Published online Jul 27, 2023. doi: 10.4240/wjgs.v15.i7.1363
Peer-review started: February 1, 2023
First decision: February 14, 2023
Revised: March 5, 2023
Accepted: May 17, 2023
Article in press: May 17, 2023
Published online: July 27, 2023
Processing time: 170 Days and 0.7 Hours
The effect of perioperative blood transfusion (PBT) on the prognosis of ampullary carcinoma (AC) is still debated.
To explore the impact of PBT on short-term safety and long-term survival in AC patients who underwent pancreaticoduodenectomy.
A total of 257 patients with AC who underwent pancreaticoduodenectomy between 1998 and 2020 in the Cancer Hospital, Chinese Academy of Medical Sciences, were retrospectively analyzed. We used Cox proportional hazard regression to identify prognostic factors of overall survival (OS) and recurrence-free survival (RFS) and the Kaplan-Meier method to analyze survival information.
A total of 144 (56%) of 257 patients received PBT. The PBT group and nonperioperative blood transfusion group showed no significant differences in demographics. Patients who received transfusion had a comparable incidence of postoperative complications with patients who did not. Univariable and multivariable Cox proportional hazard regression analyses indicated that trans
We found that PBT might be associated with decreased OS in early AC, but more validation is needed. The reasonable use of transfusion might be helpful to improve OS.
Core Tip: Considering that few researches on ampullary carcinoma (AC) and high proportion of transfusion on patients undergoing pancreatic surgery due to the sophisticated surgical procedure, we conducted a retrospective study to elucidate the influence of transfusion on short-term safety and long-term survival after curative resection of AC. We found that transfusion might be potentially associated with decreased overall survival in early AC patients. The current article might provide guidance for the reasonable use of transfusion and possible direction for further mechanism study.
- Citation: Fei H, Zhang XJ, Sun CY, Li Z, Li ZF, Guo CG, Zhao DB. Impact of perioperative blood transfusion on oncological outcomes in ampullary carcinoma patients underwent pancreaticoduodenectomy. World J Gastrointest Surg 2023; 15(7): 1363-1374
- URL: https://www.wjgnet.com/1948-9366/full/v15/i7/1363.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i7.1363
Ampullary carcinoma (AC) is a rare malignancy originating from the ampulla of Vater. The incidence of ACs has increased over the past several decades and accounts for 16% of all periampullary cancers[1,2]. Pancreaticoduodenectomy (PD) is currently the primary treatment choice for AC, and the resection rates have been reported to be more than 90% in a Japanese report[3]. Although the postoperative mortality of PD has decreased in recent decades[4], the postoperative morbidity rate remains high at 30% to 55%[5-7]. Moreover, many patients require perioperative blood transfusion (PBT) during PD due to the sophisticated surgical procedure[8,9].
A randomized trial has shown that allogeneic blood transfusions can increase the risk of colorectal cancer recurrence[10]. However, the safety and prognostic effect of PBT on patients undergoing PD remains controversial. Several studies[11,12] suggested that PBT was related to increasing rates of postoperative complications, and a previous study[13] showed that PBT was an independent risk factor for serious infections following PD. For long-term oncological outcomes, PBT in patients was associated with decreased overall survival (OS) after PD in several observational studies[14-17], but other studies[18,19] did not show this association in multivariable analysis.
With respect to AC, the subgroup analysis conducted by Park et al[20] showed that PBT was an independent risk factor for poor prognosis in AC patients (P = 0.029). However, another retrospective study did not find any adverse effect of PBT on survival in AC patients[21]. To clarify the connection between PBT and both long-term survival and short-term safety following PD of AC, we performed a retrospective analysis.
The medical records of 314 individuals who underwent PD were examined at the China National Cancer Center from 1998 to 2020. We enrolled patients in this study according to the following criteria: (1) Patients with pathologically diagnosed AC; and (2) Patients who accepted curative PD, including open approach, laparoscopic approach, and robotic approach. Patients were excluded based on the following criteria: (1) Missing adjuvant chemotherapy data (n = 40); (2) Missing differentiation data (n = 6); (3) Missing N stage data (n = 4); and (4) Missing recurrence data (n = 7). A total of 57 patients were excluded from the analysis, and 257 AC patients were included.
The collected data included sex, age, operation time, tumor size, PBT, differentiation, American Joint Committee on Cancer TNM stage (8th edition), blood vessel invasion, postoperative complications, adjuvant treatment, and outcomes. PBT was defined as any red cell concentrate transfusion intraoperatively or within the first 24 h of surgery. Surgeons and anesthetists made decisions about transfusions during the perioperative period, and generally, the transfusion criteria in our hospital included massive blood loss and hemoglobin level less than 7 g/dL. The short term was defined as the index hospitalization, and the long term was defined as discharge during the follow-up period.
Follow-up information was gathered by telephone, medical records, outpatient medical review, and population death register information system. If the patient was lost to follow-up, the follow-up time was censored. The last follow-up time was September 2021, and the duration of follow-up was 0-240 mo, with a median follow-up time of 63.0 mo. A total of 10 patients were lost to follow-up, and the follow-up rate was 96.8%. The main outcomes were OS and recurrence-free survival (RFS), and the secondary outcomes were postoperative complications. OS was defined as the time interval from diagnosis to death or, for survivors, the time interval from diagnosis to the last follow-up. RFS was defined as the time from curative surgery to locoregional or distant recurrence.
A chi-square test was used to compare the baseline information between the two groups, and then a comparison of postoperative complications was performed. To investigate the independent prognostic factors of OS and RFS, factors with P < 0.2 in univariable analysis and transfusion were included in the multivariable analysis, and the hazard ratios were provided with 95% confidence intervals (CIs). Next, we conducted subgroup analyses regarding RBC ‘dose’ (0 unit, 1-4 units, and ≥ 5 units) to research the dose-response analysis and three types of T stage (T1, T2, and T3) to research the stage analysis. All data were analyzed with SPSS software (version 21; SPSS Inc., Chicago, IL, United States). Kaplan-Meier survival analyses were performed using the survival and survminer R packages. A P value < 0.05 was considered statistically significant.
A total of 257 AC patients who underwent PD were identified and included. Patients were categorized into two groups according to transfusion status: 144 in the PBT group and 113 in the no PBT group. A total of 134 patients (93.1%) received 1-4 blood units, and 10 patients (6.9%) received 5 or more blood units (maximum of 20 units). The detailed baseline information is shown in Table 1. The two groups (PBT vs no PBT) showed no significant difference in demographics or clinicopathological features.
| Characteristic | No PBT | PBT | P value | ||
| n = 113 | 100% | n = 144 | 100% | ||
| Sex | 0.527 | ||||
| Male | 62 | 54.9% | 85 | 59.0% | |
| Female | 51 | 45.1% | 59 | 41.0% | |
| Age (yr) | 0.527 | ||||
| ≤ 60 | 60 | 53.1% | 83 | 57.6% | |
| > 60 | 53 | 46.9% | 61 | 42.4% | |
| Operation time | > 0.999 | ||||
| ≤ 6 h | 85 | 75.2% | 109 | 75.0% | |
| > 6 h | 28 | 24.8% | 35 | 25.0% | |
| Tumor size (cm) | 0.901 | ||||
| ≤ 2.0 cm | 56 | 49.6% | 70 | 48.6% | |
| > 2.0 cm | 57 | 50.4% | 74 | 51.4% | |
| Differentiation | 0.677 | ||||
| Well | 27 | 23.9% | 34 | 23.6% | |
| Moderate | 47 | 47% | 67 | 46.5% | |
| Poor | 39 | 34.5% | 43 | 29.9% | |
| T stage | 0.300 | ||||
| T1 | 22 | 19.5% | 18 | 12.5% | |
| T2 | 41 | 36.3% | 59 | 41.0% | |
| T3 | 50 | 44.2% | 67 | 46.5% | |
| N stage | 0.346 | ||||
| N0 | 82 | 72.6% | 108 | 75.0% | |
| N1 | 28 | 24.8% | 28 | 19.4% | |
| N2 | 3 | 2.7% | 8 | 5.6% | |
| Blood vessel invasion | 0.200 | ||||
| No | 87 | 77.0% | 121 | 84.0% | |
| Yes | 26 | 23.0% | 23 | 16.0% | |
| Postoperative complications | 0.699 | ||||
| No | 71 | 62.8% | 86 | 59.7% | |
| Yes | 42 | 37.2% | 58 | 40.3% | |
| Adjuvant treatment | 0.663 | ||||
| No | 87 | 77.0% | 107 | 74.3% | |
| Yes | 26 | 23.0% | 37 | 25.7% | |
Patients receiving PBT had a comparable incidence of postoperative total complications (40.3% vs 37.2%, P = 0.699) with those who did not receive PBT. Postoperative pancreatic fistula was the most frequent complication of PD, and there was also no significant difference (14.2% vs 15.3%, P = 0.802). The incidence of postpancreatectomy hemorrhage (P = 0.960), delayed gastric emptying (P = 0.847), and intra-abdominal infection (P = 0.372) showed the same results. The detailed data are depicted in Table 2.
| Characteristic | No PBT | PBT | P value | ||
| n = 113 | 100% | n = 144 | 100% | ||
| Postoperative complications | 0.699 | ||||
| No | 71 | 62.8% | 86 | 59.7% | |
| Yes | 42 | 37.2% | 58 | 40.3% | |
| Specific complications | |||||
| PPH | 10 | 8.8% | 13 | 9.0% | 0.960 |
| POPF | 16 | 14.2% | 22 | 15.3% | 0.802 |
| DGE | 11 | 9.7% | 13 | 9.0% | 0.847 |
| Intra-abdominal infection | 15 | 13.3% | 14 | 9.7% | 0.372 |
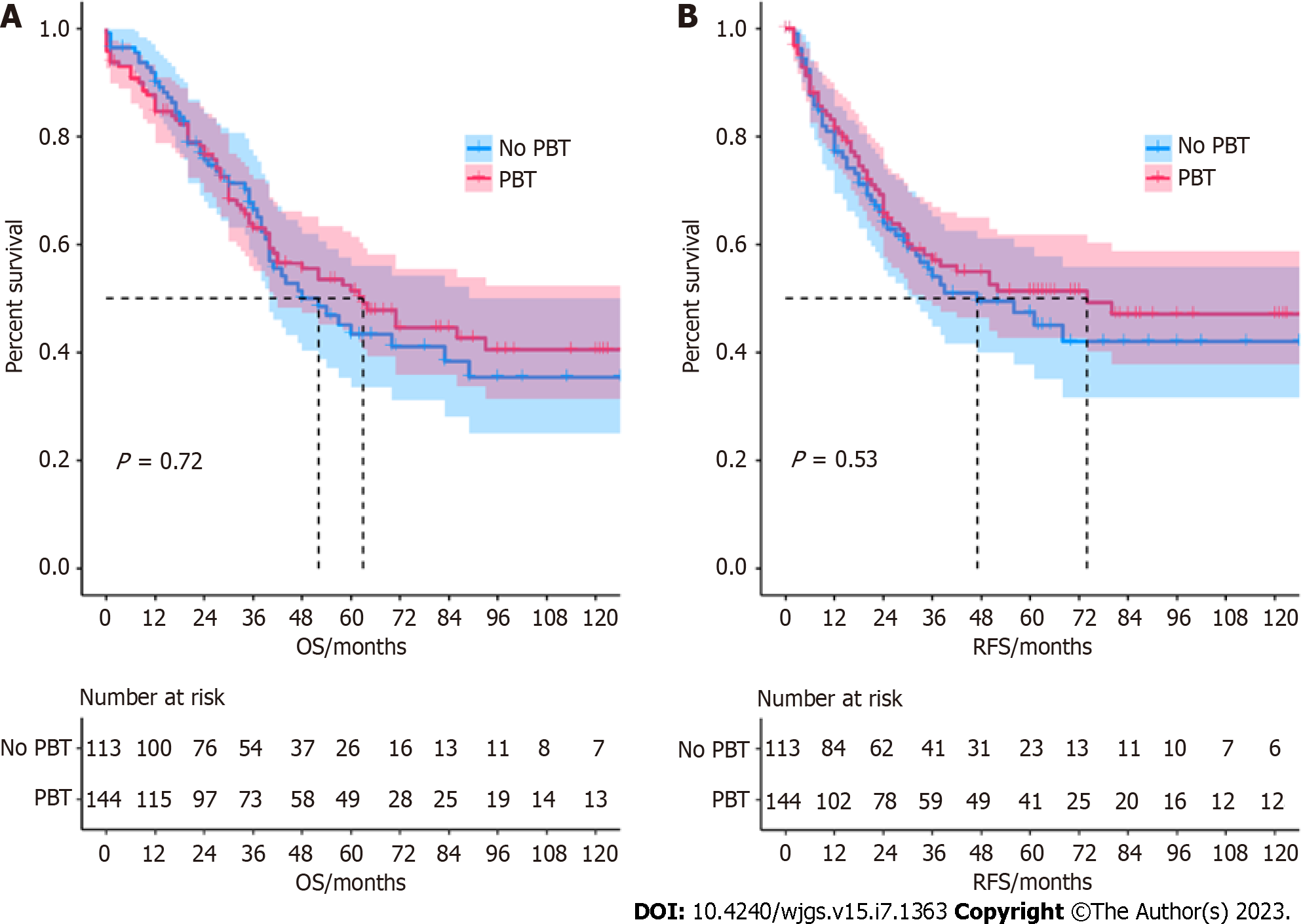
The median OS for the no PBT group and PBT group was 52 (IQR: 20-54.5) months and 64 (IQR: 12-58.5) months, respectively. The one-, three-, and five-year OS rates were 90.8%, 62.9%, 41.4% and 88.3%, 65.7%, 46.5%, RFS rates were 81.0%, 55.4%, 42.0% and 83.1%, 58.1%, 51.4%. According to the Kaplan–Meier survival curves, the no PBT group was not significantly associated with better survival outcomes than the PBT group. The Kaplan-Meier survival curve is shown in Figure 1.
After including factors with P < 0.2 in univariable analysis and transfusion, T3 stage (HR: 3.024, 95%CI: 1.245-7.341, P = 0.014) and N1 stage (HR: 2.072, 95%CI: 1.240-3.465, P = 0.021) were independent risk factors for OS, while blood vessel invasion (HR: 1.744, 95%CI: 1.077-2.824, P = 0.024) was an independent risk factor for RFS. Cox proportional hazard regression analysis showed that transfusion was not related to increased risks of OS (HR: 0.975, 95%CI: 0.676-1.405, P = 0.891) or RFS (HR: 0.983, 95%CI: 0.667-1.447, P = 0.929). The detailed data are depicted in Tables 3 and 4.
| Characteristic | Univariable analysis | Multivariable analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Perioperative blood transfusion | ||||
| No | Reference | Reference | ||
| Yes | 0.937 (0.626-1.339) | 0.723 | 0.975 (0.676-1.405) | 0.891 |
| RBC units | ||||
| 0 | Reference | |||
| 1-4 units | 0.924 (0.643-1.329) | 0.671 | ||
| ≥ 5 units | 1.139 (0.455-2.851) | 0.781 | ||
| Sex | ||||
| Male | Reference | |||
| Female | 0.868 (0.606-1.244) | 0.441 | ||
| Age (yr) | ||||
| ≤ 60 | Reference | Reference | ||
| > 60 | 0.535 (0.371-0.774) | 0.001 | 1.541 (0.698-3.400) | 0.284 |
| Operation time | ||||
| ≤ 6 h | Reference | |||
| > 6 h | 1.260 (0.840-1.892) | 0.264 | ||
| Tumor size (cm) | ||||
| ≤ 2.0 cm | Reference | Reference | ||
| > 2.0 cm | 1.337 (0.939-1.903) | 0.107 | 1.105 (0.756-1.614) | 0.608 |
| Differentiation | ||||
| Well | Reference | Reference | ||
| Moderate | 0.895 (0.603-1.330) | 0.584 | 0.969 (0.637-1.474) | 0.883 |
| Poor | 0.593 (0.360-0.977) | 0.040 | 0.967 (0.529-1.766) | 0.913 |
| T stage | ||||
| T1 | Reference | Reference | ||
| T2 | 1.407 (0.770-2.569) | 0.267 | 1.282 (0.660-2.491) | 0.463 |
| T3 | 2.796 (1.564-4.997) | 0.001 | 3.024 (1.245-7.341) | 0.014 |
| N stage | ||||
| N0 | Reference | Reference | ||
| N1 | 1.986 (1.336-2.953) | 0.001 | 2.072 (1.240-3.465) | 0.021 |
| N2 | 2.119 (0.977-4.595) | 0.057 | 1.988 (0.820-4.817) | 0.128 |
| Blood vessel invasion | ||||
| No | Reference | Reference | ||
| Yes | 1.616 (0.054-2.478) | 0.028 | 1.087 (0.669-1.766) | 0.737 |
| Postoperative complications | ||||
| No | Reference | Reference | ||
| Yes | 1.358 (0.950-1.943) | 0.093 | 1.345 (0.930-1.946) | 0.116 |
| Adjuvant treatment | ||||
| No | Reference | |||
| Yes | 1.056 (0.702-1.588) | 0.794 | ||
| Characteristic | Univariable analysis | Multivariable analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Perioperative Blood Transfusion | ||||
| No | Reference | Reference | ||
| Yes | 0.886 (0.607-1.293) | 0.531 | 0.983 (0.667-1.447) | 0.929 |
| RBC units | ||||
| 0 | Reference | |||
| 1-4 units | 0.854 (0.579-1.261) | 0.428 | ||
| ≥ 5 units | 1.282 (0.550-2.989) | 0.565 | ||
| Sex | ||||
| Male | Reference | |||
| Female | 0.976 (0.665-1.431) | 0.9 | ||
| Age (yr) | ||||
| ≤ 60 | Reference | Reference | ||
| > 60 | 0.422 (0.282-0.631) | < 0.001 | 0.886 (0.407-1.928) | 0.760 |
| Operation time | ||||
| ≤ 6 h | Reference | |||
| > 6 h | 1.194 (0.780-1.829) | 0.414 | ||
| Tumor size (cm) | ||||
| ≤ 2.0 cm | Reference | Reference | ||
| > 2.0 cm | 1.458 (0.998-2.129) | 0.051 | 1.121 (0.745-1.687) | 0.583 |
| Differentiation | ||||
| Well | Reference | Reference | ||
| Moderate | 0.892 (0.586-1.358) | 0.594 | 1.100 (0.702-1.723) | 0.679 |
| Poor | 0.511 (0.294-0.889) | 0.018 | 0.970 (0.499-1.884) | 0.928 |
| T stage | ||||
| T1 | Reference | Reference | ||
| T2 | 1.451 (0.757-2.784) | 0.262 | 1.239 (0.611-2.514) | 0.553 |
| T3 | 3.092 (1.656-5.773) | < 0.001 | 2.284 (0.874-4.968) | 0.098 |
| N stage | ||||
| N0 | Reference | Reference | ||
| N1 | 2.201 (1.449-3.343) | < 0.001 | 1.636 (0.932-2.873) | 0.087 |
| N2 | 3.248 (1.488-7.089) | 0.003 | 2.441 (0.935-6.372) | 0.068 |
| Blood vessel invasion | ||||
| No | Reference | Reference | ||
| Yes | 2.351 (1.548-3.573) | < 0.001 | 1.744 (1.077-2.824) | 0.024 |
| Postoperative complications | ||||
| No | Reference | |||
| Yes | 0.959 (0.643-1.431) | 0.839 | ||
| Adjuvant treatment | ||||
| No | Reference | Reference | ||
| Yes | 1.501 (0.999-2.256) | 0.051 | 0.711 (0.420-1.205) | 0.205 |
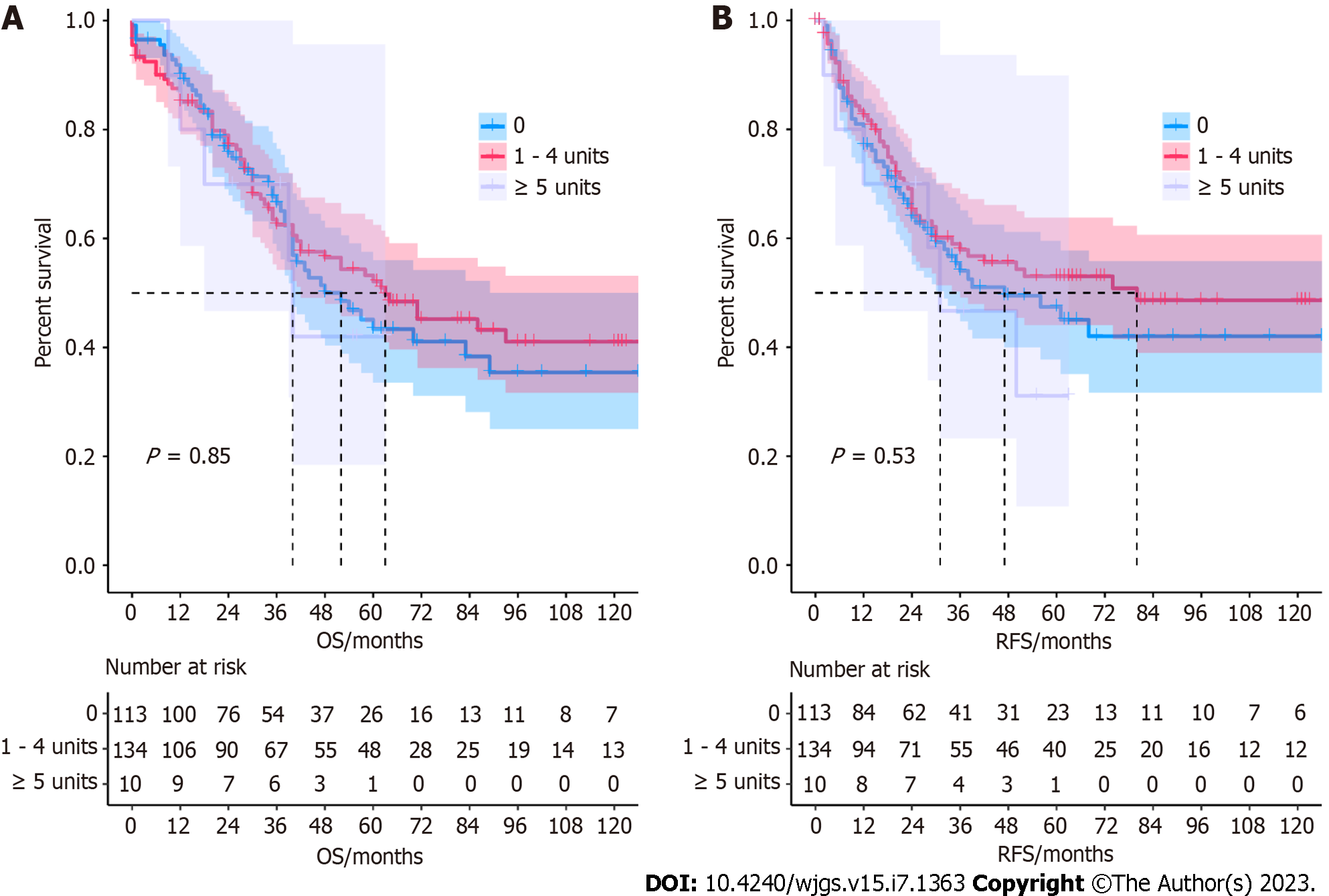
Dose-response analysis: We divided the transfusion dose into three groups (0 units, 1-4 units, and ≥ 5 units) to conduct the dose-response analysis. We found that the number of transfused blood units was not significantly associated with survival outcomes (Figure 2). The Kaplan-Meier survival curve is shown in Figure 2.
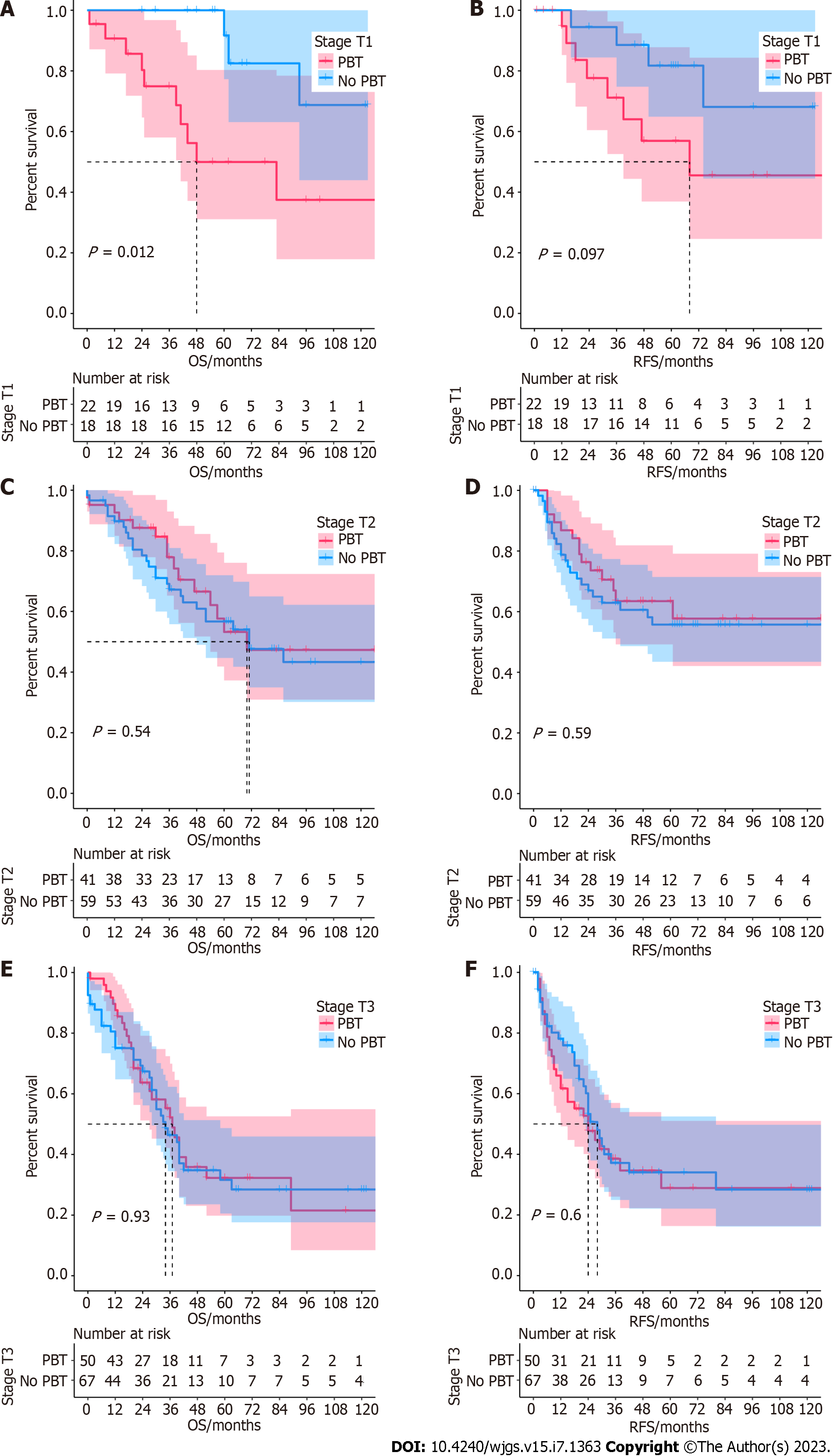
Stage analysis: Stage analyses were conducted regarding T stages (T1, T2, and T3). The survival outcomes of subgroups were also analyzed using the Kaplan-Meier method. Stage analysis revealed that transfusion was associated with worse OS (P < 0.05) in AC of stage T1 (Figure 3A), while there was no significant difference in RFS (P = 0.097) (Figure 3B). For stages T2 and T3, the two groups showed no significant difference in OS and RFS (Figure 3C-F). The Kaplan–Meier survival curve is shown in Figure 3.
The adverse effect of PBT on the prognosis of digestive system tumors is still controversial. In this study, there was no discernible difference in short-term complications and long-term survival outcomes between PBT and no PBT patients with AC who underwent PD, whereas we found that PBT might reduce the OS in early pathologic stage AC patients. Our study suggested that more reasonable transfusion may be helpful in improving OS in early AC.
In this study, transfusion was not significantly associated with increased risks of OS and RFS. The aforementioned study also did not find any adverse effect of PBT on survival in 501 AC patients[21]. Similarly, a recent study indicated that transfusion was not a significant adverse prognostic factor in 404 AC patients after PD[18]. However, another retrospective study showed that PBT seemed to have an adverse impact on the survival of patients who underwent PD[20], which included 130 AC patients, 58% of whom underwent intraoperative transfusion. Consensus conclusions have also been reached in periampullary cancer in Korea[15]. Such conflicting results might be related to several factors. First, early-stage patients accounted for only a small part in our study, while in Park’s study[20], early-stage patients accounted for a considerable proportion (40% vs 15%). Although we found that transfusion was correlated with worse prognosis in early-stage patients, this was not enough to affect the entire study. Second, decisions on transfusion were mainly made by surgeons and anesthetists during the perioperative period, and transfusion criteria usually varied in different medical organizations, which might impact the results. Third, the inadequate sample size due to the lower prevalence of AC might also be a possible reason. An analysis containing a larger sample size is needed in the future. Fourth, the presence of unknown confounding factors might influence our study in some ways.
Subgroup analysis indicated that the impact of PBT on prognosis was more pronounced in patients with lower stages. We found that the no PBT group was associated with better OS but not RFS in the T1 stage. Similar results were also reported by Cata[22] for 636 non-small cell lung cancer patients. Moreover, Wang and colleagues[23] observed that transfused patients had significantly greater rates of disease recurrence in stage I non–small cell lung cancer. Our research also showed some discrepancies in RFS with T1 stage patients but did not reach statistical significance. There were two potential reasons to explain the adverse effect of transfusion. First, previous studies suggested that transfusion of allogenic blood induced immunosuppression and lowered the activity of natural killer cells and/or helper T cells[10,24,25]. Goubran et al[26] propounded that transfusion might stimulate tumor growth through an increase in the mitogenic activity of platelet-derived growth factors. Therefore, the immunosuppressive effect of transfusion might have an adverse impact on patient survival. Another possible reason was that the higher stage AC was more aggressive, which was related to shorter survival. On the other hand, AC patients with early-stage disease had better survival outcomes. Thus, the adverse prognostic influence of transfusion could be seen[15].
This study indicated that transfusion would not increase the risk of short-term complications after PD. Likewise, Sutton et al[17] reported that PBT was not related to an increase in infectious complications. In contrast, several previous studies[13,27,28] showed that PBT among patients with PD was associated with increased rates of various postoperative complications. Ball et al[27] demonstrated that the overall morbidity rate after PD was 37%, and 30-d morbidity increased in a stepwise manner with the number of RBC transfusions. Zhang et al[13] observed that there was a significant association between PBT and serious infections after PD. There were several reasons to explain the increase in complications. First, acute hemorrhage could induce ischemia and hypoxia of tissues, which might even increase pancreatic fistula. Transfusions could be essential to avoid inducing short-term postoperative complications by improving oxygen supply and minimizing hypoxic damage to organs[28]. Second, anemia with concomitant poor nutritional status might also be an adverse factor for postoperative recovery. Third, transfusion-related immunosuppression was thought to be a reason for the increasing risk of infections after blood transfusion[29].
To the best of our knowledge, this study analyzed the impact of the largest number of transfusions in AC patients after PD, both short-term complications and long-term survival were evaluated simultaneously. Then, we implemented further subgroup analysis of the dose-response and T stage. Limitations of this study should be observed. First, this research was a single-center retrospective study with potential selection bias and other confounding factors associated with PBT that may impact OS and RFS. Second, preoperative hemoglobin level, intraoperative blood loss, and transfusion time (pre, intra, and post) were missing, which might have influenced this research. In addition, the granular data of systemic chemotherapy were also missing. Third, some subgroups contained a relatively small number of patients, which might affect the accuracy of our results. Finally, decisions on transfusion were usually made by surgeons or anesthesiologists individually, which may have impacted outcomes. This study nevertheless supports some potential clues for transfusion.
According to our study, PBT was not an independent prognostic factor for AC patients after curative PD. PBT might be associated with decreased OS in early AC. Avoiding PBT whenever possible might be helpful to improve OS. More multicenter and prospective validations are needed.
Numerous patients require transfusion due to sophisticated surgical procedures. However, the effect of perioperative blood transfusion (PBT) on the oncologic outcomes of ampullary carcinoma (AC) is still debated.
The present study attempted to explore the impact of PBT on short-term safety and long-term survival in AC patients who underwent pancreaticoduodenectomy.
This study aimed to investigate whether there was an association between PBT and poor oncologic outcomes in AC.
The clinicopathological data of AC patients who underwent surgery from January 1998 to January 2020 were analyzed. We used Cox proportional hazard regression to identify prognostic factors of overall survival (OS) and recurrence-free survival (RFS) and the Kaplan-Meier method to analyze survival information.
Patients who received transfusion had a comparable incidence of postoperative complications with patients who did not. Transfusion was not an independent predictor of OS and RFS, while PBT might be potentially associated with decreased OS in early AC.
We found that PBT might be associated with decreased OS in early AC.
There are several limitations in this retrospective study, and more multicenter and prospective validations are needed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D, D
Grade E (Poor): E
P-Reviewer: Fernández-Placencia RM, Peru; Han XJ, China; Limaiem F, Tunisia S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Hester CA, Dogeas E, Augustine MM, Mansour JC, Polanco PM, Porembka MR, Wang SC, Zeh HJ, Yopp AC. Incidence and comparative outcomes of periampullary cancer: A population-based analysis demonstrating improved outcomes and increased use of adjuvant therapy from 2004 to 2012. J Surg Oncol. 2019;119:303-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | He J, Ahuja N, Makary MA, Cameron JL, Eckhauser FE, Choti MA, Hruban RH, Pawlik TM, Wolfgang CL. 2564 resected periampullary adenocarcinomas at a single institution: trends over three decades. HPB (Oxford). 2014;16:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 223] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 3. | Ishihara S, Horiguchi A, Miyakawa S, Endo I, Miyazaki M, Takada T. Biliary tract cancer registry in Japan from 2008 to 2013. J Hepatobiliary Pancreat Sci. 2016;23:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 4. | Wu YHA, Oba A, Beaty L, Colborn KL, Rodriguez Franco S, Harnke B, Meguid C, Negrini D, Valente R, Ahrendt S, Schulick RD, Del Chiaro M. Ductal Dilatation of ≥5 mm in Intraductal Papillary Mucinous Neoplasm Should Trigger the Consideration for Pancreatectomy: A Meta-Analysis and Systematic Review of Resected Cases. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Amini N, Spolverato G, Kim Y, Pawlik TM. Trends in Hospital Volume and Failure to Rescue for Pancreatic Surgery. J Gastrointest Surg. 2015;19:1581-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 128] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 6. | Tran TB, Dua MM, Worhunsky DJ, Poultsides GA, Norton JA, Visser BC. The First Decade of Laparoscopic Pancreaticoduodenectomy in the United States: Costs and Outcomes Using the Nationwide Inpatient Sample. Surg Endosc. 2016;30:1778-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Bergeat D, Merdrignac A, Robin F, Gaignard E, Rayar M, Meunier B, Beloeil H, Boudjema K, Laviolle B, Sulpice L. Nasogastric Decompression vs No Decompression After Pancreaticoduodenectomy: The Randomized Clinical IPOD Trial. JAMA Surg. 2020;155:e202291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Ashrafizadeh A, Mehta S, Nahm CB, Doane M, Samra JS, Mittal A. Preoperative cardiac and respiratory investigations do not predict cardio-respiratory complications after pancreatectomy. ANZ J Surg. 2020;90:97-102. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Faraj W, Nassar H, Zaghal A, Mukherji D, Shamseddine A, Kanso M, Jaafar RF, Khalife M. Pancreaticoduodenectomy in the Middle East: Achieving optimal results through specialization and standardization. Hepatobiliary Pancreat Dis Int. 2019;18:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Cata JP, Wang H, Gottumukkala V, Reuben J, Sessler DI. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J Anaesth. 2013;110:690-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 337] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 11. | Medvecz A, Bernard A, Hamilton C, Schuster KM, Guillamondegui O, Davenport D. Transfusion rates in emergency general surgery: high but modifiable. Trauma Surg Acute Care Open. 2020;5:e000371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Gordon K, Figueira ERR, Rocha-Filho JA, Mondadori LA, Joaquim EHG, Seda-Neto J, da Fonseca EA, Pugliese RPS, Vintimilla AM, Auler JOC Jr, Carmona MJC, D'Alburquerque LAC. Perioperative blood transfusion decreases long-term survival in pediatric living donor liver transplantation. World J Gastroenterol. 2021;27:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 13. | Zhang L, Liao Q, Zhang T, Dai M, Zhao Y. Blood Transfusion is an Independent Risk Factor for Postoperative Serious Infectious Complications After Pancreaticoduodenectomy. World J Surg. 2016;40:2507-2512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Mavros MN, Xu L, Maqsood H, Gani F, Ejaz A, Spolverato G, Al-Refaie WB, Frank SM, Pawlik TM. Perioperative Blood Transfusion and the Prognosis of Pancreatic Cancer Surgery: Systematic Review and Meta-analysis. Ann Surg Oncol. 2015;22:4382-4391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 15. | Park HM, Park SJ, Shim JR, Lee EC, Lee SD, Han SS, Kim SH. Perioperative transfusion in pancreatoduodenectomy: The double-edged sword of pancreatic surgeons. Medicine (Baltimore). 2017;96:e9019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Abe T, Amano H, Hanada K, Minami T, Yonehara S, Hattori M, Kobayashi T, Fukuda T, Nakahara M, Ohdan H, Noriyuki T. Perioperative Red Blood Cell Transfusion Is Associated with Poor Long-term Survival in Pancreatic Adenocarcinoma. Anticancer Res. 2017;37:5863-5870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Sutton JM, Kooby DA, Wilson GC, Squires MH 3rd, Hanseman DJ, Maithel SK, Bentrem DJ, Weber SM, Cho CS, Winslow ER, Scoggins CR, Martin RC 2nd, Kim HJ, Baker JJ, Merchant NB, Parikh AA, Abbott DE, Edwards MJ, Ahmad SA. Perioperative blood transfusion is associated with decreased survival in patients undergoing pancreaticoduodenectomy for pancreatic adenocarcinoma: a multi-institutional study. J Gastrointest Surg. 2014;18:1575-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Jin J, Wang H, Peng F, Wang X, Wang M, Zhu F, Xiong G, Qin R. Prognostic significance of preoperative Naples prognostic score on short- and long-term outcomes after pancreatoduodenectomy for ampullary carcinoma. Hepatobiliary Surg Nutr. 2021;10:825-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Jin KM, Liu W, Wang K, Bao Q, Wang HW, Xing BC. The individualized selection of Pancreaticoenteric anastomosis in Pancreaticoduodenectomy. BMC Surg. 2020;20:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Park SJ, Kim SW, Jang JY, Lee KU, Park YH. Intraoperative transfusion: is it a real prognostic factor of periampullary cancer following pancreatoduodenectomy? World J Surg. 2002;26:487-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Ma CH, Lee JH, Song KB, Hwang DW, Kim SC. Predictors of early recurrence following a curative resection in patients with a carcinoma of the ampulla of Vater. Ann Surg Treat Res. 2020;99:259-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 22. | Cata JP, Chukka V, Wang H, Feng L, Gottumukkala V, Martinez F, Vaporciyan AA. Perioperative blood transfusions and survival in patients with non-small cell lung cancer: a retrospective study. BMC Anesthesiol. 2013;13:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Wang T, Luo L, Huang H, Yu J, Pan C, Cai X, Hu B, Yin X. Perioperative blood transfusion is associated with worse clinical outcomes in resected lung cancer. Ann Thorac Surg. 2014;97:1827-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Akabane S, Egi H, Takakura Y, Sada H, Kochi M, Taguchi K, Nakashima I, Sumi Y, Sato K, Yoshinaka H, Hattori M, Ohdan H. The prognostic value of organ/space surgical site infection in stage I colorectal cancer recurrence. Int J Colorectal Dis. 2020;35:1689-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Ueta H, Kitazawa Y, Sawanobori Y, Ueno T, Ueha S, Matsushima K, Matsuno K. Single blood transfusion induces the production of donor-specific alloantibodies and regulatory T cells mainly in the spleen. Int Immunol. 2018;30:53-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Goubran HA, Elemary M, Radosevich M, Seghatchian J, El-Ekiaby M, Burnouf T. Impact of Transfusion on Cancer Growth and Outcome. Cancer Growth Metastasis. 2016;9:1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Ball CG, Pitt HA, Kilbane ME, Dixon E, Sutherland FR, Lillemoe KD. Peri-operative blood transfusion and operative time are quality indicators for pancreatoduodenectomy. HPB (Oxford). 2010;12:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 28. | Ross A, Mohammed S, Vanburen G, Silberfein EJ, Artinyan A, Hodges SE, Fisher WE. An assessment of the necessity of transfusion during pancreatoduodenectomy. Surgery. 2013;154:504-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Lu L, Che J, Cheng W, Dong R, Huang J, Yang Z, Lu J. A Retrospective Study of the Relationship Between Blood Transfusion and 30-Day Postoperative Outcomes in Patients Undergoing Isolated Off-Pump Coronary Artery Bypass Grafting. Braz J Cardiovasc Surg. 2022;37:663-673. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |