Published online Apr 27, 2023. doi: 10.4240/wjgs.v15.i4.698
Peer-review started: November 17, 2022
First decision: January 23, 2023
Revised: February 5, 2023
Accepted: March 3, 2023
Article in press: March 3, 2023
Published online: April 27, 2023
Processing time: 156 Days and 14.9 Hours
Palliative endoscopic biliary drainage is the primary treatment option for the management of patients with jaundice which results from distal malignant biliary obstruction (DMBO). In this group of patients, decompression of the bile duct (BD) allows for pain reduction, symptom relief, chemotherapy administration, improved quality of life, and increased survival rate. To reduce the unfavorable effects of BD decompression, minimally invasive surgical techniques require continuous improvement.
To develop a technique for internal-external biliary-jejunal drainage (IEBJD) and assess its effectiveness in comparison to other minimally invasive procedures in the palliative treatment of patients with DMBO.
A retrospective analysis of prospectively collected data was performed, which included 134 patients with DMBO who underwent palliative BD decompression. Biliary-jejunal drainage was developed to divert bile from the BD directly into the initial loops of the small intestine to prevent duodeno-biliary reflux. IEBJD was carried out using percutaneous transhepatic access. Percutaneous transhepatic biliary drainage (PTBD), endoscopic retrograde biliary stenting (ERBS), and internal-external transpapillary biliary drainage (IETBD) were used for the treatment of study patients. Endpoints of the study were the clinical success of the procedure, the frequency and nature of complications, and the cumulative survival rate.
There were no significant differences in the frequency of minor complications between the study groups. Significant complications occurred in 5 (17.2%) patients in the IEBJD group, in 16 (64.0%) in the ERBS group, in 9 (47.4%) in the IETBD group, and in 12 (17.4%) in the PTBD group. Cholangitis was the most common severe complication. In the IEBJD group, the course of cholangitis was characterized by a delayed onset and shorter duration as compared to other study groups. The cumulative survival rate of patients who underwent IEBJD was 2.6 times higher in comparison to those of the PTBD and IETBD groups and 20% higher in comparison to that of the ERBS group.
IEBJD has advantages over other minimally invasive BD decompression techniques and can be recommended for the palliative treatment of patients with DMBO.
Core Tip: This study compared the new technique of internal-external biliary-jejunal drainage (IEBJD) for bile duct (BD) decompression in patients with obstructive jaundice with commonly used procedures through a retrospective analysis of prospectively collected data. IEBJD was used to divert bile from the BD directly into the initial loops of the small intestine to prevent duodeno-biliary reflux. The application of IEBJD was associated with a decreased incidence of significant complications, a delayed onset of cholangitis and its shorter duration, as well as an increased cumulative survival rate in patients with distal malignant biliary obstruction as compared to commonly used endoscopic ultrasound-guided retrograde and antegrade techniques and internal-external transpapillary biliary drainage.
- Citation: Susak YM, Markulan LL, Lobanov SM, Palitsya RY, Rudyk MP, Skivka LM. Effectiveness of a new approach to minimally invasive surgery in palliative treatment of patients with distal malignant biliary obstruction. World J Gastrointest Surg 2023; 15(4): 698-711
- URL: https://www.wjgnet.com/1948-9366/full/v15/i4/698.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i4.698
Patients with jaundice would be ineligible for radical treatment in 70%-85% of cases upon initial examination due to distal malignant biliary obstruction (DMBO)[1,2]. Palliative decompression of the bile ducts (BD) is currently the primary treatment option for the management of patients with obstructive jaundice. This approach allows for pain reduction, symptom relief, and, in some cases, chemotherapy administration[3]. BD decompression not only improves patients’ quality of life but also increases their survival rate[4].
In general, there are two main techniques for performing endoscopic ultrasound-guided minimally invasive BD (EUS-BD) decompression: The EUS-rendezvous approach (retrograde) and the EUS-antegrade technique[5,6], and their combination is also possible[7]. Each method has both advantages and disadvantages.
Percutaneous transhepatic biliary drainage (PTBD) is likely to result in: (1) A significant loss of bile, necessitating the oral administration of bile salts and leaving an implantable port under the skin[6]; and (2) the implantation of metastases along the trajectory of stent placement[8], as well as cholangitis development caused by stent malfunction. Nevertheless, compared to other methods, this one is relatively simple and most affordable. Endoscopic retrograde biliary stenting (ERBS) is considered a method of choice for palliative treatment of patients with DMBO[8,9]. However, it is associated with trauma to the major duodenal papilla (papilla of Vater) and pancreas, which increases the risk of bleeding and pancreatitis development[10] and causes the reflux of duodenal content to the BD[11,12]. It leads to cholangitis development and stent obstruction[13]. The proposed antireflux stents[12,14-16] have not yet found widespread use[17]. In addition, tumor ingrowth and overgrowth, as well as stent occlusion, are potential outcomes[18]. Stents are relatively expensive and difficult to repair and replace[19]. Nevertheless, they provide internal drainage of bile.
Combining the advantages of percutaneous drainage and stenting, internal-external transpapillary biliary drainage (IETBD) involves draining the duodenum while maintaining normal bile outflow. However, there is a high probability of reflux cholangitis. Researchers have polarized opinions about the effectiveness of this approach[9,20-22].
Thus, one of the main reasons for cholangitis being one of the most serious complications of minimally invasive BD decompression techniques, is stent occlusion, which is strongly associated with duodenobiliary reflux. Therefore, avoidance of duodenobiliary reflux is important in preventing stent dysfunction and cholangitis onset[23].
In order to reduce the unfavorable effects of BD decompression in patients with DMBO including cholangitis, the approaches and tools used in minimally invasive procedures, as well as the choice of method, require continuous improvement.
We aimed to develop a technique for internal-external biliary-jejunal drainage (IEBJD) and assess its effectiveness in comparison to other minimally invasive procedures in the palliative treatment of patients with DMBO.
A prospective, randomized, multicenter study was conducted in three hospitals affiliated with the Department of Surgery with a course of emergency and vascular surgery at O.O. Bogomolets National Medical University (Kyiv): Kyiv City Oleksandrivska Clinical Hospital, Kyiv City Clinical Emergency Hospital, and National Military Medical Clinical Center “Main Military Clinical Hospital”, Kyiv. A total of 134 patients who underwent palliative decompression of the BD due to DMBO between 2017 and 2021 were included in the study. Approval was obtained from the Ethics Committee of O.O. Bogomolets National Medical University (Protocol No. 25-15-65, as of November 28, 2017), and informed consent was given by all participants before the study. The inclusion criteria were: The presence of mechanical jaundice; age over 18 years; the impossibility of radical surgery; and the technical success of the minimally invasive procedure. The exclusion criteria were: Mechanical BD obstruction without jaundice; age less than 18 years; high operative risk [American Society of Anesthetists (ASA) score of 4]; multiple metastatic liver disease; ascites; hemorrhagic diathesis; coagulopathy (international normalized ratio ≥ 1.5); past history of gastrectomy and reconstruction using the Billroth II or Roux-en-Y technique.
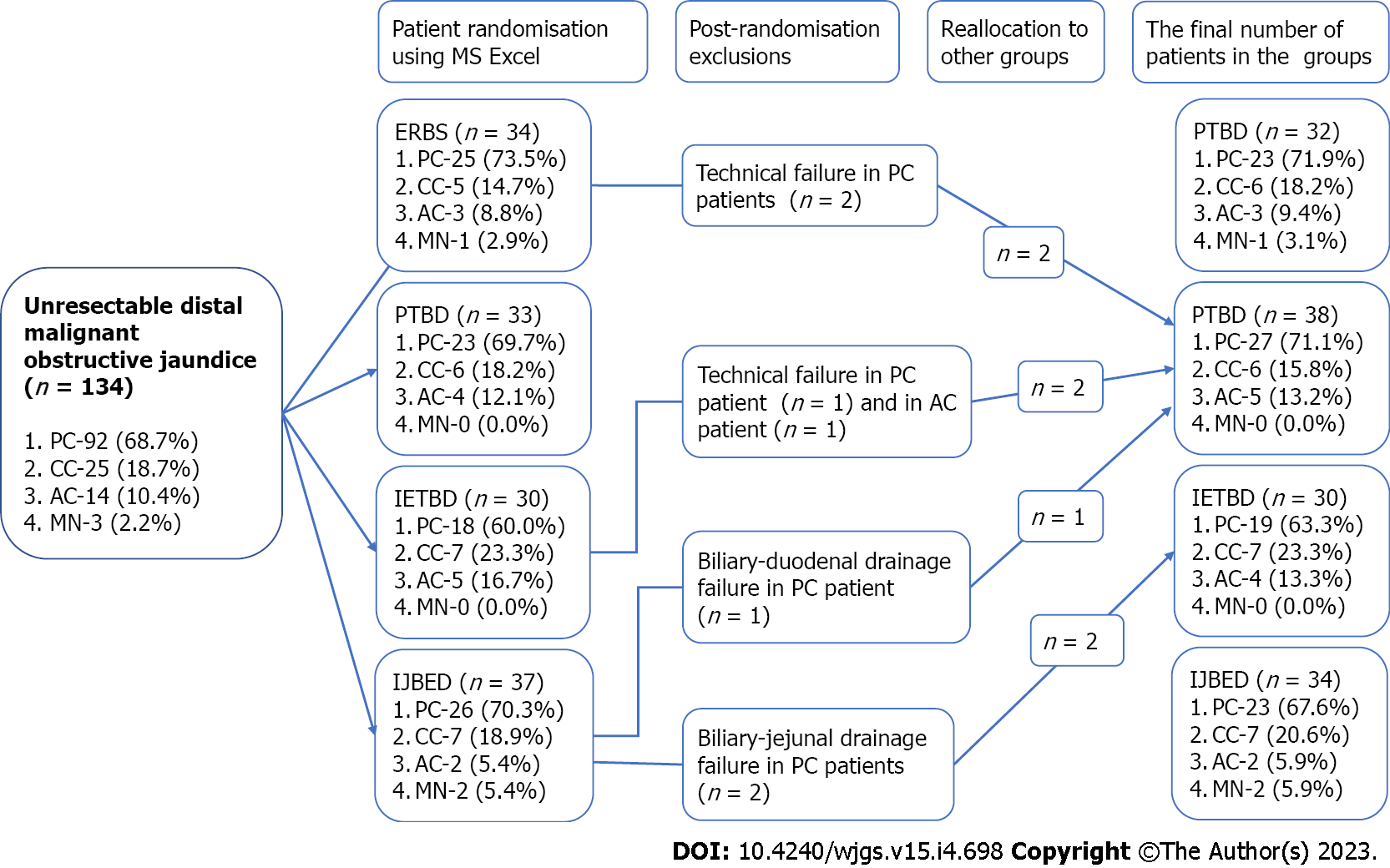
Using MS Excel, patients were randomly assigned to four treatment groups in accordance with the BD decompression procedure (Figure 1). The PTBD group included 33 patients; the IETBD group included 30 patients; the ERBS group included 34 patients; and the IEBJD group included 37 patients. However, due to technical difficulties in implementing the planned method, which was subsequently replaced with another one, the number of patients in the groups changed throughout the course of the study. In particular, two patients were not eligible for ERBS (they underwent PTBD); IETBD turned out to be impossible for two patients (they underwent PTBD); during IEBJD, we did not manage to provide drainage distally to the ligament of Treitz in two cases (they underwent IETBD); and it was impossible to insert the drain tube distally to the tumor of the pancreatic head in one patient (he underwent PTBD). All patients who were randomized to the PTBD group were treated using this BD decompression technique.
Thus, the PTBD group included 38 patients, the IETBD group included 30 patients, the ERBS group included 32 patients, and the IEBJD group included 34 patients.
The PTBD and IETBD procedures were carried out using plastic drain tubes of the Pigtail type 9Fr. For ERBS, Partially Covered Nitinol Self-Expandable Metal Stents with a diameter of 8-10 mm were used.
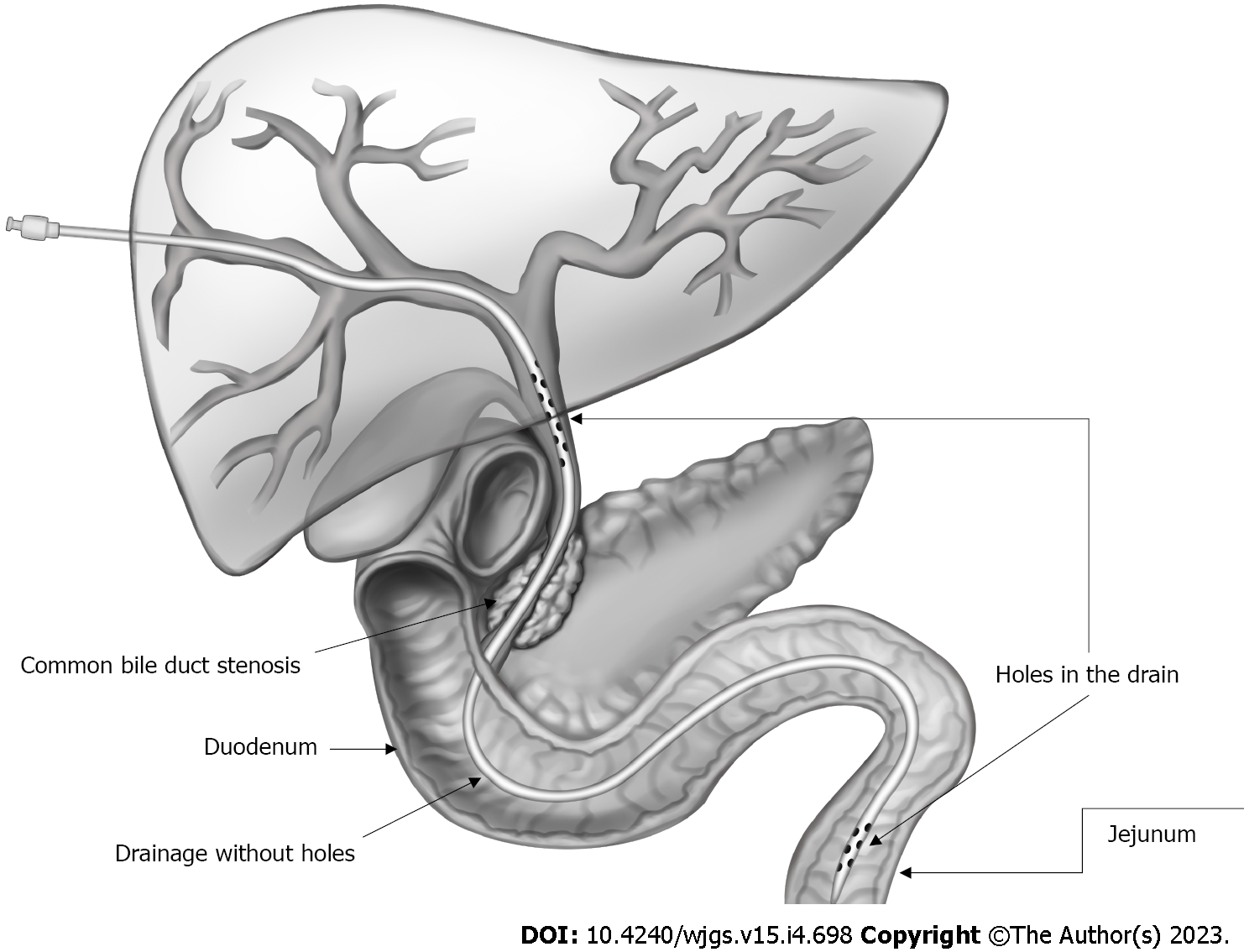
IEBJD was used to divert bile from the BD directly into the initial loops of the small intestine to prevent duodeno-biliary reflux and reflux cholangitis. In our study, the IEBJD technique was applied using a newly developed biliary-jejunal drainage system. The drain tube has two groups of lateral openings (proximal and distal), between which it is devoid of openings from the distal border of the tumor to the initial loops of the small intestine[24].
IEBJD was carried out using percutaneous transhepatic access. The end of the drain tube with the distal group of lateral openings is located behind the duodeno-jejunal bend in the initial loops of the jejunum, while the proximal group of lateral openings is located in the dilated BD above the stenosis (Figure 2).
Endpoints of the study were the clinical success of the procedure, the frequency and nature of complications from the manipulation, and the cumulative survival rate.
The procedure was considered clinically successful if the serum level of total bilirubin decreased by at least 50% as compared to the baseline value during the first 7 d after manipulation[12].
The Society of Interventional Radiology Clinical Practice Guidelines[25] classified postoperative complications as insignificant or significant.
Significant complications included acute hemobilia, pancreatitis, pneumothorax, sepsis, liver abscess, cholecystitis, biliary peritonitis, bleeding requiring blood transfusion, bile duct rupture, and cholangitis.
The clinical diagnosis of cholangitis was established on the basis of the following criteria: Body temperature above 38.5 °C, leukocyte count > 10 × 109/L, and proportion of neutrophils > 70%[26].
Postoperative pancreatitis was graded as “mild” in cases of the onset or progression of abdominal pain and an elevated serum amylase level three or more times above the reference range within 24 h after the procedure, requiring a minimum of 2-3 d of hospitalization. Pancreatitis was graded as “moderately severe” if the patient required hospitalization for 4-10 d, and as “severe” when the patient required hospitalization for more than 10 d, as well as in cases of necrosis and pseudocysts, indicating the need for percutaneous drainage or open pancreatic debridement[27].
Total bilirubin and α-amylase levels in serum were determined using an automatic biochemical analyzer, Olympus AU-800 (Olympus, Japan). Blood tests were performed using the hematological analyzer Mindray BC-2800 (China).
Statistical data processing was performed using the statistical package IBMSPPS Statistics 22. To determine whether the observations deviated from the normal curve, the Shapiro-Wilk test was used. Statistical differences were calculated using ANOVA with Tukey’s post-hoc test for multiple comparisons and a two-tailed t-test (for normally distributed variables) and non-parametric Mann-Whitney U test (for non-normally distributed variables) for single comparisons. A Pearson χ² test was used for qualitative data. A cumulative survival rate was estimated by the Kaplan-Meier method using the log-rank test. All differences with a P value of < 0.05 were considered statistically significant.
The study protocol was approved by the Ethics Committee of O. O. Bogomolets National Medical University, and informed consent was obtained from all participants before the study.
The general characteristics of study participants are summarized in Table 1. The anamnesis revealed that mechanical jaundice occurred in patients on an average of 15.2 d ± 0.2 d before the manipulation (from 10 d to 22 d). Patients did not have a statistically significant difference in terms of the average duration of jaundice before surgery.
| Indicator | Total, n = 134 | Study group | Pvalue | |||
| EIBJD, n = 29 | ERBS, n = 25 | IETBD, n = 19 | PTBD, n = 65 | |||
| Age in yr, mean ± SD | 64.1 ± 11.6 | 65.8 ± 10.1 | 61.9 ± 12.9 | 62.2 ± 13.0 | 66.0 ± 10.4 | 0.296 |
| Male/female | 69/65 | 19/15 | 17/15 | 14/16 | 19/19 | 0.894 |
| Duration of jaundice in d, mean ± SD | 15.0 ± 2.0 | 14.7 ± 1.5 | 15.5 ± 2.0 | 14.9 ± 2.6 | 14.3 ± 1.1 | 0.250 |
| Total serum bilirubin in mg/dL, mean ± SD | 11.3 ± 4.6 | 12.4 ± 4.5 | 12.3 ± 4.2 | 10.1 ± 5.3 | 10.5 ± 4.2 | 0.092 |
| Cholangitis before the procedure | 19 (14.2) | 6 (17.6) | 4 (12.5) | 4 (13.3) | 5 (13.2) | 0.928 |
| T stage | ||||||
| T2 | 6 (4.6) | 1 (2.9) | 1 (3.1) | 2 (6.7) | 2 (5.3) | 0.985 |
| T3 | 78 (58.2) | 19 (55.9) | 19 (59.4) | 18 (60.0) | 22 (57.9) | |
| T4 | 50 (37.3) | 14 (41.2) | 12 (37.5) | 10 (33.3) | 14 (36.8) | |
| N stage | ||||||
| N0 | 8 (6.7) | 3 (8.8) | 2 (6.3) | 3 (10.0) | 1 (2.6) | 0.922 |
| N1 | 97 (72.4) | 24 (70.6) | 24 (75.0) | 19 (63.3) | 30 (78.9) | |
| N2 | 11 (8.2) | 3 (8.8) | 2 (6.3) | 4 (13.3) | 2 (5.3) | |
| Nx | 17 (12.7) | 4 (11.8) | 4 (12.5) | 4 (13.3) | 5 (13.2) | |
| М stage | ||||||
| М0 | 64 (47.8) | 14(41.2) | 18 (56.3) | 16 (53.3) | 16 (42.1) | 0.858 |
| М1 | 53 (39.6) | 15 (44.1) | 11 (34.4) | 10 (33.3) | 17 (44.7) | |
| Мх | 17 (12.7) | 5 (14.7) | 3 (9.4) | 4 (13.3) | 5 (13.2) | |
| Grade | ||||||
| ІІВ | 4 (3.0) | 1 (2.9) | 1 (3.1) | 2 (6.7) | 0 (0.0) | 0.760 |
| ІІІ | 47 (35.1) | 12 (35.3) | 13 (40.6) | 10 (33.3) | 12 (31.6) | |
| ІV | 83 (61.9) | 21 (61.8) | 18 (56.3) | 18 (60.0) | 26 (68.4) | |
| Tumour etiology | ||||||
| Pancreatic cancer | 92 (68.7) | 23 (67.6) | 23 (71.9) | 19 (63.3) | 27 (71.1) | 0.757 |
| Cholangiocarcinoma | 25 (18.7) | 7 (20.6) | 5 (15.6) | 7 (23.3) | 6 (15.8) | |
| Ampullary cancer | 14 (10.4) | 2 (5.9) | 3 (9.4) | 4 (13.3) | 5 (13.2) | |
| Metastatic nodes | 3 (2.2) | 2 (5.9) | 1 (3.1) | 0 (0.0) | 0 (0.0) | |
There were no significant differences in the serum level of total bilirubin between the groups. The mean serum level of total bilirubin was 11.36 mg/dL ± 0.04 mg/dL (3.93-22.78 mg/dL).
There was no statistically significant difference between the study groups in terms of average age, sex ratio, cancer stage, TNM criteria, or etiological factors of stricture.
In the case of PTBD, the technical success was 100%, and it was 94.1% for ERBS, 93.8% for IETBD, and 91.9% for IEBJD (P = 0.365).
The clinical success was 94.1% in the IEBJD group, 93.8% in the ERBS group, 86.7% in the IETBD group, and 94.7% in the PTBD group (P > 0.582 for all).
Cholangitis, which was diagnosed at admission, subsided within 3-4 d after the procedure.
There were no technical complications (related to the specifics of the manipulation) in any study group. Minor complications occurred both singly and in combination. The groups did not differ statistically in terms of the number of patients with minor complications and their variants (Table 2).
| Complication | Study group | Pvalue | |||
| EIBJD, n = 34 | ЕRBS, n = 32 | IETBD, n = 30 | PTBD, n = 38 | ||
| Pain in the drainage area | 6 (17.6) | 5 (15.6) | 7 (23.3) | 7 (18.4) | 0.885 |
| Hyperthermia | 3 (8.8) | - | 2 (6.7) | 3 (7,9) | 0.423 |
| Bile leakage | 1 (2.9) | 2 (6.3) | 1 (3.3) | 2 (5.3) | 0.903 |
| Bleeding | 3 (8.8) | - | 3 (10,0) | 4 (10.5) | 0.325 |
| Subcapsular biloma | 1 (2.9) | 3 (9.4) | 1 (3.3) | 1 (2.6) | 0.498 |
| Shingle pain | 1 (2.9) | - | 1 (3.3) | 2 (5.3) | 0.642 |
| Total | 1 (2.9) | 3 (9.4) | 1 (3.3) | - | 0.223 |
Significant complications occurred in 5 (14.7%) patients in the IEBJD group, in 10 (31.3%) in the ERBS group, in 13 (43.3%) in the IETBD group, and in 8 (21.1%) in the PTBD group (Table 3). In the PTBD and external-internal biliary-jejunal drainage (EIBJD) groups, a significant complication of one type was observed, while in the ERBS and IETBD groups, significant complications of two types were observed in one patient.
| Indicator | Study group | Pvalue | |||
| EIBJD, n = 34 | ЕRBS, n = 32 | IETBD, n = 30 | PTBD, n = 38 | ||
| Patients with complications | 5 (14.7) | 10 (31.3) | 13 (43.3) | 8 (21.1) | 0.053 |
| Number of complications in one patient | |||||
| No | 29 (85.3) | 22 (68.8) | 17 (56.7) | 30 (78.9) | 0.072 |
| One | 5 (14.7) | 7 (21.9) | 10 (33.3) | 8 (21.1) | |
| Two | 0 (0.0) | 3 (9.4) | 3 (10.0) | 0 (0.0) | |
| Type of complication | |||||
| Cholangitis | 3 (8.8) | 8 (25.0) | 10 (33.3) | 9 (13.2) | 0.052 |
| Pancreatitis | |||||
| No | 32 (94.1) | 28 (87.5) | 27 (90.0) | 38 (100) | 0.121 |
| Mild | 2 (5.9) | 2 (6.3) | 3 (10.0) | 0 (0.0) | |
| Moderately severe | 0 (0.0) | 2 (6.3) | 0 (0.0) | 0 (0.0) | |
| Cholecystitis | 0 (0.0) | 0 (0.0) | 1 (3.3) | 3 (7.9) | 0.157 |
| Liver abscess | 0 (0.0) | 1 (3.1) | 2 (6.7) | 0 (0.0) | 0.217 |
The complication rate (P = 0.053) did not differ significantly between the groups, but it did differ significantly between the groups where the biliary decompression system connected the lumen of the duodenum to the bile ducts (IETBD and ERBS) and those where it did not (PTBD and IEBJD): 23 (37.1%) vs 13 (18.1%), respectively, P = 0.013.
The most frequent complication was cholangitis (26 cases, 19.4%). In general, there were no statistically significant differences in the cholangitis rate between the groups (P = 0.052). However, when a drain tube or stent was used to connect the lumen of the duodenum to the bile ducts, the frequency of cholangitis was significantly higher than when it was not used: 18 (29.0%) vs 8 (11.1%) (P = 0.009).
The course of cholangitis in the IEBJD group differed from that in the ERBS and IETBD groups by a longer period before its occurrence after the procedure (P < 0.05) and a shorter duration (P < 0.05) (Table 4).
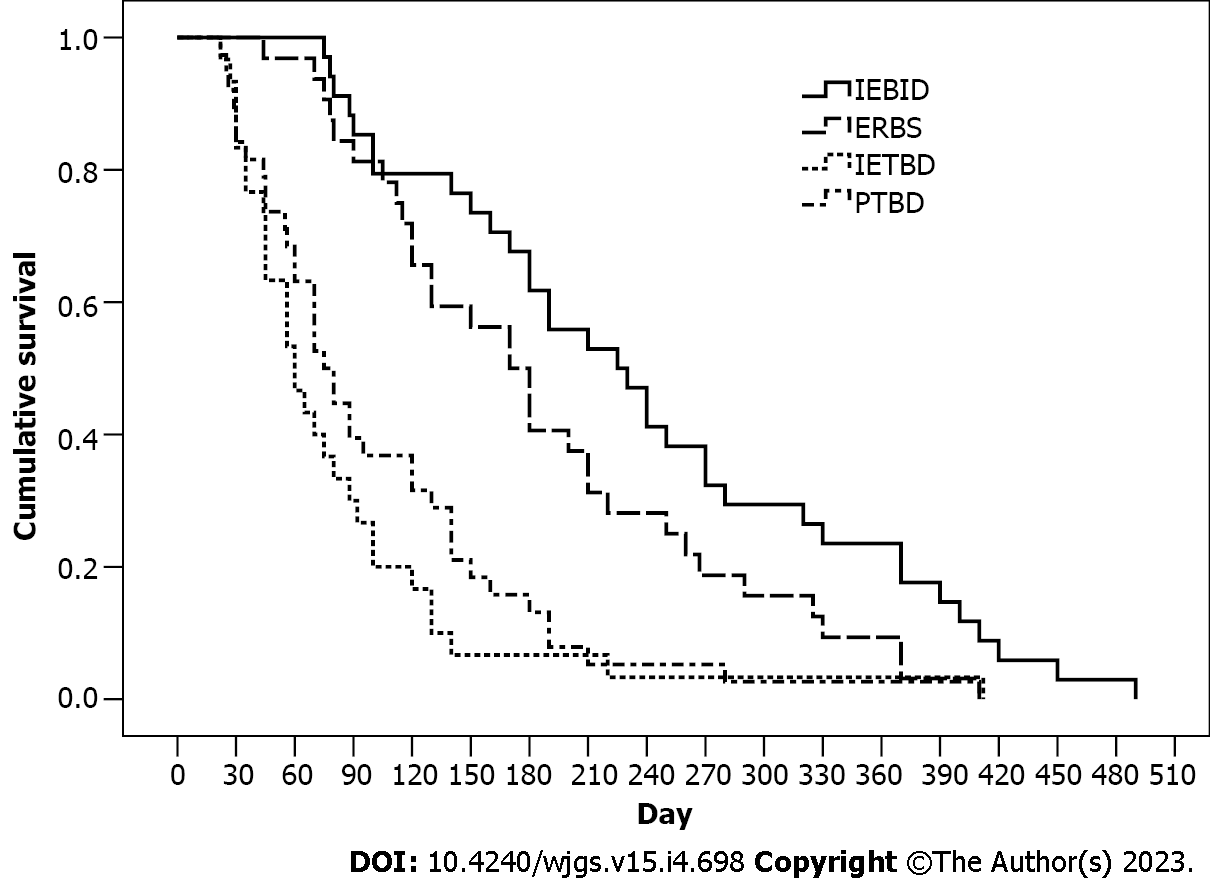
The patients who underwent IEBJD had the highest cumulative survival rate [239.3 d, 95% confidence interval (CI): 198.9-279.6 d] when compared to other groups (Figure 3). However, patients in the PTBD (102.0 d, 95%CI: 77.6-128.1 d) and IETBD (94.8 d, 95%CI: 54.1-135.5 d) groups had significantly lower cumulative survival rates (P < 0.01) than those in the ERBS group (187.8 d, 95%CI: 153.8-221.9 d).
In comparison to other groups, the mortality risk in the IEBJD group was lower 3, 6, 9, 12, and 15 mo after the start of the procedure (Table 5).
| Observation period in mo | PTBD | IETBD | ERBS |
| 3 | 0.39; 0.19-0.82, Р = 0.018 | 0.31; 0.14-0.69, Р = 0.005 | 0.75; 0.31-1.76, Р = 0.438 |
| 6 | 0.49; 0.28-0.87, Р = 0.011 | 0.34; 0.18-0.66, Р < 0.001 | 0.96; 0.56-1.69, Р = 0.982 |
| 9 | 0.36; 0.22-0.60, Р < 0.001 | 0.26; 0.14-0.49, Р < 0.001 | 0.78; 0.49-1.22, Р = 0.232 |
| 12 | 0.39; 0.24-0.64, Р < 0.001 | 0.26; 0.14-0.48, Р < 0.001 | 0.86; 0.56- 1.32, Р = 0.507 |
| 15 | 0.38; 0.23-0.62, Р < 0.001 | 0.30; 0.17-0.54, Р < 0.001 | 0.77; 0.51-1.16, Р = 0.078 |
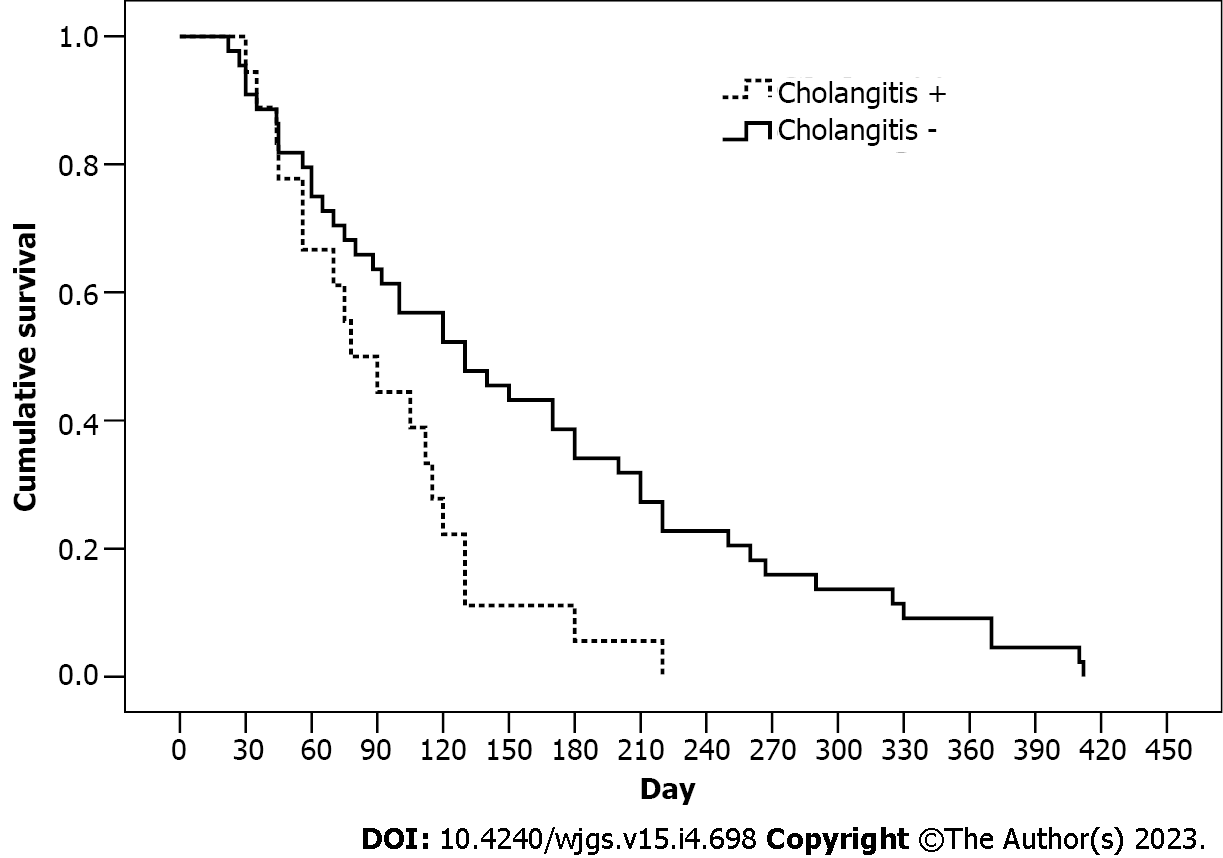
The technique aimed to increase the survival rate of patients with distal block by reducing the risk of duodeno-biliary reflux as well as the frequency and duration of reflux cholangitis. In the IEPTB and ERBS groups, there was a high probability of reflux of duodenal contents into the biliary tract through the drain tube and stent, respectively. Analysis of the impact of cholangitis episodes on the survival rate in these groups confirmed the success of the newly developed technique (Figure 4). The average survival time in patients with cholangitis episodes was 93.9 d (95%CI: 70.4-117.4 d), whereas in patients without cholangitis it was 156.1 d (95%CI: 124.9-191.3 d) (P = 0.009); the hazard ratio (HR) was 1.96 (95%CI: 1.02-3.79). However, the cholangitis factor had no effect on the survival rate in patients from the EIBJD group (HR = 1.07, 95%СI: 0.32-3.64).
Cholangitis is one of the major complications of palliative BD decompression in patients with DMBO. It is recognized as an independent risk factor for liver dysfunction, reduced quality of life, and decreased life expectancy[28].
Cholangitis can develop in cases of gastrointestinal tract infection in patients with an unresectable bilioduodenopancreatic neoplasm due to: (1) Retrograde reflux of intestinal flora during and after the procedure; (2) microbiota dissemination through the external drainage; (3) hematogenous spread of microorganisms; and (4) the contrast reaching the bile ducts. Furthermore, the infection may already be present before the procedure, despite the absence of typical cholangitis manifestations[29,30]. However, duodeno-biliary reflux is the most significant systemic cause of cholangitis. It occurs when the lumen of the duodenum is connected to the lumen of the bile duct, resulting in the disruption or even loss of the barrier function of the sphincter of Oddi[16]. The basal pressure, which is normally created by the sphincter of Oddi (135-202 mm H2O), is higher than that in the duodenum (80-120 mm H2O)[31,32]. Phase contractions of the duodenum are accompanied by an increase in pressure and simultaneous initiation of the sphincter of Oddi contractions, which, in turn, prevent reflux[31]. In contrast, the basal pressure in the common BD is usually in the range of 50-100 mm H2O and does not prevent reflux, especially in the case of connecting the lumen of the bile duct to the lumen of the duodenum[33]. Duodeno-biliary reflux occurs in 100% of patients after ERBS, as demonstrated by duodenography with barium, but it is not always associated with cholangitis[11,12]. After stenting of the BD, 98% of patients show positive bile cultures[34]. Bacteriobilia after ERBS is associated with Escherichia coli, Klebsiella pneumonia, Pseudomonas, Enterococcus cloacae, and other microorganisms that are usually resistant to commonly used antibiotics[13]. The high incidence of cholangitis after ERBS has prompted an analysis of a two-step approach to radical treatment of pancreatic head cancer. It has been demonstrated that the number of infectious complications and mortality rate are significantly higher in patients who receive two-stage treatment (BD stenting followed by radical surgery) in comparison to patients who receive one-stage treatment[13]. A meta-analysis including 1435 patients with malignant bile duct obstruction revealed a significantly lower frequency of cholangitis in the case of nasobiliary BD drainage (without duodenobiliary reflux) as compared to ERBS (HR = 0.46, P < 0.00001)[35]. In our study, cholangitis occurred in 36.0% of patients in the ERBS group during the follow-up period.
Apparently, favorable conditions for duodeno-biliary reflux and cholangitis also develop after IETBD, as evidenced by the results of the study by Xu et al[22], who diagnosed cholangitis in 52.4% of patients with IETBD, which coincides with our observations (31.6%).
Duodeno-biliary reflux after ERBS and IETBD is responsible for the reduced duration of stent patency[21,12,36]. This creates a risk of cholangitis. The presence of the food fibers, bile, bacteria, fibrin, debris, granulation tissue, and inflammatory cells in the occlusive material from removed stents confirms the effect of duodeno-biliary reflux on stent/drainage patency[37]. These sediments are usually infected with Gram-negative bacteria[38]. The biliary stent becomes occluded as a result of biofilm formation caused by bacterial colonization. Biofilm formation begins with the priming of the stent surface by various microbial proteins, followed by microbial adhesion to the stent and the formation of an exopolysaccharide matrix, embedding microbial colonies and other particles into the mature biofilm[39,40]. Over time, this leads to increased bile viscosity, slowed bile flow[41], bile stasis, increased deposition of bile salts[42], and the formation of a brown pigment stone (calcium bilirubinate)[43]. Despite the fact that the stent patency rate is frequently used as an indicator of adverse events following successful placement, we did not compare it between the groups because patients died before stent dysfunction occurred. Namely, in the IETBD and PTBD groups, the stent patency was maintained until the death in 16 (84.2%) and 59 (90.8%) patients, respectively, and the average stent patency duration was mainly determined by the life span and was 69.6 d ± 7.2 d and 84.6 d ± 6.6 d, respectively. At the same time, the average stent patency duration among patients who had drainage obstruction prior to death was 94.3 d ± 3.5 d and 155.2 d ± 20.1 d, respectively (P = 0.078). In the IEBJD and ERBS groups, the stent patency was maintained until death in 14 (48.3%) and 12 (48.0%) patients, respectively. The average stent patency duration in these groups was longer than that in the IETBD and PTBD groups: 178.9 d ± 11.5 d and 155.3 d ± 14.3 d, respectively. Among other things, this could be attributed to the longer life expectancy of patients. Nevertheless, in patients who had stent dysfunction before death, the stent patency duration was 204.1 d ± 13.1 d (between 131 d and 275 d) and 168.2 d ± 20.1 d (between 98 d and 292 d), respectively (P = 0.047). Although the stent patency duration was longer in the IEBJD group, probably due to the absence of reflux of duodenal content, we decided not to emphasize this fact for the aforementioned reasons. Nevertheless, it should be noted that the cumulative survival rate in the ERBS group with preserved stent patency and cholangitis was 157.1 d (95%CI: 132.1-182.1), while without cholangitis it was 269.6 d (95%CI: 230.3-309.0) (P = 0.005). Notably, biliary decompression was not interrupted because of drainage dysfunction in any of the patients and was usually continued for the rest of their lives.
To reduce the incidence of stent-associated cholangitis, stents with anti-reflux valves of various shapes (wine glass-shaped, funnel-shaped, or windsock-shaped) and lengths have been developed[12,16,14,15]. Preliminary data suggest that such stents may be potentially beneficial, although more research is required[17]. Despite the fact that they have patency indices comparable to valveless metal stents[15], they have not been widely used and are prone to dislocation[17]. Kuwatani et al[43] noted that, currently, there is no ideal stent with constant patency.
Our study aimed to reduce the incidence of reflux cholangitis. Therefore, we used external-internal drainage to provide bile evacuation into the initial loops of the small intestine, bypassing the duodenum. As a result, the major duodenal papilla is not damaged during the procedure, so the probability of duodeno-biliary reflux is minimal and, in our study, it was not observed. Instead, duodenal contents may enter the bile duct from the outside of the drain.
The possibility of emptying the contents of the small intestine into the choledoch cannot be ruled out, despite the fact that the basal pressure in the intestine is lower[44] or similar to that in the choledoch[45]. In our study, duodeno-biliary reflux was not observed. Furthermore, the pressure in the jejunum does not change (82 mm H2O ± 11 mm H2O) when the balloon located in the duodenum and simulating the passage of the food is inflated (up to 6 mL), in contrast to a significant increase in the pressure in the duodenum (up to 242 mm H2O ± 52 mm H2O) and in the area of duodenojejunal flexure (up to 334 mm H2O ± 48 mm H2O)[45].
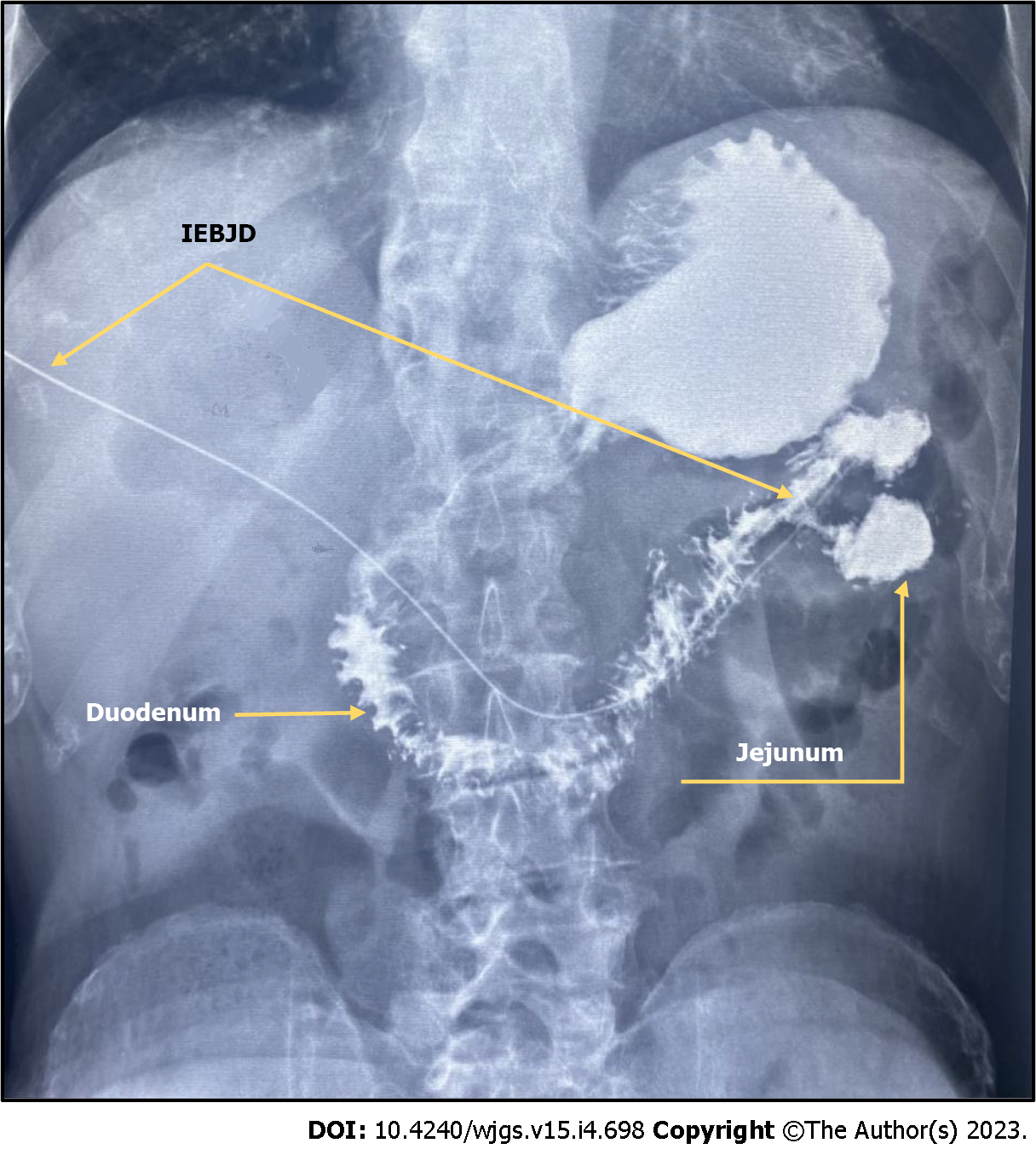
We carried out IEBJD on 34 patients with DMBO. A control barium X-ray of the stomach and duodenum did not reveal a reflux of contrast into the BD (Figure 5).
Subcapsular biloma and bleeding, two minor complications that were noted during the manipulation procedure, both subsided on their own without the need for a blood transfusion.
A decrease in the serum level of total bilirubin by more than 50% compared to baseline values was detected in 94.1% of cases. Bile leakage was not observed, unlike in the PTBD group.
In the postoperative period, significant complications occurred in 5 (14.7%) patients in the IEBJD group, in 10 (31.3%) in the ERBS group, in 13 (43.3%) in the IETBD group, and in 8 (21.1%) in the PTBD group. Although there were no significant differences between the groups (P = 0.053), the frequency of serious complications was significantly higher in the groups with the connection between the duodenal lumen and the bile ducts than in the groups without it: 23 (37.1 %) vs 13 (18.1%) patients, respectively (P = 0.013). This can also be referred to cholangitis, which is the most frequent complication: 18 (29.0%) vs 8 (11.1%) patients (P = 0.009).
The cumulative survival rate was the highest in the IEBJD group, at an average of 239.3 d (95%CI: 198.9-279.6) (P < 0.05). Three, six, nine, twelve, and fifteen months after the procedure, patients who underwent IEBJD had a lower mortality risk than those who were treated using other techniques. A lower cholangitis onset rate may account for a higher survival rate in the IEBJD group. It has been shown that cholangitis can be associated with a decrease in life expectancy: 93.9 d (95%CI: 70.4-117.4 d) in the groups with a high risk of duodenal-biliary reflux and reflux cholangitis vs 156.1 d (95%CI: 124.9-191.3 d) in the groups without cholangitis (P = 0.009) (HR = 1.96, 95%CI: 1.02-3.79). However, cholangitis had no impact on the survival rate in the IEBJD group (HR = 1.07, 95%CI: 0.32-3.64).
In patients with IEJBD, the drain tube is easier to manage in cases of cholangitis symptoms. Antibiotic therapy and drain rehabilitation helped remove cholangitis symptoms within 3-4 d, whereas other methods took 7-14 d.
Our findings suggest that IEBJD has advantages over other BD decompression techniques in the palliative treatment of patients with DMBO. However, this technique, like other external-internal drainage systems, causes difficulties for the patient since the drain exits the body and requires a drainage bag. Compared to IEBJD, ERBS has advantages in this regard. Moreover, further development of reliable anti-reflux stents would definitely prioritize ERBS use for palliative BD decompression. Nonetheless, IEBJD is currently a cost-effective treatment option, particularly for patients with a short life expectancy. The study has certain limitations, including a relatively small number of patients in the comparison groups. In addition, the study did not include patients with total bilirubin > 20.47 mg/dL and high operative risk (ASA score of 4).
Patients with distal malignant biliary obstruction (DMBO) may benefit from bile duct (BD) decompression using endoscopic biliary drainage since the procedure reduces pain, relieves symptoms, allows for the administration of chemotherapy, improves quality of life, and increases the survival rate. Cholangitis is one of the main complications of palliative BD decompression in patients with DMBO. Therefore, BD decompression techniques require further improvement to reduce the frequency of cholangitis episodes.
Duodeno-biliary reflux (DBR), among others, is regarded as one of the major systemic causes of cholangitis. The aim of the study was to develop a BD drainage technique for bile diversion from the BD directly into the initial loops of the small intestine, preventing DBR and reflux cholangitis.
To develop a technique for internal-external biliary-jejunal drainage (IEBJD) and assess its effectiveness in comparison to other minimally invasive procedures.
In our study, the IEBJD technique was applied using a newly developed biliary-jejunal drainage system. It has two groups of lateral openings (proximal and distal), between which the drainage tube is devoid of openings from the distal border of the tumor to the initial loops of the small intestine. IEBJD was carried out using percutaneous transhepatic access.
The application of the IEBJD technique contributed to a reduction in the incidence of significant postoperative complications, a delayed onset and shorter duration of postoperative cholangitis, and a considerable improvement in the cumulative survival rate of patients with DMBO.
The IEBJD technique prevents DBR and reflux cholangitis and can be recommended for the palliative treatment of patients with DMBO.
The clinical success of the newly developed IEBJD technique in a limited patient group necessitates further evaluation of its efficacy in a larger patient cohort, including those with total bilirubin > 20.47 mg/dL and high operative risk (ASA score of 4).
The authors are very grateful to everyone who took part in this study.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Ukraine
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: dos Santos JS, Brazil; Lin L, China S-Editor: Chen YL L-Editor: Filipodia; Wang TQ P-Editor: Chen YL
| 1. | Fernandez Y Viesca M, Arvanitakis M. Early Diagnosis And Management Of Malignant Distal Biliary Obstruction: A Review On Current Recommendations And Guidelines. Clin Exp Gastroenterol. 2019;12:415-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 2. | Pereira SP, Oldfield L, Ney A, Hart PA, Keane MG, Pandol SJ, Li D, Greenhalf W, Jeon CY, Koay EJ, Almario CV, Halloran C, Lennon AM, Costello E. Early detection of pancreatic cancer. Lancet Gastroenterol Hepatol. 2020;5:698-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 298] [Article Influence: 59.6] [Reference Citation Analysis (1)] |
| 3. | Vandenabeele LAM, Dhondt E, Geboes KP, Defreyne L. Percutaneous stenting in malignant biliary obstruction caused by metastatic disease: clinical outcome and prediction of survival according to tumor type and further therapeutic options. Acta Gastroenterol Belg. 2017;80:249-255. [PubMed] |
| 4. | Walter D, van Boeckel PG, Groenen MJ, Weusten BL, Witteman BJ, Tan G, Brink MA, Nicolai J, Tan AC, Alderliesten J, Venneman NG, Laleman W, Jansen JM, Bodelier A, Wolters FL, van der Waaij LA, Breumelhof R, Peters FT, Scheffer RC, Steyerberg EW, May AM, Leenders M, Hirdes MM, Vleggaar FP, Siersema PD. Higher quality of life after metal stent placement compared with plastic stent placement for malignant extrahepatic bile duct obstruction: a randomized controlled trial. Eur J Gastroenterol Hepatol. 2017;29:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Pancreatric Section, British Society of Gastroenterology; Pancreatic Society of Great Britain and Ireland; Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland; Royal College of Pathologists; Special Interest Group for Gastro-Intestinal Radiology. Guidelines for the management of patients with pancreatic cancer periampullary and ampullary carcinomas. Gut. 2005;54 Suppl 5:v1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Riaz A, Pinkard JP, Salem R, Lewandowski RJ. Percutaneous management of malignant biliary disease. J Surg Oncol. 2019;120:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Bokemeyer A, Müller F, Niesert H, Brückner M, Bettenworth D, Nowacki T, Beyna T, Ullerich H, Lenze F. Percutaneous-transhepatic-endoscopic rendezvous procedures are effective and safe in patients with refractory bile duct obstruction. United European Gastroenterol J. 2019;7:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Wang L, Lin N, Xin F, Ke Q, Zeng Y, Liu J. A systematic review of the comparison of the incidence of seeding metastasis between endoscopic biliary drainage and percutaneous transhepatic biliary drainage for resectable malignant biliary obstruction. World J Surg Oncol. 2019;17:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Lorenz JM. Management of Malignant Biliary Obstruction. Semin Intervent Radiol. 2016;33:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Inamdar S, Slattery E, Bhalla R, Sejpal DV, Trindade AJ. Comparison of Adverse Events for Endoscopic vs Percutaneous Biliary Drainage in the Treatment of Malignant Biliary Tract Obstruction in an Inpatient National Cohort. JAMA Oncol. 2016;2:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 11. | Misra SP, Dwivedi M. Reflux of duodenal contents and cholangitis in patients undergoing self-expanding metal stent placement. Gastrointest Endosc. 2009;70:317-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Lee YN, Moon JH, Choi HJ, Choi MH, Lee TH, Cha SW, Cho YD, Choi SY, Lee HK, Park SH. Effectiveness of a newly designed antireflux valve metal stent to reduce duodenobiliary reflux in patients with unresectable distal malignant biliary obstruction: a randomized, controlled pilot study (with videos). Gastrointest Endosc. 2016;83:404-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Scheufele F, Aichinger L, Jäger C, Demir IE, Schorn S, Sargut M, Erkan M, Kleeff J, Friess H, Ceyhan GO. Effect of preoperative biliary drainage on bacterial flora in bile of patients with periampullary cancer. Br J Surg. 2017;104:e182-e188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | Kim DU, Kwon CI, Kang DH, Ko KH, Hong SP. New antireflux self-expandable metal stent for malignant lower biliary obstruction: in vitro and in vivo preliminary study. Dig Endosc. 2013;25:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Hamada T, Isayama H, Nakai Y, Kogure H, Togawa O, Kawakubo K, Yamamoto N, Ito Y, Sasaki T, Tsujino T, Sasahira N, Hirano K, Tada M, Koike K. Novel antireflux covered metal stent for recurrent occlusion of biliary metal stents: a pilot study. Dig Endosc. 2014;26:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Hu B, Wang TT, Wu J, Shi ZM, Gao DJ, Pan YM. Antireflux stents to reduce the risk of cholangitis in patients with malignant biliary strictures: a randomized trial. Endoscopy. 2014;46:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Renno A, Abdel-Aziz Y, Ahmed T, Alastal Y, Toseef J, Al-Abboodi Y, Nawras A. Antireflux valve metal stent versus conventional self-expandable metal stent in distal malignant biliary obstruction: a systematic review and meta-analysis. Ann Gastroenterol. 2019;32:605-613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Park CH, Park SW, Jung JH, Jung ES, Kim JH, Park DH. Comparative Efficacy of Various Stents for Palliation in Patients with Malignant Extrahepatic Biliary Obstruction: A Systematic Review and Network Meta-Analysis. J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Shah SFH, Shah SA, Merchant SA. Investigating temporal patterns of public interest in skin whitening using Google Trends. Int J Dermatol. 2021;60:e160-e161. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Xu C, Lv PH, Huang XE, Sun L, Wang SX, Wang FA. Internal-external percutaneous transhepatic biliary drainage for patients with malignant obstructive jaundice. Asian Pac J Cancer Prev. 2014;15:9391-9394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Loew BJ, Howell DA, Sanders MK, Desilets DJ, Kortan PP, May GR, Shah RJ, Chen YK, Parsons WG, Hawes RH, Cotton PB, Slivka AA, Ahmad J, Lehman GA, Sherman S, Neuhaus H, Schumacher BM. Comparative performance of uncoated, self-expanding metal biliary stents of different designs in 2 diameters: final results of an international multicenter, randomized, controlled trial. Gastrointest Endosc. 2009;70:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 22. | Xu C, Huang XE, Wang SX, Lv PH, Sun L, Wang FA. Comparison of infection between internal-external and external percutaneous transhepatic biliary drainage in treating patients with malignant obstructive jaundice. Asian Pac J Cancer Prev. 2015;16:2543-2546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Wu CH, Lee MH, Tsou YK, Lin CH, Sung KF, Pan KT, Liu NJ. Risk Factors of Duodenobiliary Reflux-Related Dysfunction of Covered Biliary Metal Stents after Treatment of Duodenal Stricture in Patients with Malignant Biliary and Duodenal Obstruction. Curr Oncol. 2021;28:3738-3747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Susak YM, Markulan LY, Palitsya RY. [Internal-external biliary-jejunal drainage in the palliative treatment of distal obstructive jaundice]. Surg East Euro. 2021;10:205-219. |
| 25. | Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14:S199-202. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1143] [Cited by in RCA: 1338] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 26. | Yu H, Yuanyuan S, Guo Z, Xing W, Si T, Guo X, Liu F. Multifactorial analysis of biliary infection after percutaneous transhepatic biliary drainage treatment of malignant biliary obstruction. J Cancer Res Ther. 2018;14:1503-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Mine T, Morizane T, Kawaguchi Y, Akashi R, Hanada K, Ito T, Kanno A, Kida M, Miyagawa H, Yamaguchi T, Mayumi T, Takeyama Y, Shimosegawa T. Clinical practice guideline for post-ERCP pancreatitis. J Gastroenterol. 2017;52:1013-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 28. | Chen M, Wang L, Wang Y, Wei W, Yao YL, Ling TS, Shen YH, Zou XP. Risk factor analysis of post-ERCP cholangitis: A single-center experience. Hepatobiliary Pancreat Dis Int. 2018;17:55-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Melzer M, Toner R, Lacey S, Bettany E, Rait G. Biliary tract infection and bacteraemia: presentation, structural abnormalities, causative organisms and clinical outcomes. Postgrad Med J. 2007;83:773-776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Kaya M, Beştaş R, Bacalan F, Bacaksız F, Arslan EG, Kaplan MA. Microbial profile and antibiotic sensitivity pattern in bile cultures from endoscopic retrograde cholangiography patients. World J Gastroenterol. 2012;18:3585-3589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Behar J. Physiology and Pathophysiology of the Biliary Tract: The Gallbladder and Sphincter of Oddi-A Review. ISRN Physiology. 2013;2013. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Gubergrits NB, Lukashevich GM, Belyaeva NV, Fomenko PG, Borodiy KN, Linevskaya KYu. Duodenal hypertension in practice of gastroenterologist [in Russian]. Modern Gastroenterology. 2020;3:7-20. [DOI] [Full Text] |
| 33. | Pitt HA, Nakeeb A. Bile secretion and pathophysiology of biliary tract obstruction. In:Jarnagin WR. Blumgart's Surgery of the Liver, Biliary Tract and Pancreas. 6th ed. Philadelphia: Elsevier, 2017: 123-132.e1. [DOI] [Full Text] |
| 34. | Herzog T, Belyaev O, Muller CA, Mittelkotter U, Seelig MH, Weyhe D, Felderbauer P, Schlottmann R, Schrader H, Schmidt WE, Uhl W. Bacteribilia after preoperative bile duct stenting: a prospective study. J Clin Gastroenterol. 2009;43:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Lucena GCM, Barros RA. PRE-OPERATIVE BILIARY DRAINAGE IN THE PERIAMPULLARY NEOPLASIA - A SYSTEMATIC REVIEW. Arq Bras Cir Dig. 2018;31:e1372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 36. | Leung JW, Liu YL, Chan RC, Ling TK, Cheng AF. Effects of adherence factors and human bile on bacterial attachment and biliary stent blockage: an in vitro study. Gastrointest Endosc. 2002;56:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 37. | Weickert U, Venzke T, König J, Janssen J, Remberger K, Greiner L. Why do bilioduodenal plastic stents become occluded? Endoscopy. 2001;33:786-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Vaishnavi C, Samanta J, Kochhar R. Characterization of biofilms in biliary stents and potential factors involved in occlusion. World J Gastroenterol. 2018;24:112-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 39. | Donelli G, Guaglianone E, Di Rosa R, Fiocca F, Basoli A. Plastic biliary stent occlusion: factors involved and possible preventive approaches. Clin Med Res. 2007;5:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 40. | Guaglianone E, Cardines R, Vuotto C, Di Rosa R, Babini V, Mastrantonio P, Donelli G. Microbial biofilms associated with biliary stent clogging. FEMS Immunol Med Microbiol. 2010;59:410-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Sung JY, Costerton JW, Shaffer EA. Defense system in the biliary tract against bacterial infection. Dig Dis Sci. 1992;37:689-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 148] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 42. | Tohda G, Dochin M. Management of endoscopic biliary stenting for choledocholithiasis: Evaluation of stent-exchange intervals. World J Gastrointest Endosc. 2018;10:45-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Kuwatani M, Kawakubo K, Sakamoto N. Possible reasons for the regrettable results of patency of an inside stent in endoscopic transpapillary biliary stenting. Dig Endosc. 2022;34:334-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Vitebsky YD. [Fundamentals of valvular gastroenterology]. Chelyabinsk: South-Ural, 1986: 126. |
| 45. | Shafik A, Shafik IA, El Sibai O, Shafik AA. Duodeno-jejunal junction dyssynergia: description of a novel syndrome. World J Gastroenterol. 2007;13:4112-4116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |