Published online Apr 27, 2023. doi: 10.4240/wjgs.v15.i4.553
Peer-review started: December 7, 2022
First decision: February 8, 2023
Revised: February 10, 2023
Accepted: March 21, 2023
Article in press: March 21, 2023
Published online: April 27, 2023
Processing time: 136 Days and 23.8 Hours
Pneumatosis intestinalis (PI) is a striking radiological diagnosis. Formerly a rare diagnostic finding, it is becoming more frequently diagnosed due to the wider availability and improvement of computed tomography scan imaging. Once associated only with poor outcome, its clinical and prognostic significance nowadays has to be cross-referenced to the nature of the underlying condition. Multiple mechanisms of pathogenesis have been debated and multiple causes have been detected during the years. All this contributes to creating a broad range of clinical and radiological presentations. The management of patients presenting PI is related to the determining cause if it is identified. Otherwise, in particular if an association with portal venous gas and/or pneumoperitoneum is present, the eventual decision between surgery and non-operative management is challenging, even for stable patients, since this clinical condition is traditionally associated to intestinal ischemia and consequently to pending clinical collapse if not treated. Considering the wide variety of origin and outcomes, PI still remains for surgeons a demanding clinical entity. The manuscript is an updated narrative review and gives some suggestions that may help make the decisional process easier, identifying patients who can benefit from surgical intervention and those who can benefit from non-operative management avoiding unnecessary procedures.
Core Tip: Pneumatosis intestinalis (PI) represents a radiological diagnosis that must be understood correctly in order to follow the appropriate management. It is essential to identify the conditions that can evolve into transmural intestinal ischemia. It is also important to recognize those cases where PI can be managed conservatively. The integration of the clinical presentation, laboratory tests and abnormal abdominal physical examination can give indications on the path to follow. With this narrative review we have tried to provide a comprehensive analysis of the knowledge of this topic by proposing an algorithm to guide clinical decisions.
- Citation: Tropeano G, Di Grezia M, Puccioni C, Bianchi V, Pepe G, Fico V, Altieri G, Brisinda G. The spectrum of pneumatosis intestinalis in the adult. A surgical dilemma. World J Gastrointest Surg 2023; 15(4): 553-565
- URL: https://www.wjgnet.com/1948-9366/full/v15/i4/553.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i4.553
Pneumatosis Intestinalis (PI) refers to a spectrum of diseases characterized by the presence of gas in the intestinal wall[1-4]. It was firstly described the 1700s by Du Vernoy, that detected gas in the bowel wall during a cadaver dissection.
The radiographic finding of PI can indicate a spectrum of underlying processes ranging from a benign finding to a life-threating condition. It is possible to distinguish between “primary” and “secondary” PI[5-8]. Primary PI, also known as idiopathic or pneumocystis cystoides, is a pathologic condition characterized by the presence of gas-filled cysts in the sub-mucosa or sub-serosa especially of the colon[9-11]. Secondary PI is usually related to underlying pathological conditions (Table 1) and it is commonly characterized by the presence of linear or curvilinear gas balls in the intestinal wall[9,10,12-21]. Typically, the primary PI is asymptomatic and is not as frequent as the secondary PI (15% vs 85%)[1,22].
| Pathological conditions | |
| Trauma[21,64-67] | Blunt/penetrating abdominal trauma |
| Surgical anastomosis or bypass | |
| Mechanical[68] | Pyloric obstruction or stenosis |
| Duodenal obstruction or stenosis | |
| Bowel obstruction (volvulus, carcinoma, malrotation, intussusception) | |
| Autoimmune[69-71] | Lupus enteritis |
| Celiac sprue | |
| Polymyositis | |
| Dermatomyositis | |
| Polyarteritis nodosa | |
| Mixed connective tissue diseases | |
| Graft versus host disease | |
| Primary immunodeficiency | |
| Malignancies[15] | Gastrointestinal cancer |
| Leukemia | |
| Lymphoma | |
| Other malignancies | |
| Inflammation[14,72] | Inflammatory bowel disease |
| Appendicitis | |
| Diverticulitis | |
| Cholelithiasis | |
| Sarcoidosis | |
| Vascular conditions[73] | Ischemia or infarction |
| Diabetes | |
| Pulmonary disease[74,75] | Chronic obstructive pulmonary disease |
| Cystic fibrosis | |
| Asthma | |
| Drugs[13,19,76-79] | Corticosteroids |
| Chemotherapy and immunotherapy | |
| Immunosuppression | |
| Lactulose | |
| Trichloroethylene | |
| Sorbitol | |
| Alpha-glucosidase inhibitor | |
| Practolol | |
| Diagnostic/therapeutic procedures[80,81] | Endoscopy |
| Enema/colon idrotherapy | |
| Barium studies | |
| Connective tissue disease/neurological[82,83] | Scleroderma |
| Multiple sclerosis | |
| Hirschsprung disease | |
| Quadriplegia | |
| Amyloidosis | |
| Other conditions[17,84] | Hemodialysis |
| Pseudo-obstruction | |
| Whipple disease | |
| Cytomegalovirus infection | |
| COVID-19 infection |
Because of its rarity, PI is not yet completely clear from a pathophysiological, diagnostic and therapeutic point of view. Although radiographic PI is relatively common, there is no validated clinical tool to guide surgical management. This narrative review aims to summarize the existing evidence to better understand how to manage patients with this condition.
The review of the literature was conducted with the following method. A search was conducted on Pubmed for all articles published up to September 2022 with the following terms: “Pneumatosis intestinalis” OR “Portomesenteric pneumatosis” OR “intestinal pneumatosis”. A total of 206 articles were detected.
After evaluation of the full text, only 20 manuscripts were included for the draft of this review according to their pertinence in regards of the main topics. Inclusion criteria take in type of publication, study setting, reported outcome and date to publication.
Exclusion criteria were clinical case report, studies focused on specific groups. In particular, excluding case report, some of the 186 articles were excluded for being age specific (i.e., pediatric patients), other for being focused on certain procedures or pathologies (e.g., post-endoscopic procedures, pneumatosis cystoides) or, furthermore, for being of different area of interest (e.g., articles focused just on imaging appearance).
The reference list of the articles evaluated in full text was screened for any other relevant article and those articles were evaluated according to the same criteria.
The pathogenesis of PI is still unclear and probably is a combination of different theories considering how many diseases can be associated with pneumatosis[7,23,24].
Three are the main theories about the gas origin within the intestinal wall. There is the “mechanical theory” that speculates an intraluminal origin of gas: It seems to be a combination of an increased intraluminal pressure and an increased gut permeability[25]. It is possible that mucosal disruption due to inflammation or ischemia can predispose to an increase of intestinal wall permeability with the formation of small cysts in which the gas is trapped[26,27].
The second theory hypothesizes that the source of the gas is the chest through the retroperitoneum from the alveolar rupture along vascular channels[25,28]. It is demonstrated for example in patients with asthma or bronchitis, in which alveolar air runs from the mediastinum descending to the mesenteric root and vessels[29].
The last theory is the “bacterial” one. It postulates that the gas produced from gas-producing bacteria can reach the intestinal wall if associated with mucosal injury. This theory was suggested from the evidence of the high hydrogen content of the cyst, that suggests a bacterial origin[30]. It seems that bacteria cause a higher hydrogen tension than the nitrogen tension in blood, causing an exit of hydrogen in the intraluminal compartment[25].
All these theories try to explain different aspects of a complex finding, related to several diseases and several clinical conditions from asymptomatic to fatal. It is probably due to this complexity that it is a challenge for the surgeon to predict the severity of PI and the need for surgery[31-34].
Usually, PI was considered as a predictive sign of bowel ischemia, but with the improvement of the imaging techniques and its wider use, it was found also in asymptomatic patients[35,36]. For that reason, different studies tried to find a correlation between clinical findings, laboratory data and imaging, in order to distinguish between PI that needs surgery from PI that doesn’t have any clinical significance[37,38].
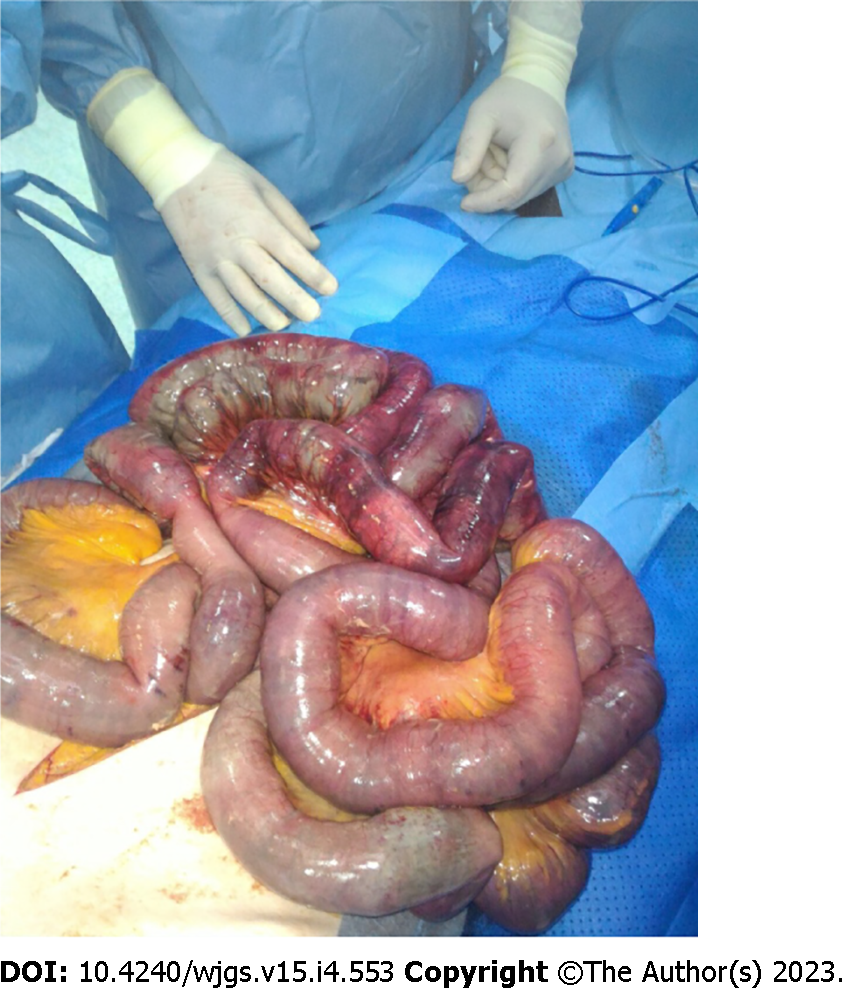
Hemodynamic instability, hypotension, sepsis, abdominal rigidity or peritonism, adynamic ileus are associated with pathological PI. These signs and symptoms are directly related to transmural intestinal infarction (Figure 1); these patients need to be evaluated from a surgeon and often need a surgical exploration[39]. The surgical challenge is the patient that is hemodynamically stable, with or without abdominal pain but not peritonitis, in which it is more difficult to decide how to proceed[37].
The more common symptoms in patients with PI associated with bowel vascular impairment are abdominal pain, weight loss, constipation or diarrhea, less frequently bleeding or ileus[40]. Despite the clinical presentation, it seems that the severity of symptoms is not correlated with the severity of the amount of intramural gas at the computed tomography (CT) scan[41,42]. It is more reasonable to believe that the clinical manifestation of PI is related to the underlying diseases[25].
Several studies tried to identify some laboratory values that could help among the management strategies. Morris et al[10] found that pH values are higher in patients treated successfully conservatively than in patients that underwent to surgery as well as lactate are lower in the non-operative group than in the operative one. Moreover, Ferrada et al[39] found that lactate, creatinine, blood urea nitrogen (BUN), potassium and white blood cells (WBC) are higher in patients with pathologic PI (underlying bowel ischemia/infarction) than in benign PI (self-limiting cause which not requires surgical intervention). On the contrary, hemoglobin, hematocrit and bicarbonate are lower in patients with pathologic PI. Treyaud et al[43] analyzed many laboratory tests, finding that only WBC correlate significatively with an underlying bowel ischemia.
Laboratory tests can also correlate with clinical outcome. Among these studies, Bani Hani et al[44] demonstrate that high lactate, low arterial CO2, low serum albumin and BUN are correlated with a worst outcome in patients with PI and in particular BUN is the most strongly associated. Also, Horowitz et al[45] tried to understand which laboratory test can predict the outcome of these patients. They found out that low bicarbonate levels (< 20 mmol/L), low pH (< 7.35) and lymphopenia (< 2.000/L) correlate with poor outcome. Although almost each laboratory test has been investigated in different studies, for some studies peritonitis and clinical exam remain the strongest predictors of outcome[39,44].
PI can be considered as a manifestation of a pathologic condition. It is not possible to discriminate the presence of PI on the basis of physical examination nor by the presence of a particular symptom. Diagnosis is typically radiological, and it is based on finding linear or circular collections of gas in the bowel wall. CT scan is the gold standard for establishing the presence of PI along with, in some cases, the associated pathological conditions[46-48]. According to some studies, radiographic location seems also to have a clinical relevance since small bowel PI has a higher incidence of transmural ischemia than PI at colonic locations[39].
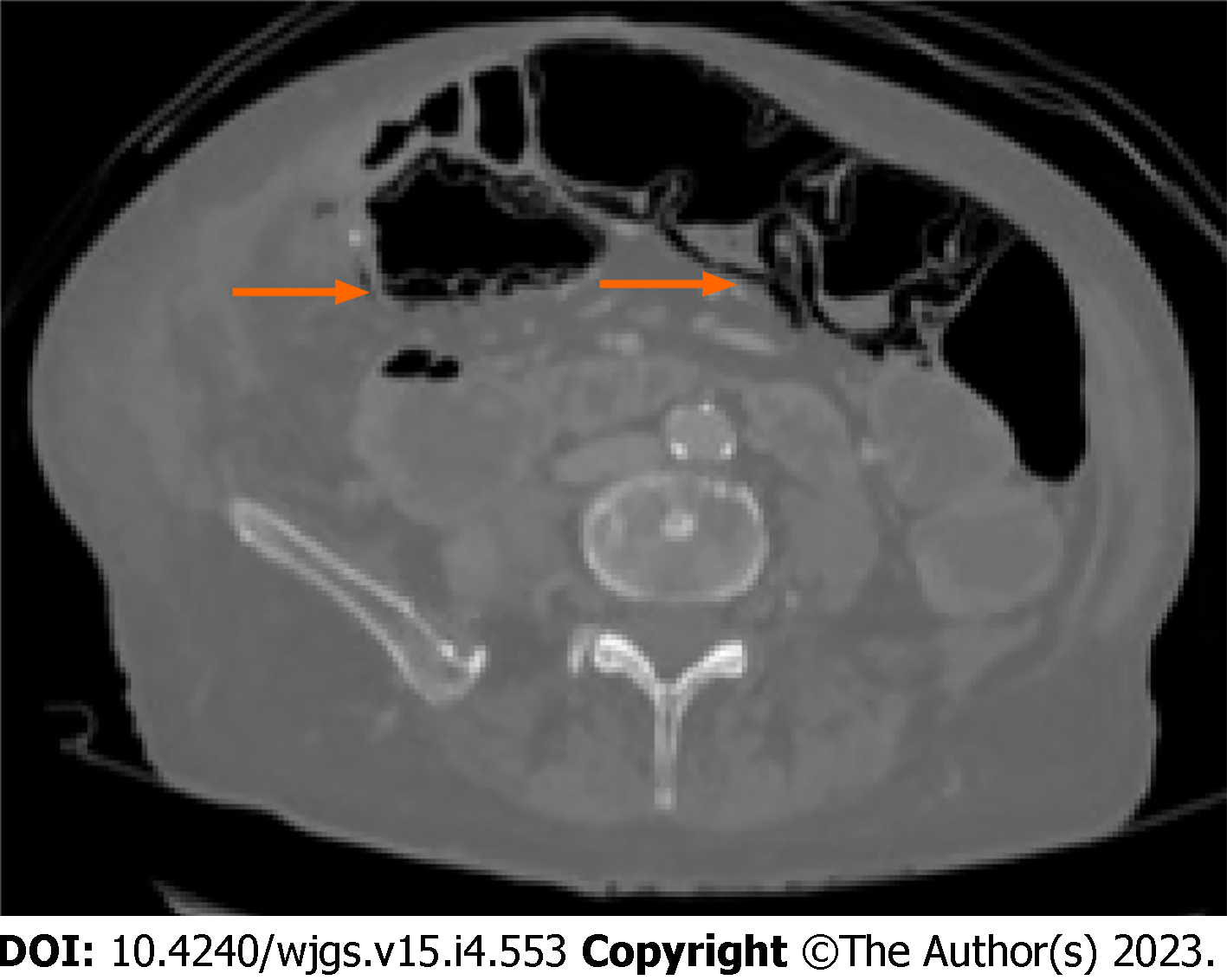
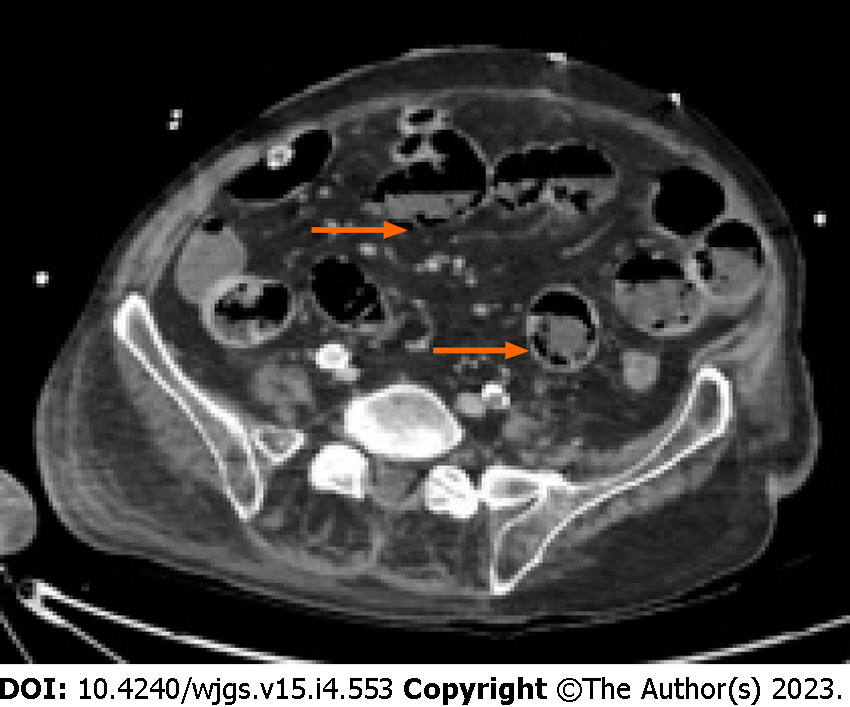
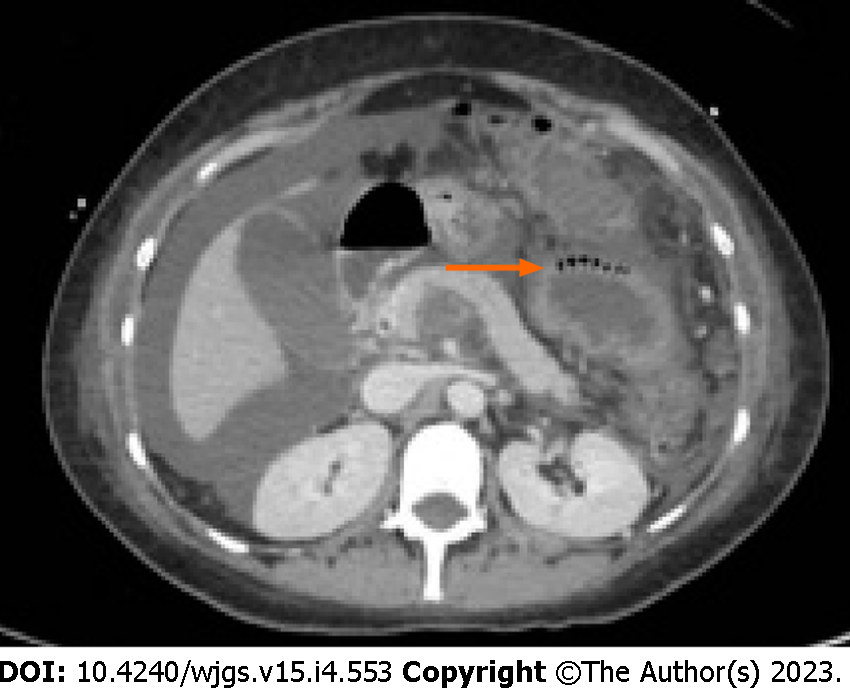
Moreover, according to some studies, also the radiological pattern of bubbles seems to be related to different underlying diseases. It is possible to recognize three different patterns: Cystoid or bubble-like pattern (Figure 2), in which gas looks like several cysts along the bowel wall and it is characteristic of the idiopathic PI; a linear pattern (Figure 3), in which gas has a curvilinear shape along the bowel and usually it is more associated with transmural infarction than the previous one; the circumferential pattern (Figure 4), in which gas appears circular along the bowel wall[49,50].
Conversely, Bani Hani et al[44] found that all the radiological distinctions between cystic or bubbly vs linear or curvilinear types of PI and the presence or absence of mesenteric stranding and thickening of bowel wall are not predictive of bowel ischemia. A recent machine learning model suggests that combined radiographic and clinical features can identify pathologic PI and aid in patient selection for surgery[37].
PI is not pathognomonic of bowel ischemia but should be a sign suspicious for alteration of the bowel vascularization. In this perspective, the treatment of PI should be guided by the underlying disease and the clinical conditions and not by the CT findings[51].
For what concerns the PI management, there should be a huge difference between symptomatic and asymptomatic patients. It is already known that PI is detectable in complete asymptomatic patients and CT scan alone cannot predict which patient will experience true intestinal ischemia[10]. Indeed, it is rare, but still possible, to find signs of PI in the CT scans of patients with mixed connective tissue diseases or bone marrow transplant, without any kind of clinical significance and in which conservative treatment with intestinal rest and antibiotics was successful[52,53]. Shinagare et al[54] reported a correlation between molecular targeted therapy (Bevacizumab, Sunitinib, Erlotinib, Cetuximab, Sorafenib, Ipilimumab) and CT scan findings of PI with no clinical significance. Other clinical conditions associated with “benign” PI are bowel infections or inflammations, neoplastic bowel wall damage, ulceration, overdistension and previous gastrointestinal surgery[24,55-57].
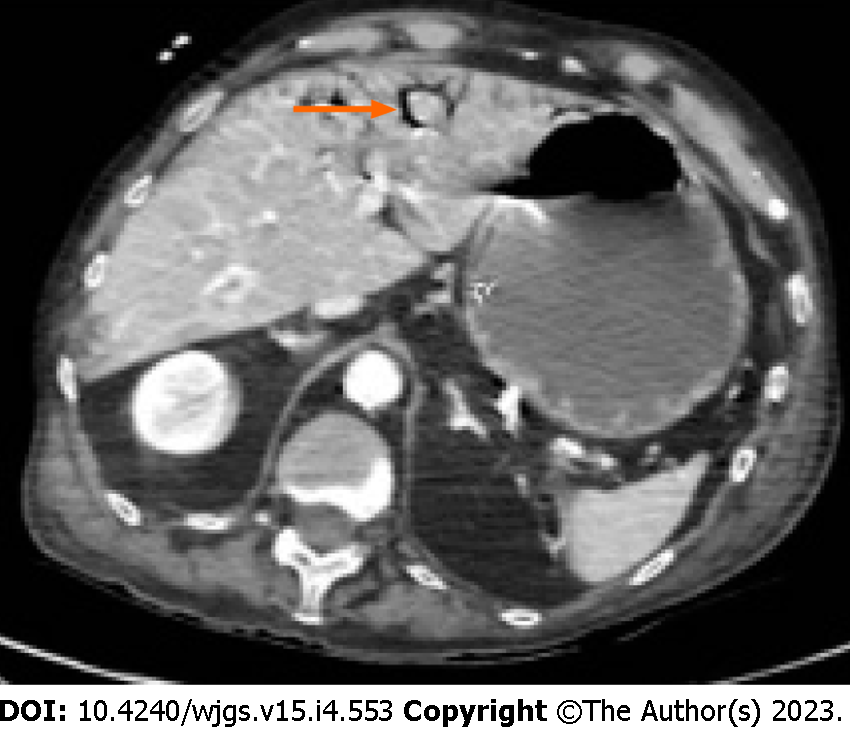
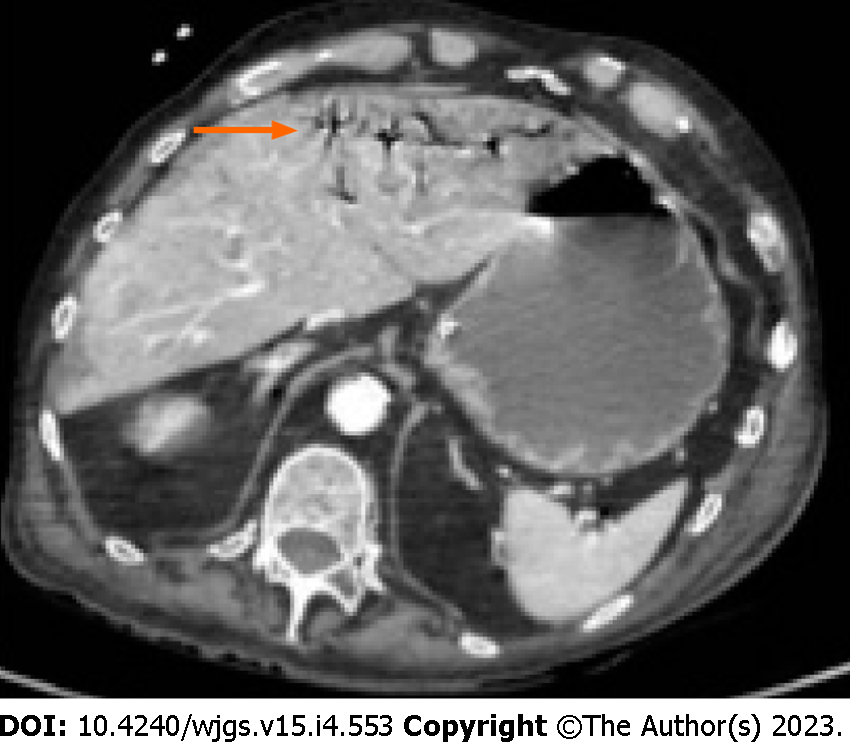
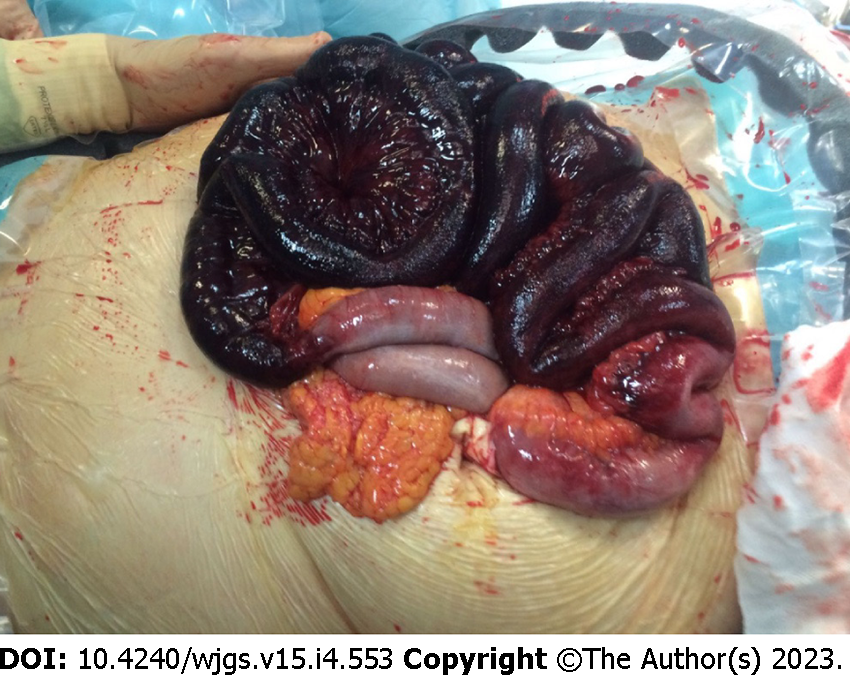
Something that can help the surgeon in the decision-making process is the presence/absence of pneumatosis portalis. Pneumatosis portalis can be localized (Figure 5) or spread to multiple portal vessels (Figure 6). According to Knechtle et al[3], the presence of portomesenteric pneumatosis (PMP) is associated with a 37% of mortality. Usually, it is an ominous prognostic sign, due to a large amount of gas that migrate from the bowel wall to the veins, and it correlates with an advanced stage of PI and ischemia[50]. Although over the years the significance of PMP was questioned several times, there are many studies that underling the relation between PMP and outcome[58-60]. Wiesner at al[55] noticed that PMP was pathognomonic of transmural infarction in the 81% of patients and if PI and PMP were detected simultaneously in the same CT-scan, patient has the 91% of possibilities to have transmural bowel ischemia. Moreover, also Lassandro group[50] found a correlation between the PMP and the transmural ischemia, observing that the 91.5% of patients with PMP at the CT scan had also a proven bowel ischemia/infarction during surgery (Figure 7).
Summarizing, the management of peritonitic patients, with high lactate or low pH, and with PMP at the CT scan can be clear but it is still very hard to determine how to manage an asymptomatic patient with suspicious linear gas balls in the bowel wall. The results of the main clinical studies are shown in Table 2.
| Author | Type of study | Patients, n | Results |
| Ferrada et al[39] | Prospective Multicenter | One hundred twenty-seven patients with PI at CT scan | Mortality in the pathologic PI group vs benign PI group: 34% vs 13.9%. Patients with pathologic PI had hemodynamic instability, sepsis, peritonitis. The radiographic location is significant: Small bowel has a higher incidence of transmural ischemia than colon. Hepatic portal venous gas is suggestive for pathologic PI |
| Treyaud et al[43] | Retrospective Monocenter | One hundred eighty-seven patients with pi at CT scan | Location of PI nor the length of intestinal involvement correlate significantly with ischemia. The radiologic features that correlate with ischemia are PMP (P =0.009) and the decreased mural contrast-enhancement (P < 0.001). Among the laboratory tests, only WBC (> 12.000/mmc) correlates with bowel ischemia (P =0.03) |
| Morris et al[10] | Retrospective Monocenter | One hundred four patients with PI at CT scan | Mortality rate: 22%; 52% of patients were treated conservatively, with a mortality rate of 6%. Mortality rate of patients with PMP was 43%. No difference found in laboratory values between groups |
| Lassandro et al[49] | Retrospective Monocenter | One hundred two patients with PI at CT scan | Fifty-two percent of patients had surgical confirmation of bowel ischemia. 42.2% of patients had a bubblelike whereas in 59% it was linear. 75.5% of patients with linear pattern had bowel infarction. Mortality rate is 30.4%; it raises to 50% when PI is associated to PMP |
| Pickhardt et al[85] | Retrospective Monocenter | Five thousand three hundred sixty-eight Colonography scans, 0.11% with colonic PI | PI with curvilinear configuration. No clear if it was a pre-existing condition. No significant complications |
| Kernagis et al[48] | Retrospective Monocenter | Fifteen patients with PI at CT scan | Nine patients (60%) of symptomatic patients had transmural bowel infarction (4 small bowel, 5 colon) |
| Wiesner et al[55] | Retrospective Monocenter | Twenty-three patients with PI or PMP at CT scan and bowel ischemia | Twenty-two percent of patients showed partial mural bowel infarction, 78% of patients showed transmural bowel infarction. 70% of bubblelike PI was associated with bowel ischemia instead of the 88% of linear pattern. 81% of patients with PMP showed transmural infarction. Overall mortality 53% |
| Shinagare et al[54] | Retrospective Monocenter | Forty-eight patients with cancer and PI at CT scan | Thirty-nine patients were receiving molecular targeted therapy. Bevacizumab and Sunitinib were the most common drugs associated with PI. Median duration of molecular targeted therapy before PI or perforation was 3 mo. Asymptomatic patients 70.8%. Conservative PI treatment 100% |
| Huzar et al[9] | Retrospective Monocenter | One thousand one hundred twenty-nine patients admitted to Burn ICU | PI at CT scan 1.3%. Mortality rate of patients with PI was 73%. Explorative laparotomy in 2-3 h from the CT scan in 94% of the patients. PI involved both small bowel and colon 60%. Nonsurvivors had greater base deficit (P = 0.03), open abdomen after surgery (P = 0.004) |
| Horowitz et al[45] | Retrospective Monocenter | Twenty-eight gynecological cancer patients and PI at CT scan | Patients symptomatic for abdominal pain 80%. Patients that did poorer were patients with preoperative acidosis, lower level of bicarbonate and lymphopenia |
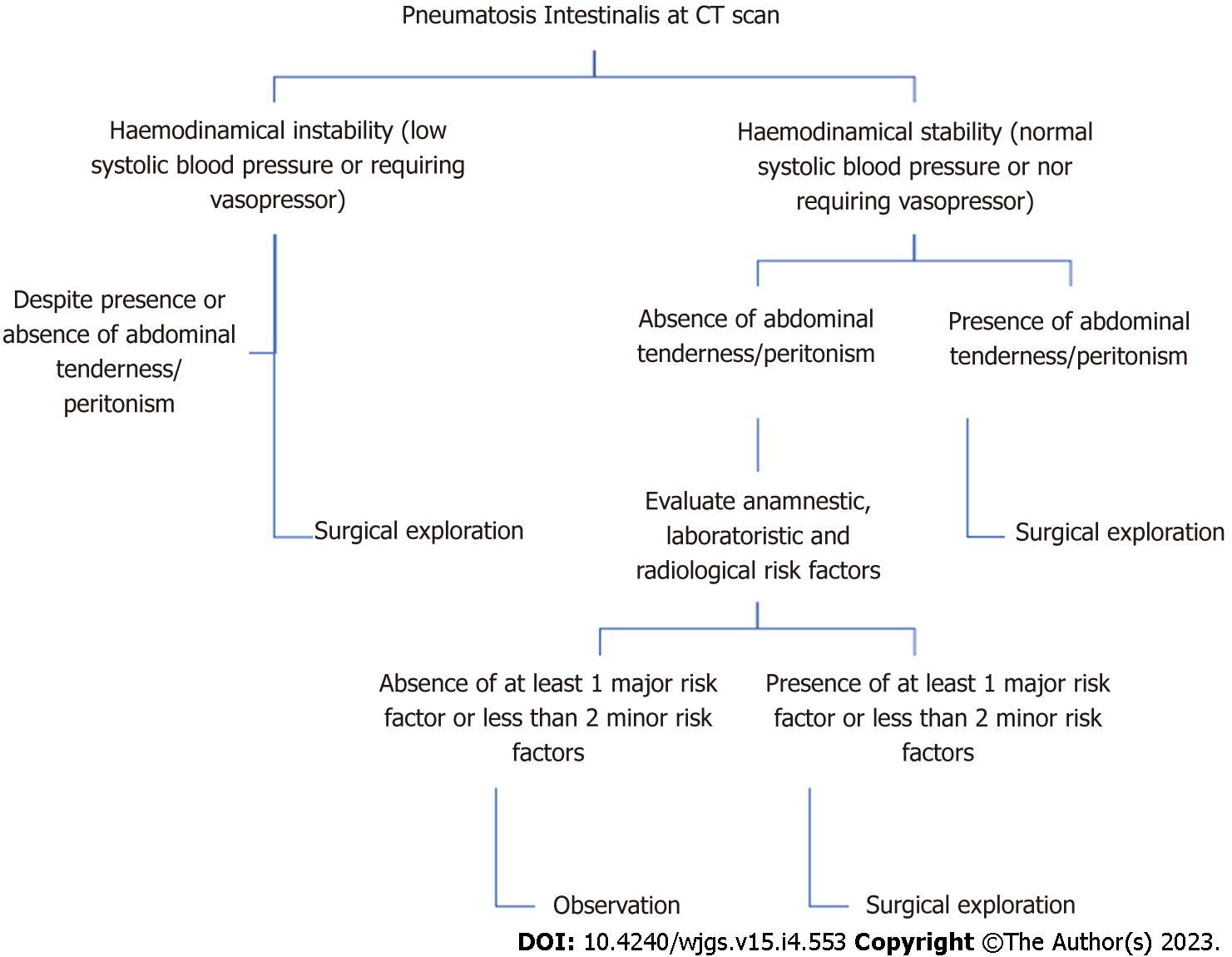
Considering the high complexity of this topic, we tried to formulate an algorithm in order to guide the surgeon in his decisional process (Figure 8). Analyzing data available in literature and data based on our experience, we selected some risk factors correlated with the presence of bowel ischemia at surgical exploration. We were able to identify some anamnestic, laboratory and radiological risk factors synthesized in Table 3.
| Risk Factors | |
| Anamnestic | Vascular disease |
| Atrial fibrillation | |
| Major laboratory risk factors (blood sample) | Lac > 4 mmol/L |
| LDH > 400 UI/L | |
| pH < 7.31 | |
| BUN > 50 mg/dL | |
| Minor laboratory risk factor (blood sample) | WBC > 15.000/L |
| Creatinine > 2 mg/dL | |
| HCO3- < 18 mmol/L | |
| Potassium 5.5 mmol/L | |
| Radiological | Portomesenteric pneumatosis |
| Pneumoperitoneum | |
| Free peritoneal fluid |
Laboratory parameters were then divided in major and minor risk factors. We wrote down a study protocol formulating an algorithm in order to help the surgeon decide if to undertake an operative or non-operative treatment. Patients are being enrolled treating them according to our algorithm (Figure 8).
In case of PI at the CT scan, distinction between hemodynamically stable or unstable patients is crucial. In case of instability surgical exploration is mandatory. In case of stability, clinical presentation plays a central role, considering as symptomatic the presence of abdominal tenderness or peritonism. If the patient is symptomatic, operative treatment is advocated. Otherwise, we rely on some anamnestic, laboratory and radiological parameters considered as risk factors (Table 3). We decided to surgically treat asymptomatic patients if the following scenario is present. At least one anamnestic and radiological risk factor plus at least one major risk factor or two minor risk factors.
Taking into account all the possible causes and outcomes, PI represents a radiological finding which has to be correctly figured out in order to pursue the right management. It is crucial to identify the underlying condition in order to discriminate between patients who are at risk of transmural infarction from those with whom this condition could be managed without surgery[36,61]. Integration between clinical presentation, laboratory tests and abnormal abdominal physical examination can give hints about the pathway to follow. The aim is to promptly treat PI on vascular basis to avoid necrosis progression and to abstain from unnecessary and potentially harmful laparotomy/laparoscopy[32,62,63]. With this narrative review we tried to give a comprehensive analysis of the knowledge of this topic proposing an algorithm to guide clinical decisions. This manuscript has some limitations. Only one of the studies included was prospective (all the other were retrospective). The algorithm proposed, even if based on guidelines concerning various conditions in the setting of emergency care, should be validated by a prospective study.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C; C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hirosawa T, Japan; Yu F, China S-Editor: Li L L-Editor: A P-Editor: Li L
| 1. | Kaya B, Celik K, Karip AB, Altun H, Ozbay Özel N, Bat O, Memişoğlu K. Pneumatosis cystoides intestinalis mimicking acute abdomen. Turk J Gastroenterol. 2014;25:426-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Maltz C. Benign pneumoperitoneum and pneumatosis intestinalis. Am J Emerg Med. 2001;19:242-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Knechtle SJ, Davidoff AM, Rice RP. Pneumatosis intestinalis. Surgical management and clinical outcome. Ann Surg. 1990;212:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 154] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 4. | Anne N, Rajput A, Dunn KB, Litwin A. Idiopathic pneumatosis intestinalis of the small intestine. Am Surg. 2008;74:1127-1129. [PubMed] |
| 5. | Amrein K, Högenauer C, Spreizer C, Spuller E, Langner C. Pneumatosis coli--an underrecognized lesion mimicking neoplastic disease. Wien Klin Wochenschr. 2011;123:515-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Schattner A, Glick Y. Gastric pneumatosis and its varied pathogenesis. QJM. 2020;113:747-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Khalil PN, Huber-Wagner S, Ladurner R, Kleespies A, Siebeck M, Mutschler W, Hallfeldt K, Kanz KG. Natural history, clinical pattern, and surgical considerations of pneumatosis intestinalis. Eur J Med Res. 2009;14:231-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Gui X, Zhou Y, Eidus L, Falck V, Gao ZH, Qin L. Is pneumatosis cystoides intestinalis gas-distended and ruptured lymphatics? Arch Pathol Lab Med. 2014;138:1059-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Huzar TF, Oh J, Renz EM, Wolf SE, King BT, Chung KK, White CE, Malin E, Lundy JB, Kim SH, Blackbourne LH, Cancio LC. Pneumatosis intestinalis in patients with severe thermal injury. J Burn Care Res. 2011;32:e37-e44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Morris MS, Gee AC, Cho SD, Limbaugh K, Underwood S, Ham B, Schreiber MA. Management and outcome of pneumatosis intestinalis. Am J Surg. 2008;195:679-82; discussion 682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 11. | Lim CX, Tan WJ, Goh BK. Benign pneumatosis intestinalis. Clin Gastroenterol Hepatol. 2014;12:xxv-xxvi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Kelly GS, Grandy B, Rice J. Diffuse Pneumatosis Coli. J Emerg Med. 2018;54:e137-e139. [PubMed] [DOI] [Full Text] |
| 13. | Yang L, Zhong X, Yang H, Wu Q, Gong Y, Wang B. Pneumatosis cystoides intestinalis associated with etoposide in hematological malignancies: a case report and a literature review. BMC Gastroenterol. 2022;22:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Gao Y, Uffenheimer M, Ashamallah M, Grimaldi G, Swaminath A, Sultan K. Presentation and outcomes among inflammatory bowel disease patients with concurrent pneumatosis intestinalis: a case series and systematic review. Intest Res. 2020;18:289-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Bilici A, Karadag B, Doventas A, Seker M. Gastric pneumatosis intestinalis associated with malignancy: an unusual case report. World J Gastroenterol. 2009;15:758-760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Meini S, Zini C, Passaleva MT, Frullini A, Fusco F, Carpi R, Piani F. Pneumatosis intestinalis in COVID-19. BMJ Open Gastroenterol. 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Balasuriya HD, Abeysinghe J, Cocco N. Portal venous gas and pneumatosis coli in severe cytomegalovirus colitis. ANZ J Surg. 2018;88:113-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Wong K, Kim DH, Khanijo S, Melamud A, Zaidi G. Pneumatosis Intestinalis in COVID-19: Case Series. Cureus. 2020;12:e10991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Goh SSN, Shelat V. Prednisolone induced pneumatosis coli and pneumoperitoneum. World J Gastroenterol. 2022;28:3739-3742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Hsueh KC, Tsou SS, Tan KT. Pneumatosis intestinalis and pneumoperitoneum on computed tomography: Beware of non-therapeutic laparotomy. World J Gastrointest Surg. 2011;3:86-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Shah A, Al Furajii H, Cahill RA. Symptomatic pneumatosis intestinalis (including portal venous gas) after laparoscopic total colectomy. World J Gastrointest Endosc. 2014;6:564-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 22. | Arikanoglu Z, Aygen E, Camci C, Akbulut S, Basbug M, Dogru O, Cetinkaya Z, Kirkil C. Pneumatosis cystoides intestinalis: a single center experience. World J Gastroenterol. 2012;18:453-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Gagliardi G, Thompson IW, Hershman MJ, Forbes A, Hawley PR, Talbot IC. Pneumatosis coli: a proposed pathogenesis based on study of 25 cases and review of the literature. Int J Colorectal Dis. 1996;11:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 79] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Gazzaniga G, Villa F, Tosi F, Pizzutilo EG, Colla S, D'Onghia S, Di Sanza G, Fornasier G, Gringeri M, Lucatelli MV, Mosini G, Pani A, Siena S, Scaglione F, Sartore-Bianchi A. Pneumatosis Intestinalis Induced by Anticancer Treatment: A Systematic Review. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | St Peter SD, Abbas MA, Kelly KA. The spectrum of pneumatosis intestinalis. Arch Surg. 2003;138:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 261] [Article Influence: 11.9] [Reference Citation Analysis (1)] |
| 26. | Feczko PJ, Mezwa DG, Farah MC, White BD. Clinical significance of pneumatosis of the bowel wall. Radiographics. 1992;12:1069-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 61] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Pieterse AS, Leong AS, Rowland R. The mucosal changes and pathogenesis of pneumatosis cystoides intestinalis. Hum Pathol. 1985;16:683-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Katada Y, Isogai J, Ina H, Tezuka M, Umehara I, Shibuya H. Potential extraperitoneal space continuous with the peri-intestinal space: CT evidence and anatomical evaluation in patients with pneumatosis intestinalis without intestinal ischemia. Surg Radiol Anat. 2009;31:707-713. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Tchabo NE, Grobmyer SR, Jarnagin WR, Chi DS. Conservative management of pneumatosis intestinalis. Gynecol Oncol. 2005;99:782-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Yale CE, Balish E. Pneumatosis cystoides intestinalis. Dis Colon Rectum. 1976;19:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Pasquier M, Waeber G. Non-surgical pneumoperitoneum. Emerg Med J. 2011;28:170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 32. | Pengermä P, Katunin J, Turunen A, Rouvelas I, Palomäki A, Kechagias A. Is surgical exploration mandatory in pneumatosis intestinalis with portomesenteric gas? ANZ J Surg. 2022;92:543-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Than VS, Nguyen MD, Gallon A, Pham MT, Nguyen DH, Boyer L, Le TD. Pneumatosis intestinalis with pneumoperitoneum: Not always a surgical emergency. Radiol Case Rep. 2020;15:2459-2463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Yuan A, Keogh C, Sandstrom A, Bricknell L, Chakraborty J, Siriwardhane M. Pneumatosis sinistralis. ANZ J Surg. 2022;92:252-254. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 35. | Atre ID, Eurboonyanun K, O'Shea A, Lahoud RM, Shih A, Kalva S, Harisinghani MG, Hedgire S. Predictors of transmural intestinal necrosis in patients presenting with acute mesenteric ischemia on computed tomography. Abdom Radiol (NY). 2022;47:1636-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Castater C, Gliga LA, Meyer C, Hazen B, Greene W, Fiza B. Successful Non-Operative Management of Extensive Pneumatosis Cystoides Intestinalis Due to Graft Versus Host Disease. Am Surg. 2022;88:1000-1002. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 37. | Clancy K, Dadashzadeh ER, Handzel R, Rieser C, Moses JB, Rosenblum L, Wu S. Machine learning for the prediction of pathologic pneumatosis intestinalis. Surgery. 2021;170:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Rieser CJ, Dadashzadeh ER, Handzel RM, Clancy KJ, Kaltenmeier CT, Moses JB, Forsythe RM, Wu S, Rosengart MR. Development and validation of a five-factor score for prediction of pathologic pneumatosis. J Trauma Acute Care Surg. 2021;90:477-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Ferrada P, Callcut R, Bauza G, O'Bosky KR, Luo-Owen X, Mansfield NJ, Inaba K, Pasley J, Bugaev N, Pereira B, Moore FO, Han J, Pasley A, DuBose J; AAST Multi-institutional Trials Committee. Pneumatosis Intestinalis Predictive Evaluation Study: A multicenter epidemiologic study of the American Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2017;82:451-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Braumann C, Menenakos C, Jacobi CA. Pneumatosis intestinalis--a pitfall for surgeons? Scand J Surg. 2005;94:47-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Adachi W, Matsushita T, Yashiro Y, Imura J, Shiozawa H, Kishimoto K. Clinical characteristics of pneumoperitoneum with pneumatosis intestinalis detected using computed tomography: A descriptive study. Medicine (Baltimore). 2020;99:e22461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Shetty DS, Naik LP, Amarapurkar AD. Bubbly bowel: A life-threatening condition. Indian J Pathol Microbiol. 2020;63:325-326. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 43. | Treyaud MO, Duran R, Zins M, Knebel JF, Meuli RA, Schmidt S. Clinical significance of pneumatosis intestinalis - correlation of MDCT-findings with treatment and outcome. Eur Radiol. 2017;27:70-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 44. | Bani Hani M, Kamangar F, Goldberg S, Greenspon J, Shah P, Volpe C, Turner DJ, Horton K, Fishman EK, Francis IR, Daly B, Cunningham SC. Pneumatosis and portal venous gas: do CT findings reassure? J Surg Res. 2013;185:581-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Horowitz NS, Cohn DE, Herzog TJ, Mutch DG, Rader JS, Bhalla S, Gibb RK. The significance of pneumatosis intestinalis or bowel perforation in patients with gynecologic malignancies. Gynecol Oncol. 2002;86:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 46. | Caudill JL, Rose BS. The role of computed tomography in the evaluation of pneumatosis intestinalis. J Clin Gastroenterol. 1987;9:223-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Pickhardt PJ, Kim DH, Taylor AJ. Asymptomatic pneumatosis at CT colonography: a benign self-limited imaging finding distinct from perforation. AJR Am J Roentgenol. 2008;190:W112-W117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Kernagis LY, Levine MS, Jacobs JE. Pneumatosis intestinalis in patients with ischemia: correlation of CT findings with viability of the bowel. AJR Am J Roentgenol. 2003;180:733-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 49. | Lassandro F, Mangoni de Santo Stefano ML, Porto AM, Grassi R, Scaglione M, Rotondo A. Intestinal pneumatosis in adults: diagnostic and prognostic value. Emerg Radiol. 2010;17:361-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Lassandro G, Picchi SG, Romano F, Sica G, Lieto R, Bocchini G, Guarino S, Lassandro F. Intestinal pneumatosis: differential diagnosis. Abdom Radiol (NY). 2022;47:1529-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Gomes AF, Fernandes S, Costa Gomes O, Coutinho J. Aeroportia and pneumatosis intestinalis: discrepancy between radiological and intraoperative findings. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 52. | van Leeuwen JC, Nossent JC. Pneumatosis intestinalis in mixed connective tissue disease. Neth J Med. 1992;40:299-304. [PubMed] |
| 53. | Cañellas CB, Irastorza CM, Olivé T, Montero AM, Burrieza GG, Gaethe JA, Rocal JL, Martínez-Ibáñez V. [Conservative treatment of pneumatosis intestinalis and pneumoperitoneum after bone marrow transplantation]. Cir Pediatr. 2008;21:219-222. [PubMed] |
| 54. | Shinagare AB, Howard SA, Krajewski KM, Zukotynski KA, Jagannathan JP, Ramaiya NH. Pneumatosis intestinalis and bowel perforation associated with molecular targeted therapy: an emerging problem and the role of radiologists in its management. AJR Am J Roentgenol. 2012;199:1259-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 55. | Wiesner W, Mortelé KJ, Glickman JN, Ji H, Ros PR. Pneumatosis intestinalis and portomesenteric venous gas in intestinal ischemia: correlation of CT findings with severity of ischemia and clinical outcome. AJR Am J Roentgenol. 2001;177:1319-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 208] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 56. | Keam B, Lee JH, Oh MD, Kim I, Yoon SS, Kim BK, Park S. Pneumatosis intestinalis with pneumoperitoneum mimicking intestinal perforation in a patient with myelodysplastic syndrome after hematopoietic stem cell transplantation. Korean J Intern Med. 2007;22:40-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 57. | Liu T, Zhang S, Mao H. Gastrointestinal malignant neoplasms disguised as pneumatosis cystoids intestinalis: A case report and literature review. Medicine (Baltimore). 2017;96:e9410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 58. | Arai M, Kim S, Ishii H, Takiguchi T, Yokota H. Portal Venous Gas in Adults: Clinical Significance, Management, and Outcomes of 25 Consecutive Patients. J Nippon Med Sch. 2021;88:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 59. | Aslam F, Apostolopoulos A, Zeeshan S. Pneumatosis intestinalis with extensive intrahepatic portal venous gas secondary to intra-abdominal sepsis: a rare occurrence. BMJ Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 60. | Gonda M, Osuga T, Ikura Y, Hasegawa K, Kawasaki K, Nakashima T. Optimal treatment strategies for hepatic portal venous gas: A retrospective assessment. World J Gastroenterol. 2020;26:1628-1637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 61. | Duron VP, Rutigliano S, Machan JT, Dupuy DE, Mazzaglia PJ. Computed tomographic diagnosis of pneumatosis intestinalis: clinical measures predictive of the need for surgical intervention. Arch Surg. 2011;146:506-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 62. | Hoover EL, Cole GD, Mitchell LS, Adams CZ Jr, Hassett J. Avoiding laparotomy in nonsurgical pneumoperitoneum. Am J Surg. 1992;164:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 63. | Al-Talib A, Al-Ghtani F, Munk R. Pneumatosis Intestinalis: Can We Avoid Surgical Intervention in Nonsurgical Patients? Case Rep Gastroenterol. 2009;3:286-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 64. | Bisgaard E, Hewgley WP, Gee KM, Pandya S, Akarichi C, Arnoldo B, Park C. Gastric Pneumatosis in a Critically Ill Pediatric Burn Patient: Case Report and Overview of Risk Factors, Diagnosis, and Management. J Burn Care Res. 2021;42:342-344. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 65. | Furuya Y, Yasuhara H, Ariki K, Yanagie H, Naka S, Nojiri T, Shinkawa H, Niwa H, Nagao T. Hepatic portal venous gas caused by blunt abdominal trauma: is it a true ominous sign of bowel necrosis? Surg Today. 2002;32:655-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 66. | Kelly BS Jr, Meyers P, Choe KA, Hurst J, Luchette FA. Traumatic pneumatosis cystoides intestinalis with portal venous air embolism. J Trauma. 1997;42:112-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 67. | Kim CT, Kim H, Wechsler B, Kim SW. Pneumatosis intestinalis (PI) following severe traumatic brain injury. Brain Inj. 2005;19:1059-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 68. | Ağaoğlu N. Pneumatosis cystoides intestinalis associated with perforated chronic duodenal ulcer and Meckel's diverticulum. Acta Chir Belg. 2005;105:415-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 69. | al-Hakeem MS, McMillen MA. Evaluation of abdominal pain in systemic lupus erythematosus. Am J Surg. 1998;176:291-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 70. | Alcocer-Gouyonnet F, Chan-Nuñez C, Hernández J, Guzmán J, Gamboa-Domínguez A. Acute abdomen and lupus enteritis: thrombocytopenia and pneumatosis intestinalis as indicators for surgery. Am Surg. 2000;66:193-195. [PubMed] |
| 71. | Dietrich CF, Hollerweger A, Dirks K, Higginson A, Serra C, Calabrese E, Dong Y, Hausken T, Maconi G, Mihmanli I, Nürnberg D, Nylund K, Pallotta N, Ripollés T, Romanini L, Săftoiu A, Sporea I, Wüstner M, Maaser C, Gilja OH. EFSUMB Gastrointestinal Ultrasound (GIUS) Task Force Group: Celiac sprue and other rare gastrointestinal diseases ultrasound features. Med Ultrason. 2019;21:299-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 72. | Bareggi E, Tonolini M, Ardizzone S. Pneumatosis intestinalis and perforation in Crohn's disease: worrisome or not? J Crohns Colitis. 2014;8:338-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 73. | Calame P, Malakhia A, Turco C, Grillet F, Piton G, Delabrousse E. Transmural Bowel Necrosis From Acute Mesenteric Ischemia and Strangulated Small-Bowel Obstruction: Distinctive CT Features. AJR Am J Roentgenol. 2020;214:90-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 74. | Choi JY, Cho SB, Kim HH, Lee IH, Lee HY, Kang HS, Lee SY. Pneumatosis intestinalis complicated by pneumoperitoneum in a patient with asthma. Tuberc Respir Dis (Seoul). 2014;77:219-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 75. | Lavelle LP, McEvoy SH, Ni Mhurchu E, Gibney RG, McMahon CJ, Heffernan EJ, Malone DE. Cystic Fibrosis below the Diaphragm: Abdominal Findings in Adult Patients. Radiographics. 2015;35:680-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 76. | Brocchi S, Parmeggiani A, Gaudiano C, Balacchi C, Renzulli M, Brandi N, Dall'Olio FG, Rihawi K, Ardizzoni A, Golfieri R. Pneumatosis intestinalis and spontaneous perforation associated with drug toxicity in oncologic patients: a case series. Acta Gastroenterol Belg. 2021;84:497-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 77. | Thein SL, Asquith P. Pneumatosis coli: complication of practolol. Br Med J. 1977;1:268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 78. | Fujimi A, Sakamoto H, Kanisawa Y, Minami S, Nagamachi Y, Yamauchi N, Ibata S, Kato J. Pneumatosis intestinalis during chemotherapy with nilotinib in a patient with chronic myeloid leukemia who tested positive for anti-topoisomerase I antibodies. Clin J Gastroenterol. 2016;9:358-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 79. | Liu H, Hsieh CT, Sun JM. Pneumatosis intestinalis after systemic chemotherapy for colorectal cancer: A case report. World J Clin Cases. 2022;10:5337-5342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 80. | Bilreiro C, Brito J. Endoscopy Induced Gastric Pneumatosis. Acta Med Port. 2017;30:252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 81. | Cho KC, Simmons MZ, Baker SR, Cappell MS. Spontaneous dissection of air into the transverse mesocolon during double-contrast barium enema. Gastrointest Radiol. 1990;15:76-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 82. | Chang CY, Marzan KA. Benign pneumatosis intestinalis in a pediatric patient with multiple risk factors including granulomatosis with polyangiitis: a case report and review of the literature. Semin Arthritis Rheum. 2015;44:423-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 83. | Duan G, Qi M, Guo Q, Song Z. Primary amyloidosis involving the gastrointestinal tract, mesentery and omentum: A case report. Exp Ther Med. 2021;22:1145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 84. | Burkett AE, Sher SB, Patel CR, Ildin-Eltoum I, Dhall D, Margaroli C, Peter S, Lee G, Bajpai P, Benson PV, Manne U, Al Diffalha S. Gastrointestinal Manifestations of COVID-19 Infection: Clinicopathologic Findings in Intestinal Resections Performed at Single Institution. Front Med (Lausanne). 2022;9:811546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 85. | Pickhardt PJ, Kim DH, Menias CO, Gopal DV, Arluk GM, Heise CP. Evaluation of submucosal lesions of the large intestine: part 2. Nonneoplastic causes. Radiographics. 2007;27:1693-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |