Published online Jul 27, 2023. doi: 10.4240/wjgs.v15.i7.1304
Peer-review started: January 30, 2023
First decision: March 24, 2023
Revised: April 6, 2023
Accepted: April 21, 2023
Article in press: April 21, 2023
Published online: July 27, 2023
Processing time: 172 Days and 0.3 Hours
Different metabolic/bariatric surgery approaches vary in their effect on weight loss and glucose levels, although the underlying mechanism is unclear. Studies have demonstrated that the gut microbiota might be an important mechanism of improved metabolism after metabolic/bariatric surgery.
To investigate the relationship between the improvement in metabolic distur
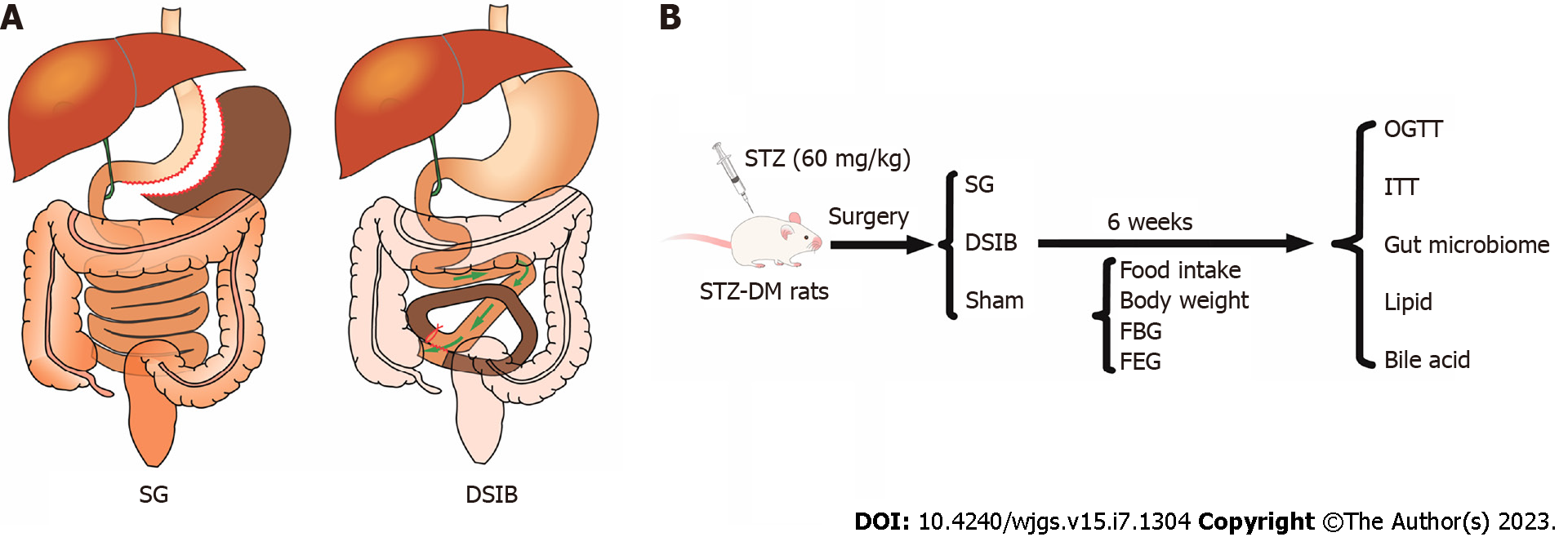
We performed sleeve gastrectomy (SG), distal small intestine bypass (DSIB) or sham surgery in nonobese rats with diabetes induced by 60 mg/kg streptozotocin (STZ-DM).
The group comparisons revealed that both SG and DSIB induced a reduction in body weight and significant improvements in glucose and lipid metabolism in the STZ-DM rats. Furthermore, DSIB exhibited a stronger glucose-lowering and lipid-reducing effect on STZ-DM rats than SG. 16S ribosomal RNA gene sequencing revealed the gut abundance of some Lactobacillus spp. increased in both the SG and DSIB groups after surgery. However, the DSIB group exhibited a more pronounced increase in the gut abundance of Lactobacillus spp. compared to the SG group, with more Lactobacillus spp. types increased in the gut.
The gut abundance of Lactobacillus was significantly correlated with the improvement in glycolipid metabolism and the change in serum fibroblast growth factor 21 levels.
Core Tip: Sleeve gastrectomy and distal small intestine bypass induced a reduction in body weight and significant improvements in glucose and lipid metabolism in the rats with streptozotocin-induced nonobese diabetes. The gut abundance of some Lactobacillus spp. increased in both the sleeve gastrectomy and distal small intestine bypass groups after surgery. The gut abundance of Lactobacillus was significantly correlated with the improvement in glycolipid metabolism and the change in serum fibroblast growth factor 21 levels.
- Citation: Luo X, Tan C, Tao F, Xu CY, Zheng ZH, Pang Q, He XA, Cao JQ, Duan JY. Differences in metabolic improvement after metabolic surgery are linked to the gut microbiota in non-obese diabetic rats. World J Gastrointest Surg 2023; 15(7): 1304-1316
- URL: https://www.wjgnet.com/1948-9366/full/v15/i7/1304.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i7.1304
The prevalence of type 2 diabetes has been increasing globally. The International Diabetes Federation estimates that 1 in 11 adults aged 20-79 years had diabetes in 2015 worldwide, with 642 million expected to be affected by 2040[1]. Diabetes often coexists with obesity. The Global Burden of Disease Obesity Collaborators estimate that a total of 603.7 million adults are obese, with obesity prevalence doubling in 73 countries between 1980 and 2015 and continuing to rise in most other countries[2]. In China, 34.0% and 16.4% of adults (≥ 18 years) were estimated to be overweight and obese, respectively, in the 2015–2019 period[3].
Metabolic/bariatric surgery (MBS), the most effective treatment for obesity, is characterized by rapid weight loss and improved metabolism. Initially, MBS was considered to include a mechanical process involving the restriction of food intake and absorption and to lead to physiological changes such as gastrointestinal hormones[4-6]. Recent studies have increasingly shown that the gut microbiota plays an important role in improving metabolism after MBS[7-9]. Currently, the most commonly performed MBS procedures are sleeve gastrectomy (SG) and gastric bypass (GBP). Studies indicate that GBP is associated with significantly better metabolic improvement than SG[10], which may be related to the bypass of a section of the small intestine during GBP.
Few studies have compared the effects of SG and small intestinal bypass on the gut microbiota or determined whether the differences in changes observed in the gut microbiota are associated with improved glycolipid metabolism. The elaboration of these aspects will aid in the elucidation of the changes imposed on the human body by these surgical approaches and will provide theoretical support for nonanatomical interventions in obesity and metabolic disorders.
In the present study, we performed SG and distal small bowel bypass (DSIB) in rats with streptozotocin-induced nonobese diabetes (STZ-DM). We investigated the difference in the efficacy between SG and DSIB to improve metabolic alterations by examining the gut microbiota. We also investigated the effect of different MBS procedures on the gut microbiota and assessed whether these changes were related to the mechanisms underlying the improvement in metabolic alterations.
Eight-week-old Sprague-Dawley (SD) male rats (269.3 ± 8.9 g) were provided by Slac Laboratory Animals Ltd. (Shanghai, China). Diabetes was induced in rats by 60 mg/kg STZ, and the rats were housed in individually ventilated cages. They were acclimatized to their environment for at least 1 wk prior to the experiment and had free access to tap water and standard rat chow. Rats were randomly divided into SG, DSIB and sham groups. Body weight, food intake, postprandial blood glucose product and fasting blood glucose (FBG) were recorded weekly after surgery. The oral glucose tolerance test (OGTT) and insulin tolerance test (ITT) were recorded preoperatively and 6 wk postoperatively. The animal experiments were conducted according to the Nanchang University Guide to Animal Experiments and approved by the Nanchang University Animal Ethics Committee. Standard animal care and laboratory guidelines were followed according to the ARRIVE guidelines.
After 14 h of fasting, the rats were operated on under anesthesia (isoflurane, 4% for induction and 2% for maintenance). The abdomen was trimmed, and the peritoneal cavity was accessed through a 4 cm midline incision.
For SG, the endopath ETS-FLEX 35 mm anastomosis (Ethicon Endo-Surgery, LLC, Cincinnati, OH) was used to transect the lateral 80% of the stomach along a large curve, leaving a sleeve-shaped gastric remnant in the lumen. For the DSIB group, the point near the ileocecal flap was used as a reference point. From this point to 40 mm distal to the flexural ligament, approximately 60% of the entire length of the small bowel was bypassed, and intestinal continuity was restored by side-to-side anastomosis between the distal jejunum and ileum. Luminal occlusion was performed by silk ligation in the first part of the bypass section. For the sham surgery, the peritoneal cavity was accessed through a 4 cm midline incision, and the bowel was gently manipulated. The abdominal cavity was closed with 3-0 silk sutures. The operative time was approximately 45 min. All rats were injected subcutaneously with 10 mL of sterile saline postoperatively and placed in separate cages to recover from anesthesia.
OGTT and ITT were performed preoperatively and 6 wk postoperatively. Rats underwent OGTT after a 14-h fast. Baseline blood glucose readings were obtained from the tail at the end of the fast. SD rats were given 20% glucose (1 g/kg) by gavage. Blood glucose was measured at 0, 15, 30, 60, 90 and 120 min, and the area under the glucose tolerance curve (AUCOGTT) was calculated. SD rats underwent ITT after 6 h of fasting. After baseline blood glucose readings, rats were injected intraperitoneally with insulin (0.5 IU/kg), and blood glucose levels were measured at 0, 15, 30, 45 and 60 min. Insulin sensitivity was assessed by the ratio of blood glucose to basal blood glucose at each time point, and the area under the insulin tolerance curve (AUCITT) was calculated.
Blood glucose levels were measured in blood collected from the tail vein of conscious rats by an electronic glucometer (Accu-Chek Performa, Roche Diagnostics, Switzerland). For FBG, food was removed at 8:00 a.m., and blood glucose levels were measured at 8:00 p.m. prior to surgery and each day after surgery, after an 8-h fast.
Rats were sacrificed after a 1 night fast, and blood was collected from the portal vein into biochemical tubes containing anticoagulant. After centrifugation at 3000 rpm for 15 min at 4 °C, the separated sera were immediately transferred to new tubes and stored at -80 °C until analysis. Serum total bile acids (TBA), serum total bilirubin, serum direct bilirubin, serum total cholesterol (CHOL), serum total triglyceride, serum high-density lipoprotein, serum low-density lipoprotein and serum nonhigh-density lipoprotein were determined using a fully automated biochemical analyzer. The analyses and tests were performed by the Biochemistry Laboratory of The Second Affiliated Hospital of Nanchang University.
Serum samples were collected and stored in aliquots at -80 °C until use. Insulin in serum was detected by enzyme-linked immunosorbent assay kits: insulin (Millipore, Billerica, MA, United States); glucagon-like peptide 1; ghrelin; PYY; leptin; and FGF21 (Uscn Life Sciences, Wuhan, China).
Microbial community DNA was extracted using MagPure fecal DNA KF kit B (Magen, China) according to the manufacturer’s instructions. DNA was quantified using a Qubit-dsDNA-BR analysis kit (Invitrogen, United States) using a Qubit fluorophotometer and checked for quality in aliquots on a 1% agarose gel. After extraction of DNA, the samples were tested. The samples that passed the test were used to construct the library. The target amplicon fragment was recovered, the interrupted sticky ends were repaired to flat ends using T4 DNA Polymerase, Klenow DNA Polymerase and T4 PNK, and then the DNA fragment was ligated to a special junction with a “T” base at the 3’ end by adding a base “A” at the 3’ end. The DNA fragment was then ligated to a special joint with a “T” base at the 3’ end. Sequencing was performed on the PacBio (Sequel) platform (BGI, Shenzhen, China), and sequences were assigned to operational taxonomic units (OTUs) based on 97% sequence similarity. The clean tags were clustered into OTUs using USEARCH software (v7.0.1090), and then the OTUs were annotated to complete the species classification of the OTUs. After obtaining the OTU representative sequences, the OTU representative sequences were compared with the database Greengene_2013_5_99 by RDP classifier (v2.2) software, and species annotation was performed with a confidence threshold set to 0.5. The overall diversity of the gut microbiota was assessed using the Shannon index, and the taxonomic classification of OTUs was annotated from the phylum level to the species level. The overall composition of the gut microbiota was further visualized using principal coordinate analysis. Linear discriminant analysis effect sizes with default parameters were used to identify differentially enriched bacterial taxa in different groups, and this analysis was performed on the Galaxy (harvard.edu) website.
Data were expressed as the mean ± standard error of the mean. All analyses were performed using GraphPad Prism version 8.4 and R 4.1.3, with the significance level set at 0.05. The area under the receiver operating characteristic curve (AUROC) was calculated using trapezoidal integration. Differences between the two groups were analyzed using a t test. Changes in body weight, change in food intake, FBG, OGTT and ITT over time were analyzed using two-way analysis of variance. Bonferroni’s test was used for pairwise comparisons between groups. Statistical significance was as follows: aP < 0.05, bP < 0.01 and cP < 0.001.
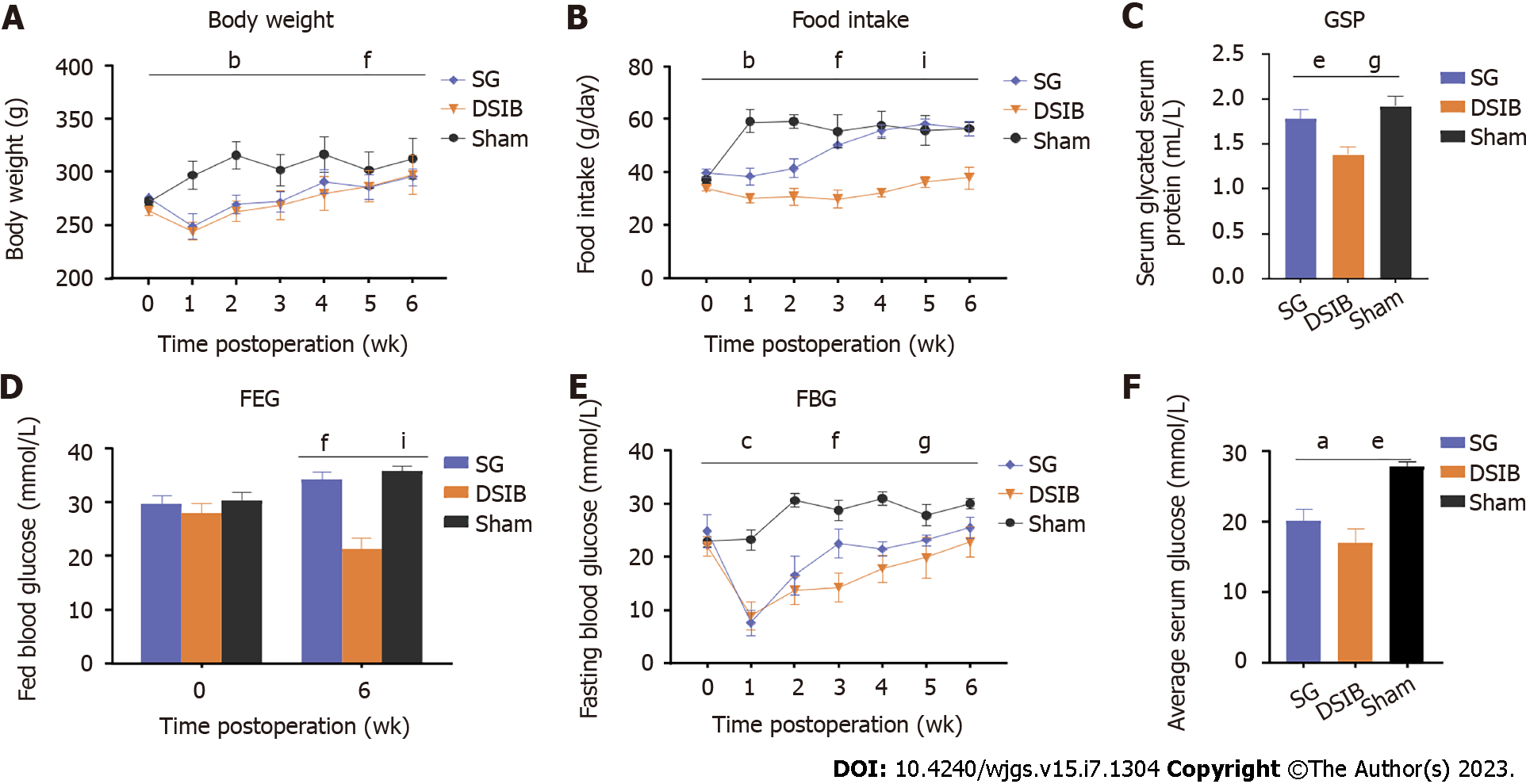
To compare the efficacy between bypass surgeries involving the gastric and intestinal tracts, we randomized SD rats with diabetes induced by STZ (60 mg/kg) to undergo SG, DSIB or sham surgery (Figure 1). Compared with the sham group, the body weight was significantly lower in the SG and DSIB groups 6 wk after surgery (Figure 2A). At 2 wk postoperatively, food intake was significantly lower in the DSIB group than in the SG and sham groups and in the SG group compared to the sham group; however, the difference in food intake gradually declined at later time points (Figure 2B). At 6 wk postoperatively, the glycated serum protein was lower in the DSIB group than in the SG and sham groups (Figure 2C). FBG was significantly lower in the SG and DSIB groups than in the sham group at 6 wk postoperatively, and the improvement was greater in the DSIB group than in the SG group (Figure 2D). The AUROC for feeding glucose after surgery was significantly lower in the DSIB group than in the SG and sham groups (Figure 2E). The feeding glucose level was significantly lower in the DSIB group than in the SG group.
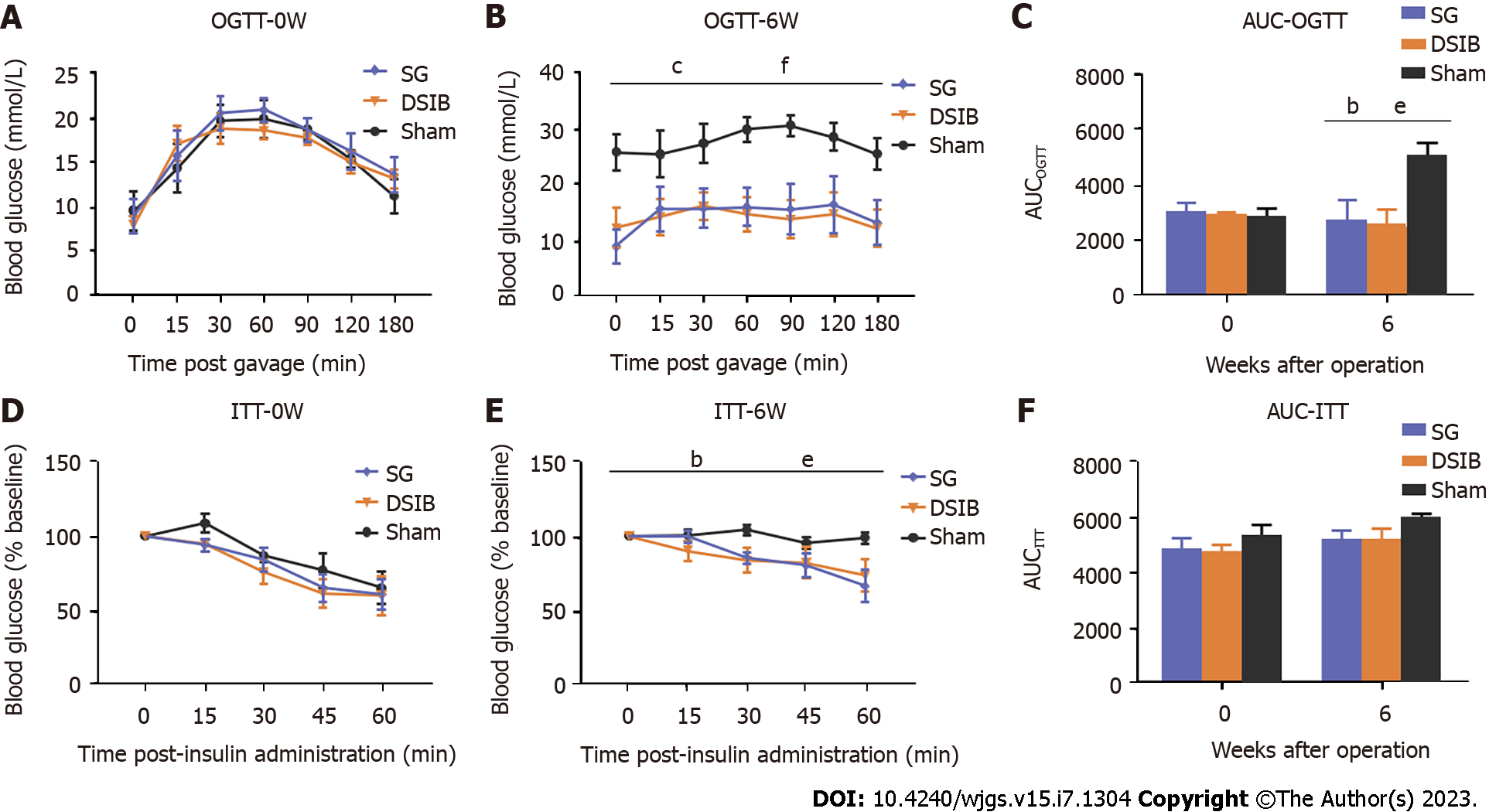
The OGTT performed at 6 wk postoperatively revealed that the AUROC for OGTT (AUCOGTT) was significantly lower in the SG and DSIB groups than in the sham group (Figure 3) and had significantly lower peaks at 30 min and 60 min (P < 0.05). At 6 wk postoperatively, the ITT indicated that the blood glucose levels at 30 min and 60 min were lower in the SG and DSIB groups than in the sham group; however, the AUCITT was not significantly different between the SG and the sham groups. Overall, the SG and DSIB groups exhibited significantly more weight loss and significantly better improvement in glucose metabolism, with DSIB showing an advantage over SG in terms of improved glucose metabolism.
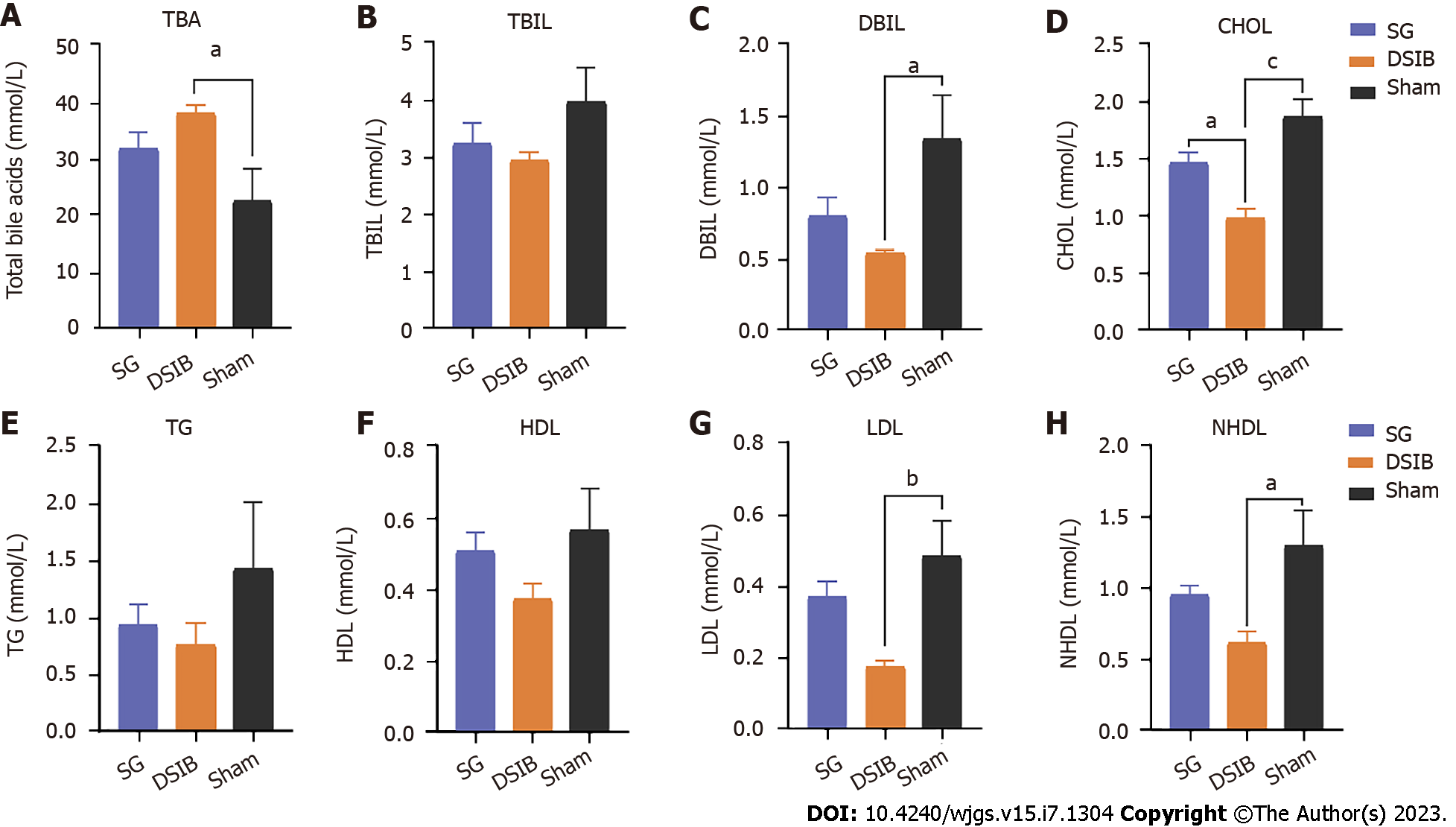
We investigated the metabolic changes in bile acids and lipids in the context of improved glucose metabolism in the SG and DSIB groups. We measured TBA and bilirubin levels in serum using high-performance liquid chromatography in the SG, DSIB and sham groups at 6 wk postoperatively. As shown in Figure 4A, the serum TBA levels were significantly higher in the DSIB group than in the sham group (P < 0.05), whereas there was no significant difference between the SG and sham groups. Additionally, the direct bilirubin levels were significantly lower in the DSIB group than in the sham group (Figure 4C) (P < 0.05).
DSIB was significantly more effective than SG in lowering blood lipid levels, and the CHOL levels were significantly lower in the DSIB group than in the other two groups (Figure 4D). Serum low-density lipoprotein and nonhigh-density lipoprotein levels were significantly lower after DSIB than after sham surgery (Figure 4D and E). Overall, DSIB led to a significant increase in serum bile acid levels and improved lipid metabolism compared to SG and sham surgery.
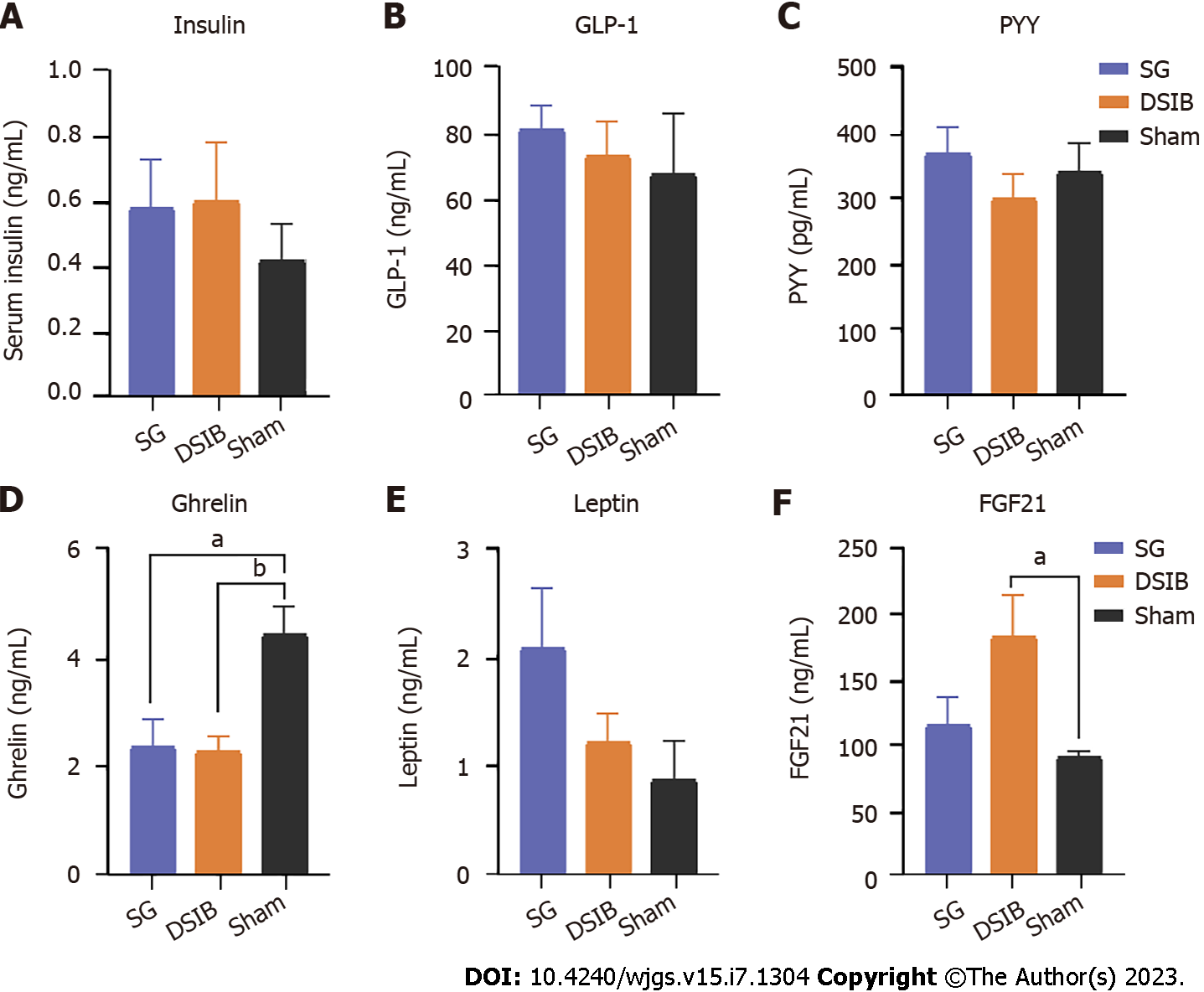
Next, we measured the levels of gastrointestinal hormones in rats undergoing SG and DSIB to compare the contribution of hormones to the metabolic improvement observed after MBS. The postoperative levels of FGF21 were significantly higher in the DSIB group than in the sham group (Figure 5). Additionally, the serum ghrelin levels were lower in the SG and DSIB groups than in the sham group (Figure 5). At 6 wk postoperatively, there were no significant differences in the serum levels of insulin, glucagon-like peptide 1 and PYY between the SG and sham groups or between the DSIB and sham groups (P > 0.05 for all).
To further elucidate the changes in gut microbiota after SG and DSIB, we collected feces from rats at 6 wk after surgery and analyzed the microbiota in samples using 16S ribosomal RNA gene sequencing. Figure 6C shows the estimation of alpha diversity in the gut microbiota of the study groups using the Shannon index. As shown in Figure 6B, the assessment of overall gut microbial variation using weighted UniFrac principal coordinates analysis indicated differences in the beta diversity of fecal flora composition among the SG, DSIB and sham groups. The Shannon index did not show differences in the overall diversity among the groups (Figure 6D and E). At the phylum level, compared to the sham group, the DSIB group showed a significant increase in the abundance of Firmicutes, whereas the SG group exhibited an increase in the abundance of Bacteroides. At the genus level, the DSIB group showed a significant increase in the abundance of Lactobacillus and a significant decrease in the abundance of Prevotella.
The identity of the gut microbiota significantly differed among the SG, DSIB and sham groups using linear discriminant analysis effect size (Figure 7A and B). Specifically, the gut microbiota in the SG group was significantly enriched in Lactobacillus helveticus and Lactobacillus hamster compared to the sham group. Additionally, the gut microbiota of the DSIB group exhibited a significant increase in the abundance of Lactobacillus spp. including Lactobacillus pontis, Lactobacillus helveticus and Lactobacillus vaginalis, and a decrease in the abundance of the genus Prevotella compared to the sham group.
In general, both the SG and DSIB groups showed an increase in the gut abundance of Lactobacillus compared to the sham group, although the specific species and the degree of change differed between the SG and DSIB groups. Specifically, the DSIB group exhibited a more pronounced and species-rich increase in the abundance of Lactobacillus spp. and a decrease in the abundance of Prevotella spp.
The correlation analysis revealed that the gut abundance of Lactobacillus was significantly correlated with body weight, CHOL and serum FGF21 levels. Additionally, the increased abundance of Lactobacillus helveticus in the SG group was associated with a decrease in the AUCOGTT and body weight, and the increased abundance of Lactobacillus pontis and Lactobacillus vaginalis in the DSIB group was significantly correlated with improved glucolipid metabolism. The decreased abundance of Prevotella spp. was associated with a decrease in the AUCOGTT and food intake in all three groups.
No study to date has compared the efficacy between SG and DSIB in improving metabolic alterations. In the present study, we found that both SG and DSIB significantly reduced body weight and improved glucolipid metabolism in STZ-DM rats and that DSIB was significantly more effective than SG in improving metabolic alterations. In addition, the gut changed significantly after surgery in both the SG and DSIB groups, with the altered gut abundance of Lactobacillus spp. observed in both groups. Unlike the SG group, the DSIB group exhibited a significant decrease in the gut abundance of Prevotella spp. The correlation analysis suggested that the gut abundance of Lactobacillus and Prevotella was significantly correlated with several metabolic indices, including blood glucose and lipid levels. Overall, these results suggest that the changes in the gut microbiota after MBS might be related to its efficacy in improving metabolic alterations.
The gut microbiota plays an important role in catabolism and energy expenditure by producing small-molecule fatty acids through the breakdown of complex nutrients, thereby controlling nutrient absorption and metabolism[11,12]. Obesity and metabolic dysfunction are often accompanied by the reorganization of the gut microbiota, and Firmicutes and Bacteroides are two of the main gut microbiota associated with obesity[13]. Some studies reported weight loss and metabolic improvement after the transfer of gut microbiota to nonsurgical germ-free animals[14,15]. In the present study, we found an increase in the gut abundance of Firmicutes after DSIB and an increase in the gut abundance of Bacteroides after SG in STZ-DM rats (Figure 6D). This finding suggests that the differences in improved glycolipid metabolism between SG and DSIB might be related to a difference in the reorganization of the gut microbiota after surgery. Both the SG and DSIB groups exhibited weight loss and improved glucose tolerance after surgery compared to the sham group, which may be due to certain changes in gut microbiota common to the SG and DSIB groups. On the other hand, the STZ-DM rats undergoing DSIB exhibited greater improvement in glucolipid alterations than those undergoing SG, suggesting that certain other gut microbes, such as Prevotella, might have been altered with DSIB, which was not observed after SG. These results indicate that the efficacy of SG and DSIB in improving glycolipid metabolism might be affected by specific microbial profiles.
Previous studies in animals have shown that the gut abundance of Lactobacillus increases significantly after SG and that SG regulates body weight and glucolipid metabolism through the activation of the short-chain fatty acid and hypoxia-inducible factor-2α pathways[7,16]. These studies indicate that Lactobacillus spp. play an important role in weight loss and improved glucolipid metabolism after MBS. At 6 wk postoperatively, we also observed a significant increase in the gut abundance of Lactobacillus spp. in both the SG and DSIB groups, including increases in two and three Lactobacillus spp. in the SG and DSIB groups, respectively. We also found that the degree of increase was significantly more pronounced in the DSIB group than in the SG group (Figure 7). We speculate that this finding might be a contributor to the superior weight loss and the improved glucolipid metabolism observed after DSIB compared to SG; however, further experimental validation is warranted.
The correlation analysis revealed that Lactobacillus showed a negative correlation with FGF21 and food intake, suggesting a close relationship between these three parameters. In agreement with studies showing that FGF21 can affect food intake[17], we observed a significant negative correlation between FGF21 and food intake. Although the SG group also showed an increase in the gut abundance of Lactobacillus, there was no significant change in food intake in these animals after SG compared to the sham group, whereas the DSIB group exhibited a sustained decrease in food intake compared to the sham group (Figure 2B). Therefore, we speculate that the increase in FGF21 levels might be the main reason for the decrease in food intake after DSIB and that DSIB might have affected serum FGF21 levels by altering the gut abundance of Lactobacillus, thereby reducing food intake.
Interestingly, at 6 wk postoperatively, the gut abundance of Prevotella in the DSIB group decreased significantly, while the gut abundance of Prevotella in the SG group did not differ from that in the sham group (Figure 6). Further correlation analysis suggested that the gut abundance of Prevotella intestinalis was significantly and positively correlated with food intake, glycated serum protein and CHOL levels and AUCOGTT (Figure 8), suggesting that Prevotella intestinalis may play an important role in improving glucose and lipid metabolism after DSIB, another potentially important mechanism underlying the superior efficacy of DSIB compared to SG. While some studies have demonstrated that Prevotella induces insulin resistance[18], others have reported that Prevotella improves glucose metabolism by promoting glycogen storage[19]. One reason for the seemingly contradictory findings might be related to the cohort characteristics. The first study was focused on diabetes, whereas the second study was conducted in a healthy population, suggesting that Prevotella may play different roles in different populations. In the present study, we observed a decrease in the gut abundance of Prevotella in the DSIB group in association with improved glycolipid metabolism. Therefore, we hypothesize that Prevotella affects glycolipid metabolism by influencing food intake and subsequently glycolipid metabolism. Further experiments are needed to verify this possibility.
In many studies, circulating bile acids have been considered metabolic signaling molecules[20]. These molecules control their own synthesis and various metabolic pathways, including the secretion of FGF19 and FGF21 in the small intestine, by targeting the transcription factor farnesoid X receptor and the membrane protein Takeda G protein-coupled receptor 5[20]. Overfeeding-mediated increases in FGF21 have been shown to increase insulin sensitivity, thereby improving glucose metabolism[21,22]. In the present study, we observed better glycemic improvement in the DSIB group than in the SG group, which might be related to the higher FGF21 levels. It has also been considered that the farnesoid X receptor may be the key to postoperative weight loss and that it is involved in fatty acid and triglyceride synthesis[23] and the promotion of adipose tissue browning[24]. This finding is consistent with our observation of increased serum bile acid levels (Figure 4A) and improved lipid metabolism (Figure 4G and H) in the DSIB group, which were significantly correlated (Figure 8). In conjunction with the correlation between the serum FGF21 levels and the gut microbiota, we hypothesize that the increased gut abundance of Lactobacillus and the reduced abundance of Prevotella after DSIB regulate bile acid concentrations to improve lipid metabolism via the regulation of FGF21 secretion.
The present study has several limitations, including the relatively limited sample size and the short study duration. To further assess the relationship between the differences in the efficacy of SG and DSIB in lowering glucose, improving lipid metabolism and leading to differential changes in gut microbiota, future studies should consider flora transplantation. In addition, the mechanisms underlying the regulation of glucose and lipid metabolism by intestinal Lactobacillus and Prevotella remain to be validated in further studies.
In conclusion, we showed that SG and DSIB exerted differential effects on weight loss and metabolic improvement. Our analyses in STZ-DM rats indicated that DSIB was associated with better improvement in glucolipid metabolism than SG, which may be related to the differential effects of these procedures on the gut microbiota. The present study findings are crucial for understanding the mechanisms underlying the improvement in glucolipid metabolism after MBS and provide additional theoretical support for the gut microbiota as an effective access point for intervention to improve metabolism.
The effectiveness of weight loss surgery is closely related to the gut microbiome, and many studies are exploring its mechanisms.
This study focused on the different effectiveness and gut microbiome differences of different weight loss surgeries to determine their key mechanisms through the different changes between the two surgeries.
The study aimed to explore the mechanisms behind the effectiveness of weight loss surgery and investigate non-surgical interventions that could improve metabolism.
We performed sleeve gastrectomy (SG), distal small intestine bypass (DSIB) or sham surgery in nonobese rats with diabetes induced by 60 mg/kg streptozotocin (STZ-DM).
The group comparisons revealed that both SG and DSIB induced a reduction in body weight and significant improvements in glucose and lipid metabolism in the STZ-DM rats. Furthermore, DSIB exhibited a stronger glucose-lowering and lipid-reducing effect on STZ-DM rats than SG. 16S ribosomal RNA gene sequencing revealed that the gut abundance of some Lactobacillus spp. increased in both the SG and DSIB groups after surgery. However, the DSIB group exhibited a more pronounced increase in the gut abundance of Lactobacillus spp. compared to the SG group, with more Lactobacillus spp. types increased in the gut.
The gut abundance of Lactobacillus was significantly correlated with the improvement in glycolipid metabolism and the change in serum FGF21 levels.
The study found that the duodenal switch procedure has better metabolic improvement and more significant changes in gut microbiota. By examining the different effectiveness and gut microbiome changes of various weight loss surgeries, this study contributed to understanding their key mechanisms.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gluvic Z, Serbia; Srinivasan AR, United States S-Editor: Zhang H L-Editor: Filipodia P-Editor: Wu RR
| 1. | Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2249] [Cited by in RCA: 3405] [Article Influence: 486.4] [Reference Citation Analysis (0)] |
| 2. | GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Ärnlöv J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Fürst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377:13-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5669] [Cited by in RCA: 5073] [Article Influence: 634.1] [Reference Citation Analysis (2)] |
| 3. | Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9:373-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 893] [Article Influence: 223.3] [Reference Citation Analysis (0)] |
| 4. | Cummings BP. Bariatric surgery works through a novel bile acid. Nat Chem Biol. 2021;17:5-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Albaugh VL, Banan B, Ajouz H, Abumrad NN, Flynn CR. Bile acids and bariatric surgery. Mol Aspects Med. 2017;56:75-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 6. | Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2421] [Cited by in RCA: 2192] [Article Influence: 128.9] [Reference Citation Analysis (0)] |
| 7. | Shao Y, Evers SS, Shin JH, Ramakrishnan SK, Bozadjieva-Kramer N, Yao Q, Shah YM, Sandoval DA, Seeley RJ. Vertical sleeve gastrectomy increases duodenal Lactobacillus spp. richness associated with the activation of intestinal HIF2α signaling and metabolic benefits. Mol Metab. 2022;57:101432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016;24:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1317] [Cited by in RCA: 1852] [Article Influence: 205.8] [Reference Citation Analysis (0)] |
| 9. | Mocanu V, Zhang Z, Deehan EC, Kao DH, Hotte N, Karmali S, Birch DW, Samarasinghe KK, Walter J, Madsen KL. Fecal microbial transplantation and fiber supplementation in patients with severe obesity and metabolic syndrome: a randomized double-blind, placebo-controlled phase 2 trial. Nat Med. 2021;27:1272-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 175] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 10. | Meneses E, Zagales I, Fanfan D, Zagales R, McKenney M, Elkbuli A. Surgical, metabolic, and prognostic outcomes for Roux-en-Y gastric bypass versus sleeve gastrectomy: a systematic review. Surg Obes Relat Dis. 2021;17:2097-2106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 11. | McNeil NI. The contribution of the large intestine to energy supplies in man. Am J Clin Nutr. 1984;39:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 464] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 12. | Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474:1823-1836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1710] [Cited by in RCA: 2060] [Article Influence: 257.5] [Reference Citation Analysis (8)] |
| 13. | Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jørgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clément K, Doré J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T; MetaHIT consortium, Bork P, Wang J, Ehrlich SD, Pedersen O. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2727] [Cited by in RCA: 3215] [Article Influence: 267.9] [Reference Citation Analysis (2)] |
| 14. | Liou AP, Paziuk M, Luevano JM Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5:178ra41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 711] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 15. | Tremaroli V, Karlsson F, Werling M, Ståhlman M, Kovatcheva-Datchary P, Olbers T, Fändriks L, le Roux CW, Nielsen J, Bäckhed F. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab. 2015;22:228-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 535] [Cited by in RCA: 563] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 16. | Fujinaga A, Ohta M, Endo Y, Nakanuma H, Kawamura M, Hirashita Y, Kawasaki T, Masuda T, Hirashita T, Gotoh K, Inomata M. Changes of Short-Chain Fatty Acids and Their Receptors in an Obese Rat Model After Sleeve Gastrectomy. Obes Surg. 2022;32:2649-2657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 17. | Fjeldborg K, Pedersen SB, Møller HJ, Richelsen B. Reduction in serum fibroblast growth factor-21 after gastric bypass is related to changes in hepatic fat content. Surg Obes Relat Dis. 2017;13:1515-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, Forslund K, Hildebrand F, Prifti E, Falony G, Le Chatelier E, Levenez F, Doré J, Mattila I, Plichta DR, Pöhö P, Hellgren LI, Arumugam M, Sunagawa S, Vieira-Silva S, Jørgensen T, Holm JB, Trošt K; MetaHIT Consortium, Kristiansen K, Brix S, Raes J, Wang J, Hansen T, Bork P, Brunak S, Oresic M, Ehrlich SD, Pedersen O. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535:376-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 1456] [Article Influence: 161.8] [Reference Citation Analysis (0)] |
| 19. | Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, Hallen A, Martens E, Björck I, Bäckhed F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015;22:971-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 927] [Cited by in RCA: 1246] [Article Influence: 124.6] [Reference Citation Analysis (2)] |
| 20. | Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1641] [Cited by in RCA: 1695] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 21. | Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, Mohammadi M, Potthoff MJ. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014;63:4057-4063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 395] [Cited by in RCA: 482] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 22. | Berglund ED, Li CY, Bina HA, Lynes SE, Michael MD, Shanafelt AB, Kharitonenkov A, Wasserman DH. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology. 2009;150:4084-4093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 268] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 23. | Yuan ZQ, Li KW. Role of farnesoid X receptor in cholestasis. J Dig Dis. 2016;17:501-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, Yoshihara E, Perino A, Jacinto S, Lukasheva Y, Atkins AR, Khvat A, Schnabl B, Yu RT, Brenner DA, Coulter S, Liddle C, Schoonjans K, Olefsky JM, Saltiel AR, Downes M, Evans RM. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 588] [Article Influence: 58.8] [Reference Citation Analysis (0)] |