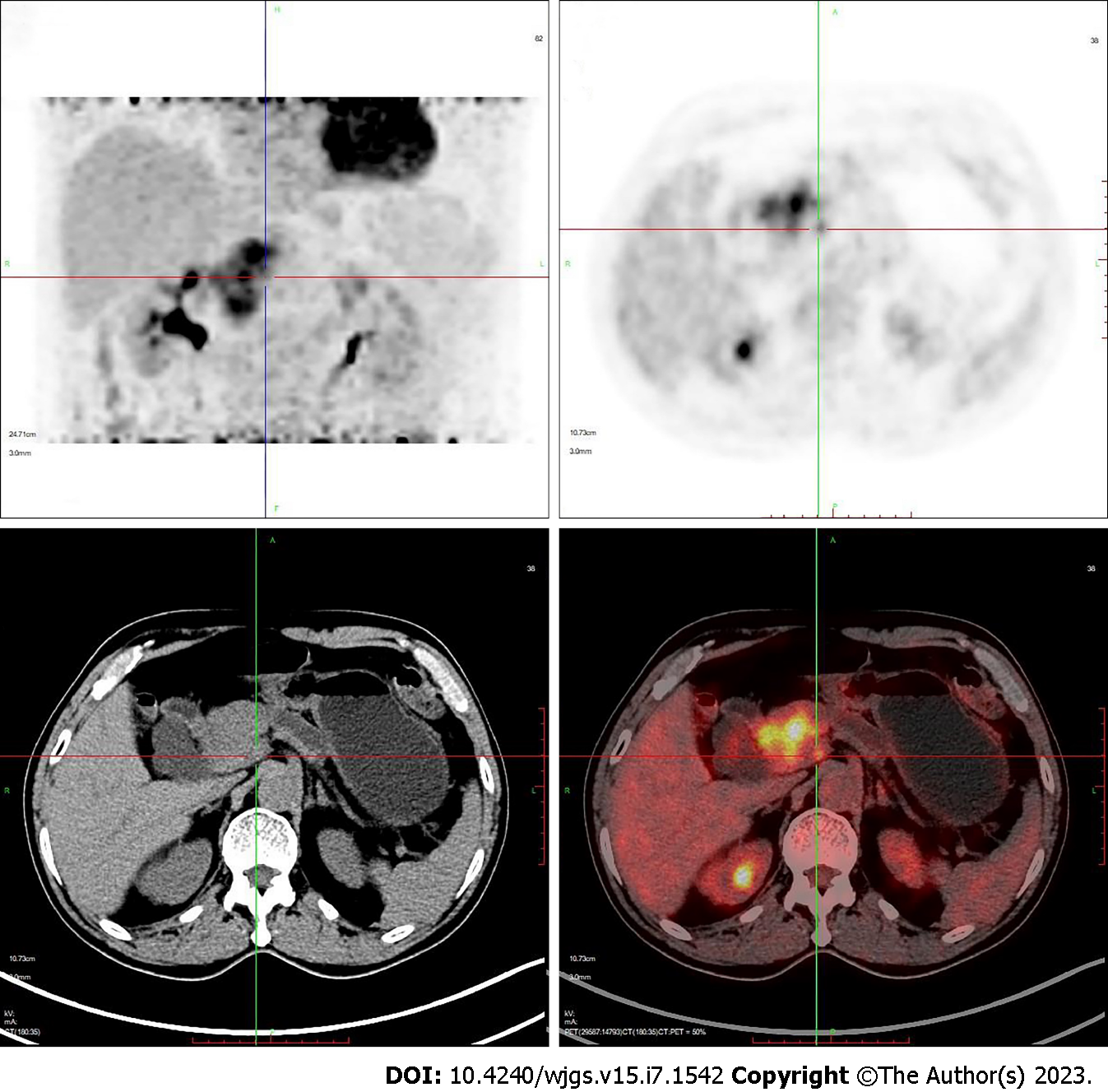
Computed tomography (CT) showed a mass in the pancreatic head with atrophy of the pancreatic tail and dilated pancreatic ducts. The intra- and extrahepatic bile ducts and common bile ducts were significantly dilated. A nodule in the anterior abdominal wall was considered to be a metastatic tumor. Positron emission tomography-CT showed a soft tissue mass in the pancreatic head (5.1 cm × 4.6 cm × 5.6 cm) with a maximum standard uptake value (SUVmax) of 11.8 and dilated pancreatic ducts. The anterior abdominal wall mass measured 1.0 cm × 0.9 cm × 0.8 cm, with SUVmax 2.6 (Figure 2).
Heterochronous malignant IPNB and malignant IPMN associated with abnormal pancreaticobiliary collaterals.
Radical pancreaticoduodenectomy was performed.
The patient was successfully treated with surgery and had a good prognosis. During 6 mo postoperative follow-up, the patient gained weight without clinical symptoms or pancreaticobiliary duct dilatation.
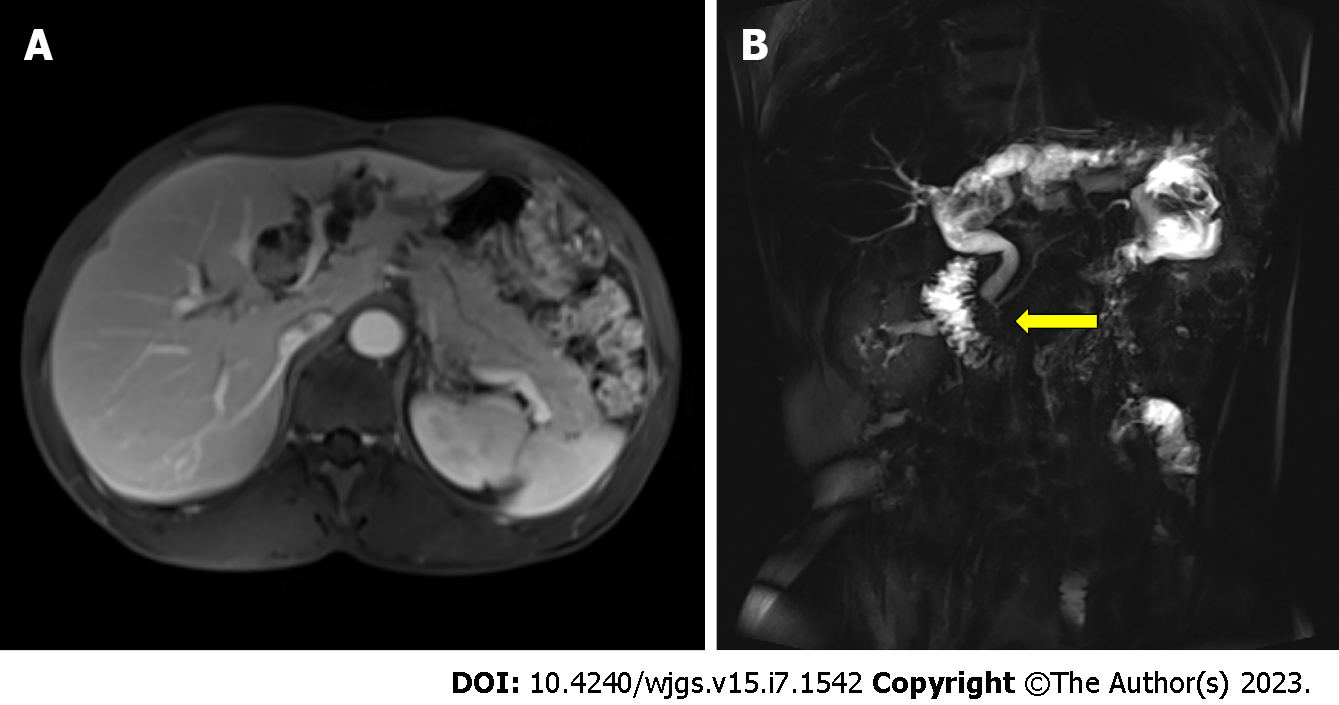
IPNB and IPMN both oversecrete mucin that eventually clogs the pancreaticobiliary duct and causes progressive dilation of the upstream ducts. Ultrasound, CT, and magnetic resonance cholangiopancreatography (MRCP) can easily detect these abnormalities, but it is not easy to distinguish between masses, wall nodules, and mucin in the ducts. Many cases are often diagnosed and treated as stones, which can easily be misdiagnosed and even affect the patient’s prognosis because of delayed diagnosis leading to tumor progression. MRCP, in this case, clearly showed a filling defect at the beginning of the left hepatic duct and dilatation of the upstream bile duct (Figure 3), and an experienced radiologist made a definitive diagnosis[15]. IPNB and IPMN tend to grow longitudinally along the lumen, so pancreatic cholangiopancreatoscopy is a recommended method that can clearly visualize a large amount of mucin being expelled from the duodenal papilla. If available, we recommend the use of SpyGlass, which provides a clearer view of the interior of the bile or pancreatic ducts[4].
In this case, the patient developed IPNB first and then IPMN after an interval of 4.5 years with similar pathological types, which raised the possibility of a common tumor origin. MRCP and gross specimen analysis suggested abnormal pancreaticobiliary coarctation (Figures 3B and 4). Pancreaticobiliary fistulae leading to simultaneous malignant IPNB and benign IPMN have been reported[11]. Therefore, we believe that IPNB developed first and led to the development of IPMN after the tumor cells were shed and implanted into the pancreatic duct during disease progression. The bile duct is anatomically higher than the pancreatic duct, and tumor cells originating from the bile duct may be shed and excreted with bile to the abnormal pancreaticobiliary commissure, leading to implantation and metastasis. However, heterochronic IPNB and IPMN are very rare. IPMN in the present case occurred in the pancreatic neck, where the pancreatic duct forms a slight distortion, and tumor cells shed into the pancreatic duct tend to be deposited there, eventually leading to IPMN. Therefore, we consider IPNB and IPMN to be a single disease caused by diffuse involvement of the pancreaticobiliary tree and recommend that the bile and pancreatic ducts should be examined in patients with papillary tumors, regardless of whether the tumor is initially found in the pancreatic or biliary system[8]. Our case corroborated this view. During the long-term follow-up of our case, we were surprised to find that the patient developed IPMN 4.5 years after surgery because we performed the operation in time to achieve long-term survival.
We were concerned that the pathological findings were all suggestive of malignancy, yet all the lymph nodes examined were negative, indicating that IPNB and IPMN, although often benign, can become malignant over time. Lymphatic clearance is not mandatory because tumors in the regional lymph nodes are less likely to metastasize. We performed mass resection while obtaining negative margins, improving the patient’s prognosis. This view is consistent with that of the known literature[16,17]. In our case, IPMN of the pancreatic head was positive for 0/16 regional lymph nodes despite malignancy, while intraoperative rapid cytopathology suggested negative resection margins, allowing preservation of the distal pancreas.
Most cases of IPNB (Table 1) have occurred in the left hepatic duct (7/12). Does this mean that the embryogenesis of the left hepatic duct and pancreatic duct is homologous or that the specific microenvironment of the left hepatic duct predisposes the development of IPNB? This requires further detailed clinical and molecular studies.
In our case, the patient was wrongly treated for distant metastasis, and chemotherapy was chosen after incisional implant metastasis was detected. However, the incisional implant metastasis was resectable along with the tumor, and the patient was able to achieve a longer survival time than chemotherapy. After receiving the patient, we developed an individualized treatment plan after careful preoperative examination, detailed multidisciplinary team discussions, and excluding contraindications to surgery. According to the European guidelines on pancreatic cystic neoplasms[18], we performed radical pancreaticoduodenectomy with resection of the incisional implant metastatic tumor. The patient recovered well after surgery and there was no tumor recurrence or metastasis detected at 6 mo follow-up. Therefore, we believe that junctional tumors similar to IPNB still require reoperation even if incisional implantation metastases have occurred. With the assurance of negative incisional margins, patients are able to achieve more prolonged overall survival.
There are reports in the literature on the effectiveness of radiotherapy in similar cases, and this may be a good therapeutic option[10]. However, surgical treatment was chosen in 10 of 11 cases of IPNB and IPMN (Table 1), and we opted for surgical treatment. After radical pancreaticoduodenectomy, the patient was cured and discharged from hospital. There was no recurrence or metastasis at 6 mo follow-ups. However, there are reports of postoperative pancreatic tumor recurrence and reoperation[14]. Therefore, the follow-up of this patient will be long-term.
We recommend CT, MRCP, and endoscopic retrograde cholangiopancreatography to diagnose IPNB and IPMN, and a SpyGlass examination can be performed if possible. IPNB and IPMN both tend to grow locally and rarely metastasize to lymph nodes. Intraoperative frozen pathology can guide the extent of resection, and negative surgical margins can significantly improve the patient’s prognosis. Long-term follow-up is warranted after IPNB or IPMN is first resected because of the potential for heterochronic morbidity.
Special thanks should go to my beloved wife and my parents for their continuous support and encouragement.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alkhatib AJ, Jordan; Kapan S, Turkey; Taura K, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Chen YL
| 1. | Fong ZV, Ferrone CR, Lillemoe KD, Fernández-Del Castillo C. Intraductal Papillary Mucinous Neoplasm of the Pancreas: Current State of the Art and Ongoing Controversies. Ann Surg. 2016;263:908-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Kim JR, Lee KB, Kwon W, Kim E, Kim SW, Jang JY. Comparison of the Clinicopathologic Characteristics of Intraductal Papillary Neoplasm of the Bile Duct according to Morphological and Anatomical Classifications. J Korean Med Sci. 2018;33:e266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Fléjou JF. [WHO Classification of digestive tumors: the fourth edition]. Ann Pathol. 2011;31:S27-S31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 191] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 4. | Ren X, Zhu CL, Qin XF, Jiang H, Xia T, Qu YP. Co-occurrence of IPMN and malignant IPNB complicated by a pancreatobiliary fistula: A case report and review of the literature. World J Clin Cases. 2019;7:102-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Joo YH, Kim MH, Lee SK, Seo DW, Yoo KS, Min YI, Chang JJ, Yu E. A case of mucin-hypersecreting intrahepatic bile duct tumor associated with pancreatic intraductal papillary mucinous tumor. Gastrointest Endosc. 2000;52:409-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Ishida M, Seki K, Honda K, Kimura T, Katayama K, Hirose K, Dojo M, Azuma T, Imamura Y, Hutchins RR, Yamaguchi A. Intraductal mucinous tumors occurring simultaneously in the liver and pancreas. J Gastroenterol. 2002;37:1073-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Yamaguchi Y, Abe N, Imase K, Mizuno H, Chinen K, Mori H, Sugiyama M, Atomi Y, Ishida H, Takahashi S. A case of mucin hypersecreting intraductal papillary carcinomas occurring simultaneously in liver and pancreas. Gastrointest Endosc. 2005;61:330-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Zalinski S, Paradis V, Valla D, Belghiti J. Intraductal papillary mucinous tumors of both biliary and pancreatic ducts. J Hepatol. 2007;46:978-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Park BH, Sun JE, Cha HJ, Kim YM, Choi HJ. Intraductal Papillary Mucinous Tumor Simultaneously Involving the Liver and Pancreas-A Case Report. Korean J Pathol. 2010;44. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Valente R, Capurso G, Pierantognetti P, Iannicelli E, Piciucchi M, Romiti A, Mercantini P, Larghi A, Federici GF, Barucca V, Osti MF, Di Giulio E, Ziparo V, Delle Fave G. Simultaneous intraductal papillary neoplasms of the bile duct and pancreas treated with chemoradiotherapy. World J Gastrointest Oncol. 2012;4:22-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Xu XW, Li RH, Zhou W, Wang J, Zhang RC, Chen K, Mou YP. Laparoscopic resection of synchronous intraductal papillary mucinous neoplasms: a case report. World J Gastroenterol. 2012;18:6510-6514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Moon DB, Lee SG, Jung DH, Park GC, Park YH, Park HW, Kim MH, Lee SK, Yu ES, Kim JH. [Synchronous malignant intraductal papillary mucinous neoplasms of the bile duct and pancreas requiring left hepatectomy and total pancreatectomy]. Korean J Gastroenterol. 2014;63:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Bansal A, Thung SN, Zhu H, Schwartz M, Lewis S. Synchronous pancreatic adenocarcinoma and intrahepatic cholangiocarcinoma arising in the context of intraductal papillary neoplasms. Clin Imaging. 2016;40:897-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Kitahama T, Yamane H, Mohri K, Fukuoka E, Yoshida T, Yamagishi T, Goto H, Furutani A, Otsubo D, Matsumoto T, Tanaka M, Fujino Y, Tominaga M. A case of intraductal papillary neoplasm of the bile duct accompanied by intraductal papillary mucinous neoplasm of the pancreas and hepatocellular carcinoma. Clin J Gastroenterol. 2021;14:1536-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Aslam A, Wasnik AP, Shi J, Sahai V, Mendiratta-Lala M. Intraductal papillary neoplasm of the bile duct (IPNB): CT and MRI appearance with radiology-pathology correlation. Clin Imaging. 2020;66:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Uemura S, Higuchi R, Yazawa T, Izumo W, Matsunaga Y, Shiihara M, Ota T, Furukawa T, Yamamoto M. Prognostic Factors for Surgically Resected Intraductal Papillary Neoplasm of the Bile Duct: A Retrospective Cohort Study. Ann Surg Oncol. 2021;28:826-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Shi J, Wan X, Xie Y, Lin J, Long J, Xu W, Liang Z, Sang X, Zhao H. CK20 and lymph node involvement predict adverse outcome of malignant intraductal papillary neoplasm of the bile duct. Histol Histopathol. 2020;35:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 896] [Article Influence: 128.0] [Reference Citation Analysis (1)] |