Published online Apr 27, 2023. doi: 10.4240/wjgs.v15.i4.643
Peer-review started: December 23, 2022
First decision: February 3, 2023
Revised: February 12, 2023
Accepted: March 30, 2023
Article in press: March 30, 2023
Published online: April 27, 2023
Processing time: 120 Days and 20.1 Hours
Gastric cancer (GC) is still a prevalent neoplasm around the world and its main treatment modality is surgical resection. The need for perioperative blood transfusions is frequent, and there is a long-lasting debate regarding its impact on survival.
To evaluate the factors related to the risk of receiving red blood cell (RBC) transfusion and its influence on surgical and survival outcomes of patients with GC.
Patients who underwent curative resection for primary gastric adenocarcinoma at our Institute between 2009 and 2021 were retrospectively evaluated. Clinicopathological and surgical characteristics data were collected. The patients were divided into transfusion and non-transfusion groups for analysis.
A total of 718 patients were included, and 189 (26.3%) patients received perioperative RBC transfusion (23 intraoperatively, 133 postoperatively, and 33 in both periods). Patients in the RBC transfusions group were older (P < 0.001), and had more comorbidities (P = 0.014), American Society of Anesthesiologists classification III/IV (P < 0.001), and lower preoperative hemoglobin (P < 0.001) and albumin levels (P < 0.001). Larger tumors (P < 0.001) and advanced tumor node metastasis stage (P < 0.001) were also associated with the RBC transfusion group. The rates of postoperative complications (POC) and 30-d and 90-d mortality were significantly higher in the RBC transfusion group than in the non-transfusion group. Lower hemoglobin and albumin levels, total gastrectomy, open surgery, and the occurrence of POC were factors associated with the RBC transfusion. Survival analysis demonstrated that the RBC transfusions group had worse disease-free survival (DFS) and overall survival (OS) compared with patients who did not receive transfusion (P < 0.001 for both). In multivariate analysis, RBC transfusion, major POC, pT3/T4 category, pN+, D1 lymphadenectomy, and total gastrectomy were independent risk factors related to worse DFS and OS.
Perioperative RBC transfusion is associated with worse clinical conditions and more advanced tumors. Further, it is an independent factor related to worse survival in the curative intent gastrectomy setting.
Core Tip: This is a retrospective study to investigate the association of perioperative red blood cell (RBC) transfusion with surgical and survival outcomes in patients with gastric cancer. Our findings demonstrated that patients who received RBC transfusion had poorer preoperative clinical conditions and more aggressive tumors, and were submitted to more invasive procedures. The rates of postoperative complications and 30-d and 90-d mortality were also significantly higher in patients who received RBC transfusions compared to those who did. Further, receiving an RBC transfusion was an independent factor associated with worse survival.
- Citation: Kawakami LE, Bonomi PB, Pereira MA, Carvalho FO, Ribeiro Jr U, Zilberstein B, Sampaio LR, Carneiro-D'Albuquerque LA, Ramos MFKP. Risk factors for blood transfusion and its prognostic implications in curative gastrectomy for gastric cancer. World J Gastrointest Surg 2023; 15(4): 643-654
- URL: https://www.wjgnet.com/1948-9366/full/v15/i4/643.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i4.643
In 2020, gastric cancer (GC) was the fifth most diagnosed neoplasm and the fourth cause of death by neoplasms[1]. Although its incidence and mortality rates have decreased in the last two decades, in 2025, GC will be accountable for more than one million cases and eight hundred thousand deaths[2]. GC is frequently associated with perioperative blood loss, whether by its biological behavior or its most important treatment, radical gastrectomy. Therefore, anemia and blood transfusion in the perioperative setting are a common concern[3,4].
The Transfusion Requirements in Critical Care trial (1999) was the first study to show worse outcomes related to excessive use of blood components in critical care patients, and since then, more restrictive use of transfusions has been recommended[5]. In the last ten years, surgeons and oncologists have studied the continuous pro-inflammatory status triggered by surgical tissue damage, postoperative complications (POC), and blood transfusions[6]. This effort confirmed the association between transfusion and higher recurrence rates in colorectal, pancreatic, and biliary tract cancers[6-8]. However, the current literature seems to struggle to find an answer for the impact of blood components on the outcomes of curative intent treatment in GC. The debate on how blood transfusion impacts survival and POC in GC is a complex topic, given the heterogeneity of results found in recent years. One side supported blood transfusion as an independently associated risk factor for inferior results; the other concluded that using blood components is a confounding factor for the worse prognosis since patients needing transfusions presented unfavorable clinical conditions previous to the surgical procedure and more advanced tumors at pathological staging compared to patients who did not receive transfusions[9-11].
Thus, this study aimed to evaluate the influence of perioperative red blood cell (RBC) transfusion on surgical and survival outcomes of patients with GC. We also examined the factors related to the risk of receiving a blood transfusion.
This is a retrospective cohort of patients with GC who underwent gastrectomy with curative intent in an oncological reference center from February 2009 to December 2021. Non-adenocarcinoma tumors (lymphoma, gastrointestinal stromal tumor, and neuroendocrine tumors) were excluded, as well as palliative surgery, diagnostic laparoscopy, previous hematological disorders, and patients with synchronic neoplasms.
The following clinical variables were evaluated: Age, sex, preoperative body mass index (BMI), neutrophil-lymphocyte ratio (NLR), hemoglobin, albumin level, and performance status based on the American Society of Anesthesiologists (ASA) classification. Charlson-Deyo comorbidity index (CCI) was used to measure comorbidities without including age and GC as comorbidity[12]. Tumor node metastasis (TNM) staging was determined according to the 8th edition of the American Joint Committee on Cancer[13].
Experienced surgeons performed surgical procedures. The surgical approach (open or laparoscopic) was carried out based on the surgeon's decision after a multidisciplinary meeting composed of the oncology, surgery, radiology, and pathology departments. The extension of gastric and lymph node (LN) dissection followed the recommendations of the Japanese Gastric Cancer Association (JGCA)[14]. The classification proposed by Baiocchi et al[15] was employed to define intraoperative complications. Intraoperative blood loss was measured in milliliters, and the length of the surgical procedure was assessed in minutes.
For analysis, the patients were divided into two groups: Patients who received an RBC transfusion and those who did not. In addition to the RBC transfusion, we also describe the transfusion of platelet concentrate (PC) and fresh frozen plasma (FFP). Regarding the moment in which the administration occurred, the following periods were considered: Intraoperative and postoperative (until the 30th day).
POC were graded according to Clavien-Dindo's classification. Clavien III to V was considered major complications[16]. Mortality at 30 and 90 d after the surgical procedure was also assessed. Adjuvant or perioperative platin-based chemotherapy was administered according to clinical indications (T3, T4, and regional LN metastasis)[17].
Surgical and oncological teams performed postoperative follow-up medical appointments every 3 mo in the first year and every 6 mo in the following years. The attending clinician assigned to each case determined recurrence based on laboratory tests, CT, or endoscopy reports. Lost to follow-up was defined as an absence for more than 12 mo in follow-up visits.
We obtained all data by reviewing the patient’s medical chart and blood center system. The hospital ethics committee approved the study (CAAE: 59337222.7.0000.0068) and it was registered online in the national research projects database (www.plataformabrasil.org.br).
The Chi-square test was used to compare categorical variables between the two groups, and the t-test or Mann-Whitney test for continuous variables. Univariate and multivariate binary regression analyses were used to identify risk factors for receiving perioperative RBC transfusion. Odds ratios (ORs) with 95%CI were calculated.
Survival was estimated using the Kaplan-Meier method, and the log-rank test was used to identify differences between the survival curves. The Cox proportional hazards model was used to identify risk factors independently associated with survival outcomes. The results are reported as hazard ratios (HRs) with 95%CIs. Disease-free survival (DFS) was calculated from the date of surgery to recurrence or death from any cause. Overall survival (OS) was the duration between the date of surgery to death. All patients alive were censored at the date of the last follow-up. All tests were two-sided, and P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS software, version 20.0 (SPSS Inc, Chicago, IL).
During the selected period, 718 patients underwent radical gastrectomy with curative intent. Among them, 189 (26.3%) patients received perioperative RBC transfusion (RBC transfusion group). The remaining 529 (73.6%) patients who did not receive any perioperative RBC formed the non-transfusion group.
Among 189 patients who received RBC transfusion, 23 underwent transfusion in the intraoperative period, 133 in the postoperative period, and 33 in both periods (intra and postoperative). Besides RBC transfusion, FFP transfusions occurred in 12 patients (6.4%) and 2 patients (1.1%) also received PC transfusion.
Patients in the RBC transfusion group had older age (P < 0.001), higher CCI (P = 0.014), ASA III/IV score (P < 0.001), and lower BMI (P = 0.016) compared with patients who did not receive a transfusion. Higher NLR (P = 0.010) and lower preoperative hemoglobin (P < 0.001) and albumin levels (P < 0.001) were also associated with the RBC transfusions group. There was no difference regarding preoperative chemotherapy between the groups (P = 0.095). Complete clinical characteristics are demonstrated in Table 1.
| Variable | Non-transfusion | Red blood cell transfusion | P value |
| n = 529 (%) | n = 189 (%) | ||
| Sex | 0.318 | ||
| Female | 215 (40.6) | 69 (36.5) | |
| Male | 314 (59.4) | 120 (63.5) | |
| Age (yr), mean ± SD | 61.3 ± 12.4 | 65.6 ± 11.6 | < 0.001 |
| Body mass index (kg/m²), mean ± SD | 24.6 ± 4.6 | 23.7 ± 11.6 | 0.019 |
| Hemoglobin (g/dL), mean ± SD | 12.5 ± 2.1 | 10.8 ± 2.2 | < 0.001 |
| Albumin (g/dL), mean ± SD | 4.0 ± 0.6 | 3.7 ± 0.7 | < 0.001 |
| Neutrophil-lymphocyte ratio, mean ± SD | 2.65 ± 2.77 | 3.26 ± 2.75 | 0.010 |
| American Society of Anesthesiologists | < 0.001 | ||
| I/II | 404 (76.4) | 11 (58.7) | |
| III/IV | 125 (23.6) | 78 (41.3) | |
| Charlson-Deyo comorbidity index | 0.014 | ||
| 0 | 360 (68.1) | 110 (58.2) | |
| ≥ 1 | 169 (31.9) | 79 (41.8) | |
| Preoperative chemotherapy | 0.095 | ||
| No | 237 (44.8) | 98 (51.9) | |
| Yes | 292 (55.2) | 91 (48.1) |
Regarding surgical procedures and postoperative features demonstrated in Table 2, total gastrectomy (P < 0.001) and open surgery (P < 0.001) were more frequent in the RBC transfusion group. There was no difference regarding the duration of surgery (P = 0.636), intraoperative complications (P = 0.209), and intraoperative blood loss (P = 0.186) between the two groups. Length of hospital stay was higher in the transfusion group (10.4 d vs 21.6 d, P < 0.001). Considering the postoperative outcomes, the rates of POC (P < 0.001) and mortality at 30 and 90 d were significantly higher in the transfusion group (P < 0.001).
| Variable | Non-transfusion | Red blood cell transfusion | P value |
| n = 529 (%) | n >= 189 (%) | ||
| Type of resection | < 0.001 | ||
| Subtotal | 337 (63.7) | 91 (48.1) | |
| Total | 192 (36.3) | 98 (51.9) | |
| Surgical access | < 0.001 | ||
| Open | 396 (74.9) | 168 (88.9) | |
| Minimally invasive | 133 (25.1) | 21 (11.1) | |
| Type of lymphadenectomy | < 0.001 | ||
| D1 | 104 (19.7) | 72 (38.1) | |
| D2 | 425 (80.3) | 117 (61.9) | |
| Operation time (min), mean ± SD | 243.5 ± 74.3 | 246.6 ± 77.3 | 0.636 |
| Intraoperative blood loss (mL), mean ± SD | 299.7 ± 336.7 | 342.0 ± 362.6 | 0.186 |
| Intraoperative complications | 0.209 | ||
| No | 508 (96.0) | 178 (94.2) | |
| Yes | 21 (4.0) | 11 (5.8) | |
| Length of postoperative stay (d), mean ± SD | 10.4 ± 7.2 | 21.6 ± 17.4 | < 0.001 |
| Postoperative complications (Clavien-Dindo) | < 0.001 | ||
| 0/I/II (minor) | 488 (92.2) | 112 (59.3) | |
| III/IV/V (major) | 41 (7.8) | 77 (40.7) | |
| Adjuvant chemotherapy | 0.001 | ||
| No | 287 (54.3) | 129 (68.3) | |
| Yes | 242 (45.7) | 60 (31.7) | |
| Mortality | |||
| 30-d | 8 (1.5) | 19 (10.1) | < 0.001 |
| 90-d | 16 (3.1) | 36 (19.0) | < 0.001 |
The pathological characteristics of the two groups are shown in Table 3. Larger tumor size (P < 0.001), intestinal Lauren type (P = 0.002), pT3/T4 (P < 0.001), and advanced pathological TNM (pTNM) stages (P < 0.001) were more frequent in the RBC transfusion group. The presence of lymphatic (P = 0.027), vascular (P = 0.017), and perineural (P = 0.001) invasions was also associated with the transfusion group.
| Variable | Non-transfusion | Red blood cell transfusion | P value |
| n >= 529 (%) | n = 189 (%) | ||
| Tumor size (cm), mean ± SD | 4.3 ± 2.6 | 6.0 ± 3.6 | < 0.001 |
| Tumor location | 0.039 | ||
| Lower | 327 (61.8) | 95 (50.3) | |
| Middle | 130 (24.6) | 60 (31.7) | |
| Upper | 64 (12.2) | 29 (15.3) | |
| Diffuse | 8 (1.5) | 5 (2.6) | |
| Lauren histologic type1 | 0.002 | ||
| Intestinal | 276 (52.6) | 124 (66.0) | |
| Diffuse/mixed | 249 (47.4) | 64 (34.0) | |
| Histological differentiation1 | 0.056 | ||
| Well/moderate | 245 (46.7) | 103 (54.8) | |
| Poor | 280 (53.3) | 85 (45.2) | |
| Lymphatic invasion | 0.027 | ||
| No | 293 (55.4) | 87 (46.0) | |
| Yes | 236 (44.6) | 102 (54.0) | |
| Vascular invasion | 0.017 | ||
| No | 364 (68.8) | 112 (59.3) | |
| Yes | 165 (31.2) | 77 (40.7) | |
| Perineural invasion | 0.001 | ||
| No | 304 (57.5) | 83 (43.9) | |
| Yes | 225 (42.5) | 106 (56.1) | |
| pT status | < 0.001 | ||
| pT1/T2 | 248 (46.9) | 54 (28.6) | |
| pT3/T4 | 281 (53.1) | 135 (71.4) | |
| Lymph nodes harvested, mean ± SD | 41.5 ± 19.4 | 39.2 ± 19.5 | 0.175 |
| pN status | 0.126 | ||
| pN0 | 244 (46.1) | 75 (39.7) | |
| pN+ | 285 (53.9) | 114 (60.3) | |
| pTNM stage | < 0.001 | ||
| I/II | 329 (62.2) | 86 (45.5) | |
| III/IV | 200 (37.8) | 103 (54.5) |
In multivariate analysis, low preoperative hemoglobin (P < 0.001), low albumin (P = 0.017), total gastrectomy (P = 0.011), open surgical access (P = 0.034), and occurrence of major POC (P < 0.001) were independent factors associated to a higher risk of receiving perioperative RBC transfusions (Table 4).
| Variable | Univariate | Multivariate | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Male vs female | 1.19 | 0.85-1.68 | 0.319 | |||
| Age ≥ 65 yr vs < 65 yr | 1.48 | 1.06-2.07 | 0.02 | 1.2 | 0.79-1.81 | 0.394 |
| Charlson ≥ 1 vs 0 | 1.53 | 1.09-2.15 | 0.015 | 1.22 | 0.76-1.97 | 0.402 |
| ASA III/IV vs I/II | 2.27 | 1.60-3.23 | < 0.001 | 1.18 | 0,72-1.94 | 0.507 |
| HB < 11 g/dL vs ≥ 11 g/dL | 4.7 | 3.30-6.70 | < 0.001 | 4.32 | 2.76-6.78 | < 0.001 |
| ALB < 3.5 g/dL vs ≥ 3.5 g/dL | 3.49 | 2.29-5.31 | < 0.001 | 1.86 | 1.12-3.09 | 0.017 |
| Total gastrectomy vs distal | 1.89 | 1.35-2.65 | < 0.001 | 1.71 | 1.13-2.60 | 0.011 |
| Open surgery vs MIS | 2.69 | 1.64-4.41 | < 0.001 | 1.91 | 1.05-3.47 | 0.034 |
| Major POC vs non/minor POC | 8.18 | 5.32-12.59 | < 0.001 | 8.83 | 5.28-14.79 | < 0.001 |
| Intraoperative intercurrence vs none | 1.49 | 0.71-3.16 | 0.293 | |||
The median follow-up time for the entire cohort of cases was 35.6 mo. During the follow-up period, 174 patients had disease recurrence and 261 died.
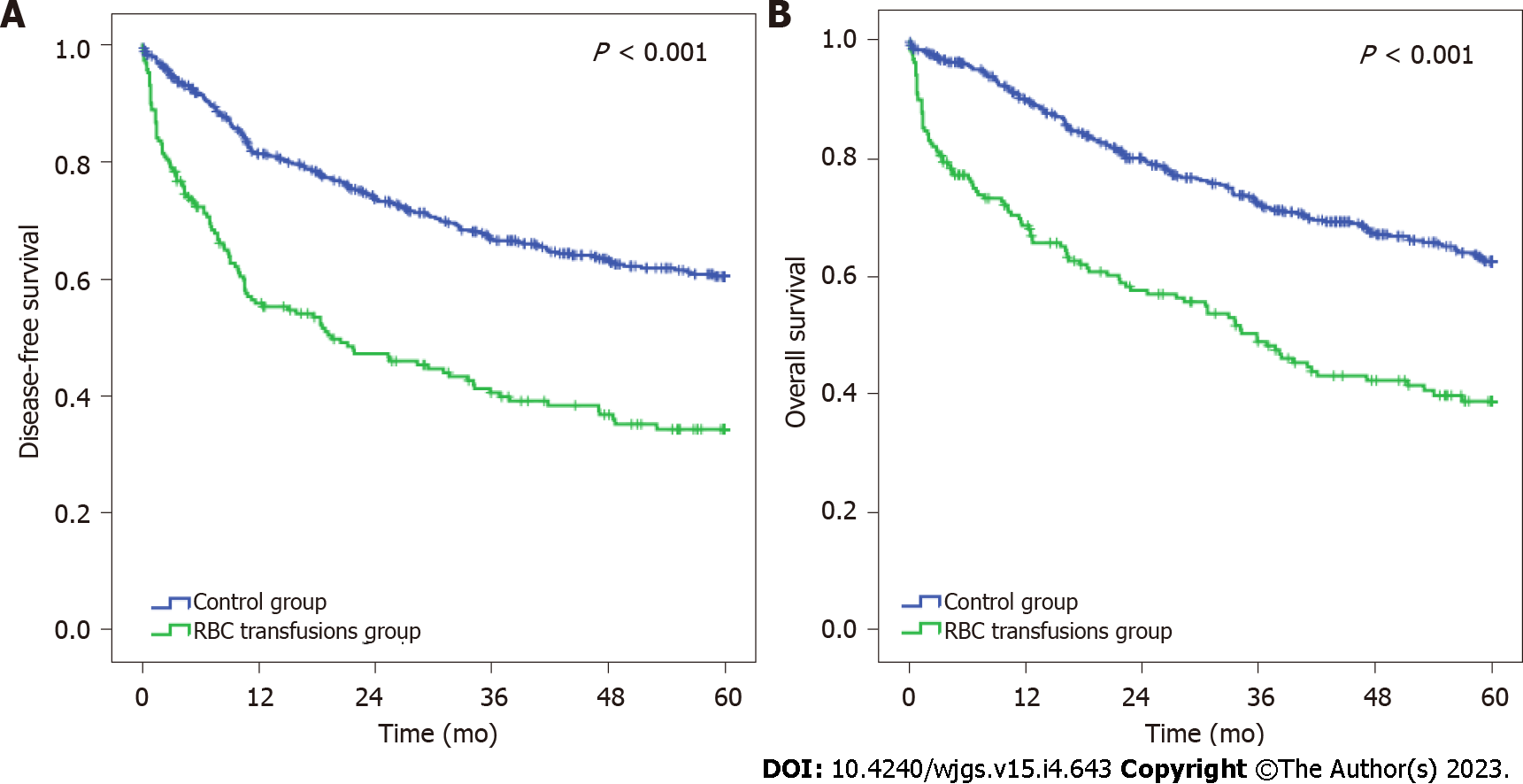
Patients who received perioperative RBC transfusions had worse DFS and OS than the non-transfusion group (P < 0.001) (Figure 1). The median DFS and OS for the RBC transfusion group were 19.5 and 35.8 mo, respectively.
In multivariate analysis, total gastrectomy, more advanced pT stage, LN metastasis, D1 Lymphadenectomy, the occurrence of POC, and perioperative RBC transfusion were independent factors associated with worse DFS (Table 5).
| Univariate | Multivariate | |||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Disease-free survival | ||||||
| Male vs female | 1.27 | 0.99-1.62 | 0.051 | |||
| Age ≥ 65 yr vs < 65 yr | 1.25 | 0.99-1.57 | 0.059 | |||
| Charlson ≥ 1 vs 0 | 1.33 | 1.05-1.68 | 0.018 | 1.22 | 0.93-1.60 | 0.159 |
| ASA III/IV vs I/II | 1.83 | 1.44-2.33 | < 0.001 | 1.24 | 0.93-1.66 | 0.149 |
| Total gastrectomy vs distal | 1.77 | 1.41-2.33 | < 0.001 | 1.45 | 1.14-1.84 | 0.002 |
| D1 vs D2 | 1.61 | 1.25-2.07 | < 0.001 | 1.4 | 1.06-1.85 | 0.017 |
| pT3/T4 vs pT1/T2 | 2.87 | 2.19-3.76 | < 0.001 | 2.05 | 1.50-2.79 | < 0.001 |
| pN+ vs pN0 | 2.81 | 2.17-3.65 | < 0.001 | 1.97 | 1.47-2.65 | < 0.001 |
| Major POC vs non/minor POC | 2.86 | 2.19-3.73 | < 0.001 | 2.15 | 1.62-2.86 | < 0.001 |
| Non-CMT vs received CMT | 1 | 0.79-1.26 | 0.995 | |||
| Perioperative RBC transfusion vs non | 2.39 | 1.88-3.02 | < 0.001 | 1.49 | 1.14-1.94 | 0.003 |
| Overall survival | ||||||
| Male vs female | 1.25 | 0.97-1.61 | 0.084 | |||
| Age ≥ 65 yr vs < 65 yr | 1.41 | 1.11-1.80 | 0.006 | 1.24 | 0.95-1.61 | 0.113 |
| Charlson ≥ 1 vs 0 | 1.35 | 1.06-1.73 | 0.017 | 1.11 | 0.83-1.49 | 0.473 |
| ASA III/IV vs I/II | 2.02 | 1.57-2.61 | < 0.001 | 1.36 | 1.01-1.85 | 0.048 |
| Total gastrectomy vs distal | 1.65 | 1.29-2.10 | < 0.001 | 1.33 | 1.03-1.71 | 0.027 |
| Lymphadenectomy D1 vs D2 | 1.81 | 1.39-2.35 | < 0.001 | 1.51 | 1.11-2.05 | 0.008 |
| pT3/T4 vs pT1/T2 | 2.92 | 2.19-3.90 | < 0.001 | 2.19 | 1.57-3.06 | < 0.001 |
| pN+ vs pN0 | 2.7 | 2.06-3.56 | < 0.001 | 1.87 | 1.37-2.56 | < 0.001 |
| Major POC vs non/minor POC | 3.33 | 2.53-4.38 | < 0.001 | 2.65 | 1.97-3.56 | < 0.001 |
| Non-CMT vs received CMT | 1.05 | 0.83-1.34 | 0.678 | |||
| Perioperative RBC transfusion vs non | 2.33 | 1.81-2.99 | < 0.001 | 1.34 | 1.02-1.77 | 0.038 |
ASA, type of gastrectomy, lymphadenectomy, pT, pN, POC, and perioperative RBC transfusion were factors significantly associated with OS in the multivariate model (Table 5).
Perioperative RBC transfusion remained an independently associated risk factor for both DFS [hazard ratio (HR) = 1.49, 95%CI: 1.14-1.94, P = 0.003] and OS (HR = 1.34, 95%CI: 1.02-1.77, P = 0.038).
During the progression of GC, cachexia and uncontrolled tumor bleeding may induce severe anemia leading to life-threatening conditions and worse clinical outcomes. In this scenario, perioperative RBC transfusion is indicated to improve performance and decrease morbidity in the postoperative period[4,18,19]. On the other hand, recent advances in immunology have questioned the role of immunosuppression triggered by transfusion and its impact on tumor recurrence in gastrointestinal tract neoplasms[20-22].
In our retrospective cohort composed of 718 patients, perioperative RBC transfusions were related to worse DFS and OS. It is crucial to recognize expressive baseline differences between patients who received RBC transfusions and those who did not. Patients in the transfusion group were older and presented more unfavorable clinical conditions. Further, a higher frequency of total gastrectomy, open surgery, and advanced tumors was observed in the transfusion group. Other studies also reported the same heterogeneity noted in the analyzed population[9-11,23]. Even though, after multivariate analysis, we found that perioperative RBC transfusion was an independent factor related to recurrence and survival.
Although there is a vast amount of literature investigating the impact of blood components on the oncologic prognosis of GC patients, the current data still present discordant results[11,24,25]. This situation will probably be extended since it is difficult to perform a randomized controlled trial (RCT) in this scenario since in many situations the need for transfusion is a life-threatening condition. Further, most meta-analyses stress that current studies lack high-quality data[26]. In the recent retrospective studies that found no impact of RBC transfusions on long-term survival, some of them applied propensity-score matched analysis; however, preoperative hemoglobin and intraoperative blood loss remain as factors that could not be matched between groups[11,27]. Despite the knowledge of the relevance of anemia in the perioperative setting, conflicting data persist around the impact of intraoperative blood loss on OS and DFS of GC patients[28-30]. Grasso et al[31] carried out prospective studies comparing different hemoglobin threshold values for the indication of transfusion may be an alternative to define this issue.
The current hypothesis that explains the biological association between blood components and poorer oncological outcomes is that transfusion-related immunomodulation (TRIM) acts as a propagating factor for the TH2 immune response, favoring a pro-tumoral environment through inhibition of interleukin (IL)-2 and stimulation of suppressor T cells, allowing tumor spread and recurrence[20-22]. The recent application of immunotherapy in gastrointestinal tract cancers provided additional data by demonstrating that TRIM could be acting as an opponent and negatively impacting its effectiveness and survival[32]. Another important topic related to the immune response is the data provided by Lange et al[33] that showed no difference in using leukocyte depletion in long-term survival, underlining that specific constituents of allogeneic blood may mediate the TRIM effect. This same result was detected when RBC transfusions were applied to other neoplasms[34].
Preoperative hemoglobin and albumin were independent factors associated with RBC transfusions. Since GC causes feeding and bleeding disturbances, aggressive protocols for improving hematologic and nutritional preoperative status must be paramount in clinical compensation ahead of surgical treatment. Current data support those policies in clinical and financial terms since Jericó et al[35] demonstrated reduced direct and indirect spent resources, lower hospital length of stay, and readmissions succeeding radical gastrectomy[36].
Interestingly, D1 lymphadenectomy was associated with worse DFS and OS. D2 lymphadenectomy is considered a more aggressive procedure and the standard in GC treatment. However, even though 42% of our population were composed of patients with more advanced stages III/IV, some of them did not have the clinical conditions to perform D2 lymphadenectomy. So, the employment of D1 lymphadenectomy rarely was an oncological indication as proposed in the 2018 JGCA guideline for GC treatment[14]. It was mainly indicated for patients with unfavorable medical conditions to reduce POC and mortality, as previously reported by our service[37].
Open surgical access was associated with the transfusion group. Minimally invasive surgery causes less tissue damage to the abdominal wall with reports of less intraoperative blood loss on several RCTs[38-40]. However, intraoperative blood loss, although higher in the transfusion group, did not show a significant difference between our groups. Intraoperative blood loss is a variable that is difficult to measure in clinical practice. The retrospective nature of the study also makes accurate measurement difficult. Despite this possible bias, we also found that there was no difference between the groups in the occurrence of intraoperative complications, a variable that is very well documented. Baiocchi et al[15] reported a low 2% incidence of intraoperative complications in GC surgery. In our study, a 4.45% incidence of intraoperative complications was reported, represented mainly by intraoperative bleeding. Those numbers indicate adequate documentation of the intraoperative complications in our medical reports and eventually, intraoperative blood loss did not differ between the groups. Therefore, our best efforts should focus on better patient perioperative management to avoid RBC transfusion[41].
Regarding POC, the multivariate analysis indicated that major POC presented the highest OR related to RBC transfusion among the eight selected variables. It must also be emphasized that the transfusion in the postoperative period was more frequent than in the pre and intraoperative periods. The importance of POC was already stressed in 2020 through a meta-analysis evaluating their repercussions on GC survival[42]. A plausible reason for cancer recurrence is the pro-inflammatory state caused by surgical trauma, where IL-6 suppresses the specific and non-specific immune responses. This mechanism could be synergically associated with IL-2 suppression caused by TRIM since a retrospective analysis found a signature of cytokines (including IL-2 and IL-6) and angiogenic factors associated with poor DFS and OS[43]. Further, POCs may prevent patients to return to the intended oncological treatment, a known prognostic factor[44].
The performance of retrospective studies has some limitations inherent to its design. Despite the relevant number of patients included for a Western center, the numerous variables evaluated are confounding factors for the adequate definition of the association between RBC transfusion and prognosis. We chose multivariate logistic regression to adjust the potential bias of covariates. Ultimately, perioperative transfusion of RBC was an independent prognostic factor together with known prognostic factors such as pTNM stage, demonstrating a good accuracy of the performed analyses. As another limitation, we must point out that our data were collected over 13 years, so variations and advances in oncological treatments and surgical techniques may cause additional heterogeneity.
In GC patients undergoing curative surgeries, poor clinical status, more extensive surgical procedures, and advanced tumor stages are common features in patients receiving RBC transfusions. In addition to being associated with higher rates of POC and mortality, receiving an RBC transfusion proves to be an independent factor associated with worse survival.
Anemia and intraoperative blood loss are frequent issues in gastric cancer (GC) surgical treatment. The current literature still debates the impact of perioperative blood transfusion on GC survival.
Red blood cell (RBC) transfusions are sometimes required for patients undergoing surgery for GC. However, the prognostic impact of perioperative RBC transfusion in GC is controversial.
We analyzed the influence of RBC transfusions on the prognosis of patients with gastric adenocarcinoma undergoing gastrectomy with curative intention.
We retrospectively evaluated all GC patients who underwent gastrectomy between 2009 and 2021. Patients were divided into transfusion group and non-transfusion group for analysis. RBC transfusions that occurred intraoperatively and postoperatively within 30 d were considered.
A total of 718 patients were included, and 189 (26.3%) patients received RBC transfusions. Patients who received transfusions had unfavorable clinical and pathological characteristics, and underwent more extensive surgical procedures. Patients who received RBC transfusions had worse survival compared to those who did not. In multivariate analysis, receiving an RCB transfusion was an independent factor associated with poor disease-free survival (DFS) and overall survival (OS).
Even though the patients who receive RCB transfusion have worse clinical conditions, we found that perioperative transfusion represents an independent factor associated with poor prognosis, with worse DFS and OS.
The application of blood component transfusion in randomized clinical trials presents ethical limitations; however, the current design of retrospective studies still interferes with controlling confounding factors. With this study, we endorse a favorable position for increasing preoperative and postoperative care to avoid RBC transfusion. Further, our findings provide additional data for future meta-analysis.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kinami S, Japan; Manojlovic N, Serbia S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Zhang H
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64650] [Article Influence: 16162.5] [Reference Citation Analysis (176)] |
| 2. | Ilic M, Ilic I. Epidemiology of stomach cancer. World J Gastroenterol. 2022;28:1187-1203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 239] [Cited by in RCA: 235] [Article Influence: 78.3] [Reference Citation Analysis (22)] |
| 3. | Kouyoumdjian A, Trepanier M, Al Shehhi R, Cools-Lartigue J, Ferri LE, Lee L, Mueller CL. The Effect of Preoperative Anemia and Perioperative Transfusion on Surgical Outcomes After Gastrectomy for Gastric Cancer. J Surg Res. 2021;259:523-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Huang XZ, Yang YC, Chen Y, Wu CC, Lin RF, Wang ZN, Zhang X. Preoperative Anemia or Low Hemoglobin Predicts Poor Prognosis in Gastric Cancer Patients: A Meta-Analysis. Dis Markers. 2019;2019:7606128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3699] [Cited by in RCA: 3355] [Article Influence: 129.0] [Reference Citation Analysis (0)] |
| 6. | McSorley ST, Tham A, Dolan RD, Steele CW, Ramsingh J, Roxburgh C, Horgan PG, McMillan DC. Perioperative Blood Transfusion is Associated with Postoperative Systemic Inflammatory Response and Poorer Outcomes Following Surgery for Colorectal Cancer. Ann Surg Oncol. 2020;27:833-843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Kanda T, Wakiya T, Ishido K, Kimura N, Nagase H, Kubota S, Fujita H, Hagiwara Y, Hakamada K. Intraoperative Allogeneic Red Blood Cell Transfusion Negatively Influences Prognosis After Radical Surgery for Pancreatic Cancer: A Propensity Score Matching Analysis. Pancreas. 2021;50:1314-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Wang Q, Du T, Lu C. Perioperative blood transfusion and the clinical outcomes of patients undergoing cholangiocarcinoma surgery: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2016;28:1233-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Liu X, Ma M, Huang H, Wang Y. Effect of perioperative blood transfusion on prognosis of patients with gastric cancer: a retrospective analysis of a single center database. BMC Cancer. 2018;18:649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Xu D, Fang X, Li Y, Zhang Z, Li Q. Perioperative blood transfusion is one of the factors that affect the prognosis of gastric cancer. J BUON. 2018;23:672-677. [PubMed] |
| 11. | Xiao H, Liu W, Quan H, Ouyang Y. Peri-Operative Blood Transfusion Does Not Influence Overall and Disease-Free Survival After Radical Gastrectomy for Stage II/III Gastric Cancer: a Propensity Score Matching Analysis. J Gastrointest Surg. 2018;22:1489-1500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32099] [Cited by in RCA: 38306] [Article Influence: 1008.1] [Reference Citation Analysis (0)] |
| 13. | Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM. AJCC Cancer Staging Manual (8th edition). Springer International Publishing: American Joint Commission on Cancer, 2017. |
| 14. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1338] [Article Influence: 334.5] [Reference Citation Analysis (2)] |
| 15. | Baiocchi GL, Giacopuzzi S, Marrelli D, Reim D, Piessen G, Matos da Costa P, Reynolds JV, Meyer HJ, Morgagni P, Gockel I, Lara Santos L, Jensen LS, Murphy T, Preston SR, Ter-Ovanesov M, Fumagalli Romario U, Degiuli M, Kielan W, Mönig S, Kołodziejczyk P, Polkowski W, Hardwick R, Pera M, Johansson J, Schneider PM, de Steur WO, Gisbertz SS, Hartgrink H, van Sandick JW, Portolani N, Hölscher AH, Botticini M, Roviello F, Mariette C, Allum W, De Manzoni G. International consensus on a complications list after gastrectomy for cancer. Gastric Cancer. 2019;22:172-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 16. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24843] [Article Influence: 1183.0] [Reference Citation Analysis (0)] |
| 17. | Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, Vogel A, Smyth EC; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:1005-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 687] [Article Influence: 229.0] [Reference Citation Analysis (0)] |
| 18. | Tang GH, Hart R, Sholzberg M, Brezden-Masley C. Iron deficiency anemia in gastric cancer: a Canadian retrospective review. Eur J Gastroenterol Hepatol. 2018;30:1497-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers. 2018;4:17105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 1026] [Article Influence: 146.6] [Reference Citation Analysis (0)] |
| 20. | Youssef LA, Spitalnik SL. Transfusion-related immunomodulation: a reappraisal. Curr Opin Hematol. 2017;24:551-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 21. | Cata JP, Wang H, Gottumukkala V, Reuben J, Sessler DI. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J Anaesth. 2013;110:690-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 336] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 22. | Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2007;21:327-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 491] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 23. | Elmi M, Mahar A, Kagedan D, Law CH, Karanicolas PJ, Lin Y, Callum J, Coburn NG, Hallet J. The impact of blood transfusion on perioperative outcomes following gastric cancer resection: an analysis of the American College of Surgeons National Surgical Quality Improvement Program database. Can J Surg. 2016;59:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Xiao H, Xiao Y, Chen P, Quan H, Luo J, Huang G. Association Among Blood Transfusion, Postoperative Infectious Complications, and Cancer-Specific Survival in Patients with Stage II/III Gastric Cancer After Radical Gastrectomy: Emphasizing Benefit from Adjuvant Chemotherapy. Ann Surg Oncol. 2021;28:2394-2404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Song JH, Shin HJ, Lee S, Park SH, Cho M, Kim YM, Hyung WJ, Kim HI. No detrimental effect of perioperative blood transfusion on recurrence in 2905 stage II/III gastric cancer patients: A propensity-score matching analysis. Eur J Surg Oncol. 2022;48:2132-2140. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Agnes A, Lirosi MC, Panunzi S, Santocchi P, Persiani R, D'Ugo D. The prognostic role of perioperative allogeneic blood transfusions in gastric cancer patients undergoing curative resection: A systematic review and meta-analysis of non-randomized, adjusted studies. Eur J Surg Oncol. 2018;44:404-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 27. | Hsu FK, Chang WK, Lin KJ, Liu CY, Fang WL, Chang KY. The Associations between Perioperative Blood Transfusion and Long-Term Outcomes after Stomach Cancer Surgery. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Wen ZL, Xiao DC, Zhou X. Does Intraoperative Blood Loss Affect the Short-Term Outcomes and Prognosis of Gastric Cancer Patients After Gastrectomy? Front Surg. 2022;9:924444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 29. | Zhao B, Huang X, Lu H, Zhang J, Luo R, Xu H, Huang B. Intraoperative blood loss does not independently affect the survival outcome of gastric cancer patients who underwent curative resection. Clin Transl Oncol. 2019;21:1197-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Nakanishi K, Kanda M, Kodera Y. Long-lasting discussion: Adverse effects of intraoperative blood loss and allogeneic transfusion on prognosis of patients with gastric cancer. World J Gastroenterol. 2019;25:2743-2751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 31. | Grasso M, Pacella G, Sangiuliano N, De Palma M, Puzziello A. Gastric cancer surgery: clinical outcomes and prognosis are influenced by perioperative blood transfusions. Updates Surg. 2019;71:439-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Mispelbaum R, Hattenhauer ST, Brossart P, Heine A. Red blood cell transfusions impact response rates to immunotherapy in patients with solid malignant tumors. Front Immunol. 2022;13:976011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 33. | Lange MM, van Hilten JA, van de Watering LM, Bijnen BA, Roumen RM, Putter H, Brand A, van de Velde CJ; cooperative clinical investigators of the Cancer Recurrence And Blood Transfusion (CRAB) study and the Transfusion Associated Complications = Transfusion Induced Complications? (TACTIC) study. Leucocyte depletion of perioperative blood transfusion does not affect long-term survival and recurrence in patients with gastrointestinal cancer. Br J Surg. 2009;96:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Geng Y, Liu L. Impact of Allogeneic Leukocyte-Depleted Red Blood Cell Transfusion on Inflammatory Response and Blood Coagulation in Patients with Recurrence of Colon Cancer after Operation. Evid Based Complement Alternat Med. 2021;2021:6957569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 35. | Jericó C, Puértolas N, Osorio J, Miranda C, Santamaría M, Artigau E, Galofré G, Garsot E, Luna A, Aldeano A, Olona C, Pulido L, Pera M; Spanish EURECCA Esophagogastric Cancer Group. Cost analysis of a patient blood management program for patients undergoing gastric cancer surgery. Eur J Surg Oncol. 2023;49:293-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 36. | Osorio J, Jericó C, Miranda C, Santamaría M, Artigau E, Galofré G, Garsot E, Luna A, Puértolas N, Aldeano A, Olona C, Molinas J, Feliu J, Videla S, Tebe C, Pera M. Improved postoperative outcomes and reduced transfusion rates after implementation of a Patient Blood Management program in gastric cancer surgery. Eur J Surg Oncol. 2021;47:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Ramos MFKP, Pereira MA, Dias AR, Yagi OK, Zaidan EP, Ribeiro-Júnior U, Zilberstein B, Cecconello I. Surgical outcomes of gastrectomy with D1 lymph node dissection performed for patients with unfavorable clinical conditions. Eur J Surg Oncol. 2019;45:460-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Hyung WJ, Yang HK, Park YK, Lee HJ, An JY, Kim W, Kim HI, Kim HH, Ryu SW, Hur H, Kim MC, Kong SH, Cho GS, Kim JJ, Park DJ, Ryu KW, Kim YW, Kim JW, Lee JH, Han SU; Korean Laparoendoscopic Gastrointestinal Surgery Study Group. Long-Term Outcomes of Laparoscopic Distal Gastrectomy for Locally Advanced Gastric Cancer: The KLASS-02-RCT Randomized Clinical Trial. J Clin Oncol. 2020;38:3304-3313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 266] [Article Influence: 53.2] [Reference Citation Analysis (1)] |
| 39. | Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, Wang K, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Hu Y, Liu H, Zheng C, Li P, Xie J, Liu F, Li Z, Zhao G, Yang K, Liu C, Li H, Chen P, Ji J, Li G; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA. 2019;321:1983-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 527] [Article Influence: 87.8] [Reference Citation Analysis (1)] |
| 40. | Yang Y, Chen Y, Hu Y, Feng Y, Mao Q, Xue W. Outcomes of laparoscopic versus open total gastrectomy with D2 lymphadenectomy for gastric cancer: a systematic review and meta-analysis. Eur J Med Res. 2022;27:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 41. | Huang H, Cao M. Development and validation of a nomogram to predict intraoperative blood transfusion for gastric cancer surgery. Transfus Med. 2021;31:250-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Li J, Zhang Y, Hu DM, Gong TP, Xu R, Gao J. Impact of postoperative complications on long-term outcomes of patients following surgery for gastric cancer: A systematic review and meta-analysis of 64 follow-up studies. Asian J Surg. 2020;43:719-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Ock CY, Nam AR, Bang JH, Kim TY, Lee KH, Han SW, Im SA, Bang YJ, Oh DY. Signature of cytokines and angiogenic factors (CAFs) defines a clinically distinct subgroup of gastric cancer. Gastric Cancer. 2017;20:164-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Ramos MFKP, de Castria TB, Pereira MA, Dias AR, Antonacio FF, Zilberstein B, Hoff PMG, Ribeiro Jr U, Cecconello I. Return to Intended Oncologic Treatment (RIOT) in Resected Gastric Cancer Patients. J Gastrointest Surg. 2020;24:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |