Published online Jun 27, 2023. doi: 10.4240/wjgs.v15.i6.1149
Peer-review started: March 8, 2023
First decision: March 15, 2023
Revised: March 18, 2023
Accepted: April 14, 2023
Article in press: April 14, 2023
Published online: June 27, 2023
Processing time: 99 Days and 8.4 Hours
Pseudomyxoma peritonei (PMP) is a rare peritoneal malignant tumor syndrome. Cytoreductive surgery combined with hyperthermic intraperitoneal chemo
To determine if bevacizumab combined with cyclophosphamide and oxaliplatin (Bev+CTX+OXA) is effective for treatment of advanced PMP. The primary study endpoint was progression-free survival (PFS).
Retrospective analysis was conducted on the clinical data of patients with advanced PMP who received Bev+CTX+OXA regimen (bevacizumab 7.5 mg/kg ivgtt d1, oxaliplatin 130 mg/m2 ivgtt d1 and cyclophosphamide 500 mg/m2 ivgtt d1, q3w) in our center from December 2015 to December 2020. Objective response rate (ORR), disease control rate (DCR) and incidence of adverse events were evaluated. PFS was followed up. Kaplan-Meier method was used to draw survival curve, and log-rank test was used for comparison between groups. Multivariate Cox proportional hazards regression model was used to analyze the independent influencing factors of PFS.
A total of 32 patients were enrolled. After 2 cycles, the ORR and DCR were 3.1% and 93.7%, respectively. The median follow-up time was 7.5 mo. During the follow-up period, 14 patients (43.8%) had disease progression, and the median PFS was 8.9 mo. Stratified analysis showed that the PFS of patients with a preoperative increase in CA125 (8.9 vs 2.1, P = 0.022) and a completeness of cytoreduction score of 2-3 (8.9 vs 5.0, P = 0.043) was significantly longer than that of the control group. Multivariate analysis showed that a preoperative increase in CA125 was an independent prognostic factor for PFS (HR = 0.245, 95%CI: 0.066-0.904, P = 0.035).
Our retrospective assessment confirmed that the Bev+CTX+OXA regimen is effective in second- or posterior-line treatment of advanced PMP and that adverse reactions can be tolerated. A preoperative increase in CA125 is an independent prognostic factor of PFS.
Core Tip: For systemic chemotherapy of advanced pseudomyxoma peritonei (PMP), there are currently few studies and insufficient evidence. In this study, the bevacizumab combined with cyclophosphamide and oxaliplatin regimen was used for advanced PMP for the first time. The scheme used in this study was based on clinical experience and had achieved good results.
- Citation: Zhang Y, Zhao X, Gao C, Lin LY, Li Y. Treatment outcome analysis of bevacizumab combined with cyclophosphamide and oxaliplatin in advanced pseudomyxoma peritonei. World J Gastrointest Surg 2023; 15(6): 1149-1158
- URL: https://www.wjgnet.com/1948-9366/full/v15/i6/1149.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i6.1149
Pseudomyxoma peritonei (PMP) is a rare peritoneal malignant tumor syndrome with an incidence of approximately 2 to 4 per 1 million[1]. It is characterized by accumulation and redistribution of mucus produced by mucinous tumor cells in the abdominal cavity, mainly from appendiceal mucinous tumors. Cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) is the standard treatment for PMP[2,3]. Our previous work showed obvious clinical benefits after stan
Cyclophosphamide (CTX) is a nitrogen mustard alkylating agent that has been used in the treatment of a variety of solid tumors. Application of CTX for treating PMP can be traced back to the 1950s[9]. Recent studies have reported that the DCR of CTX combined with capecitabine for PMP is 87.0%[10]. To date, there has been no report on the use of bevacizumab combined with CTX and oxaliplatin (hereinafter referred to as the Bev+CTX+OXA regimen) to treat PMP. This single-center, retrospective study aimed to evaluate the efficacy, safety, and prognosis of the Bev+CTX+OXA regimen for patients with unresectable PMP.
This was a retrospective study involving clinical data of patients with advanced PMP who received the Bev+CTX+OXA regimen in the Department of Peritoneal Cancer Surgery in Beijing Shijitan Hospital affiliated with Capital Medical University from December 2015 to December 2020.
The inclusion criteria were as follows: (1) Pathologic confirmation of PMP; (2) Incomplete CRS + HIPEC treatment or recurrence and metastasis after complete CRS + HIPEC treatment that could not be operated on again; (3) Received at least first-line or above chemotherapy; (4) Karnofsky performance status > 60 points; (5) Measurable target lesions; (6) Received at least 2 cycles of treatment with the Bev+CTX+OXA regimen; and (7) Complete clinical pathology and follow-up data.
Exclusion criteria were: (1) Concomitant other malignant tumors; (2) Unable to complete the efficacy evaluation; (3) PMP from a noncolorectal origin; and (4) Follow-up time < 3 mo. In this study, application of chemotherapy regimens was carried out with the informed consent of patients and their families.
The following drugs were used: Bevacizumab (bevacizumab, Bev, Avastin, Germany/Roche Diagnostics GmbH, 400 mg (16 mL)/bottle), 7.5 mg/kg, d1, ivgtt (60-90 min), q3w; oxaliplatin (oxaliplatin, L-OHP, Jiangsu Hengrui Pharmaceuticals Co., Ltd., National Medicine Standard H20000337, 50 mg/bottle), 130 mg/m2, d1, ivgtt (120 min), q3w; and CTX [CTX (endoxan), CTX, Baxter Oncology GmbH, 200 mg/bottle], 500 mg/m2, ivgtt (approximately 30 min), q3w. Patients received this regimen until the disease progressed or an intolerable adverse reaction occurred or the patient withdrew informed consent. When patients had drug-related grade III or above adverse reactions during treatment, the dose was reduced by 25%; if it was still not tolerated, we adjusted to single-agent maintenance therapy or changed the chemotherapy regimen. Such cases were censored.
The primary study endpoint was PFS, as defined as the time from when a patient started receiving treatment to disease progression, death, or the follow-up deadline. The last follow-up date was July 4, 2021.
All patients received baseline examinations before treatment, including routine blood, liver and kidney function, tumor marker, electrocardiogram, and CT scans of measurable target lesions. Imaging evaluation was carried out before and every 2 cycles of treatment, and we identified the most defined and clearly assessable lesions that we chose as target lesions. Efficacy was evaluated according to "Response Evaluation Criteria in Solid Tumors" (RECIST) version 1.1 criteria by a radiologist with special expertise to define complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). We calculated the objective response rate (ORR) by (CR + PR)/total number of cases × 100% and the DCR by (CR + PR + SD)/total number of cases × 100%. Short-term efficacy in all patients was determined at the end of the second cycle. Serum tumor markers were evaluated once a month. The level of serum tumor markers at the beginning of treatment and the lowest level of serum tumor markers during treatment were used to evaluate chemotherapy response. The safety evaluation adopted National Cancer Institute Common Terminology Criteria.
SPSS 19.0 software (SPSS Inc., Chicago, IL, United States) and R studio 4.1.0 software (http://www.rstudio.com/) were used for statistical analysis. Measurement data are expressed as the median (range) or x ± s, and enumeration data are expressed as the rate. The Kaplan-Meier method was used to draw survival curves, and the log-rank test was used for comparisons between groups. The Cox proportional hazard regression model was employed to perform univariate analysis, and factors with P < 0.1 were included in multivariate analysis. The Wilcoxon paired signed rank test was used to compare changes in tumor markers before and after treatment. Bilateral P < 0.05 was considered statistically significant.
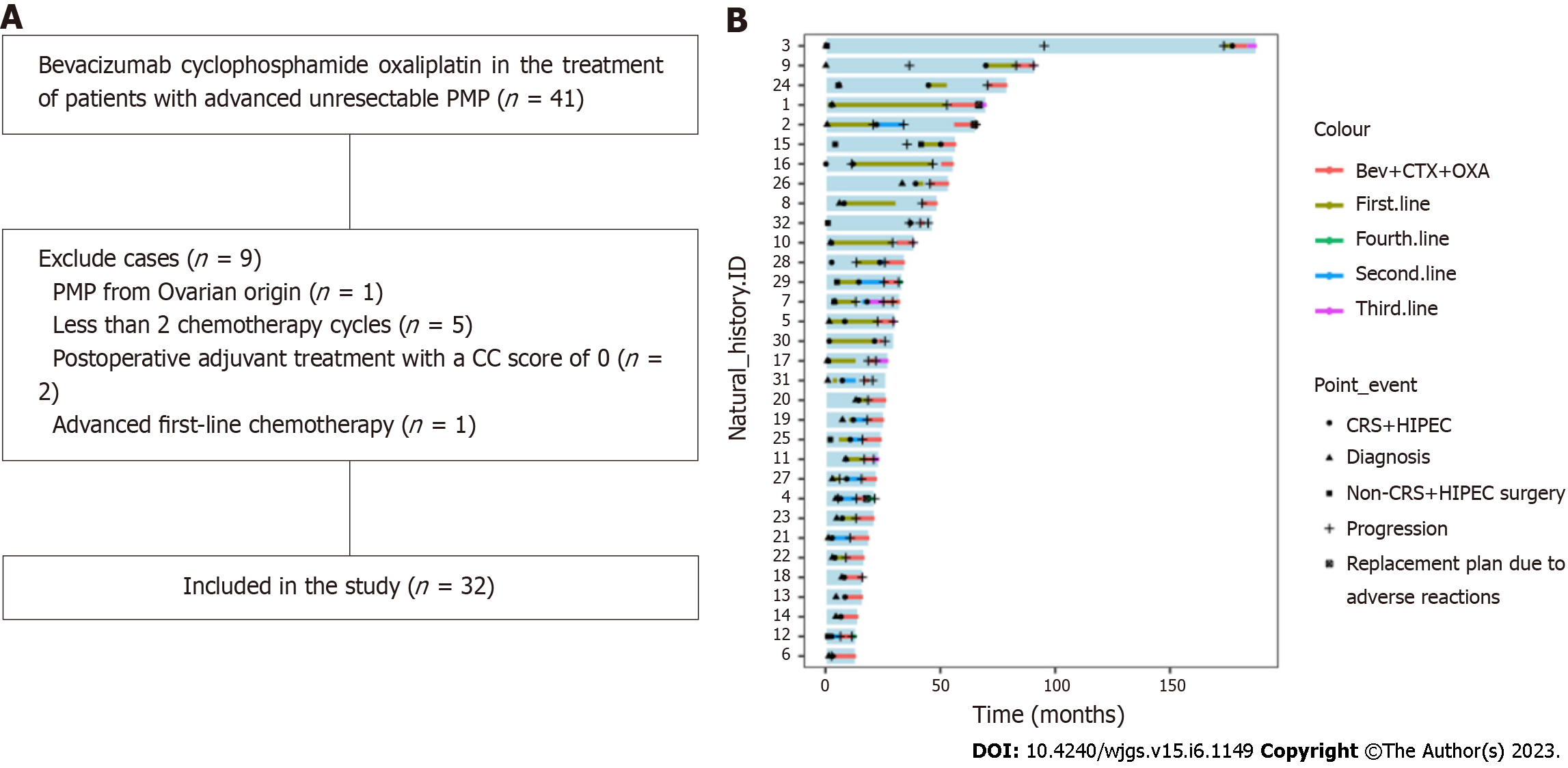
A total of 41 patients with advanced unresectable PMP received the Bev+CTX+OXA regimen, and 9 cases were excluded according to the inclusion and exclusion criteria. Finally, 32 patients were enrolled in the study (Figure 1A), and the swimmer plot of the 32 patients was shown in Figure 1B. Among them, 24 (75%) were males and 8 (25%) females, with a median age of 57.5 (34-74) years. The main clinicopathological characteristics are shown in Table 1.
| Characteristic | No. of patients (%) |
| Sex | |
| Male | 24 (75) |
| Female | 8 (25) |
| Age (years), median (rang) | 57.5 (34-74) |
| BSA (m2), median (rang) | 1.69 (1.27-2.07) |
| KPS score, median (rang) | 90 (60-100) |
| PCI score, median (rang) | 31 (16-39) |
| Ca199 before chemotherapy, median (rang), U/mL | 46.09 (4.68-10707.5) |
| CEA before chemotherapy, median (rang), ng/mL | 16.63 (1.08-632.27) |
| Ca125 before chemotherapy, median (rang), U/mL | 26.25 (5.3-146.7) |
| CC score | |
| 0-1 | 8 (25) |
| 2-3 | 24 (75) |
| Histological diagnosis | |
| Low-grade | 12 (37.5) |
| High-grade | 18 (56.2) |
| High-grade with signet ring cells | 2 (6.3) |
| Lymph node metastasis | |
| Yes | 2 (6.3) |
| No | 30 (93.7) |
| Vascular tumor thrombus | |
| Yes | 2 (6.3) |
| No | 30 (93.7) |
| Nerve invasion | |
| Yes | 2 (6.3) |
| No | 30 (93.7) |
| VEGF expression | |
| Positive | 19 (59.4) |
| Negative | 3 (9.4) |
| Unknown | 10 (31.2) |
| Microsatellite status | |
| MSS | 14 (43.8) |
| MSI-L | 1 (3.1) |
| Unknown | 17 (53.1) |
| Past use of bevacizumab | |
| Yes | 13 (40.6) |
| No | 19 (59.4) |
| First-line chemotherapy (months), median (rang) | 4.72 (0.01-34.73) |
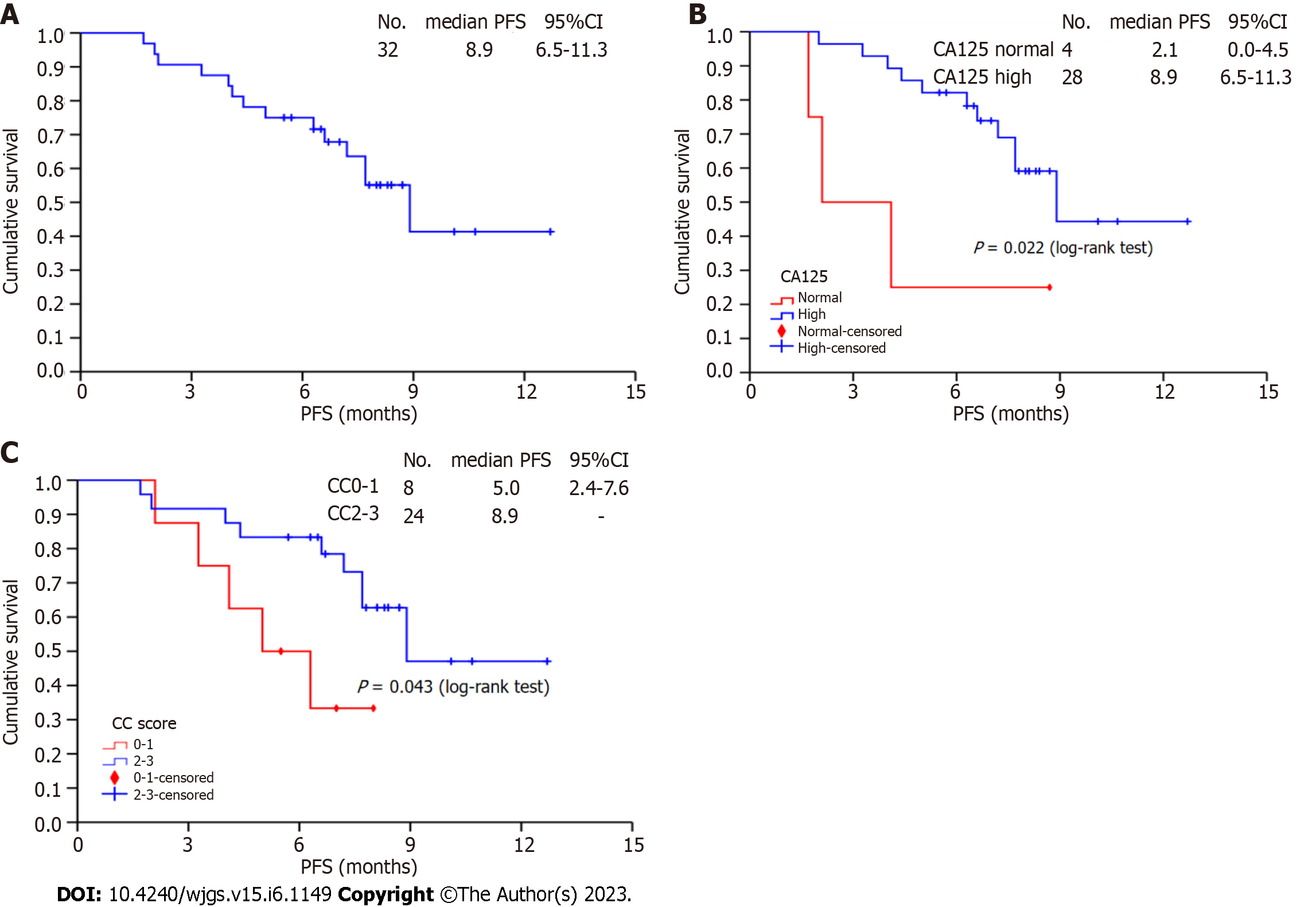
The median chemotherapy cycle of 32 patients was 4 (2-11) cycles. After 2 cycles, 1 (3.1%) case of PR, 29 (90.6%) cases of SD, and 2 (6.3%) cases of PD were observed; the ORR and DCR were 3.1% and 93.7%, respectively. The median follow-up time was 7.5 mo. During the follow-up period, 14 (43.8%) patients experienced disease progression, and the median PFS was 8.9 mo (95%CI: 6.53-11.18), as shown in Figure 2A. By the end of follow-up, no deaths had occurred. The stratified analysis showed that patients with a preoperative increase in CA125 (8.9 vs 2.1, P = 0.022, Figure 2B) and a completeness of cytoreduction (CC) score of 2-3 (8.9 vs 5.0, P = 0.043, Figure 2C) had prolonged PFS, which was significantly different from the control group.
Adverse events occurred in 24 (75.0%) patients. The most common adverse events were neutropenia, anemia, and nausea and vomiting. One (3.1%) patient was allergic to oxaliplatin, and we replaced oxaliplatin with irinotecan. Five (15.6%) patients had grade 3 adverse events that were improved through dose reduction and symptomatic treatment, including 2 (6.3%) cases of neutropenia, 4 (12.5%) cases of anemia, 1 (3.1%) case of nausea and vomiting, and 1 (3.1%) case of proteinuria. In 2 (2.3%) patients, we replaced oxaliplatin with carboplatin due to grade 3 peripheral neurotoxicity (Table 2).
| Adverse events | Total | Grade 1-2 | Grade 3-5 |
| Total | 24 (75.0) | 20 (62.5) | 5 (15.6) |
| Neutropenia | 14 (43.8) | 12 (37.5) | 2 (6.3) |
| Thrombocytopenia | 1 (3.1) | 1 (3.1) | 0 (0) |
| Anemia | 16 (50.0) | 12 (37.5) | 4 (12.5) |
| Peripheral neurotoxicity | 8 (25.0) | 6 (18.8) | 2 (6.3) |
| Fatigue | 9 (28.1) | 9 (28.1) | 0 (0) |
| Nausea and vomiting | 15 (46.9) | 14 (43.8) | 1 (3.1) |
| Liver damage | 7 (21.9) | 7 (21.9) | 0 (0) |
| Renal impairment | 9 (28.1) | 9 (28.1) | 0 (0) |
| Proteinuria | 5 (15.6) | 4 (12.5) | 1 (3.1) |
| Hypertension | 5 (15.6) | 5 (15.6) | 0 (0) |
| Allergy | 1 (3.1) | 1 (3.1) | 0 (0) |
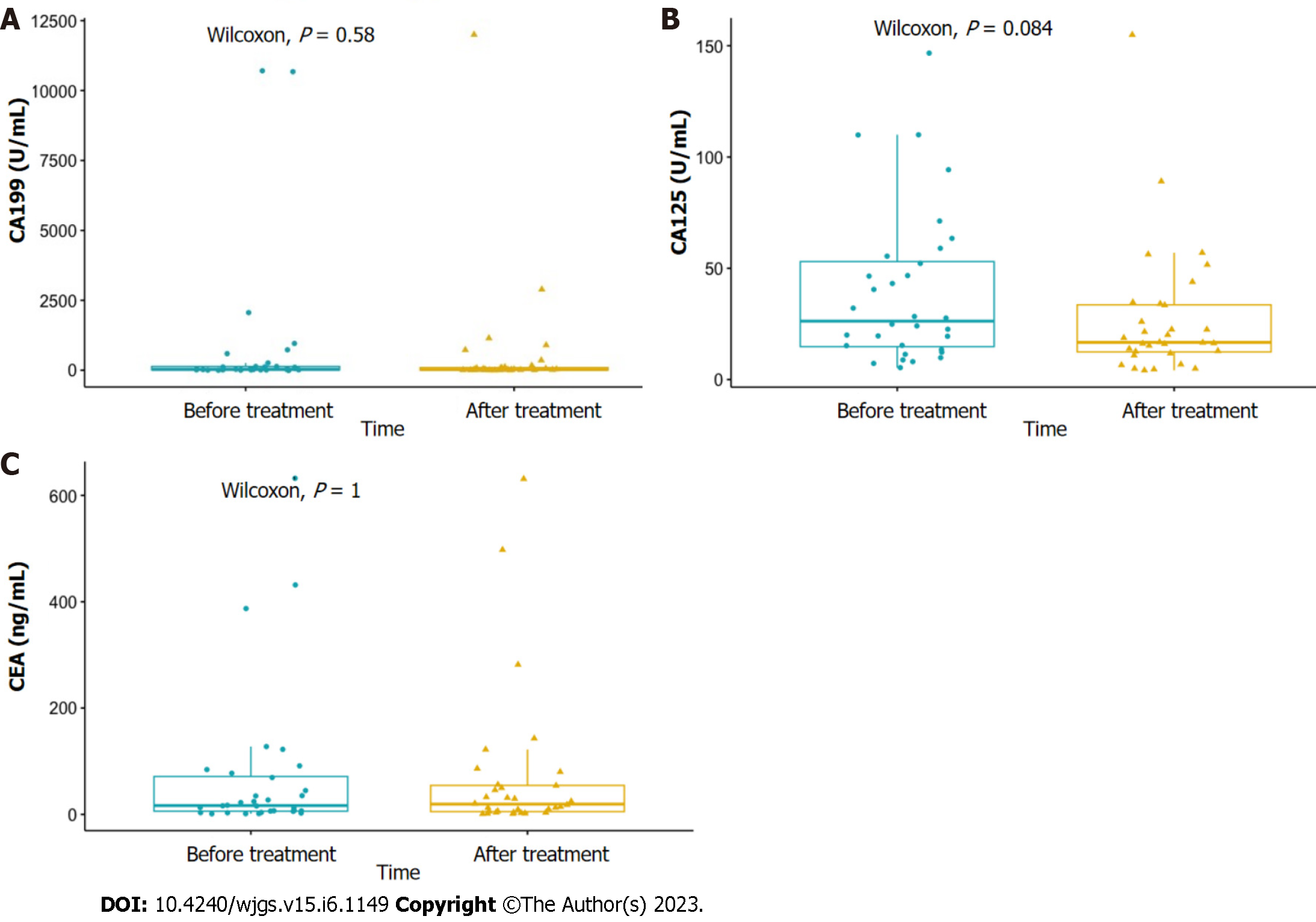
The mean values of serum CA199, carcinoembryonic antigen (CEA) and CA125 levels of 32 patients before chemotherapy were 844.17 ± 462.33 U/mL, 72.95 ± 25.22 ng/mL and 39.51 ± 6.15 U/mL, respectively. The mean minimum values during the treatment were 668.54 ± 384.65 U/mL, 71.65 ± 25.12 ng/mL and 27.41 ± 5.29 U/mL respectively. Both had a downward trend compared with that before treatment, but the difference was not statistically significant (Figure 3).
Univariate analysis showed that the following two factors were related to PFS (P < 0.1): Preoperative increase of CA125 (P = 0.035), CC score was 2-3 points (P = 0.054). Multivariate analysis showed that preoperative increase of CA125 was an independent prognostic factor of PFS (HR = 0.245, 95%CI: 0.066-0.904, P = 0.035) (Table 3).
| Prognostic factors | Univariate analysis | Multivariate analysis | |||||
| HR | 95%CI | P value | HR | 95%CI | P value | ||
| Sex (female vs male) | 0.522 | 0.143-1.906 | 0.325 | - | - | ||
| Age (< 60 vs ≥ 60) | 0.630 | 0.208-1.910 | 0.414 | - | - | ||
| Preoperative CEA (increased vs normal) | 1.383 | 0.309-6.193 | 0.671 | - | - | - | |
| Preoperative CA199 (increased vs normal) | 1.289 | 0.446-3.725 | 0.639 | ||||
| Preoperative CA125 (increased vs normal) | 0.245 | 0.066-0.904 | 0.035 | 0.245 | 0.066-0.904 | 0.035 | |
| KPS (≥ 80 vs < 80) | 0.946 | 0.119-7.493 | 0.958 | ||||
| CC (2-3 vs 0-1) | 0.319 | 0.100-1.012 | 0.054 | 0.351 | 0.106-1.164 | 0.087 | |
| Pathology (high-grade with signet ring cells vs high-grade vs low-grade) | 1.247 | 0.463-3.357 | 0.662 | ||||
| Lymph node metastasis (yes vs no) | 0.044 | 0.000-435.823 | 0.506 | ||||
| Vascular tumor thrombus (yes vs no) | 0.044 | 0.000-435.823 | 0.506 | ||||
| Nerve invasion (yes vs no) | 0.043 | 0.000-196.970 | 0.464 | ||||
| VEGF expression (+ vs -) | 0.764 | 0.157-3.712 | 0.739 | ||||
| CA199 before chemotherapy (increased vs normal) | 0.764 | 0.266-2.197 | 0.618 | ||||
| CEA before chemotherapy (increased vs normal) | 0.743 | 0.232-2.379 | 0.616 | ||||
| CA125 before chemotherapy (increased vs normal) | 1.401 | 0.489-4.014 | 0.530 | ||||
For systemic chemotherapy of advanced PMP, there are currently few studies and insufficient evidence. In this study, the Bev+CTX+OXA regimen was used for advanced PMP for the first time. The results showed that although the ORR was only 3.1%, the DCR reached 93.7%. This result is higher than the DCR with Pietrantonio et al’s FOLFOX4 and Hiraide et al's mFOLFOX6 regimens, suggesting that this regimen has a certain effect on patients with advanced PMP[6,7]. We consider the following reasons. First, CTX was added to this regimen for the first time. Some studies have shown that CTX has a certain immunomodulatory effect[11]. Research suggests that low-dose CTX can induce secretion of interferon-γ, thereby enhancing the antitumor immune response in mice, which may be one of the underlying mechanisms[12,13]. Second, studies have shown that screening for gene mutations related to vascular endothelial growth factor (VEGF) signal transduction and administering anti-VEGF therapy may provide new options for treatment of patients with refractory/relapsed advanced PMP[14-16]. In this study, 59.4% of tumors were positive for VEGF expression. The higher DCR may be related to inhibition of VEGF and its downstream pathways by addition of bevacizumab. It is worth noting that 59.4% of the patients in this study had previously used bevacizumab; considering the clear evidence for bevacizumab in cross-line treatment of a variety of solid tumors, we did not remove it. The results of the study also showed that whether bevacizumab has been used in the past did not affect PFS, suggesting that in second- or posterior-line treatment of patients with advanced PMP, cross-line application of bevacizumab may still bring survival benefits.
In terms of adverse events, 24 (75.0%) patients had adverse events, 2 (6.3%) patients had grade 3 neutropenia, and 4 (12.5%) patients had grade 3 anemia. These rates are slightly higher than those of Pietrantonio et al[7] and Hiraide et al[6], but lower than that of Raimondi[10]. This may be related to the fact that our enrolled population had received at least first-line chemotherapy in the past, which may have caused a decline in bone marrow hematopoietic function. In terms of proteinuria and peripheral neurotoxicity, the rate of grade 3 adverse events in this study was not high, and the grade 1-2 adverse events were all alleviated by symptomatic treatment, suggesting that the regimen can be tolerated.
During the treatment period of this study, serum CEA, CA125, and CA199 levels exhibited a downward trend. Although the difference was not statistically significant, this trend is still worth noting. The research of Randall et al[17] showed that in patients with epithelial ovarian cancer and peritoneal cancer continuously treated with bevacizumab, RECIST and CA125 are related in disease evaluation. Approximately 10% of patients may be found disease progression earlier through CA125. Hiraide et al[6] and others also used tumor markers as a method to monitor the efficacy. This provides a certain basis for monitoring efficacy in patients with no measurable lesions in the future. The median PFS in this study was 8.9 mo, which was lower than that with the FOLFOX4[6] and mFOLFOX6[7] regimens. However, considering that the mediean follow-up time of this study was only 7.5 mo, the median chemotherapy cycle was 4 cycles; thus, further follow-up is still needed to assess the PFS with this program. At the same time, 62.5% of patients with high-grade pathological types were included in this study, and patients with CC scores 2-3 accounted for 75%. These poor baseline data may limit the improvement in PFS. Stratified analysis and multivariate analysis showed that a preoperative increase in serum CA125 is an independent prognostic factor of prolonged PFS in this study. However, this trend was not observed in patients with elevated CA125 at the beginning of this regimen, which may be related to the surgical cytoreduction and previous chemotherapy that caused a significant decrease in serum CA125 before this regimen. The patients in this study had symptoms of abdominal and pelvic effusion during initial treatment. Previous studies have shown that an increase in CA125 is related to the degree of ascites. Anti-VEGF treatment can inhibit neovascularization and has obvious benefits for ascites control. This may be one of the reasons for the prolonged PFS of these patients. On the other hand, stratified analysis showed that the PFS of the patients with CC scores of 2-3 was prolonged, but the CC score in multivariate analysis was not an independent prognostic factor. This may be related to the large proportion of patients with CC scores of 2-3, and further research is needed for verification.
This study has certain limitations. First, this study was a single-center retrospective study. The previous treatment plan, clinical pathological data and biological characteristics of the enrolled patients were heterogeneous, which will lead to patient selection bias in the results. Second, the sample size was small, and the follow-up time was short, leading to some results that may be contrary to theory. In general, selection of beneficial regimens needs to be verified by expanding the sample and extending the follow-up time. Third, this study did not establish a control group.
In summary, the Bev+CTX+OXA regimen is effective in second- or posterior-line treatment of advanced PMP, and adverse reactions can be tolerated. A preoperative increase in CA125 is an independent prognostic factor of PFS.
Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy is its standard treatment. But for systemic chemotherapy of advanced pseudomyxoma peritonei (PMP), there are currently few studies and insufficient evidence.
Regimens for colorectal cancer are often used clinically, but there is no uniform standard for late-stage treatment.
The purpose of this single-center, retrospective study was to determine if bevacizumab combined with cyclophosphamide and oxaliplatin (Bev+CTX+OXA) is effective for treatment of advanced PMP.
Retrospective analysis was conducted on the clinical data of patients with advanced PMP who received Bev+CTX+OXA regimen from December 2015 to December 2020. Objective response rate (ORR), disease control rate (DCR) and incidence of adverse events were evaluated. Progression-free survival (PFS) was followed up.
A total of 32 patients were enrolled, after 2 cycles, ORR and DCR were 3.1% and 93.7% respectively. The median follow-up time was 7.5 mo. During the follow-up period, 14 patients (43.8%) had disease progression, and the median progression-free survival (PFS) was 8.9 mo. Stratified analysis showed that the PFS of patients with preoperative increase of CA125 (8.9 vs 2.1, P = 0.022) and completeness of cytoreduction score of 2-3 (8.9 vs 5.0, P = 0.043) were significantly longer than those of the control group. Multivariate analysis showed that preoperative increase of CA125 was an independent prognostic factor for PFS (HR = 0.245, 95%CI: 0.066-0.904, P = 0.035).
Bev+CTX+OXA regimen is certain effective in the posterior-line treatment of advanced PMP, and the adverse reactions can be tolerated. The preoperative increase of CA125 is an independent prognostic factor of PFS.
More sample size should be conduct in the future to validate the conclusion of our study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ahn KS, South Korea; Silsirivanit A, Thailand S-Editor: Fan JR L-Editor: A P-Editor: Guo X
| 1. | Lin YL, Xu DZ, Li XB, Yan FC, Xu HB, Peng Z, Li Y. Consensuses and controversies on pseudomyxoma peritonei: a review of the published consensus statements and guidelines. Orphanet J Rare Dis. 2021;16:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 2. | Govaerts K, Lurvink RJ, De Hingh IHJT, Van der Speeten K, Villeneuve L, Kusamura S, Kepenekian V, Deraco M, Glehen O, Moran BJ; PSOGI. Appendiceal tumours and pseudomyxoma peritonei: Literature review with PSOGI/EURACAN clinical practice guidelines for diagnosis and treatment. Eur J Surg Oncol. 2021;47:11-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (1)] |
| 3. | Li Y, Zhou YF, Liang H, Wang HQ, Hao JH, Zhu ZG, Wan DS, Qin LX, Cui SZ, Ji JF, Xu HM, Wei SZ, Xu HB, Suo T, Yang SJ, Xie CH, Yang XJ, Yang GL. Chinese expert consensus on cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal malignancies. World J Gastroenterol. 2016;22:6906-6916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 4. | Li XB, Ma R, Ji ZH, Lin YL, Zhang J, Yang ZR, Chen LF, Yan FC, Li Y. Perioperative safety after cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy for pseudomyxoma peritonei from appendiceal origin: Experience on 254 patients from a single center. Eur J Surg Oncol. 2020;46:600-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Chicago Consensus Working Group. The Chicago Consensus on Peritoneal Surface Malignancies: Management of Appendiceal Neoplasms. Ann Surg Oncol. 2020;27:1753-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 6. | Hiraide S, Komine K, Sato Y, Ouchi K, Imai H, Saijo K, Takahashi M, Takahashi S, Shirota H, Ishioka C. Efficacy of modified FOLFOX6 chemotherapy for patients with unresectable pseudomyxoma peritonei. Int J Clin Oncol. 2020;25:774-781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Pietrantonio F, Maggi C, Fanetti G, Iacovelli R, Di Bartolomeo M, Ricchini F, Deraco M, Perrone F, Baratti D, Kusamura S, Tamborini E, Castano A, Consonni PV, Bossi I, Gavazzi C, Milione M, Pelosi G, de Braud F. FOLFOX-4 chemotherapy for patients with unresectable or relapsed peritoneal pseudomyxoma. Oncologist. 2014;19:845-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Pietrantonio F, Berenato R, Maggi C, Caporale M, Milione M, Perrone F, Tamborini E, Baratti D, Kusamura S, Mariani L, Niger M, Mennitto A, Gloghini A, Bossi I, Settanni G, Busico A, Bagnoli PF, Di Bartolomeo M, Deraco M, de Braud F. GNAS mutations as prognostic biomarker in patients with relapsed peritoneal pseudomyxoma receiving metronomic capecitabine and bevacizumab: a clinical and translational study. J Transl Med. 2016;14:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Mittal R, Chandramohan A, Moran B. Pseudomyxoma peritonei: natural history and treatment. Int J Hyperthermia. 2017;33:511-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 10. | Raimondi A, Corallo S, Niger M, Antista M, Randon G, Morano F, Milione M, Kusamura S, Baratti D, Guaglio M, Cremolini C, Marmorino F, Di Bartolomeo M, Deraco M, De Braud F, Pietrantonio F. Metronomic Capecitabine With Cyclophosphamide Regimen in Unresectable or Relapsed Pseudomyxoma Peritonei. Clin Colorectal Cancer. 2019;18:e179-e190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Traverso I, Fenoglio D, Negrini S, Parodi A, Battaglia F, Kalli F, Conteduca G, Tardito S, Traverso P, Indiveri F, Filaci G. Cyclophosphamide inhibits the generation and function of CD8 (+) regulatory T cells. Hum Immunol. 2012;73:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Huang XM, Zhang NR, Lin XT, Zhu CY, Zou YF, Wu XJ, He XS, He XW, Wan YL, Lan P. Antitumor immunity of low-dose cyclophosphamide: changes in T cells and cytokines TGF-beta and IL-10 in mice with colon-cancer liver metastasis. Gastroenterol Rep (Oxf). 2020;8:56-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Huang X, Zou Y, Lian L, Wu X, He X, Huang Y, Lan P. Changes of T cells and cytokines TGF-β1 and IL-10 in mice during liver metastasis of colon carcinoma: implications for liver anti-tumor immunity. J Gastrointest Surg. 2013;17:1283-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Liu W, Liu L, Wang R, Gong G, Ding X, Yang B, Bao Y, Wang Z, Zhang B, Zhao D, Wu F, Ding Y. Bevacizumab Combined With Oxaliplatin/Capecitabine in Patient With Refractory and Recurrent Mucinous Adenocarcinoma of the Appendix: A Case Report. Front Oncol. 2019;9:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Hirano S, Gohda Y, Miyazaki H, Hayama N, Shimizu S, Igari T, Yano H. A case of pseudomyxoma peritonei successfully treated with trifluridine/tipiracil (TAS-102) and bevacizumab after palliative debulking surgery. Chin Clin Oncol. 2021;10:29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Choe JH, Overman MJ, Fournier KF, Royal RE, Ohinata A, Rafeeq S, Beaty K, Phillips JK, Wolff RA, Mansfield PF, Eng C. Improved Survival with Anti-VEGF Therapy in the Treatment of Unresectable Appendiceal Epithelial Neoplasms. Ann Surg Oncol. 2015;22:2578-2584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Randall LM, Sill MW, Burger RA, Monk BJ, Buening B, Sorosky JI. Predictive value of serum CA-125 levels in patients with persistent or recurrent epithelial ovarian cancer or peritoneal cancer treated with bevacizumab on a Gynecologic Oncology Group phase II trial. Gynecol Oncol. 2012;124:563-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |