Published online Jul 27, 2023. doi: 10.4240/wjgs.v15.i7.1317
Peer-review started: March 14, 2023
First decision: April 7, 2023
Revised: April 24, 2023
Accepted: May 11, 2023
Article in press: May 11, 2023
Published online: July 27, 2023
Processing time: 129 Days and 5.1 Hours
The prevention and treatment of Hirschsprung-associated enterocolitis (HAEC) is a serious challenge in pediatric surgery. Exploring the mechanism of HAEC is conducive to the prevention of this disease.
To explore the possible mechanism of glycyrrhizic acid (GA) and its therapeutic effect on HAEC.
We developed a model of enteritis induced by trinitrobenzenesulfonic acid (TNBS) in zebrafish, and treated it with different concentrations of GA. We analyzed the effect of GA on the phenotype and inflammation of zebrafish.
After treatment with TNBS, the area of the intestinal lumen in zebrafish was significantly increased, but the number of goblet cells in the intestinal lumen was significantly reduced, but these did not increase the mortality of zebrafish, indicating that the zebrafish enteritis model was successfully developed. Different concentrations of GA protected zebrafish with enteritis. In particular, high concentrations of GA were important for the prevention and control of HAEC because it significantly reduced the intestinal luminal area, increased the number of goblet cells in the intestinal lumen, and reduced the levels of interleukin (IL)-1β and IL-8.
GA significantly reduced the intestinal luminal area, increased the number of intestinal goblet cells, and decreased IL-1β and IL-8 in zebrafish, and is important for prevention and control of HAEC.
Core Tip: As an important late-stage inflammatory mediator, high mobility group box 1 protein (HMGB1) is involved in the occurrence, development and outcome of many chronic inflammatory diseases and autoimmune diseases. Glycyrrhizic acid (GA) is the most widely used HMGB1 inhibitor, which can directly bind to HMGB1 and inhibit its chemotactic and mitotic activity. Here, we constructed a zebrafish Hirschsprung-associated enterocolitis (HAEC) model and used different concentrations of GA for intervention. Through the intervention study, we understood the effects of HMGB1 inhibitors-GA on the phenotype and inflammatory state of HAEC zebrafish, and the study results provided a new reference for the prevention and treatment of HAEC.
- Citation: Liu MK, Chen YJ, Chen F, Lin ZX, Zhu ZC, Lin Y, Fang YF, Wu DM. Intervention effects and related mechanisms of glycyrrhizic acid on zebrafish with Hirschsprung-associated enterocolitis. World J Gastrointest Surg 2023; 15(7): 1317-1330
- URL: https://www.wjgnet.com/1948-9366/full/v15/i7/1317.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i7.1317
Hirschsprung’s disease (HD), also known as congenital aganglionosis, is a congenital disorder of intestinal motility[1]. Hirschsprung-associated enterocolitis (HAEC) is the most common and most serious complications of HD[2]. The clinical manifestations of HAEC are mainly hyperpyrexia, abdominal distension and foul-smelling watery stools. In more serious cases, vomiting, intestinal bleeding, lethargy, and shock may occur[3,4]. The incidence of HAEC is reported to be 20%-58%, the recurrence rate is up to 50%, and the case fatality rate is 1%-30%[5,6]. HAEC is a serious challenge in pediatric surgery, and can occur and recur at any stage of human development from infancy to adulthood.
At present, the pathogenesis of HAEC is not clear. Previous studies have suggested that HAEC is caused by me
High mobility group box 1 (HMGB1) is a highly conserved non-histone nuclear protein prevalent in eukaryotic cells. The amino acid sequence homology between human and rodents is 98%[10]. Toll-like receptor 4 (TLR4) is not only a signal of danger released by the body to recognize microbial infection, inflammation or damage in intestine, but an important pattern recognition receptor that mediates innate immunity and induces adaptive immunity. Its role in the inflammatory response of intestinal tissue has attracted much attention, but its mechanism and regulation have yet to be studied[11,12]. In recent years, studies have found that HMGB1 is an important mediator of inflammation involved in the occurrence and development of many chronic inflammatory and autoimmune diseases. One of the key endogenous ligands of TLR4 regulates the inflammation and abnormal immune response mediated by the TLR4 signaling pathway[13,14]. TLR4 plays an important role in the occurrence and development of enteritis, but what role it plays and whether regulating the HMGB1/TLR4 pathway can improve the chronic inflammatory response of the intestine has rarely been reported[15-17]. In order to understand the relationship between the HMGB1/TLR4 pathway and HAEC, we used glycyrrhizic acid (GA), an inhibitor of HMGB1, to explore the role and possible mechanisms of HMGB1 in HAEC, with the aim of providing a theoretical and experimental basis for the prevention and treatment of HAEC.
AB type wild zebrafish were purchased from the China Zebrafish Resource Center; Alcian blue solution from Shanghai Aladdin Biochemical Technology Co. Ltd; GA ammonium salt from Shanghai Yuanye Biotechnology Co. Ltd; dimethyl sulfoxide (DMSO) from Beijing Solarbio Science & Technology Co. Ltd; trinitrobenzenesulfonic acid (TNBS) from Dalian Meilunbio Co. Ltd; NaCl, KCl, calcium chloride dihydrate and magnesium sulfate heptahydrate from Sinopharm Chemical Reagent Co. Ltd; refrigerator by Qingdao Haier Co. Ltd; light incubator from Shanghai Yiheng Scientific Instrument Co. Ltd; pipette from Eppendorf; stereo microscope and stereo fluorescence microscope from Nikon.
CZ59 and CZ1211ret type adult zebrafish were selected for mating, and 3 d post-fertilization (dpf) zebrafish embryos were obtained. Healthy zebrafish embryos with fluorescent phenotype were selected and incubated in embryo medium supplemented with 75 μg/mL TNBS for 5 d. Finally, 8 dpf zebrafish with enteritis were obtained. This study was approved by Institutional Animal Care and Use Ethics Committee.
First, larvae were anesthetized, and fixed in 4% paraformaldehyde (PFA) for 12 h at 4 °C in a refrigerator. We removed PFA, and soaked the larvae in a mixture of 0.5 mL 0.5% KOH and 3% H2O2 for 2 h, and washed three times with 0.5 mL phosphate buffer solution-tween-20 (PBST). The larvae were washed with acid alcohol and stained with Alcian blue for 24 h. Larvae were washed three times with acid alcohol. Lastly, they were washed once with 75% alcohol, 50% alcohol, 25% alcohol, and PBS.
Eight days post-fertilization, zebrafish with enteritis were divided into different groups and placed in Petri dishes. They were treated with different concentrations of GA for 3 d (from 8 to 11 dpf) and dressing was changed every day. The grouping was performed according to the pre experimental results, as follows: (1) Model control group (DMSO) included 50 zebrafish in two Petri dishes; (2) Research group with low concentration of GA (DMSO + 1.25 μg/mL GA) included 50 zebrafish in two Petri dishes; (3) Research group with medium concentration of GA (DMSO + 12.5 μg/mL GA) included 50 zebrafish in two Petri dishes; (4) Research group with high concentration of GA (DMSO + 50 μg/mL GA) included 50 zebrafish in two Petri dishes; and (5) Blank control group (E3 solution) included 50 zebrafish in two Petri dishes.
The following solutions were prepared for each group as follows. Research group with high concentration of GA: 10 mg GA ammonium salt was added to 500 mL DMSO for solution 1, and 60 μL solution 1 was added to 24 mL E3. Research group with medium concentration of GA: 100 mL solution 1 was added to 300 mL DMSO for solution 2, and 60 μL solution 2 was added to 24 mL E3. Research group with low concentration of GA: 40 mL solution 2 was added to 360 mL DMSO for solution 3, and 60 μL solution 3 was added to 24 mL E3. Model control group: 60 μL DMSO was mixed with 24 mL E3. Blank control group: 24 mL E3.
After 3 d of GA administration, each group of zebrafish was divided into two categories: Normal phenotype and intestinal lumen enlargement according to the degree of malformation, and the proportion of different phenotypes in each group was counted. The intestinal lumen of zebrafish was photographed using a stereo microscope and stereo fluorescence microscope. The intestinal luminal area of each zebrafish was calculated, and the number of fluorescent neutrophils and fluorescence intensity in the same area were identified.
Collection of zebrafish for research: 10 larvae were selected from each group. Alcian blue staining: Selected larvae were anesthetized and fixed in 4% PFA at 4 °C for 24 h. We removed PFA, and soaked the larvae in a mixture of 0.5 mL 0.5% KOH and 3% H2O2 for 2 h. We washed the larvae three times with PBST. After addition of 200 μL acidic alcohol and an equal amount of Alcian blue solution, the larvae were incubated overnight at 4 °C. The larvae were washed again three times with PBST. Photography and counting: The goblet cells were stained with Alcian blue solution, and the number was counted to determine the damage to the intestinal mucosa.
Baby fish were collected after 3 d of GA treatment. Altogether 16 tubes of fish were collected, with four tubes from each group and 10 fish in each tube. Beads and 500 μL Trizol were added to three tubes of each group. The total RNA of the fish was extracted, and the concentration of total RNA was measured. Quantitative polymerase chain reaction (qPCR) was used to analyze expression of genes of interest (Table 1).
| Gene | Primer sequence (5’-3’) | Length |
| ef1α | F: CTTCTCAGGCTGACTGTGC | 358 bp |
| R: CCGCTAGCATTACCCTCC | ||
| IL-1β | F: CCAGCTCTGAAATGATGGCAT | 139 bp |
| R: TCGCATCTGTAGCTCATTGC | ||
| IL-8 | F: ATTGAAACAGAAAGCCGA | 150 bp |
| R: CTTAACCCATGGAGCAGA |
The area and fluorescence intensity of the intestinal lumen were analyzed using Image J software. GraphPad Prism 8 software was used for data analysis. One-way ANOVA was used to compare the means of groups. P < 0.05 indicated statistically significant differences.
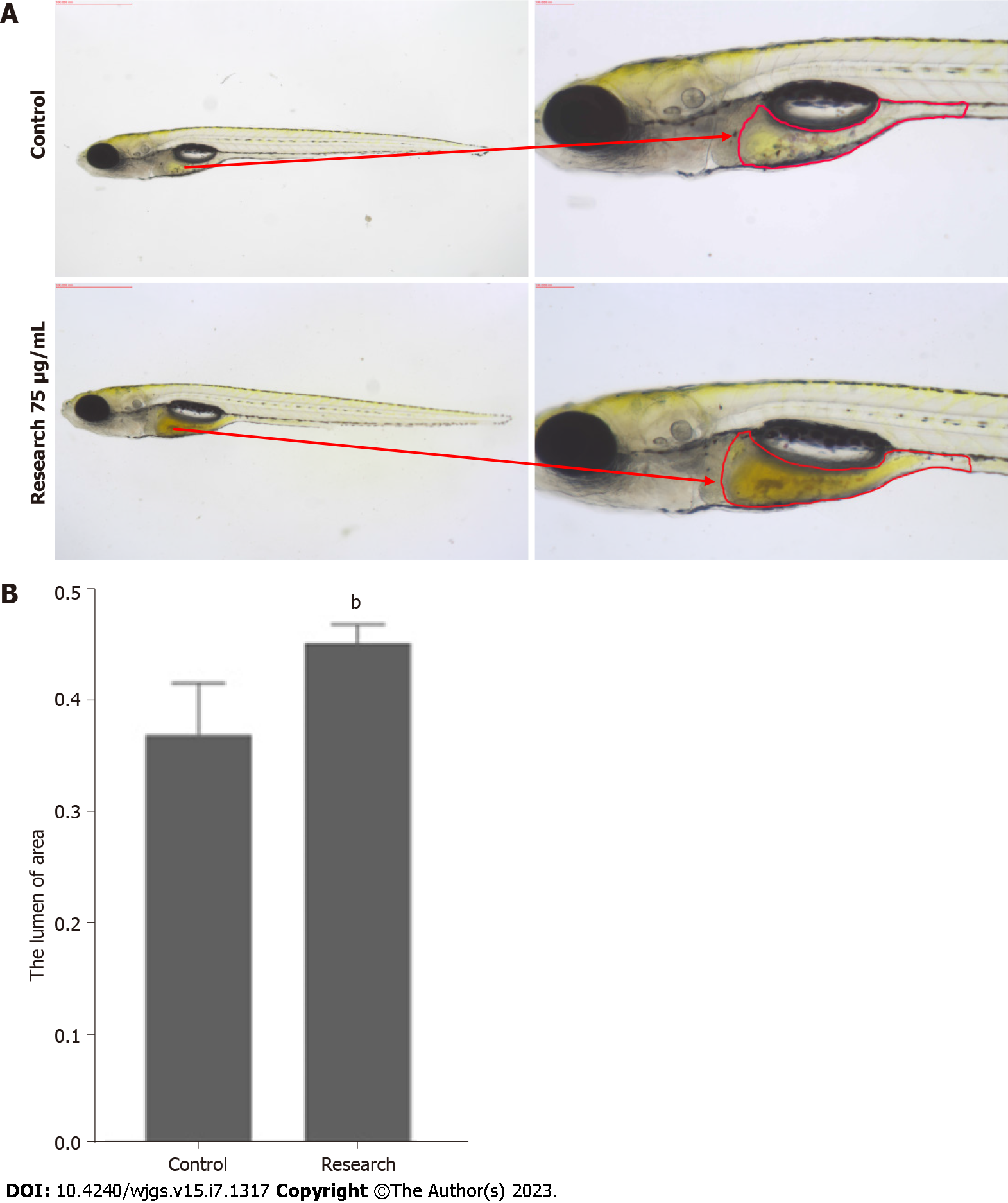
By observing the phenotypes of zebrafish in the control group and research group, compared with the former, the area of the intestinal lumen of the latter was increased (Figure 1A). The area of the intestinal lumen of the two groups of zebrafish was significantly different (Figure 1B) (P < 0.05).
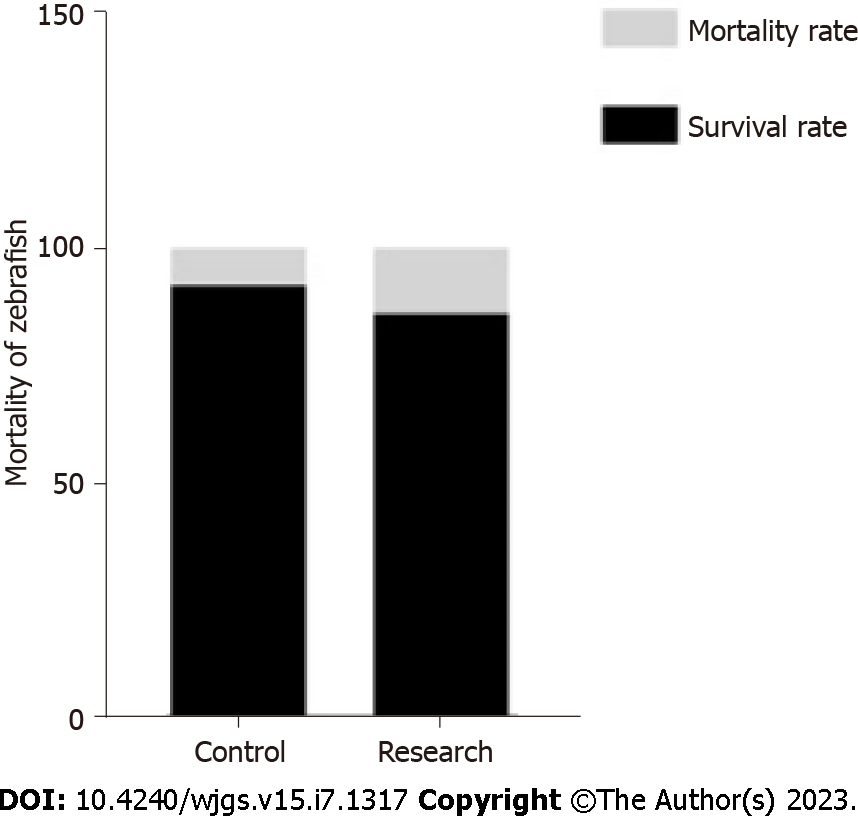
There was no significant difference in mortality between the research and control groups, and the survival rate of zebrafish was not increase after being exposed to TNBS (Figure 2). A concentration of 75 μg/mL TNBS was safe.
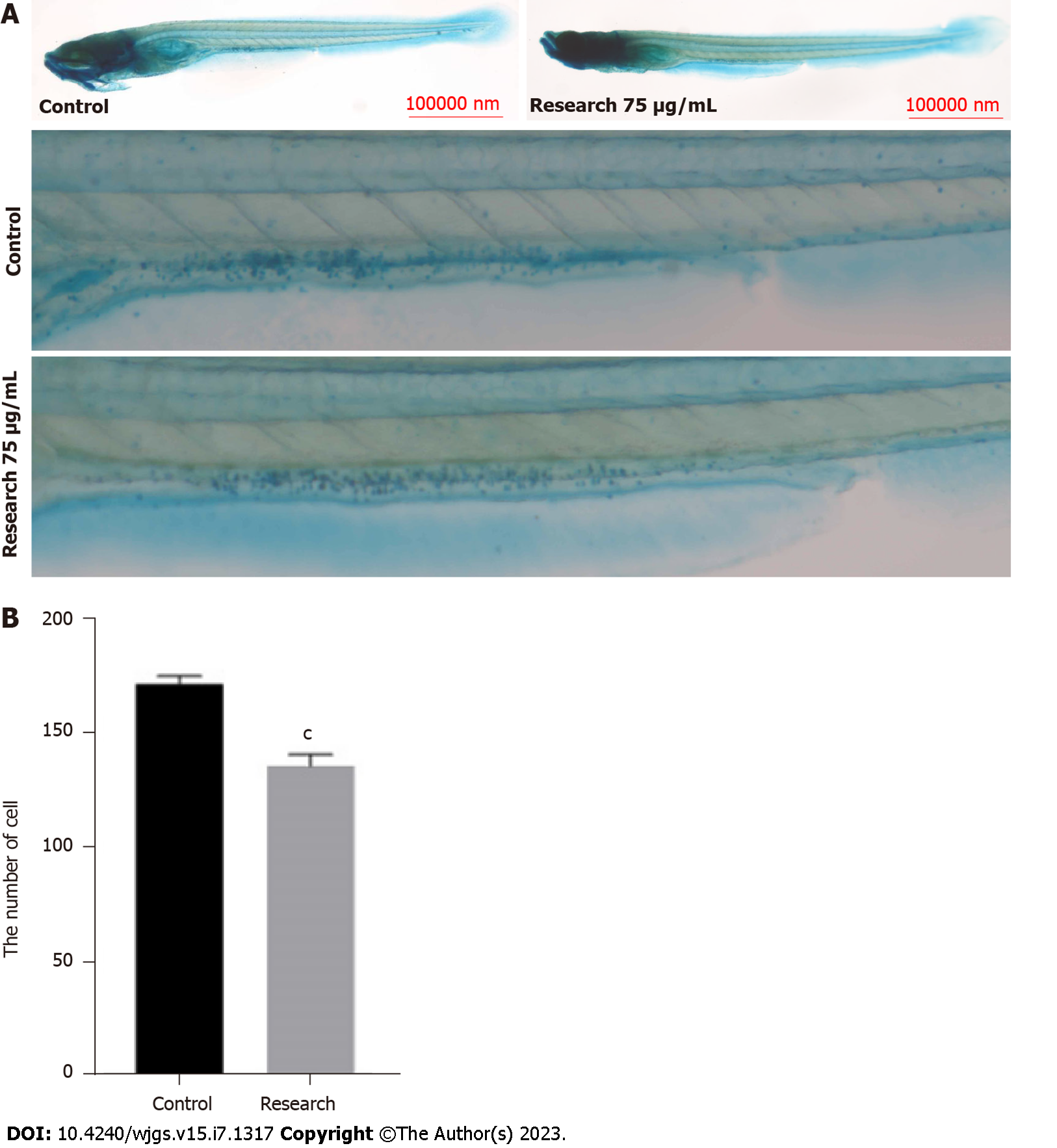
Compared with zebrafish in the control group, the research groups had a significantly increased number of goblet cells in the intestinal lumen (P < 0.05) (Figures 3A and B).
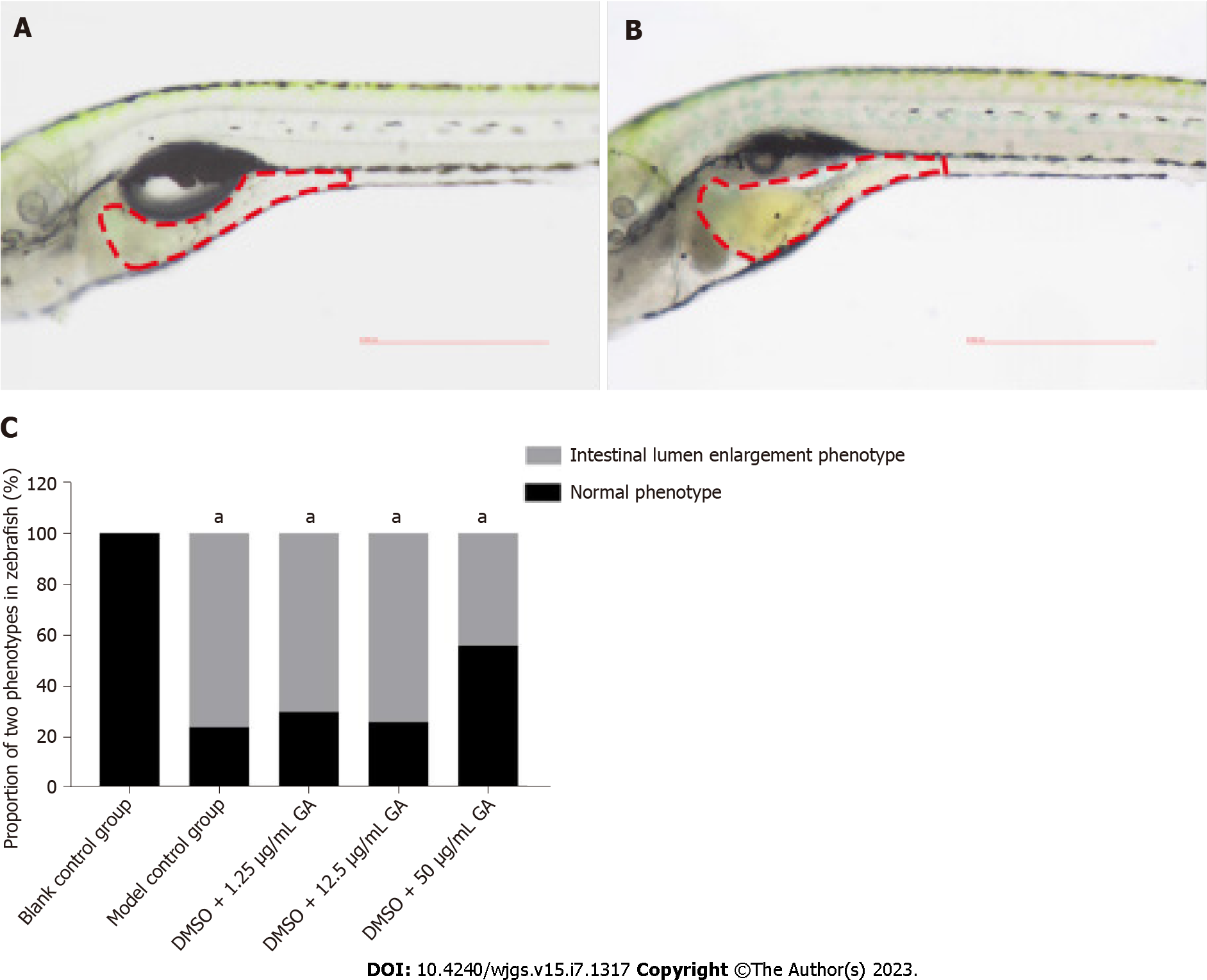
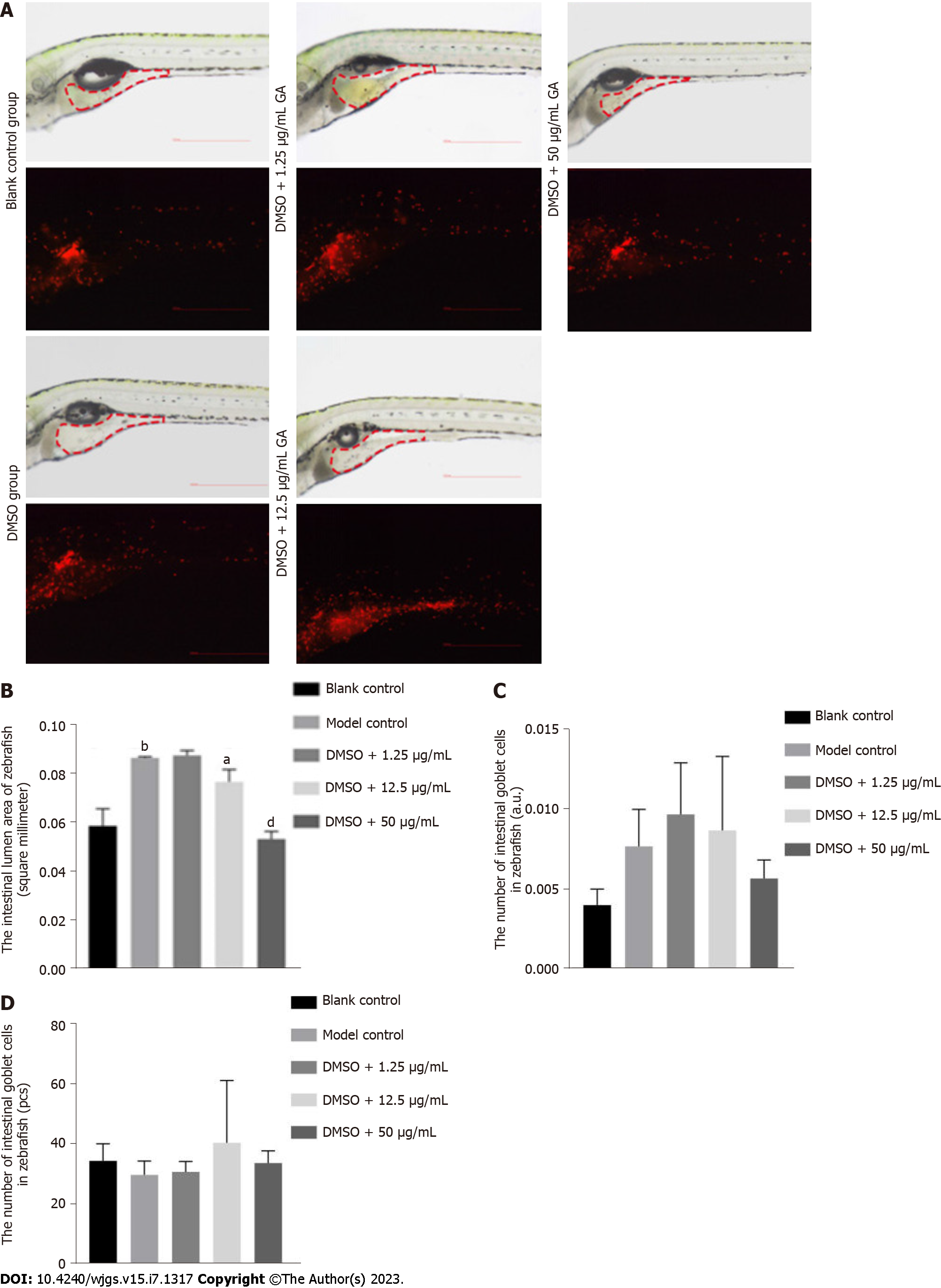
After 3 d of GA administration, the phenotypes in each group were divided into two categories according to the degree of malformation: Normal phenotype and intestinal luminal enlargement (Figures 4A and B). The proportion of phenotypes in each group is shown in Table 2. Comparison between the blank control and model control groups revealed that TNBS induced colitis in zebrafish (Figure 4C). It can be seen from the DMSO + 50 μg/mL GA group that GA had a protective effect against TNBS-induced colitis.
| Group | Normal phenotype | Intestinal lumen enlargement |
| Blank control group | 0/50 | 0/50 |
| Model control group | 11/45 | 34/45 |
| DMSO + 1.25 μg/mL GA group | 13/43 | 30/43 |
| DMSO + 12.5 μg/mL GA group | 12/46 | 34/46 |
| DMSO + 50 μg/mL GA group | 18/32 | 14/32 |
After 3 d of GA administration, the intestinal lumen of zebrafish was photographed using stereo and stereo fluorescence microscopes (Figure 5A), and the number of fluorescent neutrophils and fluorescence intensity in the same area were identified. The area of the intestinal lumen of zebrafish in the blank control group was substantially different from that of zebrafish in the model control group (P = 0.0024), indicating that TNBS induced colitis in zebrafish (Figure 5B). Comparison between the research group with a medium concentration of GA (P = 0.0292) and the research group with a high concentration of GA (P < 0.0001) revealed that GA had a protective effect against TNBS-induced colitis. No difference was found in the number of fluorescent neutrophils and fluorescence intensity in the intestinal lumen of zebrafish of all groups (Figures 5C and D).
After 3 d of GA administration, the zebrafish were stained with Alcian blue solution (Figure 6A). The number of goblet cells in the intestinal lumen of zebrafish was counted. There was a significant difference in the number of goblet cells in the intestinal lumen of zebrafish in the blank control group (P < 0.0001) and the model control group, indicating that TNBS induced colitis in zebrafish (Figure 6B). Comparison between the model control group and research groups with low, medium and high concentrations of GA (P = 0.0449, P = 0.0104, and P = 0.0003, respectively) showed that GA had a protective effect against TNBS-induced colitis, which was significantly embodied in the research group with high concentration of GA.
Baby fish were collected after 3 d of GA administration. The total RNA of the fish was extracted, and the concentration measured. qPCR was used to analyze expression of the genes of interest.
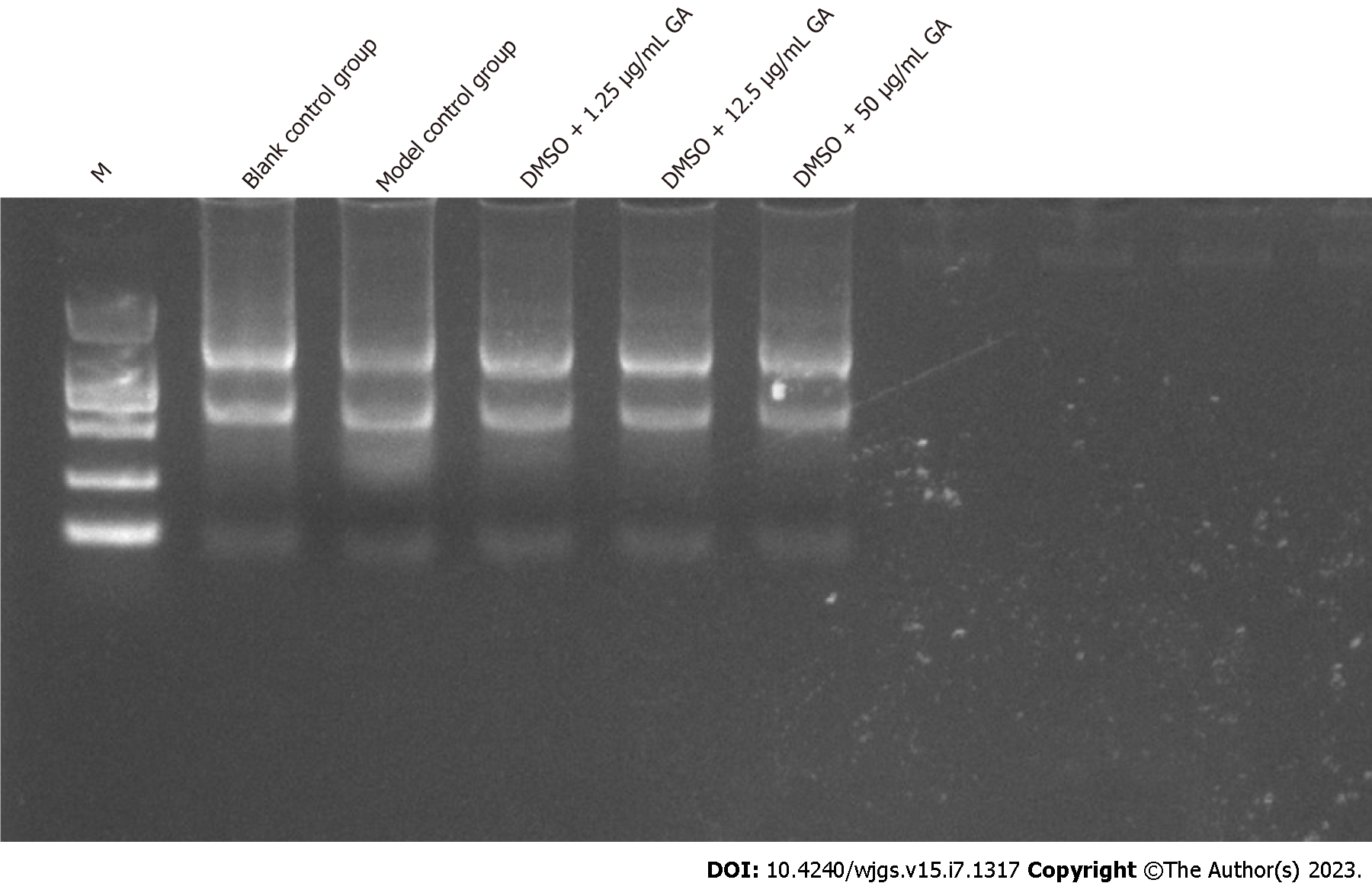
The concentration of RNA extracted from larvae after 3 d of GA administration was 214.584 ng/μL (blank control group), 451.88 ng/μL (model control group), 374.74 ng/μL (DMSO + 1.25 μg/mL GA group), 140.052 ng/μL (DMSO + 12.5 μg/mL GA group), and 139.372 ng/μL (DMSO + 50 μg/mL GA). A260/A280 ratio was over 1.8, which indicated that the RNA was pure. According to the electrophoresis results (Figure 7), two RNA bands were observed. RNA was not degraded and it was pure. Therefore, subsequent experiments were performed.
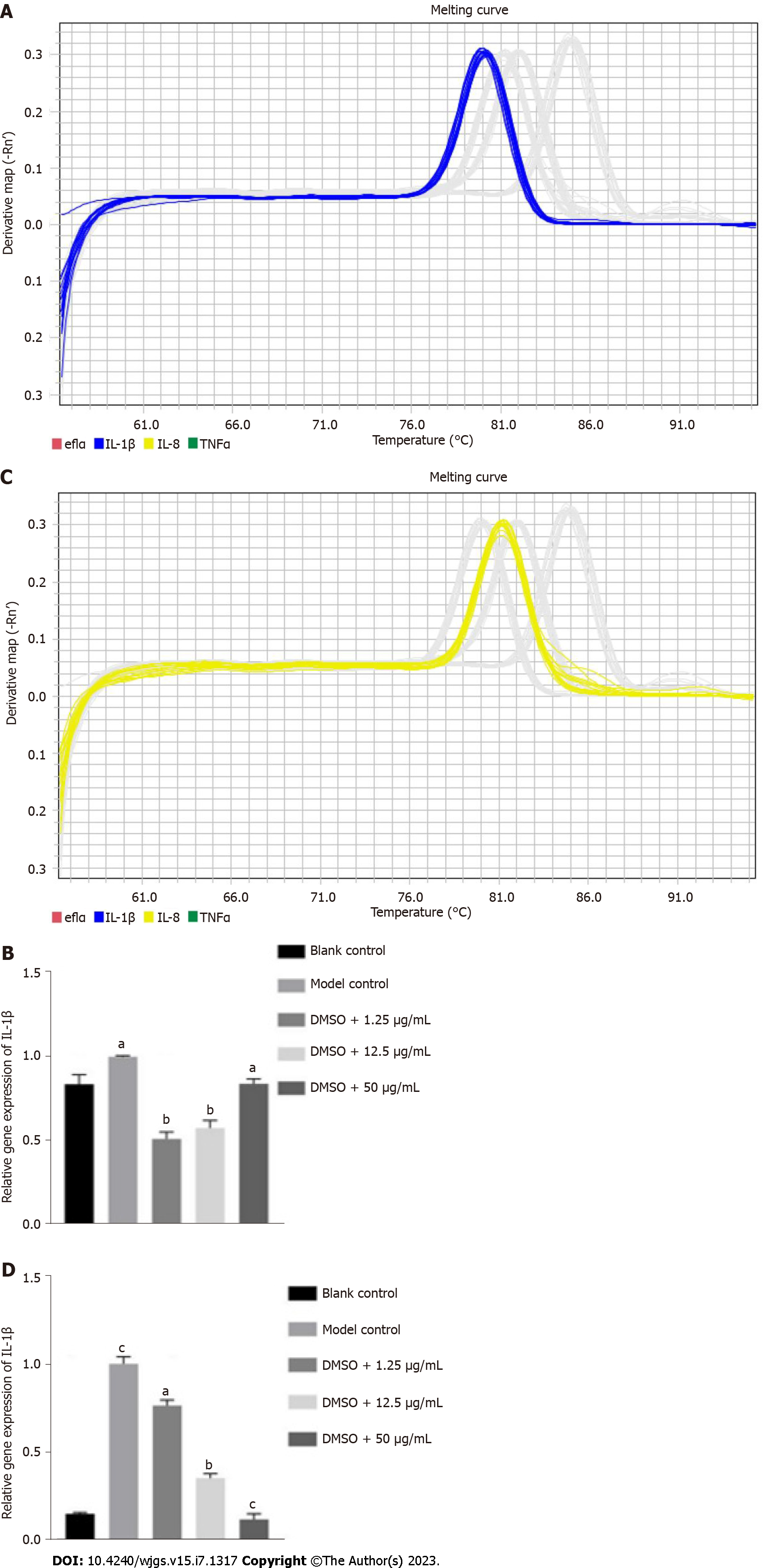
There was a significant difference in the expression of IL-1β between the blank control group (P = 0.0005) and model control group, indicating that TNBS induced colitis in zebrafish (Figures 8A and B). Significant differences in the expression of IL-1β were also found between the model control group and research groups with low, medium and high concentrations of GA (P = 0.0259, P = 0.0021, and P = 0.0161, respectively), which revealed that GA had a protective effect against TNBS-induced colitis. There was a significant difference in the expression of IL-8 between the blank control group (P = 0.0440) and the model control group, indicating that TNBS was able to induce colitis in zebrafish (Figures 8C and D). Significant differences in the expression level of IL-8 were also found between the model control group and research groups with low, medium and high concentrations of GA (P = 0.0023, P = 0.0045, and P = 0.0004, respectively), which suggested that GA had a protective effect against TNBS-induced colitis.
HAEC is a complication that can occur both preoperatively and postoperatively, and is a common cause of death in children with HD. With the deepening of research on HAEC, the main theories on its etiology have gradually transitioned from mechanical obstruction, infection, and mucin abnormalities to intestinal mucosal immunodeficiency, damaged defense barriers, and abnormal gene expression, but there is still no clear explanation of its pathogenesis[18-20]. As a protein widely distributed in the nucleus, HMGB1, under normal conditions, plays a role in the replication, transcription, recombination and repair of DNA to maintain the structural stability of the nucleosomes[21]. When stimulated by inflammatory mediators, HMGB1 is actively secreted by immune cells such as mononuclear phagocytes and neutrophils, and the damaged and dead cells also passively release HMGB1 extracellularly[22]. Extracellular HMGB1 binds to corresponding cell surface receptors and exerts biological effects. TLR4 is a transmembrane receptor in the immune system that activates downstream signaling molecules (for example, nuclear factor-kB) by recognizing pathogen-associated molecular patterns, and eventually release inflammatory factors like IL-1β[23].
The human gastrointestinal neuroendocrine regulatory system consists of two parts: The intestinal endocrine cells and the enteric nervous system. Intestinal endocrine cells make up 1% of all intestinal cells and can produce large amounts of hormones/peptides with at least 15 species. These hormones act through cells, neurons, blood, or synaptic transmission. The enteric nervous system is regulated by the central nervous system and the autonomic nervous system, which regulates intestinal homeostasis by its interaction with endocrine cells[24]. Zebrafish have a similar intestinal structure with enteric nervous system and intestinal endocrine cells. Depending on the morphology and gene expression, the intestines of zebrafish can be divided into foregut, midgut and hindgut. The intestinal nerve cells of zebrafish are present between the circular and longitudinal muscle layer. There are finger-like cellular protrusions (also called filopodia) in the intestine of zebrafish[25], which consist of various types of differentiated intestinal cells, such as enterocytes, intestinal goblet cells, and intestinal endocrine cells[26]. In this study, a TNBS-induced enteritis model in zebrafish was constructed in order to explore the relationship between the HMGB1/TLR4 pathway and HAEC.
The regulation of HMGB1 to intervene in the occurrence and development of HMGB1-associated diseases has become a research hotspot. Inhibitors of HMGB1 such as HMGB1 A-Box, ethyl pyruvate, and GA can interfere with the ex
The prevention and treatment of HAEC is a serious challenge in pediatric surgery. Exploring the mechanism of HAEC is conducive to the prevention of this disease. In this study, a TNBS-induced enteritis model in zebrafish was developed, and zebrafish were treated with different concentrations of GA. The effects of GA on the phenotype and inflammation of zebrafish were analyzed, and showed that different concentrations of GA had a protective effect on zebrafish with enteritis. In particular, high concentrations of GA significantly reduced the intestinal luminal area of zebrafish, increased the number of goblet cells in the intestinal lumen, and reduced the levels of IL-1β and IL-8. These findings provide a theoretical and experimental basis for the prevention and treatment of HAEC.
Hirschsprung’s disease (HD)-associated enterocolitis (HAEC) is the most common and serious complication of HD. The intestinal structure of zebrafish is similar to that of humans. The zebrafish model can effectively fill the gap between in vitro and mammalian experiments, and improve the existing drug research and development process.
At present, the pathogenesis of HAEC is not clear. Therefore, clarifying the pathogenic mechanism or characteristic pathogenic pathway of HAEC and blocking the vicious cycle of the pathogenic pathway may be an effective way to prevent and treat HAEC.
This study aimed to explore the therapeutic effect and possible mechanism of action of glycyrrhizic acid (GA) on HAEC, and provide an important theoretical and experimental basis for the prevention and treatment of HAEC.
In this study, fish fry at 3 d post-fertilization (dpf) were treated with trinitrobenzene sulfonic acid (TNBS), and 8 dpf enteritis model fish were obtained after 5 d of continuous treatment between 8 and 11 dpf, and changed daily for 3 d. To analyze the effect of GA on the phenotype and inflammatory status of zebrafish, zebrafish can be divided into normal phenotype and intestinal enlargement phenotype according to malformation.
In zebrafish, the intestinal luminal area of TNBS-treated zebrafish was increased compared with that of TNBS-untreated zebrafish. Although TNBS increased the number of goblet cells in the intestinal lumen and promoted intestinal inflammation, TNBS treatment did not decrease the survival rate of zebrafish. After GA treatment, the intestinal luminal area, number of goblet cells in the intestinal lumen, and levels of interleukin (IL)-1β and IL-8 were significantly changed.
TNBS was used to construct a zebrafish enteritis model, and different concentrations of GA were used to treat enteritis. In the analysis of the effects of GA on the phenotype and inflammatory state of zebrafish, different concentrations of GA had protective effects on zebrafish with enteritis.
The protective effect of GA on zebrafish enteritis was confirmed, but its specific mechanism still needs to be further explored.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dimitriadis E, Australia; Horne ZD, United States S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Chantakhow S, Khorana J, Tepmalai K, Boonchooduang N, Chattipakorn N, Chattipakorn SC. Alterations of Gut Bacteria in Hirschsprung Disease and Hirschsprung-Associated Enterocolitis. Microorganisms. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Roorda D, Oosterlaan J, van Heurn E, Derikx JPM. Risk factors for enterocolitis in patients with Hirschsprung disease: A retrospective observational study. J Pediatr Surg. 2021;56:1791-1798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Lewit RA, Kuruvilla KP, Fu M, Gosain A. Current understanding of Hirschsprung-associated enterocolitis: Pathogenesis, diagnosis and treatment. Semin Pediatr Surg. 2022;31:151162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Takeda M, Miyano G, Nakazawa-Tanaka N, Shigeta Y, Lane GJ, Doi T, Takahashi T, Urao M, Okazaki T, Ochi T, Koga H, Yamataka A. Forty-Year Experience Alleviating Postoperative Hirschsprung-Associated Enterocolitis by Complete Full-Thickness Posterior Rectal Cuff Excision. The Anorectal Line Eliminates Problematic Anastomoses. J Laparoendosc Adv Surg Tech A. 2021;31:1436-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Hagens J, Reinshagen K, Tomuschat C. Prevalence of Hirschsprung-associated enterocolitis in patients with Hirschsprung disease. Pediatr Surg Int. 2022;38:3-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Li Q, Zhang Z, Xiao P, Ma Y, Yan Y, Jiang Q, Low Y, Li L. Surgical approach and functional outcome of redo pull-through for postoperative complications in Hirschsprung's disease. Pediatr Surg Int. 2021;37:1401-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Svetanoff WJ, Lopez J, Aguayo P, Hendrickson RJ, Oyetunji TA, Rentea RM. The impact of botulinum injection for hospitalized children with Hirschsprung-associated enterocolitis. Pediatr Surg Int. 2021;37:1467-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Svetanoff WJ, Lopez JJ, Briggs KB, Fraser JA, Fraser JD, Oyetunji TA, Peter SDS, Rentea RM. Management of Hirschsprung associated enterocolitis-How different are practice strategies? An international pediatric endosurgery group (IPEG) survey. J Pediatr Surg. 2022;57:1119-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Moesch M, Usemann J, Bruder E, Romero P, Schwab C, Niesler B, Tapia-Laliena MA, Khasanov R, Nisar T; Study Group NIG Retro, Holland-Cunz S, Keck S. Associations of Mucosal Nerve Fiber Innervation Density with Hirschsprung-Associated Enterocolitis: A Retrospective Three-Center Cohort Study. Eur J Pediatr Surg. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Cai J, Lin Z. Correlation of blood high mobility group box-1 protein with mortality of patients with sepsis: A meta-analysis. Heart Lung. 2021;50:885-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Olona A, Hateley C, Muralidharan S, Wenk MR, Torta F, Behmoaras J. Sphingolipid metabolism during Toll-like receptor 4 (TLR4)-mediated macrophage activation. Br J Pharmacol. 2021;178:4575-4587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 12. | Jeyaseelan S, Chu HW, Young SK, Freeman MW, Worthen GS. Correction for Jeyaseelan et al, "Distinct Roles of Pattern Recognition Receptors CD14 and Toll-Like Receptor 4 in Acute Lung Injury". Infect Immun. 2022;90:e0001422. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Wang L, Botchway BOA, Liu X. The Repression of the HMGB1-TLR4-NF-κB Signaling Pathway by Safflower Yellow May Improve Spinal Cord Injury. Front Neurosci. 2021;15:803885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Li Y, Guo Z, Zhang G, Tian X, Li Q, Luo Z. Neonatal Streptococcus Pneumoniae pneumonia induces airway SMMHC expression through HMGB1/TLR4/ERK. Immunol Lett. 2021;240:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Kovler ML, Gonzalez Salazar AJ, Fulton WB, Lu P, Yamaguchi Y, Zhou Q, Sampah M, Ishiyama A, Prindle T Jr, Wang S, Jia H, Wipf P, Sodhi CP, Hackam DJ. Toll-like receptor 4-mediated enteric glia loss is critical for the development of necrotizing enterocolitis. Sci Transl Med. 2021;13:eabg3459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 16. | Hu LH, Liu JY, Yin JB. Eriodictyol attenuates TNBS-induced ulcerative colitis through repressing TLR4/NF-kB signaling pathway in rats. Kaohsiung J Med Sci. 2021;37:812-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Xiong T, Zheng X, Zhang K, Wu H, Dong Y, Zhou F, Cheng B, Li L, Xu W, Su J, Huang J, Jiang Z, Li B, Zhang B, Lv G, Chen S. Ganluyin ameliorates DSS-induced ulcerative colitis by inhibiting the enteric-origin LPS/TLR4/NF-κB pathway. J Ethnopharmacol. 2022;289:115001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 76] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 18. | Le-Nguyen A, Righini-Grunder F, Piché N, Faure C, Aspirot A. Factors influencing the incidence of Hirschsprung associated enterocolitis (HAEC). J Pediatr Surg. 2019;54:959-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Cheng Z, Zhao L, Dhall D, Ruegger PM, Borneman J, Frykman PK. Bacterial Microbiome Dynamics in Post Pull-Through Hirschsprung-Associated Enterocolitis (HAEC): An Experimental Study Employing the Endothelin Receptor B-Null Mouse Model. Front Surg. 2018;5:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Klein M, Varga I. Hirschsprung's Disease-Recent Understanding of Embryonic Aspects, Etiopathogenesis and Future Treatment Avenues. Medicina (Kaunas). 2020;56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Yang H, Wang H, Andersson U. Targeting Inflammation Driven by HMGB1. Front Immunol. 2020;11:484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 419] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 22. | Andersson U, Tracey KJ, Yang H. Post-Translational Modification of HMGB1 Disulfide Bonds in Stimulating and Inhibiting Inflammation. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 23. | Guan Y, Chen JQ, Li XY, Jiang SN. ClyA enhances LPS-induced IL-1β secretion in human macrophages through TLR4 and NLRP3 signaling. J Biol Regul Homeost Agents. 2021;35:495-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Cirmi S, Randazzo B, Russo C, Musumeci L, Maugeri A, Montalbano G, Guerrera MC, Lombardo GE, Levanti M. Anti-inflammatory effect of a flavonoid-rich extract of orange juice in adult zebrafish subjected to Vibrio anguillarum-induced enteritis. Nat Prod Res. 2021;35:5350-5353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Kuil LE, Chauhan RK, Cheng WW, Hofstra RMW, Alves MM. Zebrafish: A Model Organism for Studying Enteric Nervous System Development and Disease. Front Cell Dev Biol. 2020;8:629073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 26. | Graves CL, Chen A, Kwon V, Shiau CE. Zebrafish harbor diverse intestinal macrophage populations including a subset intimately associated with enteric neural processes. iScience. 2021;24:102496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Hubert P, Roncarati P, Demoulin S, Pilard C, Ancion M, Reynders C, Lerho T, Bruyere D, Lebeau A, Radermecker C, Meunier M, Nokin MJ, Hendrick E, Peulen O, Delvenne P, Herfs M. Extracellular HMGB1 blockade inhibits tumor growth through profoundly remodeling immune microenvironment and enhances checkpoint inhibitor-based immunotherapy. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 28. | Yu R, Jiang S, Tao Y, Li P, Yin J, Zhou Q. Inhibition of HMGB1 improves necrotizing enterocolitis by inhibiting NLRP3 via TLR4 and NF-κB signaling pathways. J Cell Physiol. 2019;234:13431-13438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 29. | Zhu ZH, Li X, He LF, Cai HF, Ye B, Wu ZM. Glycyrrhizic acid, as an inhibitor of HMGB1, alleviates bleomycin-induced pulmonary toxicity in mice through the MAPK and Smad3 pathways. Immunopharmacol Immunotoxicol. 2021;43:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Niu Z, Lin J, Hao C, Xu X, Wang C, Dai K, Deng X, Deng M, Guo Y, Yao W. Glycyrrhizic Acid Attenuates Pulmonary Fibrosis of Silicosis by Inhibiting the Interaction between HMGB1 and BRG1 through PI3K/Akt/mTOR Pathway. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Zhang XL, Li B, Zhang X, Zhu J, Xie Y, Shen T, Tang W, Zhang J. 18β-Glycyrrhetinic acid monoglucuronide (GAMG) alleviates single-walled carbon nanotubes (SWCNT)-induced lung inflammation and fibrosis in mice through PI3K/AKT/NF-κB signaling pathway. Ecotoxicol Environ Saf. 2022;242:113858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Zhang Q, Wang Y, Wang Z, Mohammed EAH, Zhao Q, He D. Synthesis and anti-inflammatory activities of glycyrrhetinic acid derivatives containing disulfide bond. Bioorg Chem. 2022;119:105542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Zhou S, Liu G, Si Z, Yu L, Hou L. Glycyrrhizin, an HMGB1 inhibitor, Suppresses Interleukin-1β-Induced Inflammatory Responses in Chondrocytes from Patients with Osteoarthritis. Cartilage. 2021;13:947S-955S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |