Published online Jul 27, 2023. doi: 10.4240/wjgs.v15.i7.1474
Peer-review started: November 23, 2022
First decision: February 23, 2023
Revised: March 5, 2023
Accepted: May 8, 2023
Article in press: May 8, 2023
Published online: July 27, 2023
Processing time: 240 Days and 1.3 Hours
Acupuncture promotes the recovery of gastrointestinal function and provides analgesia after major abdominal surgery. The effects of transcutaneous electrical acupoint stimulation (TEAS) remain unclear.
To explore the potential effects of TEAS on the recovery of gastrointestinal function after gastrectomy and colorectal resection.
Patients scheduled for gastrectomy or colorectal resection were randomized at a 2:3:3:2 ratio to receive: (1) TEAS at maximum tolerable current for 30 min immediately prior to anesthesia induction and for the entire duration of surgery, plus two 30-min daily sessions for 3 consecutive days after surgery (perioperative TEAS group); (2) Preoperative and intraoperative TEAS only; (3) Preoperative and postoperative TEAS only; or (4) Sham sti
In total, 441 patients were randomized; 405 patients (58.4 ± 10.2 years of age; 247 males) received the planned surgery. The time to the first bowel sounds did not differ among the four groups (P = 0.90; log-rank test). On postoperative day 1, the rest pain scores differed significantly among the four groups (P = 0.04; Kruskal–Wallis test). Post hoc comparison using the Bonferroni test showed lower pain scores in the perioperative TEAS group (1.4 ± 1.2) than in the sham sti
TEAS provided analgesic effects in adult patients undergoing major abdominal surgery, and it can be added to clinical practice as a means of accelerating postoperative rehabilitation of these patients.
Core Tip: Transcutaneous electrical acupoint stimulation at an alternating 2/100-Hz frequency and maximum tolerable current to the bilateral Neiguan (P6), Hegu (LI4), Zusanli (ST36), and Sanyinjiao (SP6) did not promote functional recovery of the gastrointestinal tract after major abdominal surgery but alleviated postoperative pain.
- Citation: Hou YT, Pan YY, Wan L, Zhao WS, Luo Y, Yan Q, Zhang Y, Zhang WX, Mo YC, Huang LP, Dai QX, Jia DY, Yang AM, An HY, Wu AS, Tian M, Fang JQ, Wang JL, Feng Y. Transcutaneous electrical acupoint stimulation in adult patients receiving gastrectomy/colorectal resection: A randomized controlled trial. World J Gastrointest Surg 2023; 15(7): 1474-1484
- URL: https://www.wjgnet.com/1948-9366/full/v15/i7/1474.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i7.1474
Despite the use of early enteral feeding and prokinetic agents, postoperative ileus remains a common complication of colorectal cancer[1-3]. Acupuncture is an effective therapy for postoperative ileus[4], as well as nausea and vomiting[5], in patients undergoing laparoscopic surgery for colorectal cancer. A previous study showed that acupuncture accelerated gastrointestinal (GI) function recovery after appendectomy, possibly by increasing the release of gastrin and inhibiting the secretion of vasoactive intestinal peptide[6,7]. A recent meta-analysis on the effect of acupuncture on early bowel function recovery after gastric and colorectal cancer surgery (including gastrectomy, colorectal resection, and ileostomy/colostomy closure) indicated that acupuncture shortens the time to first exhaustion and defecation[8].
Transcutaneous electrical acupoint stimulation (TEAS) provides electrical stimulation to the acupoints without piercing the skin[9]. Animal studies have shown that, similar to electroacupuncture, TEAS can produce analgesic effects, possibly by inhibiting the phosphorylation of c-Jun N-terminal kinase in the dorsal root ganglion[10]. In clinical studies, TEAS has been shown to reduce pain in both outpatient and inpatient settings[11-14]. A meta-analysis of 682 patients showed that patients who were treated with TEAS experienced less pain and used fewer opioid analgesics on the 1st day after surgery than controls (non-acupoint control and sham treatment)[14]. In patients undergoing skin expansion treatment[11], TEAS decreased the overall and maximum pain scores, and it is effective for treating chronic pain such as osteoarthritic knee pain[12].
TEAS has been shown to reduce the incidence of postoperative nausea and vomiting and decrease antiemetic use in patients undergoing gynecological surgery[13]. In a randomized controlled trial of 110 patients undergoing cesarean section[15], TEAS at the ST36 acupoint shortened the time to first bowel sound, first anal exhaust, and first defecation after surgery. However, the effective stimulation paradigm remains unknown as most studies on perioperative TEAS treatment were relatively underpowered[13,15-17]. In addition, most previous studies were single-center studies with limited external validity.
In this trial, we examined the potential effects of TEAS on the recovery of GI function and its analgesic effects after gastrectomy/colorectal resection.
This multicenter randomized controlled trial was conducted at five medical centers across China between June 2014 and October 2015 (Peking University People’s Hospital, Beijing Friendship Hospital, Capital Medical University, Beijing Chaoyang Hospital, Capital Medical University, Second Affiliated Hospital of Zhejiang Chinese Medical University, and First Affiliated Hospital of Wenzhou Medical University). Another center (Peking University Third Hospital) was included in the protocol but did not enroll any subjects. Details of the protocol were published previously[18]. This trial was approved by the Ethics Committee of Peking University People’s Hospital (#2013 (09)) on June 9, 2013 and by the Ethical Committees of all participating centers. Written informed consent was obtained from all the patients. The trial was registered on the Chinese Clinical Trial Registry (ChiCTR-TRC-14004435). This study was conducted in accordance with the principles of the Declaration of Helsinki.
A randomization sequence was generated using a commercial randomization system (CIMS® Brightechm, Chengdu, China) and stratified by the surgical site (stomach or colorectum). Subjects were randomized at a 2:3:3:2 ratio to receive either: (1) TEAS at maximum tolerable current for 30 min immediately prior to anesthesia induction and for the entire duration of surgery between Neiguan (P6) and Hegu (LI4) on both sides, as well as two 30-min daily sessions for 3 consecutive days after surgery between P6 and LI4 and between Zusanli (ST36) and Sanyinjiao (SP6) (perioperative TEAS group) (Figure 1); (2) Preoperative and intraoperative TEAS; (3) Preoperative and postoperative TEAS; or (4) Sham stimulation. The TEAS was generated using a HANS 100 B stimulator (four conductors, eight electrodes; Jisheng Co., Nanjing, China). Concealment was achieved using a remote web-based real-time allocation system to allocate specific participants after enrollment.
Adult patients (18-75 years of age) scheduled for gastrectomy or colorectal resection were eligible. Other inclusion criteria were as follows: (1) Body mass index of 18-31 kg/m2; and (2) American Society of Anesthesiologists grade I–III. Subjects with one or more of the following conditions were excluded: (1) Sensory impairment or infection or scar near the selected acupoints; (2) Mental or neurological disease, limb nerve injury, or a history of spinal surgery; (3) Cardiac pacemakers; (4) Liver or kidney dysfunction (alanine aminotransferase, aspartate aminotransferase, blood urea nitrogen, or creatinine 1.5 times higher than the upper normal limit); (5) Preoperative pain numerical rating scale (NRS) score > 0, or a history of steroid or long-term analgesic use; (6) Heavy drinkers, defined as > 3 standard drinks (each containing 14 g pure ethanol) per day for women and > 4 standard drinks per day for men[19]; (7) Patients who did not understand NRS scores or refused to use patient-controlled intravenous analgesia (PCIA); (8) Preoperative serum K+ at > 5.5 mmol/L or < 3.0 mmol/L or hemoglobin < 7 g/dL; (9) Pre-planned colostomy during surgery; and (10) Pre-planned return to the intensive care unit after surgery.
Anesthesia was induced with intravenous midazolam (0.03 mg/kg), propofol (1.5–2.5 mg/kg), sufentanil (0.3–0.4 μg/kg), and rocuronium (0.8 mg/kg) and maintained at a bispectral index of 45–55 with remifentanil (0.05–0.2 μg/kg/min), propofol, and rocuronium. Upon closing the peritoneal cavity, 5–10 μg sufentanil or 0.05 mg fentanyl and 5 mg tropisetron were administered prophylactically. Patients started to receive PCIA (250 μg sufentanil in 250 mL saline; 1 mL/h, 3 mL/bolus, 10 min interval) immediately prior to transfer to a post-anesthesia care unit.
Nausea and pain severity were scored using a 10-point numerical NRS. Rescue tropisetron (5 mg) was given intravenously when the nausea score was ≥ 7 or upon repeated vomiting. Rescue pethidine (50 mg) was given intramuscularly when the NRS pain score remained at ≥ 4 after five consecutive sufentanil bolus via the PCIA.
Bowel sounds were examined through auscultation of the lower abdomen by trained nurses at 6-h interval [3 am, 9 am, 3 pm, and 9 pm on postoperative days (PODs) 1-3]. Each auscultation session lasted at least 3 min. A postoperative diary was maintained by the patient’s family members and included the time to oral water intake, solid food intake, first flatulence, and ambulation. The patients’ family members were educated before surgery to maintain their records. Pain intensity during the resting and active states was scored using a 10-point NRS at 9 am and 3 pm on POD 1–3. The cumulative sufentanil dosage used in PCIA was also recorded. Patients were asked to complete an SF-8 questionnaire via telephone before surgery and 1 mo after discharge.
The primary outcome was the time to the first bowel sound, calculated from the end of surgery. The first bowel sound was verified by two assessments. Secondary outcomes included time to first flatus, time to water intake, time to solid food tolerance (defined as no nausea and vomiting within 4 h after consumption of solid food), time to ambulation, postoperative NRS pain score, PCIA sufentanil dosage, rate and severity of postoperative nausea and vomiting, postoperative and preoperative quality of life assessment, and surgical complications. Surgical complications were graded using the Clavien-Dindo grading system[20].
A preliminary trial that included 72 patients was conducted at the Peking University People’s Hospital. The result showed that the time to the first bowel sound was 60.3 ± 9.8 h in the sham stimulation group and 51.6 ± 17.8 h in the perioperative TEAS group. The larger standard deviation in the two groups (17.8) was used to calculate the sample size. Assuming 90% power and alpha at 0.05, 73, 110, 110, and 73 subjects were required in the sham stimulation, perioperative TEAS, preoperative and intraoperative TEAS, and preoperative and postoperative TEAS groups, respectively. Considering dropout, we planned to enroll 80, 120, 120, and 80 participants in the four groups, respectively.
Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, United States). The Peking University Clinical Research Institute managed all the data and was responsible for sample size calculations, data entry, and statistical analyses. The primary endpoint was analyzed in a modified intention-to-treat population that included all patients who underwent the planned surgery. Normally distributed continuous variables were analyzed using analysis of variance, followed by the Bonferroni test for post hoc pairwise comparisons. Continuous variables that did not follow a normal distribution were analyzed using the Kruskal–Wallis H test. Categorical variables were analyzed using a χ2 test. Time to first bowel sounds was analyzed using the log-rank test. The NRS scores at rest and during activity were analyzed using the study site (gastric or colorectal) and surgical method (open or laparoscopic) as stratification factors. Statistical significance was set at P < 0.05.
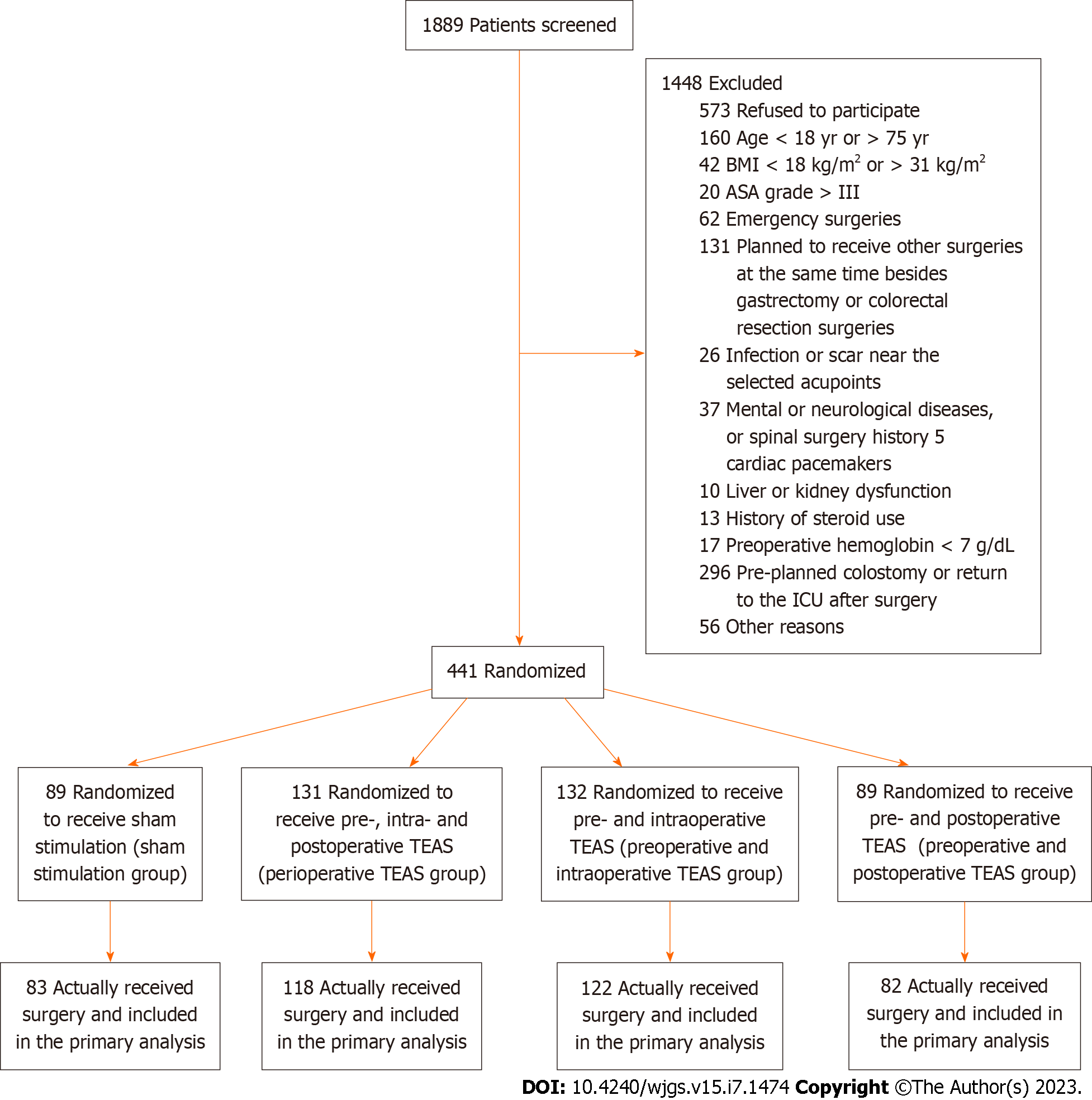
A total of 1889 patients were screened; 1448 patients were excluded for the reasons specified in Figure 2. In total, 441 patients were randomized and 405 received surgeries (58.4 ± 10.2 years of age; 247 males): 83 in the sham stimulation group; 118 in the perioperative TEAS group; 122 in the preoperative and intraoperative TEAS group; and 82 in the preoperative and postoperative TEAS group. The four groups were generally comparable in terms of demographic and baseline characteristics (Table 1). Regarding surgeries, 170 patients underwent gastrectomy (137 open gastrectomy and 33 laparoscopic gastrectomy), and 235 patients underwent colorectal surgery (126 open colorectal surgery and 109 laparoscopic colorectal surgery).
| Characteristic | Perioperative TEAS, n = 118 | Preoperative and intraoperative TEAS, n = 122 | Preoperative and postoperative TEAS, n = 82 | Sham stimulation, n = 83 |
| Mean age in yr | 59.0 ± 10.6 | 57.6 ± 10.1 | 58.0 ± 10.6 | 59.2 ± 9.7 |
| Male sex, n (%) | 75 (63.6) | 72 (59.0) | 50 (61) | 50 (60.2) |
| Mean BMI, kg/m2 | 23.2 ± 3.0 | 23.1 ± 3.0 | 23.2 ± 3.0 | 23.3 ± 3.2 |
| Type of surgery, n (%) | ||||
| Gastrectomy | 50 (42.4) | 52 (42.6) | 33 (40.2) | 35 (42.2) |
| Colorectal surgery | 68 (57.6) | 70 (57.4) | 49 (59.8) | 48 (57.8) |
The median time to first bowel sound was 53.8 h (interquartile range: 38.8–86.8 h) in the sham stimulation group, 52.2 h (30.0–80.3 h) in the perioperative TEAS group, 55.7 h (32.3–80.9 h) in the preoperative and intraoperative TEAS group, and 51.1 h (30.3–81.3 h) in the preoperative and postoperative TEAS group (log-rank test, P = 0.90). In the subgroup analysis that included gastric or colorectal surgery only, the time to the first bowel sound did not differ between the four treatment groups (colorectal subgroup: log-rank test, P = 0.85; gastric subgroup: log-rank test, P = 0.84).
The median time to first flatus was 68.1 h (53.6–94.0 h) in the sham stimulation group, 71.9 h (55.6–98.1 h) in the perioperative TEAS group, 81.3 h (60.5–107.6 h) in the preoperative and intraoperative TEAS group, and 83.2 h (52.5–120.0 h) in the preoperative and postoperative TEAS group (P = 0.15). The median time to water intake was 86.6 h (52.4–119.3 h) in the sham stimulation group, 90.7 h (64.6–139.5 h) in the perioperative TEAS group, 92.5 h (53.3–135.8 h) in the preoperative and intraoperative TEAS group, and 91.4 h (43.7–139.9 h) in the preoperative and postoperative TEAS group (P = 0.63). The time to solid food tolerance was 141.0 h (117.2–192.8 h) in the sham stimulation group, 140.4 h (116.0-183.1 h) in the perioperative TEAS group, 141.3 h (120.6–181.7 h) in the preoperative and intraoperative TEAS group, and 141.2 h (115.3–188.1 h) in the preoperative and postoperative TEAS group (P = 0.55). The time to ambulation was 46.2 h (39.6–89.2 h) in the perioperative TEAS group, 62.6 h (40.1–90.1 h) in the sham stimulation group, 56.7 h (31.3–98.1 h) in the preoperative and intraoperative TEAS group, and 50.7 h (40.3–88.8 h) in the preoperative and postoperative TEAS group (P = 0.54).
Compared with the sham stimulation group, the perioperative TEAS group had significantly lower pain NRS scores in the resting state at 3 pm on POD 1 (Kruskal–Wallis test, P = 0.04) and 3 pm on POD 2 (Kruskal–Wallis test, P = 0.04). No significant differences in the resting-state NRS scores were observed at any other timepoint. The NRS pain score in the active state at 3 pm on POD 1 was also lower in the perioperative TEAS group than in the sham stimulation group (Kruskal–Wallis test, P = 0.03) (Figure 3). Subgroup analysis revealed similar results in the colorectal surgery subgroup but not in the gastrectomy subgroup (Figure 4).
The rate of postoperative vomiting was 7.2% in the sham stimulation group, 8.5% in the perioperative TEAS group, 5.7% in the preoperative and intraoperative TEAS groups, and 11.0% in the preoperative and postoperative TEAS groups (P = 0.58). There were no differences in the NRS scores for nausea among the four treatment groups on POD 1-3 (Table 2).
| POD | Perioperative TEAS, n = 108 | Preoperative and intraoperative TEAS, n = 106 | Preoperative and postoperative TEAS, n = 73 | Sham stimulation, n = 77 | P value | |
| 1 | 9 am | 0.6 ± 1.5 | 0.5 ± 1.5 | 0.7 ± 2.0 | 0.5 ± 1.1 | 0.95 |
| 3 pm | 0.4 ± 1.0 | 0.2 ± 0.7 | 0.5 ± 1.7 | 0.4 ± 1.1 | 0.71 | |
| 2 | 9 am | 0.4 ± 1.0 | 0.1 ± 0.4 | 0.5 ± 1.6 | 0.5 ± 1.6 | 0.18 |
| 3 pm | 0.2 ± 0.8 | 0.1 ± 0.4 | 0.4 ± 1.5 | 0.3 ± 1.3 | 0.21 | |
| 3 | 9 am | 0.2 ± 0.6 | 0.2 ± 1.0 | 0.1 ± 0.6 | 0.3 ± 1.1 | 0.89 |
| 3 pm | 0.1 ± 0.2 | 0.1 ± 0.7 | 0.1 ± 0.2 | 0.2 ± 1.0 | 0.67 | |
The SF-8 questionnaire was completed by 354 subjects 1 mo after discharge, and the mean scores did not differ among the four groups: 19.7 ± 6.9 in the sham stimulation group; 18.3 ± 6.1 in the perioperative TEAS group; 18.8 ± 5.6 in the preoperative and intraoperative TEAS group; and 19.4 ± 5.9 in the preoperative and postoperative TEAS group (P = 0.41).
Grade III or higher complications occurred in 7 patients: One type III complication (wound healing was poor for debridement); two type IIIb complications (anastomotic stricture, second operation for gastric anastomotic leakage); one type IVa complication (cerebral infarction); one type IVb complication (pulmonary embolism and pulmonary infection); and one type V complication (death). There was no difference in the incidence of complications among the four treatment groups [1.7% (2/118), 2.5% (3/122), 1.2% (1/82), and 1.2% (1/83) in the perioperative TEAS, preoperative and intraoperative TEAS, preoperative and postoperative TEAS, and sham stimulation groups, respectively; P = 0.92].
In this trial, the use of 2/100 Hz TEAS did not affect the time to the first bowel sound after surgery. The NRS pain score was significantly lower in the perioperative group than in the sham stimulation group on POD 1 and POD 2.
In the current study, bowel function recovery indices did not differ among the four groups. However, an earlier trial conducted in 110 women receiving TEAS showed improved bowel function recovery after caesarean section[15]. This difference may be because the GI tract was unaltered during the caesarean section[21]. Intensive manipulations, such as incision and anastomosis, can cause severe injuries to the GI tract. The recovery of postoperative bowel function depends on many factors including intestinal mucosal barrier reconstruction, parasympathetic nervous activation, inflammatory response reduction, and homeostasis maintenance[22].
Another reason for this is that opioids were used during and after surgery in our study. By activating opioid receptors in the GI tract, opioid peptides inhibit acetylcholine release and submucosal secretomotor neurons, thereby reducing the propulsive motility of the bowel and dehydrating bowel contents[23,24]. It has been shown that opioids can delay bowel function recovery, and peripherally acting μ opioid receptor antagonists can reduce ileus after bowel resection[25]. Therefore, we speculate that the intensive mechanical interference caused by surgery and the pharmacological effects of opioids contribute to the inhibition of bowel function recovery after major abdominal surgeries. Although TEAS can provide an analgesic effect through coordination of the central nervous system, it is not powerful enough to compensate for both mechanical and pharmacological disturbances.
Consistent with a previous study showing the analgesic effect of TEAS[14] and a reduction in opioid consumption for electrotherapy[26], we found that perioperative TEAS achieved lower NRS pain scores on POD 1 and 2 during rest and activity. These mechanisms involve both peripheral and central aspects[27,28] and may be caused by the release of neuropeptides such as enkephalin, endorphin, and dynorphin in the brain and spinal cord[29,30]. Acupuncture can activate the enteric nervous system[31] and modulate the brain–gut axis[32]. Acupuncture treatment reduces c-Fos, substance P, serotonin, and N-methyl-D-aspartate receptor expression levels and elevates serotonin receptor/transporter and leu-enkephalin expression levels in the gut and spinal cord[33]. A previous study showed that the maximum tolerable rectal sensation and distension pressure in patients with irritable bowel syndrome were significantly increased by acu-TEAS compared to sham TEAS, and the secretion of β-endorphin increased after acu-TEAS[34].
The analgesic effect of perioperative TEAS treatment is more effective in colorectal surgery than in gastrectomy. This difference may be due to the different pH environments and flora in the stomach and colorectum, thus affecting the therapeutic effects of TEAS.
This study is innovative for several reasons. First, the design is rigorous; the large sample size and multicenter randomized controlled design strengthen the results. Second, this study explored the optimum stimulation mode and duration for perioperative analgesia, which is an expansion of the existing research on acupuncture and TEAS. Perioperative TEAS reduced the pain score in patients undergoing colorectal surgery but not in those undergoing gastrectomy.
Our study also had some limitations. One limitation is that the enhanced recovery after surgery (ERAS) principles were not fully utilized in this study (most notably, strict preoperative fluid and electrolyte therapy) since the study was conducted between 2014 and 2015. With the continuous development of ERAS, recovery after GI surgery has significantly accelerated[35]. In 2019, Huang et al[36] conducted a randomized controlled trial in 64 patients who underwent laparoscopic colorectal cancer resection, and perioperative anesthesia management was performed according to ERAS guidelines[36]. The results showed that postoperative anal exhaust time in the control group was 53.64 h, which is shorter than the 68.1 h found in our study. However, different outcomes among different TEAS groups should still exist, considering the randomized controlled design.
In this randomized clinical trial, we found that 2/100 Hz TEAS could provide analgesic effects in patients undergoing major abdominal surgery, and it can be added to the clinical practice as a means of accelerating postoperative rehabilitation. Future research should focus on different stimulation frequencies and acupoints for the treatment effects of TEAS as well as its comparison with acupuncture.
Postoperative ileus delays patient recovery. Acupuncture can accelerate the recovery of gastrointestinal (GI) function after abdominal surgery; however, the effect of transcutaneous electrical acupoint stimulation (TEAS) is unknown.
The effective stimulation paradigm of TEAS treatment for postoperative GI function remains unknown since most studies on perioperative TEAS treatment have been relatively underpowered, and the majority of previous studies were from a single center, with limited external validity.
To explore the potential effects of TEAS on the recovery of GI function and its analgesic effects in patients undergoing gastrectomy or colorectal resection.
The 441 patients were randomized; 405 actually received surgeries (58.4 ± 10.2 years of age; 247 men): 83 in the sham stimulation group; 118 in the perioperative TEAS group; 122 in the preoperative and intraoperative TEAS group; and 82 in the preoperative and postoperative TEAS group. The primary outcome was the time to the first bowel sound. Secondary outcomes included the time to first flatus, time to water intake, time to solid food tolerance, time to ambulation, postoperative numerical rating scale pain score, patient-controlled intravenous analgesia sufentanil dosage, rate and severity of postoperative nausea and vomiting, postoperative and preoperative quality of life assessments, and surgical complications.
The time to the first bowel sounds did not differ among the four groups (P = 0.90; log-rank test). The resting pain score on postoperative day 1 differed significantly among the four groups (P = 0.04; Kruskal-Wallis test). Subgroup analysis showed that compared with the sham stimulation group the perioperative TEAS group had significantly reduced resting pain score on postoperative day 1 (1.4 ± 1.2 vs 1.7 ± 1.1; P = 0.04; Bonferroni test).
TEAS provided analgesic effects but did not promote GI function recovery in adult patients undergoing gastrectomy or colorectal resection. This is the first large-sample multicenter randomized controlled trial to explore the treatment effects of TEAS on bowel function recovery after major abdominal surgery.
Future research should focus on different stimulation frequencies and acupoints for the treatment effects of TEAS as well as its comparison with acupuncture.
The authors thank Dr. Yong-Pei Yu, Dr. Xiao-Yan Yan, and Prof. Chen Yao from Peking University Clinical Research Institute for their guidance in statistical analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cabezuelo AS, Spain; Nakaji K, Japan; Paparoupa M, Germany S-Editor: Fan JR L-Editor: Filipodia P-Editor: Xu ZH
| 1. | Willis MA, Toews I, Soltau SL, Kalff JC, Meerpohl JJ, Vilz TO. Preoperative combined mechanical and oral antibiotic bowel preparation for preventing complications in elective colorectal surgery. Cochrane Database Syst Rev. 2023;2:CD014909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 2. | Iyer S, Saunders WB, Stemkowski S. Economic burden of postoperative ileus associated with colectomy in the United States. J Manag Care Pharm. 2009;15:485-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 3. | Chapman SJ, Pericleous A, Downey C, Jayne DG. Postoperative ileus following major colorectal surgery. Br J Surg. 2018;105:797-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 4. | Wang Y, Yang JW, Yan SY, Lu Y, Han JG, Pei W, Zhao JJ, Li ZK, Zhou H, Yang NN, Wang LQ, Yang YC, Liu CZ. Electroacupuncture vs Sham Electroacupuncture in the Treatment of Postoperative Ileus After Laparoscopic Surgery for Colorectal Cancer: A Multicenter, Randomized Clinical Trial. JAMA Surg. 2023;158:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 5. | Shin HC, Kim JS, Lee SK, Kwon SH, Kim MS, Lee EJ, Yoon YJ. The effect of acupuncture on postoperative nausea and vomiting after pediatric tonsillectomy: A meta-analysis and systematic review. Laryngoscope. 2016;126:1761-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Chen J, Li W, Wang KY, Kong XD. Effect of acupuncture at zusanli and shangjuxu on recovery of gastrointestinal function in rats after appendix operation. Zhenjiu Linchuang Zazhi. 2015;31:87-89. [DOI] [Full Text] |
| 7. | Zhu J, Chen YF. [Progress of studies on regulative effect of acupuncture on activities of vasoactive intestinal peptide]. Zhen Ci Yan Jiu. 2011;36:453-456. [PubMed] |
| 8. | Liu YH, Dong GT, Ye Y, Zheng JB, Zhang Y, Lin HS, Wang XQ. Effectiveness of Acupuncture for Early Recovery of Bowel Function in Cancer: A Systematic Review and Meta-Analysis. Evid Based Complement Alternat Med. 2017;2017:2504021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Greif R, Laciny S, Mokhtarani M, Doufas AG, Bakhshandeh M, Dorfer L, Sessler DI. Transcutaneous electrical stimulation of an auricular acupuncture point decreases anesthetic requirement. Anesthesiology. 2002;96:306-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Wang JQ, Mao L, Han JS. Comparison of the antinociceptive effects induced by electroacupuncture and transcutaneous electrical nerve stimulation in the rat. Int J Neurosci. 1992;65:117-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Huang Y, Bian WW, Hou LL. [Effects of transcutaneous electrical acupoint stimulation on pain of patients in expansion process of skin soft tissue dilator on forehead by water injection]. Zhonghua Shao Shang Za Zhi. 2019;35:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Cheing GL, Tsui AY, Lo SK, Hui-Chan CW. Optimal stimulation duration of tens in the management of osteoarthritic knee pain. J Rehabil Med. 2003;35:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Yu X, Zhang F, Chen B. The effect of TEAS on the quality of early recovery in patients undergoing gynecological laparoscopic surgery: a prospective, randomized, placebo-controlled trial. Trials. 2020;21:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 14. | Wu MS, Chen KH, Chen IF, Huang SK, Tzeng PC, Yeh ML, Lee FP, Lin JG, Chen C. The Efficacy of Acupuncture in Post-Operative Pain Management: A Systematic Review and Meta-Analysis. PLoS One. 2016;11:e0150367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 149] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 15. | Mu L, Gao H, Zhao ML, Ren HF, Ma HS. [Effect of transcutaneous electrical acupoint stimulation on recovery of gastrointestinal function after cesarean section]. Zhongguo Zhen Jiu. 2019;39:259-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 16. | Yeh ML, Chung YC, Chen KM, Chen HH. Pain reduction of acupoint electrical stimulation for patients with spinal surgery: a placebo-controlled study. Int J Nurs Stud. 2011;48:703-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Lan F, Ma YH, Xue JX, Wang TL, Ma DQ. Transcutaneous electrical nerve stimulation on acupoints reduces fentanyl requirement for postoperative pain relief after total hip arthroplasty in elderly patients. Minerva Anestesiol. 2012;78:887-895. [PubMed] |
| 18. | Hou Y, Yan Q, An H, Wang J, Tian M, Zhao W, Wu A, Feng Y. The use and protective effects of transcutaneous electrical acupoint stimulation during abdominal surgery: study protocol for a multicenter randomized parallel controlled trial. Trials. 2019;20:462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Witkiewitz K, Wilson AD, Pearson MR, Hallgren KA, Falk DE, Litten RZ, Kranzler HR, Mann KF, Hasin DS, O'Malley SS, Anton RF. Temporal Stability of Heavy Drinking Days and Drinking Reductions Among Heavy Drinkers in the COMBINE Study. Alcohol Clin Exp Res. 2017;41:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24853] [Article Influence: 1183.5] [Reference Citation Analysis (0)] |
| 21. | Springer JE, Elkheir S, Eskicioglu C, Doumouras AG, Kelly S, Yang I, Forbes S. The effect of simethicone on postoperative ileus in patients undergoing colorectal surgery (SPOT), a randomized controlled trial. Int J Surg. 2018;56:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Venara A, Neunlist M, Slim K, Barbieux J, Colas PA, Hamy A, Meurette G. Postoperative ileus: Pathophysiology, incidence, and prevention. J Visc Surg. 2016;153:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 194] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 23. | Farmer AD, Holt CB, Downes TJ, Ruggeri E, Del Vecchio S, De Giorgio R. Pathophysiology, diagnosis, and management of opioid-induced constipation. Lancet Gastroenterol Hepatol. 2018;3:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 24. | Galligan JJ, Sternini C. Insights into the Role of Opioid Receptors in the GI Tract: Experimental Evidence and Therapeutic Relevance. Handb Exp Pharmacol. 2017;239:363-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 25. | Wolff BG, Michelassi F, Gerkin TM, Techner L, Gabriel K, Du W, Wallin BA; Alvimopan Postoperative Ileus Study Group. Alvimopan, a novel, peripherally acting mu opioid antagonist: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial of major abdominal surgery and postoperative ileus. Ann Surg. 2004;240:728-34; discussion 734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 235] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 26. | Tedesco D, Gori D, Desai KR, Asch S, Carroll IR, Curtin C, McDonald KM, Fantini MP, Hernandez-Boussard T. Drug-Free Interventions to Reduce Pain or Opioid Consumption After Total Knee Arthroplasty: A Systematic Review and Meta-analysis. JAMA Surg. 2017;152:e172872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 27. | Deer TR, Jain S, Hunter C, Chakravarthy K. Neurostimulation for Intractable Chronic Pain. Brain Sci. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 28. | Lin T, Gargya A, Singh H, Sivanesan E, Gulati A. Mechanism of Peripheral Nerve Stimulation in Chronic Pain. Pain Med. 2020;21:S6-S12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 29. | Han JS. [Research on Acupuncture Anesthesia-analgesia]. Zhen Ci Yan Jiu. 2016;41:377-387. [PubMed] |
| 30. | Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003;26:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 680] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 31. | Hu S, Zhao ZK, Liu R, Wang HB, Gu CY, Luo HM, Wang H, Du MH, Lv Y, Shi X. Electroacupuncture activates enteric glial cells and protects the gut barrier in hemorrhaged rats. World J Gastroenterol. 2015;21:1468-1478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Li H, He T, Xu Q, Li Z, Liu Y, Li F, Yang BF, Liu CZ. Acupuncture and regulation of gastrointestinal function. World J Gastroenterol. 2015;21:8304-8313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 114] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (3)] |
| 33. | Lee IS, Cheon S, Park JY. Central and Peripheral Mechanism of Acupuncture Analgesia on Visceral Pain: A Systematic Review. Evid Based Complement Alternat Med. 2019;2019:1304152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 34. | Leung WW, Jones AY, Ng SS, Wong CY, Lee JF. Acupuncture transcutaneous electrical nerve stimulation reduces discomfort associated with barostat-induced rectal distension: a randomized-controlled study. World J Gastroenterol. 2013;19:381-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, Rockall TA, Young-Fadok TM, Hill AG, Soop M, de Boer HD, Urman RD, Chang GJ, Fichera A, Kessler H, Grass F, Whang EE, Fawcett WJ, Carli F, Lobo DN, Rollins KE, Balfour A, Baldini G, Riedel B, Ljungqvist O. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS(®)) Society Recommendations: 2018. World J Surg. 2019;43:659-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1071] [Cited by in RCA: 1232] [Article Influence: 205.3] [Reference Citation Analysis (0)] |
| 36. | Huang W, Long W, Xiao J, Zhao G, Yu T. Effect of electrically stimulating acupoint, Zusanli (ST 36), on patient's recovery after laparoscopic colorectal cancer resection: a randomized controlled trial. J Tradit Chin Med. 2019;39:433-439. [PubMed] |