Published online May 27, 2023. doi: 10.4240/wjgs.v15.i5.892
Peer-review started: December 3, 2022
First decision: February 1, 2023
Revised: February 27, 2023
Accepted: March 27, 2023
Article in press: March 27, 2023
Published online: May 27, 2023
Processing time: 174 Days and 6.7 Hours
Surgery remains the primary treatment for localized colorectal cancer (CRC). Improving surgical decision-making for elderly CRC patients necessitates an accurate predictive tool.
To build a nomogram to predict the overall survival of elderly patients over 80 years undergoing CRC resection.
Two hundred and ninety-five elderly CRC patients over 80 years undergoing surgery at Singapore General Hospital between 2018 and 2021 were identified from the American College of Surgeons – National Surgical Quality Improvement Program (ACS-NSQIP) database. Prognostic variables were selected using uni
Eight predictors: Age, Charlson comorbidity index, body mass index, serum albumin level, distant metastasis, emergency surgery, postoperative pneumonia, and postoperative myocardial infarction, were included in the nomogram. The AUC values for the 1-year survival were 0.843 and 0.826 for the training and validation cohorts, respectively. The AUC values for the 3-year survival were 0.788 and 0.750 for the training and validation cohorts, respectively. C-index values of the training cohort (0.845) and validation cohort (0.793) suggested the excellent discriminative ability of the nomogram. Calibration curves demonstrated a good consistency between the predictions and actual observations of overall survival in both training and validation cohorts. A significant difference in overall survival was seen between elderly patients stratified into low- and high-risk groups (P < 0.001).
We constructed and validated a nomogram predicting 1- and 3-year survival probability in elderly patients over 80 years undergoing CRC resection, thereby facilitating holistic and informed decision-making among these patients.
Core Tip: This is the first predictive nomogram evaluating the survival outcomes among elderly colorectal cancer patients over 80 years. This nomogram has incorporated age, Charlson comorbidity index, body mass index, serum albumin level, the presence of metastatic disease, emergency surgery, as well as postoperative pneumonia and myocardial infarction. Our study is the first to link these variables together in predicting overall survival in elderly colorectal cancer patients over 80 years. This novel nomogram that accurately predicts survival probabilities may facilitate preoperative treatment decisions in the advancing age group.
- Citation: Chok AY, Zhao Y, Chen HLR, Tan IEH, Chew DHW, Zhao Y, Au MKH, Tan EJKW. Elderly patients over 80 years undergoing colorectal cancer resection: Development and validation of a predictive nomogram for survival. World J Gastrointest Surg 2023; 15(5): 892-905
- URL: https://www.wjgnet.com/1948-9366/full/v15/i5/892.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i5.892
The world’s population is rapidly aging, with currently over 84 million people aged 75 and above[1]. Population aging will impact cancer control, as around 50% of all cancers affect the older population[2]. Colorectal cancer (CRC) is the second most prevalent malignancy, with an incidence of 1.36 million cases yearly[3]. CRC is the third leading cause of cancer-related mortality, with 50% of new CRC diagnoses being made in patients aged over 70, and 20% in those over 80 years[4]. In Singapore, the incidence of CRC has risen over the last four decades and is now the most common malignancy in the country[5].
Surgery remains the primary treatment for localized CRC. With increasing life expectancy and advances in surgical techniques, there is a growing number of elderly patients over 80 years undergoing CRC resection nowadays[6]. Besides the technical considerations of surgical resectability of the primary CRC, elderly patients continue to pose challenges for the surgeon, as they often have significant comorbidities, such as cardiovascular or pulmonary diseases, which would increase operative risks and potentially lead to postoperative morbidity and mortality[6-8]. Other factors, such as emergency presentation[9] and poor nutritional status, may also result in adverse perioperative outcomes and overall survival (OS).
Therefore, for elderly CRC patients over 80 years, a more individualized approach is essential in the decision-making process, weighing the risks and benefits of surgery on a case-by-case basis[10,11]. Although some studies have suggested that advanced age is a risk factor following specific surgical procedures[12-14], certain results are procedure-specific and limited to the experiences of single centers. Moreover, no study has fully established the impact of advanced age and clinical risk factors on the probability of survival at one year or longer among elderly patients undergoing CRC resection[15-17].
In this study, we developed and validated a predictive nomogram to quantify the probability of OS at one and three years among elderly CRC patients over 80 years, to enable patients, caregivers, and surgeons to make better-informed decisions.
This study was approved by our institutional review board (IRB No. 2022/2438). Data from the American College of Surgeons–National Surgical Quality Improvement Program (ACS-NSQIP) Parti
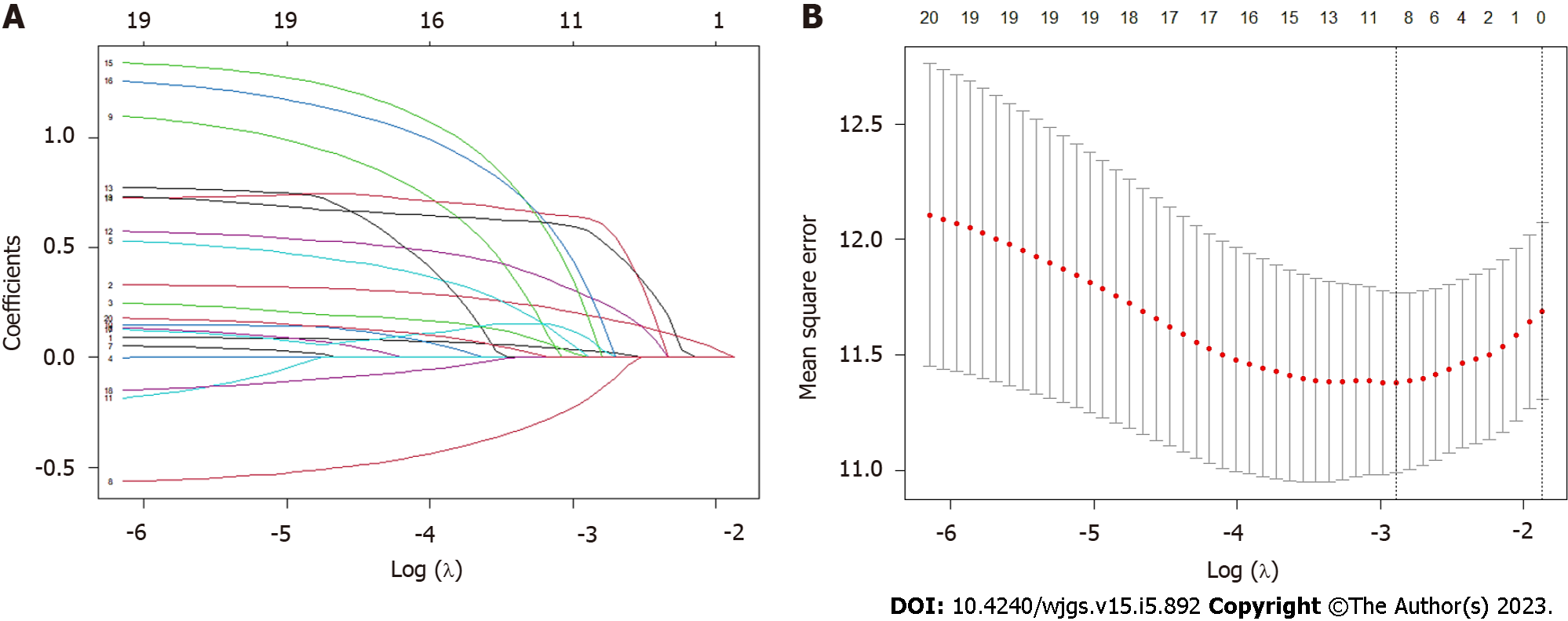
Clinical variables from NSQIP database were: age, American Society of Anesthesiologists classification (ASA), body mass index (BMI), chronic disease history, preoperative medical conditions, serum albumin level, surgical information, tumor-node-metastasis (TNM) staging, postoperative complications, and 30-d mortality. Diagnosis information was collected from our electronic health record system (Sunrise Clinical Manager version 5.8, Eclipsys Corp., Atlanta, GA, United States). Charlson comorbidity index (CCI) was calculated based on a patient’s diagnosis using the 10th revision of the International Statistical Classification of Diseases and Related Health Problems codes. The primary endpoint was OS, which was defined as the time from surgery completion to death of all causes or to the date of the last outpatient clinic follow-up in 2022. Patients who were alive at the time of the last follow-up were censored. Clinical features for constructing a nomogram were screened in three steps. Clinical perspective was the most critical consideration for variable screening. Based on our clinical experiences, we first selected those confirmed factors with a strong association with OS. Secondly, univariate Cox regression was used to identify variables statistically associated with OS. The list of candidate clinical features is presented in Supplementary Table 1. Variables with a P value < 0.20 of univariate analysis were selected. Lastly, the least absolute shrinkage and selection operator (LASSO) regression algorithm was employed to screen all selected features. The 10-fold cross-validation was used to confirm the significant clinical variables and optimal tuning parameter (λ) of LASSO Cox regression.
The nomogram was constructed using clinically significant risk factors identified in the univariate and multivariate Cox proportional hazards analyses and important features recommended by LASSO. The 1- and 3-year OS probabilities were predicted by the nomogram. The performance of the nomogram was evaluated using the concordance index (C-index) and area under the receiver operating characteristic (ROC) curve (AUC)[18]. Similar to AUC, the C-index quantified the discrimination performance of the nomogram. C-index and AUC values ranged from 0 to 1, with 0.5 representing random chance and 1.0 indicating a perfect fit. Values greater than 0.7 suggested a reasonable and accurate model prediction. Calibration curves based on the bootstrap re-sampling method were used to assess the goodness-of-fit of the nomogram[19]. Calibration was determined by comparing the 1- and 3-year OS probabilities predicted by the nomogram to the observed OS probabilities.
The total risk points for each elderly CRC patient were computed using the nomogram. The optimal cut-off risk point was determined by the “survivalROC” model using the Kaplan-Meier estimator[20]. A time-dependent survival ROC curve was plotted to evaluate the prediction of OS based on the total risk points. All elderly patients were stratified into low- and high-risk groups according to the optimal risk threshold. Survival curves of low- and high-risk groups were generated with a hazard ratio (HR) and the P value of the log-rank test.
All statistical calculations were performed using R programming language (version 4.2.1). Continuous variables were shown as median [interquartile range (IQR)]. Categorical variables were presented as frequency distributions (percentage). The Wilcoxon-Mann-Whitney and χ2 or Fisher’s exact tests were used to analyze continuous and categorical variables, respectively. P values of < 0.05 indicated statistical significance.
The baseline demographic, clinicopathologic, and surgical characteristics of 295 elderly CRC patients are shown in Table 1. All patients were randomly divided into a training cohort (n = 177) and a validation cohort (n = 118) in a ratio of 6:4. The median duration of follow-up was 22.68 (IQR: 13.54-37.00) months for the entire cohort. In total, there were 135 male patients (45.8%) and 160 female patients (54.2%) with a median age of 83 (IQR: 81-86) years. Two hundred and sixty-nine patients (91.2%) were between 80 and 89 years old, whereas 26 (8.8%) were nonagenarians. The training and validation cohorts possessed nearly identical characteristics (P > 0.05), with the proportion of patients with significant comorbidities, including congestive heart failure and diabetes mellitus, similar between both groups. Within the entire study cohort, 206 patients (69.8%) underwent surgery on an elective basis. Minimally invasive approach (MIS) was used for 160 patients (54.2%), while the remaining 135 patients (45.8%) underwent open surgery. Right hemicolectomy was performed in 110 patients (37.3%), and anterior resection was performed in 138 patients (46.8%). No stoma formation was required in 205 patients (69.5%). The majority of patients in the study cohort had non-metastatic disease (94.2%, n = 278), with the largest proportion having stage III disease (43.7%, n = 129). None of the patients received adjuvant chemotherapy. In terms of perioperative outcome, 19 patients (6.4%) developed postoperative pneumonia, and 6 patients (2.0%) had an anastomotic leak. The perioperative 30-d mortality was 2.0%.
| Variable | Total, n (%) | Training cohort, n (%) | Validation cohort, n (%) | P value |
| Total case | 295 | 177 | 118 | |
| Follow-up period (mo) | ||||
| Median (IQR) | 22.68 (13.54, 37.00) | 23.43 (14.04, 37.00) | 21.00 (12.93, 37.75) | 0.798 |
| Age (yr) | ||||
| Median (IQR) | 83 (81, 86) | 82 (81, 86) | 83 (81, 86) | 0.738 |
| 80-89 | 269 (91.19) | 161 (90.96) | 108 (91.53) | 0.867 |
| 90-99 | 26 (8.81) | 16 (9.04) | 10 (8.47) | |
| Sex | ||||
| Male | 135 (45.76) | 81 (45.76) | 54 (45.76) | 0.998 |
| Female | 160 (54.24) | 96 (54.24) | 64 (54.24) | |
| Race | ||||
| Chinese | 274 (92.88) | 165 (93.22) | 109 (92.37) | 0.491 |
| Malay | 6 (2.03) | 3 (1.69) | 3 (2.54) | |
| Indian | 9 (3.06) | 4 (2.26) | 5 (4.24) | |
| Others | 6 (2.03) | 5 (2.83) | 1 (0.85) | |
| ASA classification | ||||
| 1 | 2 (0.68) | 1 (0.56) | 1 (0.85) | 0.599 |
| 2 | 90 (30.51) | 52 (29.38) | 38 (32.20) | |
| 3 | 187 (63.39) | 112 (63.28) | 75 (63.56) | |
| 4 | 16 (5.42) | 12 (6.78) | 4 (3.39) | |
| BMI (kg/m2) | ||||
| Median (IQR) | 22.43 (19.82, 25.68) | 22.51 (20.08, 25.82) | 22.23 (19.31, 25.19) | 0.480 |
| ≥ 18.5 | 252 (85.42) | 154 (87.01) | 98 (83.05) | 0.346 |
| < 18.5 | 43 (14.58) | 23 (12.99) | 20 (16.95) | |
| Smoking | ||||
| No | 281 (95.25) | 170 (96.05) | 111 (94.07) | 0.434 |
| Yes | 14 (4.75) | 7 (3.95) | 7 (5.93) | |
| Congestive heart failure within 30 d | ||||
| No | 289 (97.97) | 174 (98.31) | 115 (97.46) | 0.686 |
| Yes | 6 (2.03) | 3 (1.69) | 3 (2.54) | |
| COPD | ||||
| No | 291 (98.64) | 174 (98.31) | 117 (99.15) | 0.652 |
| Yes | 4 (1.36) | 3 (1.69) | 1 (0.85) | |
| Diabetes mellitus | ||||
| No | 217 (73.56) | 133 (75.14) | 84 (71.19) | 0.451 |
| Yes | 78 (26.44) | 44 (24.86) | 34 (28.81) | |
| Preoperative dialysis dependent | ||||
| No | 291 (98.64) | 174 (98.31) | 117 (99.15) | 0.652 |
| Yes | 4 (1.36) | 3 (1.69) | 1 (0.85) | |
| CCI | ||||
| 0 | 2 (0.68) | 1 (0.56) | 1 (0.85) | 0.118 |
| 1-2 | 98 (33.22) | 50 (28.25) | 48 (40.68) | |
| 3-4 | 62 (21.02) | 40 (22.60) | 22 (18.64) | |
| ≥ 5 | 133 (45.08) | 86 (48.59) | 47 (39.83) | |
| Serum albumin (g/dL) | ||||
| Median (IQR) | 3.6 (3.2, 3.9) | 3.6 (3.1, 3.9) | 3.6 (3.2, 3.9) | 0.316 |
| ≥ 3.0 | 261 (88.48) | 160 (90.40) | 101 (85.59) | 0.209 |
| 2.5-3.0 | 27 (9.15) | 12 (6.78) | 15 (12.71) | |
| < 2.5 | 7 (2.37) | 5 (2.82) | 2 (1.70) | |
| Priority of operation | ||||
| Elective | 206 (69.83) | 121 (68.36) | 85 (72.03) | 0.501 |
| Emergency | 89 (30.17) | 56 (31.64) | 33 (27.97) | |
| Method of operation | ||||
| Open | 135 (45.76) | 80 (45.20) | 55 (46.61) | 0.812 |
| Minimally invasive surgery | 160 (54.24) | 97 (54.80) | 63 (53.39) | |
| Type of operation | ||||
| Right hemicolectomy | 110 (37.29) | 65 (36.72) | 45 (38.14) | 0.942 |
| Left hemicolectomy | 8 (2.71) | 5 (2.83) | 3 (2.54) | |
| High anterior resection | 86 (29.15) | 51 (28.81) | 35 (29.66) | |
| Low anterior resection | 52 (17.63) | 30 (16.95) | 22 (18.64) | |
| Subtotal/total colectomy | 13 (4.40) | 10 (5.65) | 3 (2.54) | |
| Abdominoperineal resection | 12 (4.07) | 7 (3.95) | 5 (4.24) | |
| Hartmann's procedure | 14 (4.75) | 9 (5.09) | 5 (4.24) | |
| Stoma | ||||
| No | 205 (69.49) | 123 (69.49) | 82 (69.49) | 0.529 |
| Loop ileostomy | 23 (7.80) | 10 (5.65) | 13 (11.02) | |
| End ileostomy | 6 (2.03) | 5 (2.82) | 1 (0.85) | |
| Ileo-colostomy | 8 (2.71) | 5 (2.82) | 3 (2.54) | |
| Loop colostomy | 19 (6.44) | 12 (6.78) | 7 (5.93) | |
| End colostomy | 34 (11.53) | 22 (12.44) | 12 (10.17) | |
| TNM staging | ||||
| I | 61 (20.68) | 40 (22.60) | 21 (17.80) | 0.274 |
| II | 88 (29.83) | 50 (28.25) | 38 (32.20) | |
| III | 129 (43.73) | 80 (45.20) | 49 (41.53) | |
| IV | 17 (5.76) | 7 (3.95) | 10 (8.47) | |
| Postoperative anastomotic leak | ||||
| No | 289 (97.97) | 173 (97.74) | 116 (98.31) | 0.999 |
| Yes | 6 (2.03) | 4 (2.26) | 2 (1.69) | |
| Postoperative pneumonia | ||||
| No | 276 (93.56) | 165 (93.22) | 111 (94.07) | 0.771 |
| Yes | 19 (6.44) | 12 (6.78) | 7 (5.93) | |
| Postoperative myocardial infarction | ||||
| No | 288 (97.63) | 172 (97.18) | 116 (98.31) | 0.706 |
| Yes | 7 (2.37) | 5 (2.82) | 2 (1.69) | |
| Postoperative 30-d readmission | ||||
| No | 255 (86.44) | 154 (87.01) | 101 (85.59) | 0.729 |
| Yes | 40 (13.56) | 23 (12.99) | 17 (14.41) | |
| Postoperative 30-d mortality | ||||
| No | 289 (97.97) | 175 (98.87) | 114 (96.61) | 0.222 |
| Yes | 6 (2.03) | 2 (1.13) | 4 (3.39) | |
| Postoperative 1-yr mortality | ||||
| No | 206 (69.83) | 124 (70.06) | 82 (69.49) | 0.918 |
| Yes | 89 (30.17) | 53 (29.94) | 36 (30.51) |
All candidate clinical features with their univariate Cox regression P values are listed in Supp
| Univariate | Multivariate | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age1 | 1.08 (1.02-1.14) | 0.005 | 1.10 (1.04-1.17) | < 0.001 |
| Age | ||||
| 80-89 | Reference | |||
| 90-99 | 1.31 (0.63-2.73) | 0.470 | - | - |
| BMI (kg/m2) | ||||
| ≥ 18.5 | Reference | Reference | ||
| < 18.5 | 1.93 (1.12-3.33) | 0.018 | 1.93 (1.05-3.53) | 0.034 |
| Serum albumin (g/dL) | ||||
| ≥ 3.0 | Reference | Reference | ||
| 2.5-3.0 | 1.75 (0.90-3.44) | 0.101 | 1.18 (0.55-2.54) | 0.672 |
| < 2.5 | 7.15 (2.82-18.2) | < 0.001 | 5.04 (1.82-13.9) | 0.002 |
| TNM staging | ||||
| I, II, and III | Reference | Reference | ||
| IV | 3.95 (2.07-7.55) | < 0.001 | 2.06 (0.95-4.49) | 0.068 |
| CCI1 | 1.34 (1.21-1.48) | < 0.001 | 1.41 (1.25-1.59) | < 0.001 |
| Priority of operation | ||||
| Elective | Reference | Reference | ||
| Emergency | 1.81 (1.13-2.88) | 0.013 | 1.35 (0.78-2.33) | 0.278 |
| Postoperative pneumonia | ||||
| No | Reference | Reference | ||
| Yes | 3.46 (1.77-6.78) | < 0.001 | 2.99 (1.43-6.23) | 0.004 |
| Postoperative myocardial infarction | ||||
| No | Reference | Reference | ||
| Yes | 6.01 (2.17-16.6) | < 0.001 | 4.09 (1.37-12.2) | 0.012 |
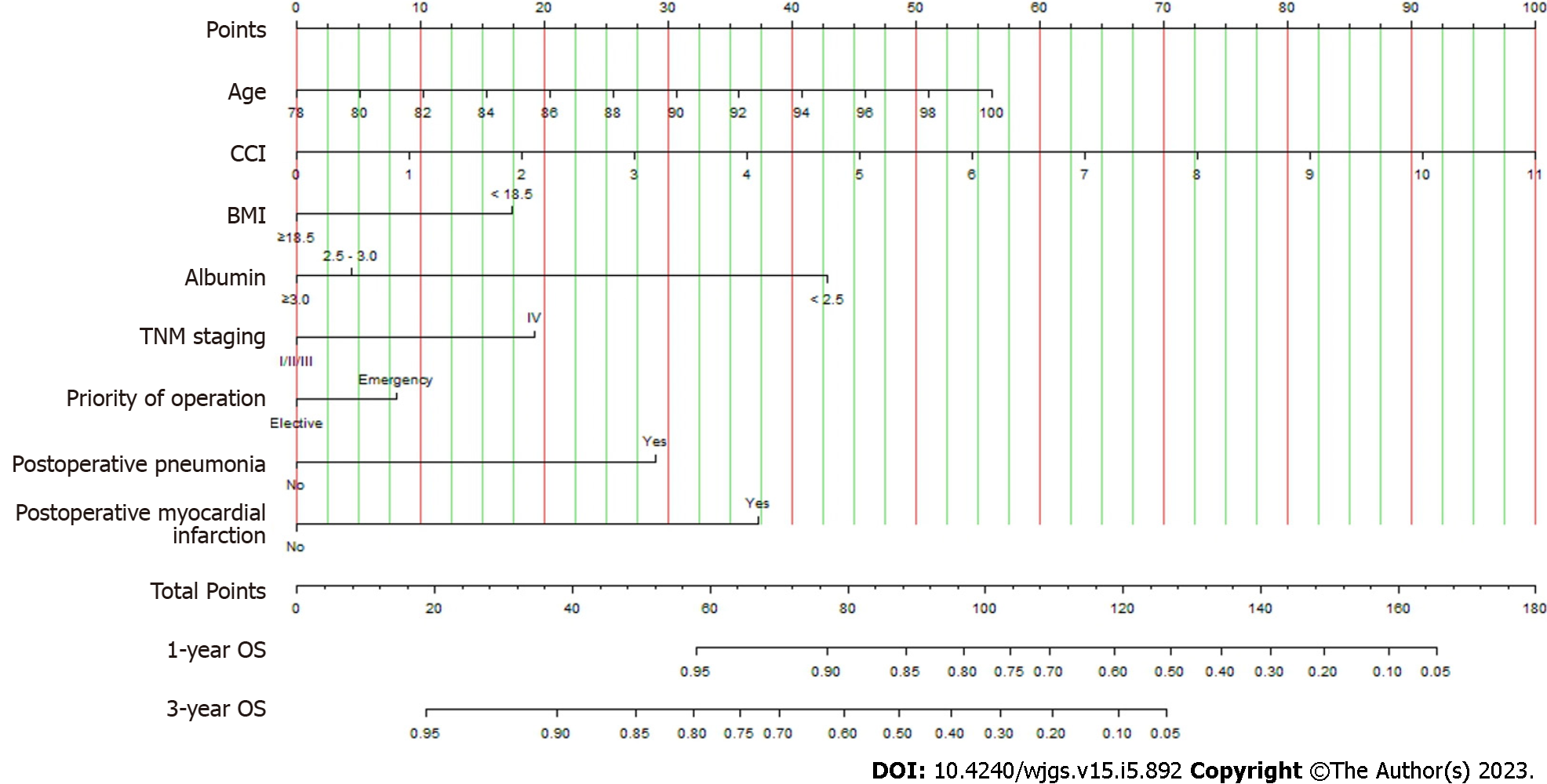
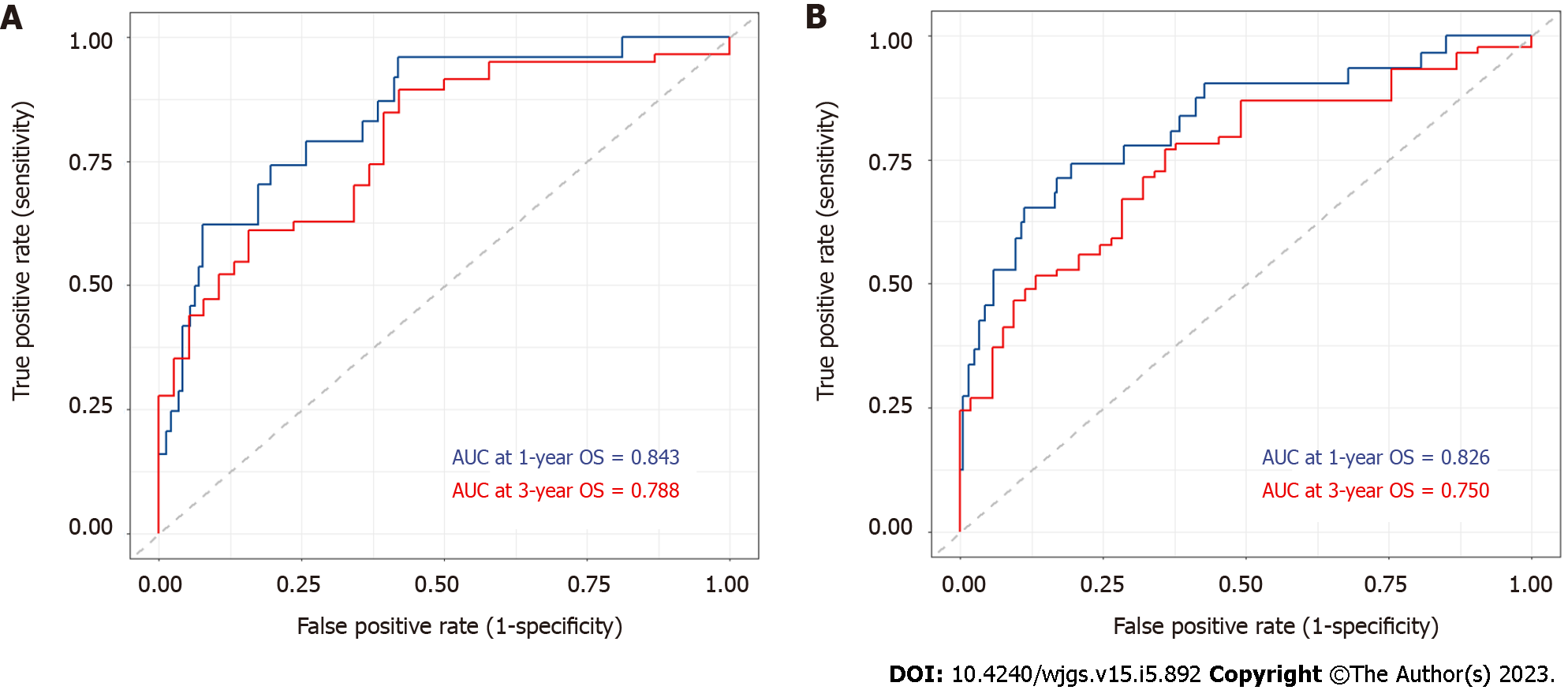
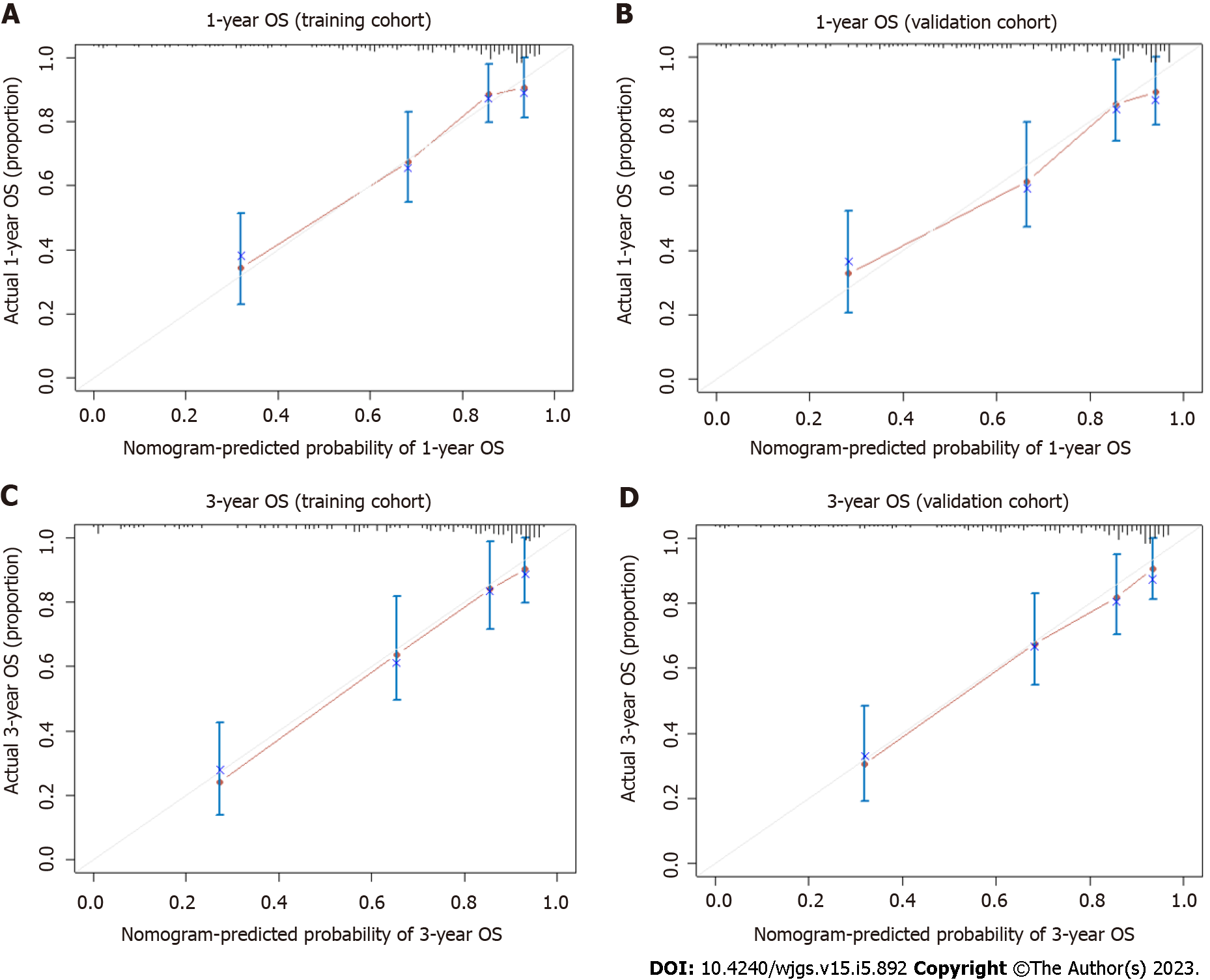
A nomogram applicable to all elderly CRC patients was created using eight selected predictors’ point scales, with the sum of the eight variables’ points defining the total number of points. Estimated 1- and 3-year OS probabilities could be obtained by drawing a vertical line from the “Total Points” axis down to the two-outcome probability axis (Figure 2). The AUC of the nomogram for predicting 1-year OS was 0.843 (95%CI: 0.827-0.935) in the training cohort and 0.826 (95%CI: 0.816-0.912) in the validation cohort, while AUC for predicting 3-year OS was 0.788 (95%CI: 0.762-0.889) in the training cohort and 0.750 (95%CI: 0.734-0.883) in the validation cohort (Figure 3). The C-index value was 0.845 (95%CI: 0.789-0.889) in the training cohort and 0.793 (95%CI: 0.754-0.887) in the validation cohort. Both AUC and C-index values indicated the constructed nomogram provided favorable discrimination. The calibration curves of the nomogram were evaluated by plotting the predicted 1- and 3-year OS against the observed 1- and 3-year OS. A 45-degree line would be obtained if the predictions were accurately calibrated. The 1- and 3-year calibration curves in both training and validation cohorts showed a good concordance between the predicted and observed OS probabilities (Figure 4).
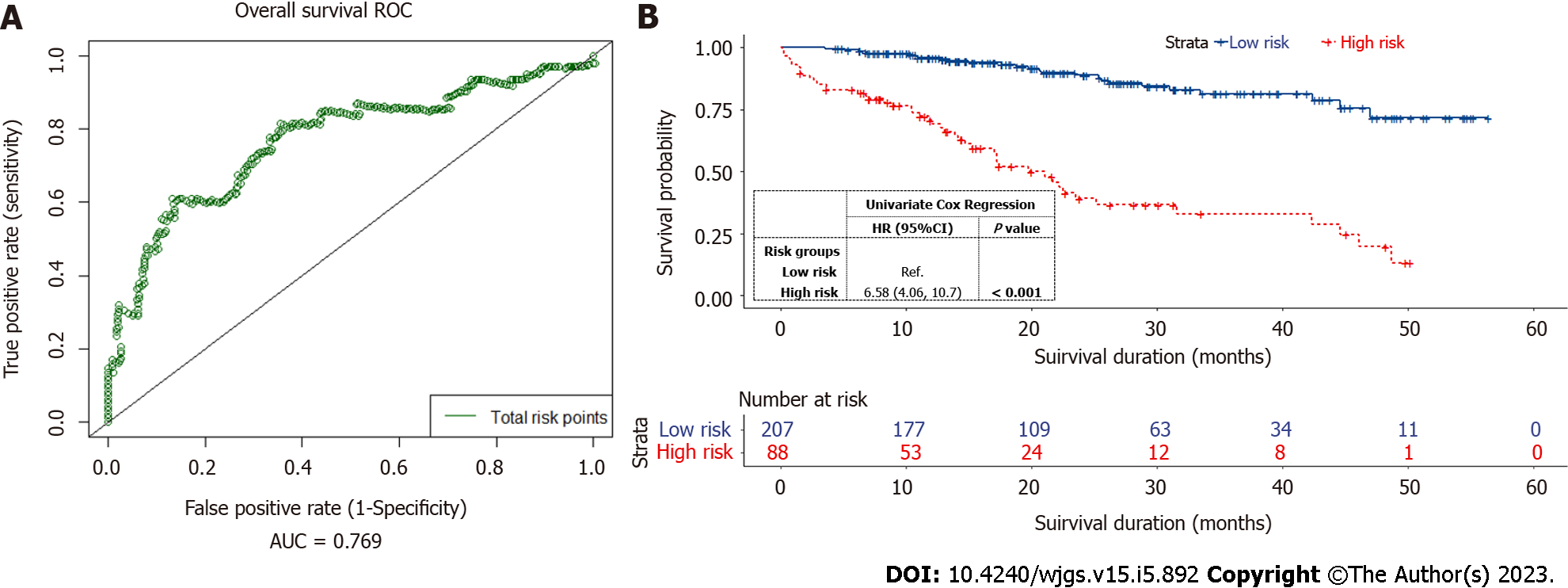
The total risk points of each elderly CRC patient were calculated based on the nomogram. The optimal risk cut-off point of 81 was determined using the Kaplan-Meier estimation[20]. On the nomogram, the risk threshold of 81 points approximately corresponded to the 1-year OS probability of 87% and the 3-year OS probability of 56%. A time-dependent 3-year survival ROC curve was generated for all patients using the total risk points computed by the nomogram (Figure 5A). The AUC of the total risk points (0.769, 95%CI: 0.724-0.883) indicated that the optimal risk threshold was adequate for risk stratification in elderly CRC patients. All patients were categorized into low-risk (total risk points < 81) or high-risk (total risk points ≥ 81) groups. The Kaplan-Meier curves accurately distinguished the low- and high-risk groups (Figure 5B). The 3-year OS probabilities of elderly CRC patients in the high-risk groups were significantly lower (HR = 6.58, 95%CI: 4.06-10.7, P < 0.001).
Increasing age is a well-known risk factor for the development of CRC, with a majority of patients diagnosed after 70 years old[21]. Moreover, elderly patients tend to have a higher prevalence of frailty, comorbidities, and mortality risk from other causes[22]. Nevertheless, there is still significant heterogeneity in terms of physiological capacity and performance status among the elderly population. Considering the increased life expectancy of an aging population as well as new advances in surgical technology and perioperative care, it is, therefore, necessary to stratify the risk associated with elderly patients undergoing surgery. As the proportion of elderly CRC patients continues to rise, there is a greater need to comprehend the risks associated with surgical resection.
In the present study, we developed and validated a nomogram based on clinical risk factors predicting the probabilities of 1- and 3-year OS in elderly CRC patients over 80 years undergoing surgical resection using the ACS-NSQIP data. Although there are existing nomograms[23,24] predicting cancer-specific and OS among CRC patients, this is the first predictive nomogram evaluating the survival outcomes among elderly CRC patients over the age of 80. Our ACS-NSQIP dataset was comprehensive and well-organized, allowing us to obtain critical clinical data regarding the association between risk factors and OS after colorectal resection. We identified eight variables as independent prognostic factors based on clinical observations and LASSO regression, which efficiently processed demographic and clinical feature selection as a statistical strategy for high-dimensional data.
This nomogram incorporated age, CCI, BMI, serum albumin level, TNM staging, priority of surgery, postoperative pneumonia, and postoperative myocardial infarction. Some characteristics included in the nomogram construction have previously been reported to have a significant correlation with mortality and OS, but our study is the first to link them together in predicting OS in elderly CRC patients over 80 years. We found that every additional year of age beyond 80 was associated with a 10% increase in mortality risk. In terms of the comorbidity profile, an increase of one point in the CCI score was associated with a 41% increase in mortality risk. Of note, CCI has been reported as an independent prognostic factor in older CRC patients[25]. Elderly CRC patients with high CCI scores tended to have a lower OS[25]. In addition, surgical outcomes of the geriatric population have been stratified using frailty assessments involving age and CCI[26,27]. A systematic review revealed that frailty was associated with an increased incidence of postoperative complications, mortality, readmissions, reoperations, and prolonged hospital length of stay, but age by itself was not associated with any adverse outcomes[28]. Suboptimal nutritional status reflected by BMI < 18.5 kg/m2 and serum albumin level < 2.5 g/dL were also independent risk factors for poorer OS. It has been reported that lower BMI and serum albumin levels were nutritional risk factors associated with shorter survival in cancer patients[29,30]. Lymph node metastasis is another risk factor for OS. It is well-known that lymph node metastasis is associated with worse outcomes in CRC patients with poor prognoses[31]. Emergency surgery was identified to be significantly associated with poorer OS in our elderly CRC cohort. Among the 17 elderly patients with stage IV disease who underwent CRC resection, 12 had surgery performed in an emergency setting. Some studies have highlighted the need for improved risk stratification based on emergency because surgeries performed urgently are more likely to have distinct morbidity and mortality rates than surgeries performed electively[32,33].
In this study, MIS was found to have a positive impact on OS in elderly CRC patients in the univariate Cox regression (P = 0.01). The LASSO regression, however, eliminated the method of operation, indicating that it was not a predictor of OS in CRC patients over 80 years undergoing surgery. The majority (90.6%) of the 160 elderly patients in the MIS group underwent laparoscopic surgery, whereas 15 patients (9.4%) underwent robotic surgery. Laparoscopic surgery for colon cancer has been shown to be associated with improved postoperative outcomes with similar long-term oncological outcomes in recent years[34,35]. It has been recommended as the preferred approach for elective surgery[36]. While the long-term oncological outcomes of laparoscopic rectal cancer surgery have yet to be conclusive, it does confer improved postoperative outcomes and has been included in society guidelines to be considered in centers with technical expertise and experience. Although laparoscopic surgery is associated with superior postoperative outcomes such as reduced wound pain and ileus, without compromising long-term oncological outcomes[37-39], its role among elderly patients remains unclear given the longer operating time and the effect of pneumoperitoneum on the cardiorespiratory system.
Elderly patients, after surgery, are at increased risk of postoperative complications such as surgical site infections, pneumonia, cardiac complications, and anastomotic leak. In our study cohort, primary anastomosis was performed in 69.5% of the patients. There were 6 cases (2.03%) had the postoperative anastomotic leak. These findings are consistent with other recent studies. Hashimoto et al[40] reported an anastomosis rate of 86.0% with a leak rate of 2.3%, while Zeng et al[41] reported an anastomosis rate of 62.9% with a leak rate of 2.1%. Furthermore, it has been estimated that a patient older than 80 years is more than five times as likely to suffer from postoperative pulmonary complications compared to a patient younger than 50 years[42]. Unsurprisingly, postoperative pneumonia and myocardial infarction were identified as prognostic factors of OS in elderly CRC patients. Therefore, the identification of elderly patients at risk of postoperative cardiopulmonary complications can facilitate the early involvement of the multidisciplinary team in pre-habilitation and postoperative care, including adequate pain control, chest physiotherapy, and early mobilization. In line with our predictive nomogram, the successful mitigation of postoperative pneumonia and myocardial infarction risks can result in higher probabilities of improved OS at one and three years among elderly patients undergoing CRC resection.
We consolidated the eight selected predictors into the nomogram and evaluated the performance using bootstrapping and cross-validation methods in calculating AUC, C-index, and calibration curves. Both AUC and C-index values were replicated well in the training and validation sets. The calibration curves of 1- and 3-year OS in both sets displayed favorable agreement between the predicted and observed survival probabilities. We further stratified elderly CRC patients into low- and high-risk groups according to their total risk points and optimal threshold values. The Kaplan-Meier method and Cox proportional hazards model revealed statistically significant differences between the two risk groups in terms of OS. Our results demonstrate that the nomogram accurately identifies the high-risk population and predicts OS, thereby facilitating appropriate clinical decision-making. It provides a distinct visual representation that enables information sharing between clinicians and patients. For example, it is clear that advanced age is not the only predictive factor influencing OS. In addition, an elderly patient in the high-risk group with multiple comorbidities and poor nutrition, may benefit from a period of pre-habilitation and optimization prior to CRC resection.
Our study has some limitations, including its retrospective nature and selection bias. First, elderly CRC patients who are physically fit are more likely to undergo surgery. In our study cohort, octogenarians comprised a more significant percentage (91.2%) than nonagenarians. Nevertheless, the primary data was complete with a median follow-up duration of 22.68 mo. Secondly, our data were limited in size and derived from a single institution, which may limit the generalizability of the nomogram. Despite these limitations, the ability of our constructed nomogram to accurately predict survival probability in elderly CRC patients over 80 years undergoing colorectal resection has substantial clinical implications. In this advancing age group, it is challenging to make management decisions in light of the risks associated with surgery. Therefore, the application of the nomogram lies in its capacity to guide the individualization of clinical decisions in complex scenarios.
In summary, colorectal surgery in elderly CRC patients is associated with a lower likelihood of survival. We used data from ACS-NSQIP to construct and validate an original nomogram for the postoperative survival of elderly CRC patients over 80 years. By accurately predicting 1- and 3-year survival probabilities, our novel nomogram, which incorporated age, CCI, BMI, serum albumin level, distant metastasis, emergency surgery, postoperative pneumonia, and postoperative myocardial infarction, may facilitate preoperative clinical decisions for patients, caregivers, and clinicians.
Colorectal surgery is associated with a decreased probability of survival in elderly cancer patients. Several factors can affect the postoperative survival of elderly colorectal cancer (CRC) patients.
A precise predictive tool is required to enhance the decision-making process for elderly CRC patients undergoing colorectal resection.
To construct and validate a nomogram to predict the overall survival of elderly CRC patients over 80 years undergoing colorectal surgery.
This retrospective study included 295 elderly CRC patients over 80 years undergoing colorectal resection. Variables were selected using regression methods, and a nomogram for 1- and 3-year overall survival was constructed from 60% of the cohort and validated on the remaining 40%. The performance of the nomogram was evaluated using various metrics, and the risk group was stratified based on the risk points of the nomogram.
The nomogram, which comprised age, comorbidities, body mass index, serum albumin level, distant metastasis, emergency surgery, postoperative pneumonia, and postoperative myocardial infarction, demonstrated excellent discriminative ability and consistency between predictions and actual observations. The risk group was stratified based on the nomogram's risk points, and a significant difference in overall survival was observed between low- and high-risk groups.
This novel nomogram provides a valuable tool for informed decision-making in elderly CRC patients undergoing colorectal resection.
We developed a nomogram using demographic and clinical variables to estimate the survival of elderly CRC patients undergoing colorectal surgery. This nomogram may guide treatment decisions, facilitate patient counseling, and enhance surgical outcomes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Wan XH, China; Zhao Z, China S-Editor: Gao CC L-Editor: A P-Editor: Wu RR
| 1. | Kasai DT. Preparing for population ageing in the Western Pacific Region. Lancet Reg Health West Pac. 2021;6:100069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, van Ballegooijen M, Goede SL, Ries LA. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1504] [Article Influence: 100.3] [Reference Citation Analysis (1)] |
| 3. | Balducci L. Studying cancer treatment in the elderly patient population. Cancer Control. 2014;21:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2526] [Cited by in RCA: 2912] [Article Influence: 364.0] [Reference Citation Analysis (3)] |
| 5. | Wong MT, Eu KW. Rise of colorectal cancer in Singapore: an epidemiological review. ANZ J Surg. 2007;77:446-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Turrentine FE, Wang H, Simpson VB, Jones RS. Surgical risk factors, morbidity, and mortality in elderly patients. J Am Coll Surg. 2006;203:865-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 662] [Cited by in RCA: 751] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 7. | Grosso G, Biondi A, Marventano S, Mistretta A, Calabrese G, Basile F. Major postoperative complications and survival for colon cancer elderly patients. BMC Surg. 2012;12 Suppl 1:S20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Dekker JW, Gooiker GA, Bastiaannet E, van den Broek CB, van der Geest LG, van de Velde CJ, Tollenaar RA, Liefers GJ; Steering Committee of the ‘Quality Information System Colorectal Cancer’ Project. Cause of death the first year after curative colorectal cancer surgery; a prolonged impact of the surgery in elderly colorectal cancer patients. Eur J Surg Oncol. 2014;40:1481-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Balducci L, Ershler WB. Cancer and ageing: a nexus at several levels. Nat Rev Cancer. 2005;5:655-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 172] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Gooiker GA, Dekker JW, Bastiaannet E, van der Geest LG, Merkus JW, van de Velde CJ, Tollenaar RA, Liefers GJ. Risk factors for excess mortality in the first year after curative surgery for colorectal cancer. Ann Surg Oncol. 2012;19:2428-2434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Kolfschoten NE, Wouters MW, Gooiker GA, Eddes EH, Kievit J, Tollenaar RA, Marang-van de Mheen PJ; Dutch Surgical Colorectal Audit group. Nonelective colon cancer resections in elderly patients: results from the dutch surgical colorectal audit. Dig Surg. 2012;29:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Hallowell PT, Stellato TA, Schuster M, Graf K, Robinson A, Jasper JJ. Avoidance of complications in older patients and Medicare recipients undergoing gastric bypass. Arch Surg. 2007;142:506-10; discussion 510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Surgery for colorectal cancer in elderly patients: a systematic review. Colorectal Cancer Collaborative Group. Lancet. 2000;356:968-974. [PubMed] |
| 14. | Pedrazzani C, Cerullo G, De Marco G, Marrelli D, Neri A, De Stefano A, Pinto E, Roviello F. Impact of age-related comorbidity on results of colorectal cancer surgery. World J Gastroenterol. 2009;15:5706-5711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Ogata T, Yoshida N, Sadakari Y, Iwanaga A, Nakane H, Okawara K, Endo K, Kaneshiro K, Hirokata G, Aoyagi T, Shima H, Taniguchi M. Colorectal cancer surgery in elderly patients 80 years and older: a comparison with younger age groups. J Gastrointest Oncol. 2022;13:137-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Hashimoto S, To K, Wada H, Sakakibara Y, Ozeki K, Komaki M, Kondo M. Total Risk Points Predict Short- and Long-term Outcomes Following Colorectal Cancer Resection in Older Patients. Cancer Diagn Progn. 2022;2:360-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Bos ACRK, Kortbeek D, van Erning FN, Zimmerman DDE, Lemmens VEPP, Dekker JWT, Maas HAAM. Postoperative mortality in elderly patients with colorectal cancer: The impact of age, time-trends and competing risks of dying. Eur J Surg Oncol. 2019;45:1575-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Harrell FE Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543-2546. [PubMed] |
| 19. | Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1306] [Cited by in RCA: 2307] [Article Influence: 135.7] [Reference Citation Analysis (0)] |
| 20. | Heagerty PJ, Saha-Chaudhuri P, Saha-Chaudhuri MP. Package ‘survivalROC’. San Francisco: GitHub 2013. [cited 30 November 2022]. In: GitHub [Internet]. Available from: https://github.com/. |
| 21. | Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374:1196-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2275] [Cited by in RCA: 2237] [Article Influence: 139.8] [Reference Citation Analysis (0)] |
| 22. | Fu J, Ruan H, Zheng H, Cai C, Zhou S, Wang Q, Chen W, Fu W, Du J. Impact of old age on resectable colorectal cancer outcomes. PeerJ. 2019;7:e6350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Yu C, Zhang Y. Establishment of prognostic nomogram for elderly colorectal cancer patients: a SEER database analysis. BMC Gastroenterol. 2020;20:347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 24. | Liu J, Huang X, Yang W, Li C, Li Z, Zhang C, Chen S, Wu G, Xie W, Wei C, Tian C, Huang L, Jeen F, Mo X, Tang W. Nomogram for predicting overall survival in stage II-III colorectal cancer. Cancer Med. 2020;9:2363-2371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 25. | Tominaga T, Nonaka T, Takeshita H, Kunizaki M, Sumida Y, Hidaka S, Sawai T, Nagayasu T. The Charlson Comorbidity Index as an Independent Prognostic Factor in Older Colorectal Cancer Patients. Indian J Surg. 2018;80:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Robinson TN, Eiseman B, Wallace JI, Church SD, McFann KK, Pfister SM, Sharp TJ, Moss M. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg. 2009;250:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 373] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 27. | Niemeläinen S, Huhtala H, Andersen J, Ehrlich A, Haukijärvi E, Koikkalainen S, Koskensalo S, Kössi J, Mattila A, Pinta T, Uotila-Nieminen M, Vihervaara H, Hyöty M, Jämsen E. The Clinical Frailty Scale is a useful tool for predicting postoperative complications following elective colon cancer surgery at the age of 80 years and above: A prospective, multicentre observational study. Colorectal Dis. 2021;23:1824-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Michaud Maturana M, English WJ, Nandakumar M, Li Chen J, Dvorkin L. The impact of frailty on clinical outcomes in colorectal cancer surgery: a systematic literature review. ANZ J Surg. 2021;91:2322-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Seebacher V, Rockall A, Nobbenhuis M, Sohaib SA, Knogler T, Alvarez RM, Kolomainen D, Shepherd JH, Shaw C, Barton DP. The impact of nutritional risk factors and sarcopenia on survival in patients treated with pelvic exenteration for recurrent gynaecological malignancy: a retrospective cohort study. Arch Gynecol Obstet. 2022;305:1343-1352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Cui L, Yu H, Sun Q, Miao Y, Jiang K, Fang X. Effects of body mass index and serum albumin on overall survival in patients with cancer undergoing pancreaticoduodenectomy: a single-center retrospective cohort study. World J Surg Oncol. 2022;20:221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 31. | Naxerova K, Reiter JG, Brachtel E, Lennerz JK, van de Wetering M, Rowan A, Cai T, Clevers H, Swanton C, Nowak MA, Elledge SJ, Jain RK. Origins of lymphatic and distant metastases in human colorectal cancer. Science. 2017;357:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 375] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 32. | Mullen MG, Michaels AD, Mehaffey JH, Guidry CA, Turrentine FE, Hedrick TL, Friel CM. Risk Associated With Complications and Mortality After Urgent Surgery vs Elective and Emergency Surgery: Implications for Defining "Quality" and Reporting Outcomes for Urgent Surgery. JAMA Surg. 2017;152:768-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 33. | Tolstrup MB, Watt SK, Gögenur I. Morbidity and mortality rates after emergency abdominal surgery: an analysis of 4346 patients scheduled for emergency laparotomy or laparoscopy. Langenbecks Arch Surg. 2017;402:615-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 34. | Draeger T, Völkel V, Gerken M, Klinkhammer-Schalke M, Fürst A. Long-term oncologic outcomes after laparoscopic vs open rectal cancer resection: a high-quality population-based analysis in a Southern German district. Surg Endosc. 2018;32:4096-4104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Ueda Y, Shiraishi N, Kawasaki T, Akagi T, Ninomiya S, Shiroshita H, Etoh T, Inomata M. Short- and long-term outcomes of laparoscopic surgery for colorectal cancer in the elderly aged over 80 years old vs non-elderly: a retrospective cohort study. BMC Geriatr. 2020;20:445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 36. | Moghadamyeghaneh Z, Carmichael JC, Mills S, Pigazzi A, Nguyen NT, Stamos MJ. Variations in Laparoscopic Colectomy Utilization in the United States. Dis Colon Rectum. 2015;58:950-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 37. | Clinical Outcomes of Surgical Therapy Study Group; Nelson H, Sargent DJ, Wieand HS, Fleshman J, Anvari M, Stryker SJ, Beart RW Jr, Hellinger M, Flanagan R Jr, Peters W, Ota D. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2606] [Cited by in RCA: 2518] [Article Influence: 119.9] [Reference Citation Analysis (0)] |
| 38. | Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW Jr, Hellinger M, Flanagan R Jr, Peters W, Nelson H; Clinical Outcomes of Surgical Therapy Study Group. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg. 2007;246:655-62; discussion 662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 802] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 39. | Bonjer HJ, Hop WC, Nelson H, Sargent DJ, Lacy AM, Castells A, Guillou PJ, Thorpe H, Brown J, Delgado S, Kuhrij E, Haglind E, Påhlman L; Transatlantic Laparoscopically Assisted vs Open Colectomy Trials Study Group. Laparoscopically assisted vs open colectomy for colon cancer: a meta-analysis. Arch Surg. 2007;142:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 389] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 40. | Hashimoto S, Hamada K, Sumida Y, Araki M, Wakata K, Kugiyama T, Shibuya A, Nishimuta M, Morino S, Baba M, Kiya S, Ozeki K, Nakamura A. Short- and long-term survival after curative resection for colorectal cancer in nonagenarian patients. Asian J Surg. 2022;45:208-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Zeng WG, Liu MJ, Zhou ZX, Hu JJ, Wang ZJ. Outcomes of colorectal cancer surgery in nonagenarian patients: a multicenter retrospective study. J Gastrointest Oncol. 2021;12:1568-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Smetana GW, Lawrence VA, Cornell JE; American College of Physicians. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144:581-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 530] [Article Influence: 27.9] [Reference Citation Analysis (0)] |