Published online Jun 27, 2023. doi: 10.4240/wjgs.v15.i6.1202
Peer-review started: December 26, 2022
First decision: February 21, 2023
Revised: March 21, 2023
Accepted: April 25, 2023
Article in press: April 25, 2023
Published online: June 27, 2023
Processing time: 171 Days and 10.8 Hours
Anastomotic leakage (AL) following rectal cancer surgery is an important cause of mortality and recurrence. Although transanal drainage tubes (TDTs) are expected to reduce the rate of AL, their preventive effects are controversial.
To reveal the effect of TDT in patients with symptomatic AL after rectal cancer surgery.
A systematic literature search was performed using the PubMed, Embase, and Cochrane Library databases. We included randomized controlled trials (RCTs) and prospective cohort studies (PCSs) in which patients were assigned to two groups depending on the use or non-use of TDT and in which AL was evaluated. The results of the studies were synthesized using the Mantel-Haenszel random-effects model, and a two-tailed P value > 0.05 was considered statistically significant.
Three RCTs and two PCSs were included in this study. Symptomatic AL was examined in all 1417 patients (712 with TDT), and TDTs did not reduce the symptomatic AL rate. In a subgroup analysis of 955 patients without a diverting stoma, TDT reduced the symptomatic AL rate (odds ratio = 0.50, 95% confidence interval: 0.29–0.86, P = 0.012).
TDT may not reduce AL overall among patients undergoing rectal cancer surgery. However, patients without a diverting stoma may benefit from TDT placement.
Core Tip: Anastomotic leakage (AL) following rectal cancer surgery is a serious problem, and a transanal drainage tube (TDT) is expected to reduce AL. However, the preventive effects of TDT placement are controversial. Thus, we performed a meta-analysis of three randomized controlled trials and two prospective cohort studies. A systematic literature search was performed, and the results of the meta-analysis were synthesized using the Mantel-Haenszel random-effects model. Overall, TDT did not significantly reduce the symptomatic AL rate, but it did among patients without a diverting stoma.
- Citation: Fujino S, Yasui M, Ohue M, Miyoshi N. Efficacy of transanal drainage tube in preventing anastomotic leakage after surgery for rectal cancer: A meta-analysis. World J Gastrointest Surg 2023; 15(6): 1202-1210
- URL: https://www.wjgnet.com/1948-9366/full/v15/i6/1202.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i6.1202
Colorectal cancer (CRC) is a major cause of death in many countries and regions[1], and surgical resection of primary tumors is an important treatment for CRC[2]. With the development of surgical devices and procedures, from open to laparoscopic to robot-assisted surgeries, surgical outcomes have improved[3,4]. However, anastomotic leakage (AL) following surgery remains a serious complication related to mortality and recurrence, and the rate of AL is higher for rectal cancer surgery than that for colon cancer surgery[5,6].
To avoid AL, a combination of prophylactic procedures has been used, such as bowel preparation before surgery, anastomosis blood flow evaluation[7,8], and especially transanal drainage tubes (TDTs) and diverting stomas[8,9]. In recent years, preoperative therapies, such as chemoradiotherapy (CRT) or radiotherapy followed by chemotherapy, have been aggressively performed for advanced rectal cancer, and higher-risk patients are undergoing surgery after radiotherapy[10,11]. A diverting stoma is recommended for patients at high risk for AL[12], but stoma-related complications, such as high-output syndrome, skin irritation, stoma necrosis, and parastomal hernia, decrease the patient’s quality of life and may lead to rehospitalization[13]. Therefore, many clinical studies have been performed to determine whether TDT can prevent AL; however, the results are controversial and most studies were retrospective[14-17]. Recently, the two most randomized controlled trials (RCTs) on the role of TDT in the prevention of AL were reported by Zhao et al[18] and Tamura et al[19]. The only related RCT published before these studies was reported by Bülow et al[20], but surgical procedures and pre
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines[21]. The inclusion criteria were as follows: (1) An RCT or PCS for patients with a TDT; (2) Patients assigned to two groups depending on the use or non-use of TDT; and (3) The primary endpoint was the AL rate. Studies were excluded if one of the following occurred: (1) It was retrospective; (2) It was a review or case report; (3) Data were duplicated; (4) No comparisons were performed with a non-TDT group; (5) Full text could not be obtained; or (6) The TDT was not located at least several centimeters above the anastomosis. This study was not registered to public database.
We targeted patients with rectal cancer who underwent surgery for resection of the primary tumor with anastomosis. This is because the outcome is difficult to understand if the patient population is expanded, for instance, including those with inflammatory bowel disease. The outcome was the incidence of symptomatic AL after TDT.
A systematic literature search for this study was performed using the advanced search of MEDLINE/PubMed, Embase, and Cochrane Library databases from inception until December 12, 2022, without language restrictions. The following search terms were used in all database searches: “transanal OR trans anal” AND “drainage OR tube OR stent” AND “rectal cancer”. The titles and abstracts of all the retrieved records were reviewed independently by two investigators (Fujino S and Miyoshi N). All disagreements were resolved by consensus with a third investigator (Yasui M). The information extracted included the name of the first authors, year of publication, study design, study setting, types of operation, randomization procedure, TDT-related information (material, diameter, placement, duration), number of cases of AL, and grades of AL.
The results were synthesized using the Mantel-Haenszel random-effects model. Data were expressed as odds ratios (ORs) and 95% confidence intervals (CIs). A funnel plot was used to evaluate potential publication bias and other possible biases. A two-tailed P value > 0.05 was considered statistically significant. A sensitivity analysis detected the influence of individual studies on the pooled OR by omitting one study at a time and recalculating the pooled OR. Subgroup analyses determined the effect of TDT in patients without a diverting stoma. Data were analyzed using R software (CRAN, R 3.6.2; cran.r-project.org) and the meta package (v4.17-0)[22]. The statistical methods of this study were reviewed by Miyoshi N.
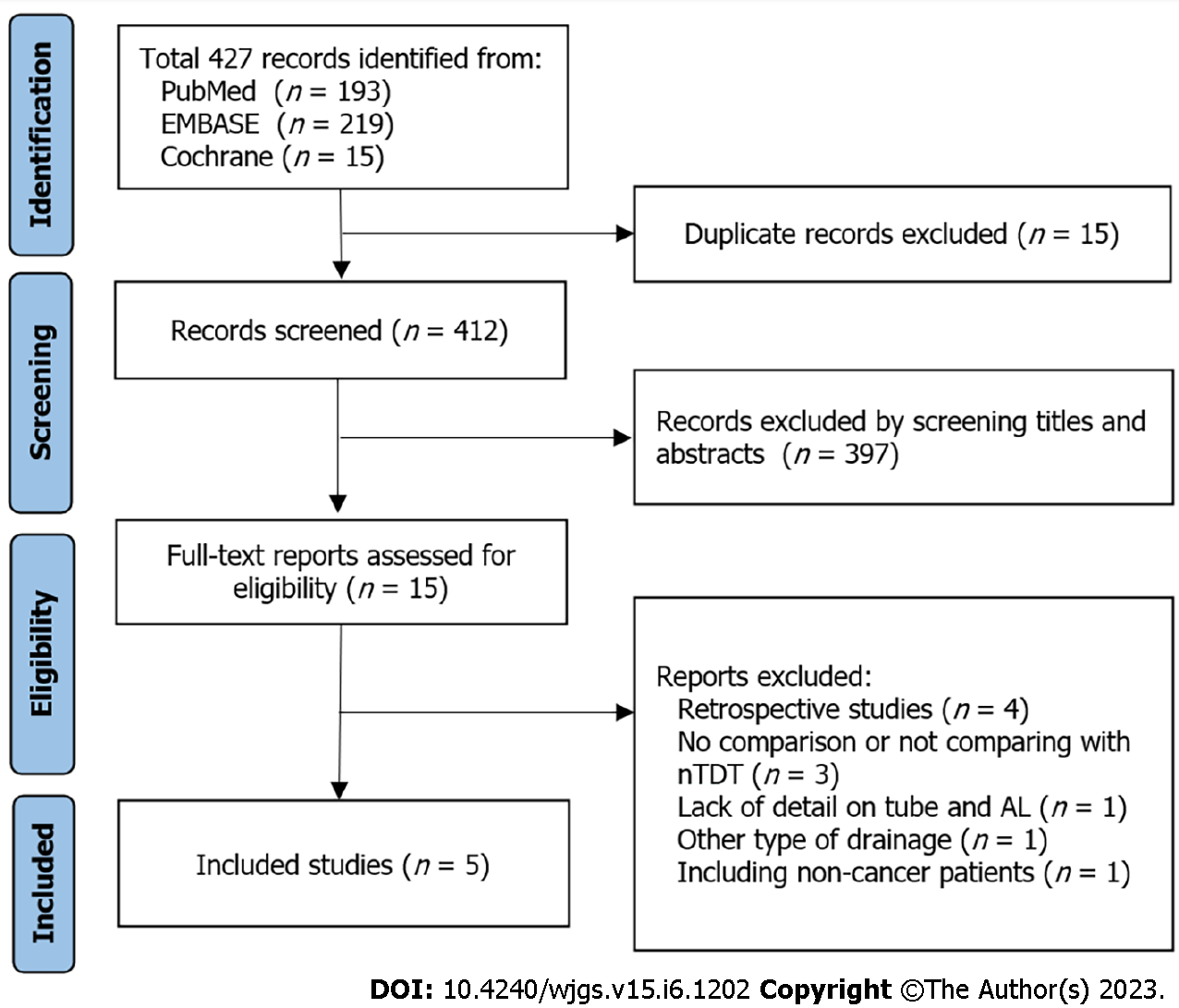
Overall, 412 records were identified from the selected databases. We carefully evaluated each of them according to the inclusion and exclusion criteria. Finally, three RCTs[18,19,23] and two PCSs[24,25] were included in this study (Figure 1). The characteristics of the study population are summarized in Table 1. None of the studies revealed differences between the TDT and non-TDT groups in terms of sex, age, diverting stoma, or preoperative CRT. Patients undergoing preoperative CRT were excluded from three studies, and patients undergoing diverting stoma were excluded from two studies.
| Zhao etal[18] | Tamura etal[19] | Xiao etal[23] | Challine etal[24] | Zhao etal[25] | ||
| Country | China | Japan | China | France | China | |
| Published year | 2021 | 2021 | 2011 | 2020 | 2013 | |
| Study design | RCT | RCT | RCT | PCS | PCS | |
| Study setting | Multicenter | Multicenter | Single center | Single center | Singlecenter | |
| Age | TDT | 62 (54-69)1 | 69 (40-90)1 | 59 ± 112 | 64 ± 122 | ≥ 60/< 60, 30/51 |
| Non-TDT | 62 (52-69)1 | 69 (39-91)1 | 58 ± 122 | 60 ± 122 | ≥ 60/< 60, 36/41 | |
| Sex (male/female) | TDT | 177/103 | 51/28 | 115/85 | 51/21 | 47/34 |
| Non-TDT | 169/111 | 50/28 | 121/77 | 51 / 21 | 43/34 | |
| Preoperative treatment (radiochemotherapy) | TDT | Excluded | 10 (12.7%) | Excluded | 41 (56.9%) | Excluded |
| Non-TDT | Excluded | 19 (24.3%) | Excluded | 47 (65.3%) | Excluded | |
| DS | TDT | 72 (25.7%) | 34 (43.0%) | Excluded | Unknown but equal rate by matching | Excluded |
| Non-TDT | 89 (31.8%) | 37 (47.4%) | Excluded | Unknown but equal rate by matching | Excluded | |
| Type of tube | Silicone tube, 28 Fr | Latex tube, 20-24 Fr | Silicone tube commonly used for abdominal drainage | Foley catheter, Ch 22 | Rubber tube, 26 Fr | |
| Duration | 3-7 d | At least 5 d | 5-7 d | At least 4 d | 5-6 d | |
| Significant side effects relating to anal tube | Anal pain | None | Perianastomotic bleeding | None | None | |
| AL (A/B/C) | TDT | NA/14/4 | 2/5/1 | NA/6/2 | 12/9/4 | NA/0/2 |
| Non-TDT | NA/11/8 | 3/7/1 | NA/3/16 | 9/5/2 | NA/0/7 | |
| AL in the patients wihout a DS (A/B/C) | TDT | NA/8/4 | NA | NA/6/2 | NA | NA/0/2 |
| Non-TDT | NA/7/8 | NA | NA/3/16 | NA | NA/0/7 |
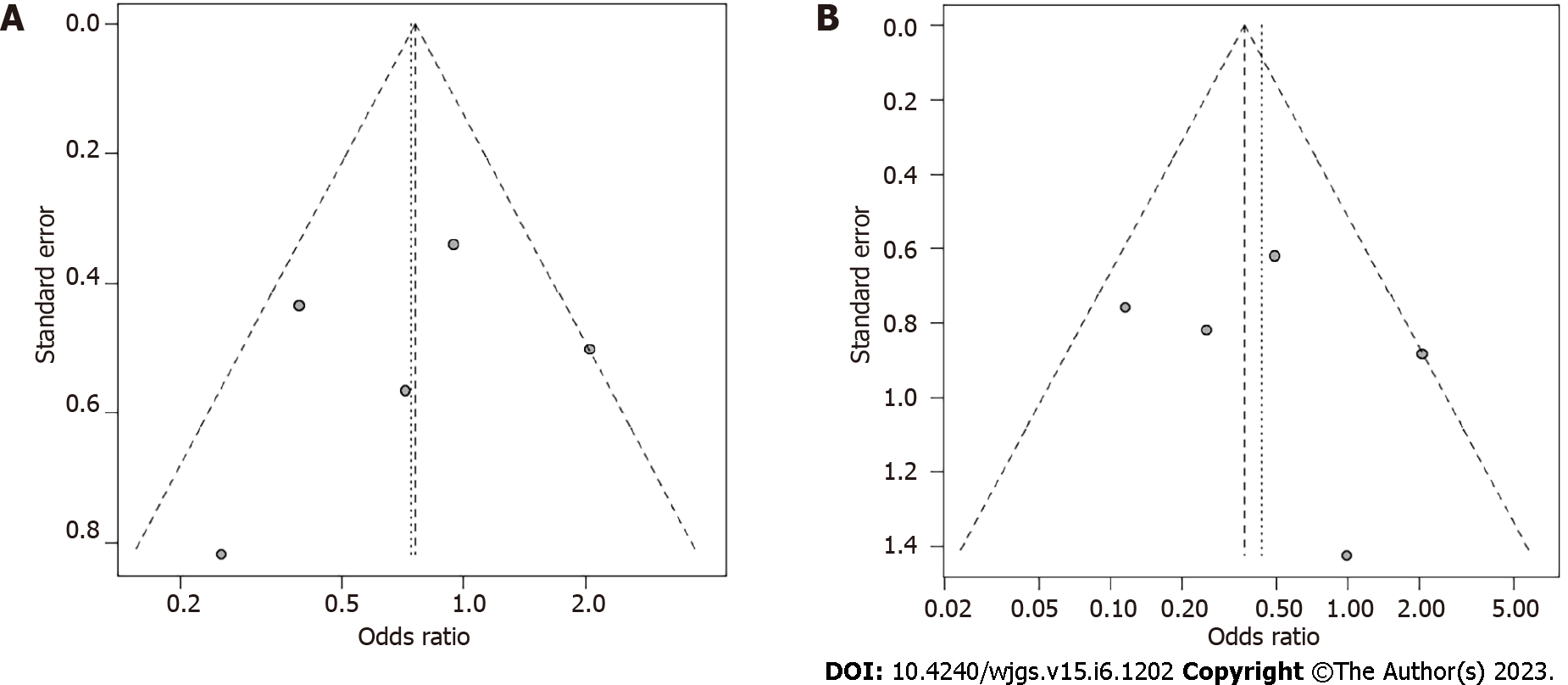
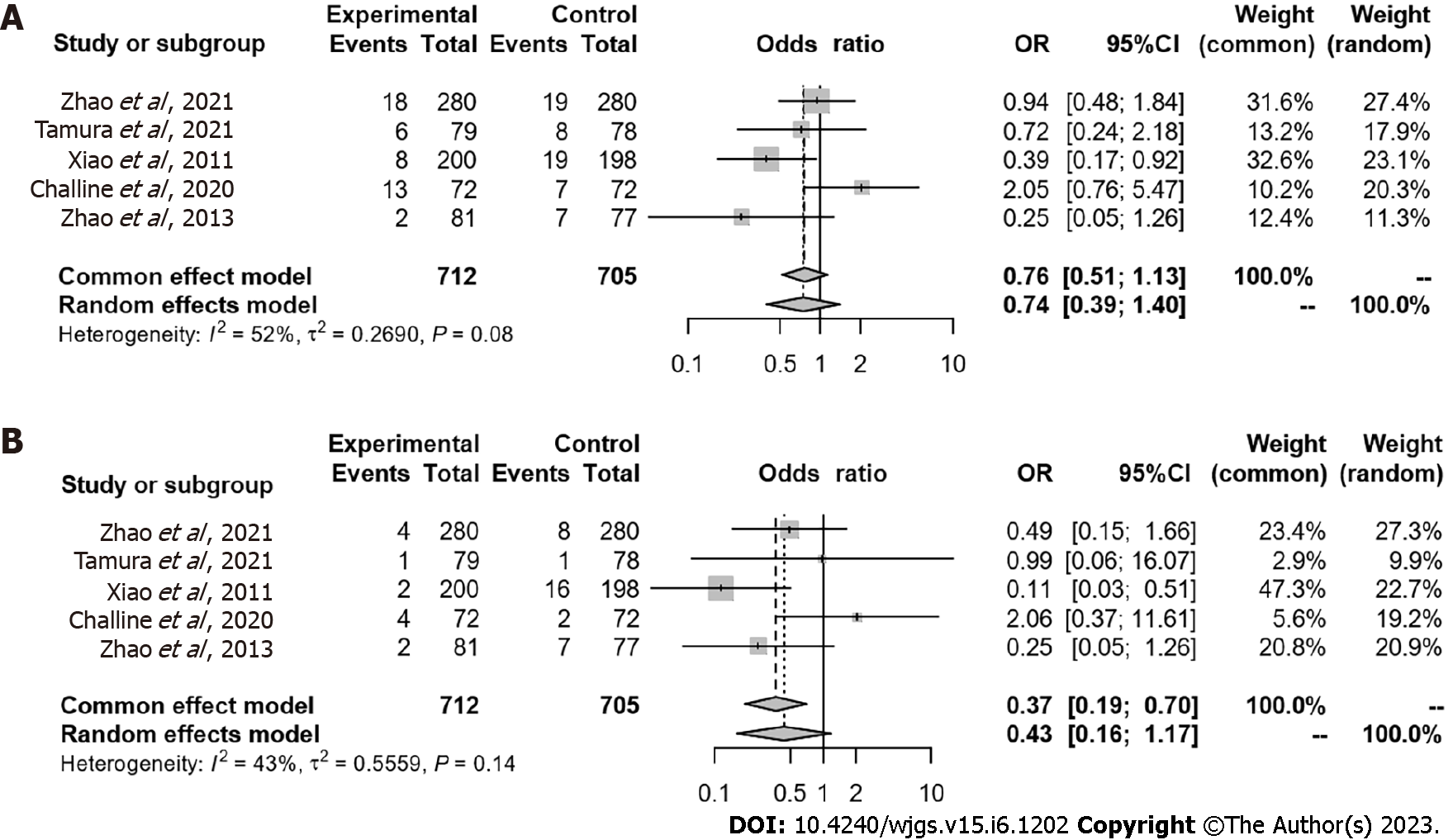
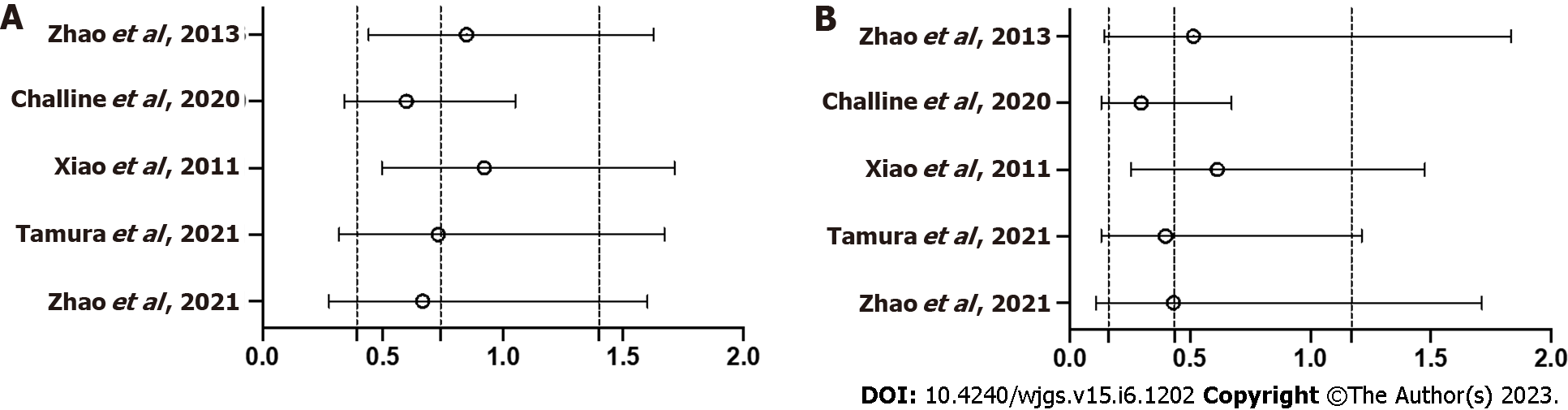
Symptomatic AL was examined in all 1417 patients: 712 with TDT and 705 without TDT. Funnel plots based on AL grades are shown in Figure 2. Symptomatic AL was observed in 47 patients (6.6%) with TDT and 60 (8.5%) without TDT. TDT did not reduce the symptomatic AL rate (OR = 0.74, 95%CI: 0.39-1.40, P = 0.355) (Figure 3A). AL that required re-operation, i.e., grade C, was observed in 13 patients (1.8%) with TDT and 34 (4.8%) without TDT. TDT did not reduce the grade C AL rate (OR = 0.43, 95%CI: 0.16-1.17, P = 0.099) (Figure 3B). Sensitivity analysis showed that the pooled estimate of the effect of TDT for AL in all patients did not vary substantially (Figure 4).
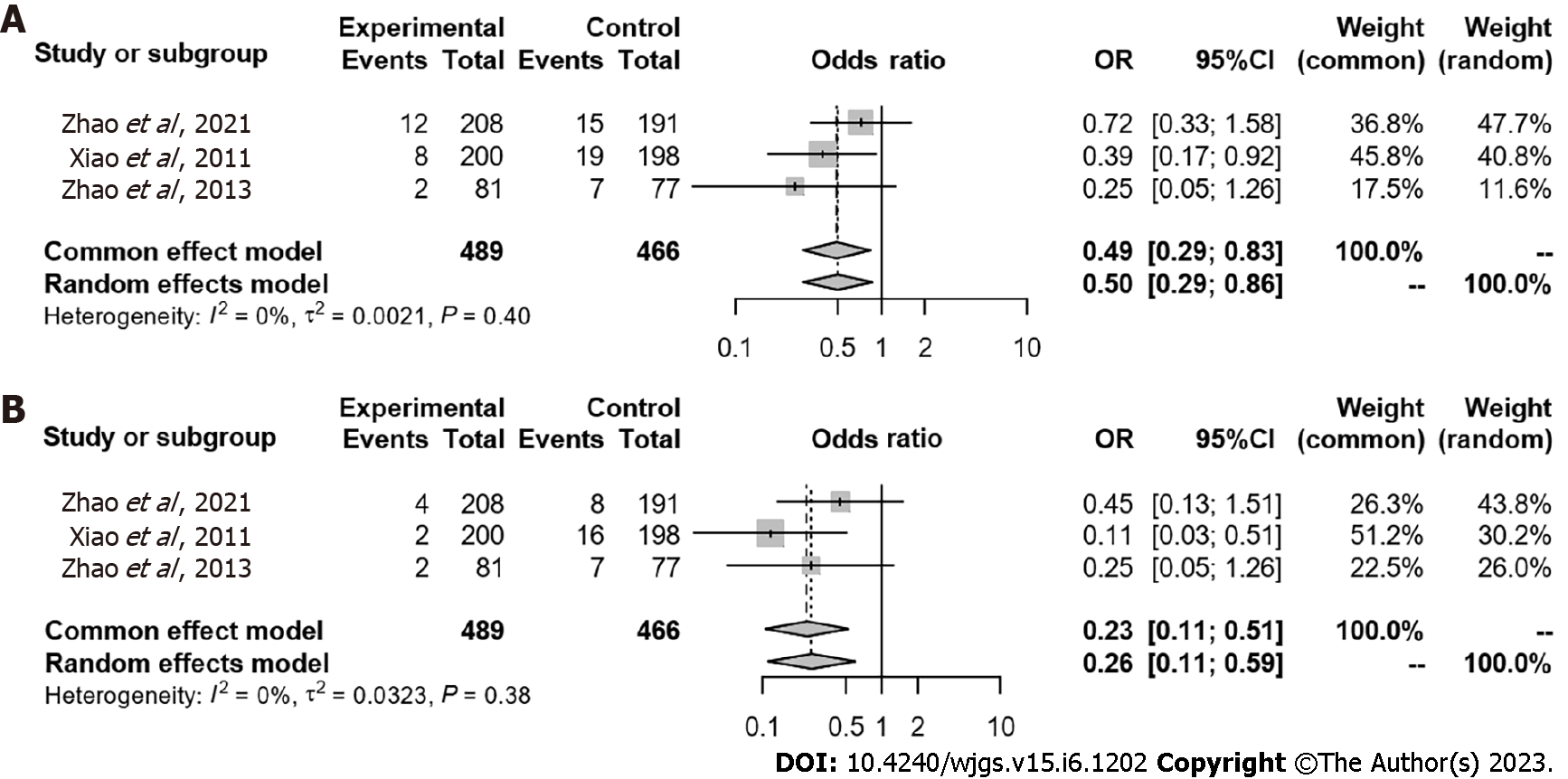
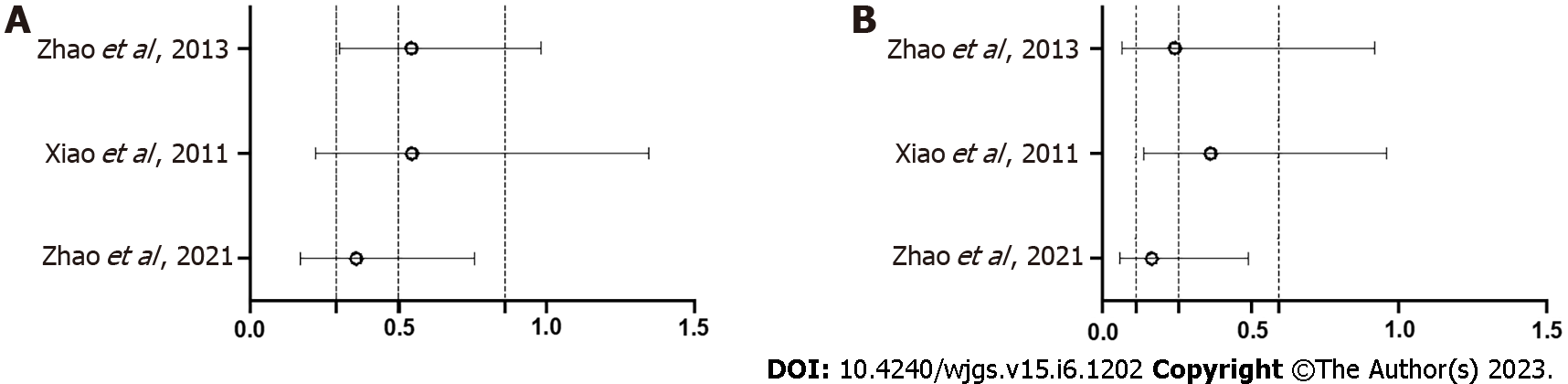
In two studies, incidence of AL in patients without a diverting stoma was not mentioned. Therefore, a total of 955 patients without a diverting stoma were identified in three studies[18,23,25]: 489 with TDT and 466 without TDT. Symptomatic AL was observed in 22 patients (4.5%) with TDT and 41 (8.8%) without TDT. TDT reduced the symptomatic AL rate (OR = 0.50, 95%CI: 0.29-0.86, P = 0.012) (Figure 5A). Grade C AL was observed in eight patients (1.6%) with TDT and 31 (6.7%) without TDT. TDT also reduced the grade C AL rate (OR = 0.26, 95%CI: 0.11-0.59, P = 0.001) (Figure 5B). Sensitivity analysis revealed that the pooled estimate of the effect of TDT for AL in patients without a diverting stoma did not vary substantially (Figure 6).
The development of surgical methods and the intensification of combination therapies with radiation therapy, chemotherapy, etc., constantly changes the background of the patients that physicians encounter. However, we must continue efforts to improve surgical outcomes because they are directly related to patient outcomes[5,6]. Regarding the background of the five trials included in this meta-analysis, patients who had received preoperative treatment were excluded in three. as the stated reason was that radiotherapy is a risk factor for AL[18]. In addition, patients with diverting stomas were excluded from two studies and allowed in three studies. The decision to use a diverting stoma depended on the surgeon, that is, diverting stomas were used in patients whom surgeons considered at a high risk for AL. Thus, the results of these studies should be interpreted carefully, recognizing the limitations inherent in the patient samples. In this meta-analysis, TDT did not reduce the rate of AL in any of the patients. Therefore, we attempted to clarify the role of TDT by subgroup analysis. Accordingly, we revealed that TDT significantly reduced the incidence of AL among patients without a diverting stoma.
Thus, based on patients’ background and the analysis results, a diverting stoma should be used in high-risk patients, but TDT is sufficient in patients who are not at a high risk of AL, without the use of a diverting stoma. We expect that further research will be conducted to determine which patients are at a high risk and are eligible for diverting stoma augmentation. The time from preoperative radiation therapy to surgery varies among patients[10], and other risk factors for AL, such as sex, age, tumor size, and tumor location have been reported[26,27]. The role of TDT may be to steadily reduce AL in patients for whom a stoma may be avoided, rather than to place a stoma in such high-risk patients.
Besides, there are also some meta-analyses including tow RCTs[18,19] reported in 2021. Zhao et al[18] analyzed only 3 RCTs[18,19,23] and concluded that TDTs do not reduce the incidence of AL, but may reduce the grade C AL[28]. Deng et al[29] analyzed 7 studies, including retrospective studies, and concluded that TDTs do not reduce the incidence of AL in all patients. They also performed subgroup analyses and the AL rate was significantly low in patients without neoadjuvant therapy and diverting stoma but mentioned that TDT may be useless for those in high-risk situations. Zhang et al[30] analyzed 13 studies including retrospective studies and concluded that TDT reduced the incidence of AL in the patients without diverting stoma. Although each study was conducted in a different, separately selected group, we can conclude, as we did, that the benefit of TDT for all patients is low, but the benefit of TDT for a limited number of patients is high. Therefore, we would like to reiterate that the role of TDT would not be to avoid diverting stoma, but to steadily decrease AL in low-risk patients who were thought to be able to avoid diverting stoma.
Finally, in the five included studies, complications of TDT were anal pain and anal bleeding, whereas no intestinal injuries due to the tube were observed. However, such injuries were previously reported[31], and patients should be carefully monitored to determine when and where to place a TDT and to confirm its position using radiography.
As the limitations of this study, the patients’ background was different in studies, and the criteria for high-risk patients with a diverting stoma was not standardized. Additionally, the number of studies included in our review was small, and there may have been some bias. However, rather than viewing TDTs as substitutes for diverting stomas, one may need to identify high-risk patients, in whom a stoma should be used, and non-high-risk patients, in whom a TDT should be placed to prevent AL and improve surgical outcomes for patients with rectal cancer.
TDTs did not reduce AL in any of the patients with rectal cancer who underwent primary tumor resection with anastomosis. However, patients who do not undergo diverting stoma augmentation based on the surgeon’s decision may benefit from TDT placement.
Anastomotic leakage (AL) following rectal cancer surgery remains a serious problem, and transanal drainage tubes (TDTs) and diverting stomas have been performed to avoid AL. However, the efficiency of TDTs results is controversial.
Recently, the two randomized controlled trials (RCTs) on the role of TDT were reported. Therefore, we performed an updated meta-analysis to incorporate them.
We aimed to reveal the role of TDTs in preventing AL after rectal cancer surgery.
A systematic literature search was performed using databases and meta-analyses were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines.
TDT did not reduce the symptomatic AL rate in all patients, but TDT reduced the symptomatic AL rate in patients without a diverting stoma.
TDT may not reduce AL in all patients undergoing rectal cancer surgery. However, patients without a diverting stoma may benefit from TDT placement.
Rather than viewing TDTs as substitutes for diverting stomas, we must identify high-risk patients, in whom a stoma should be used, and non-high-risk patients, in whom a TDT should be placed to prevent AL.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Samala Venkata V, United States; Sun Z, China S-Editor: Wang JJ L-Editor: A P-Editor: Yu HG
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64442] [Article Influence: 16110.5] [Reference Citation Analysis (176)] |
| 2. | Yoshino T, Arnold D, Taniguchi H, Pentheroudakis G, Yamazaki K, Xu RH, Kim TW, Ismail F, Tan IB, Yeh KH, Grothey A, Zhang S, Ahn JB, Mastura MY, Chong D, Chen LT, Kopetz S, Eguchi-Nakajima T, Ebi H, Ohtsu A, Cervantes A, Muro K, Tabernero J, Minami H, Ciardiello F, Douillard JY. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol. 2018;29:44-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 421] [Article Influence: 70.2] [Reference Citation Analysis (0)] |
| 3. | Khan JS, Ahmad A, Odermatt M, Jayne DG, Ahmad NZ, Kandala N, West NP. Robotic complete mesocolic excision with central vascular ligation for right colonic tumours - a propensity score-matching study comparing with standard laparoscopy. BJS Open. 2021;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Safiejko K, Tarkowski R, Koselak M, Juchimiuk M, Tarasik A, Pruc M, Smereka J, Szarpak L. Robotic-Assisted vs. Standard Laparoscopic Surgery for Rectal Cancer Resection: A Systematic Review and Meta-Analysis of 19,731 Patients. Cancers (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 5. | Boström P, Haapamäki MM, Rutegård J, Matthiessen P, Rutegård M. Population-based cohort study of the impact on postoperative mortality of anastomotic leakage after anterior resection for rectal cancer. BJS Open. 2019;3:106-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 6. | Koedam TWA, Bootsma BT, Deijen CL, van de Brug T, Kazemier G, Cuesta MA, Fürst A, Lacy AM, Haglind E, Tuynman JB, Daams F, Bonjer HJ; COLOR COLOR II study group. Oncological Outcomes After Anastomotic Leakage After Surgery for Colon or Rectal Cancer: Increased Risk of Local Recurrence. Ann Surg. 2022;275:e420-e427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 93] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 7. | Chaouch MA, Kellil T, Jeddi C, Saidani A, Chebbi F, Zouari K. How to Prevent Anastomotic Leak in Colorectal Surgery? A Systematic Review. Ann Coloproctol. 2020;36:213-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Gomez-Rosado JC, Valdes-Hernandez J, Cintas-Catena J, Cano-Matias A, Perez-Sanchez A, Del Rio-Lafuente FJ, Torres-Arcos C, Lara-Fernandez Y, Capitan-Morales LC, Oliva-Mompean F. Feasibility of quantitative analysis of colonic perfusion using indocyanine green to prevent anastomotic leak in colorectal surgery. Surg Endosc. 2022;36:1688-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Matthiessen P, Hallböök O, Rutegård J, Simert G, Sjödahl R. Defunctioning stoma reduces symptomatic anastomotic leakage after low anterior resection of the rectum for cancer: a randomized multicenter trial. Ann Surg. 2007;246:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 861] [Cited by in RCA: 795] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 10. | Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, Roodvoets AGH, Nagtegaal ID, Beets-Tan RGH, Blomqvist LK, Fokstuen T, Ten Tije AJ, Capdevila J, Hendriks MP, Edhemovic I, Cervantes A, Nilsson PJ, Glimelius B, van de Velde CJH, Hospers GAP; RAPIDO collaborative investigators. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:29-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 951] [Article Influence: 237.8] [Reference Citation Analysis (0)] |
| 11. | Conroy T, Bosset JF, Etienne PL, Rio E, François É, Mesgouez-Nebout N, Vendrely V, Artignan X, Bouché O, Gargot D, Boige V, Bonichon-Lamichhane N, Louvet C, Morand C, de la Fouchardière C, Lamfichekh N, Juzyna B, Jouffroy-Zeller C, Rullier E, Marchal F, Gourgou S, Castan F, Borg C; Unicancer Gastrointestinal Group and Partenariat de Recherche en Oncologie Digestive (PRODIGE) Group. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:702-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 726] [Article Influence: 181.5] [Reference Citation Analysis (0)] |
| 12. | Mrak K, Uranitsch S, Pedross F, Heuberger A, Klingler A, Jagoditsch M, Weihs D, Eberl T, Tschmelitsch J. Diverting ileostomy versus no diversion after low anterior resection for rectal cancer: A prospective, randomized, multicenter trial. Surgery. 2016;159:1129-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 13. | Babakhanlou R, Larkin K, Hita AG, Stroh J, Yeung SC. Stoma-related complications and emergencies. Int J Emerg Med. 2022;15:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 14. | Wang FG, Yan WM, Yan M, Song MM. Outcomes of transanal tube placement in anterior resection: A meta-analysis and systematic review. Int J Surg. 2018;59:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Wang FG, Yan WM, Yan M, Song MM. Comparison of anastomotic leakage rate and reoperation rate between transanal tube placement and defunctioning stoma after anterior resection: A network meta-analysis of clinical data. Eur J Surg Oncol. 2019;45:1301-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Choy KT, Yang TWW, Heriot A, Warrier SK, Kong JC. Does rectal tube/transanal stent placement after an anterior resection for rectal cancer reduce anastomotic leak? A systematic review and meta-analysis. Int J Colorectal Dis. 2021;36:1123-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Rondelli F, Avenia S, De Rosa M, Rozzi A, Rozzi S, Chillitupa CIZ, Bugiantella W. Efficacy of a transanal drainage tube versus diverting stoma in protecting colorectal anastomosis: a systematic review and meta-analysis. Surg Today. 2023;53:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Zhao S, Zhang L, Gao F, Wu M, Zheng J, Bai L, Li F, Liu B, Pan Z, Liu J, Du K, Zhou X, Li C, Zhang A, Pu Z, Li Y, Feng B, Tong W. Transanal Drainage Tube Use for Preventing Anastomotic Leakage After Laparoscopic Low Anterior Resection in Patients With Rectal Cancer: A Randomized Clinical Trial. JAMA Surg. 2021;156:1151-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 19. | Tamura K, Matsuda K, Horiuchi T, Noguchi K, Hotta T, Takifuji K, Iwahashi M, Iwamoto H, Mizumoto Y, Yamaue H. Laparoscopic anterior resection with or without transanal tube for rectal cancer patients - A multicenter randomized controlled trial. Am J Surg. 2021;222:606-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Bülow S, Bulut O, Christensen IJ, Harling H; Rectal Stent Study Group. Transanal stent in anterior resection does not prevent anastomotic leakage. Colorectal Dis. 2006;8:494-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40131] [Article Influence: 10032.8] [Reference Citation Analysis (2)] |
| 22. | Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1954] [Cited by in RCA: 3121] [Article Influence: 520.2] [Reference Citation Analysis (0)] |
| 23. | Xiao L, Zhang WB, Jiang PC, Bu XF, Yan Q, Li H, Zhang YJ, Yu F. Can transanal tube placement after anterior resection for rectal carcinoma reduce anastomotic leakage rate? A single-institution prospective randomized study. World J Surg. 2011;35:1367-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Challine A, Cazelles A, Frontali A, Maggiori L, Panis Y. Does a transanal drainage tube reduce anastomotic leakage? A matched cohort study in 144 patients undergoing laparoscopic sphincter-saving surgery for rectal cancer. Tech Coloproctol. 2020;24:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Zhao WT, Hu FL, Li YY, Li HJ, Luo WM, Sun F. Use of a transanal drainage tube for prevention of anastomotic leakage and bleeding after anterior resection for rectal cancer. World J Surg. 2013;37:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Tanaka K, Okuda J, Yamamoto S, Ito M, Sakamoto K, Kokuba Y, Yoshimura K, Watanabe M. Risk factors for anastomotic leakage after laparoscopic surgery with the double stapling technique for stage 0/I rectal carcinoma: a subgroup analysis of a multicenter, single-arm phase II trial. Surg Today. 2017;47:1215-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Hoek VT, Buettner S, Sparreboom CL, Detering R, Menon AG, Kleinrensink GJ, Wouters MWJM, Lange JF, Wiggers JK; Dutch ColoRectal Audit group. A preoperative prediction model for anastomotic leakage after rectal cancer resection based on 13.175 patients. Eur J Surg Oncol. 2022;48:2495-2501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 28. | Zhao S, Hu K, Tian Y, Xu Y, Tong W. Role of transanal drainage tubes in preventing anastomotic leakage after low anterior resection: a meta-analysis of randomized controlled trials. Tech Coloproctol. 2022;26:931-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (2)] |
| 29. | Deng SY, Xing JD, Liu MX, Xu K, Tan F, Yao ZD, Zhang N, Yang H, Zhang CH, Cui M, Su XQ. Effect of the transanal drainage tube on preventing anastomotic leakage after laparoscopic surgery for rectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2022;37:1739-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Zhang YX, Jin T, Yang K. The role of transanal drainage tube in preventing the anastomotic leakage in rectal cancer surgery without a defunctioning stoma: A meta-analysis. Surgeon. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 31. | Hiraki M, Tanaka T, Okuyama K, Kubo H, Ikeda O, Kitahara K. Colon perforation caused by transanal decompression tube after laparoscopic low anterior resection: A case report. Int J Surg Case Rep. 2021;80:105640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |