Published online Feb 27, 2023. doi: 10.4240/wjgs.v15.i2.249
Peer-review started: July 19, 2022
First decision: August 19, 2022
Revised: September 1, 2022
Accepted: November 28, 2022
Article in press: November 28, 2022
Published online: February 27, 2023
Processing time: 222 Days and 18.1 Hours
Post-hepatectomy liver failure (PHLF) is one of the main causes of postoperative mortality and is challenging to predict early in patients after liver resection. Some studies suggest that the postoperative serum phosphorus might predict outcomes in these patients.
To perform a systematic literature review on hypophosphatemia and evaluate it as a prognostic factor for PHLF and overall morbidity.
This systematic review was performed according to preferred reporting items for systematic reviews and meta-analyses statement. A study protocol for the review was registered in the International Prospective Register of Systematic Reviews database. PubMed, Cochrane and Lippincott Williams & Wilkins databases were systematically searched up to March 31, 2022 for studies analyzing postoperative hypophosphatemia as a prognostic factor for PHLF, overall postoperative morbidity and liver regeneration. The quality assessment of the included cohort studies was performed according to the Newcastle-Ottawa Scale.
After final assessment, nine studies (eight retrospective and one prospective cohort study) with 1677 patients were included in the systematic review. All selected studies scored ≥ 6 points according to the Newcastle-Ottawa Scale. Cutoff values of hypophosphatemia varied from < 1 mg/dL to ≤ 2.5 mg/dL in selected studies with ≤ 2.5 mg/dL being the most used defining value. Five studies analyzed PHLF, while the remaining four analyzed overall complications as a main outcome associated with hypophosphatemia. Only two of the selected studies analyzed postoperative liver regeneration, with reported better postoperative liver regeneration in cases of postoperative hypophosphatemia. In three studies hypophosphatemia was associated with better postoperative outcomes, while six studies revealed hypophosphatemia as a predictive factor for worse patient outcomes.
Changes of the postoperative serum phosphorus level might be useful for predicting outcomes after liver resection. However, routine measurement of perioperative serum phosphorus levels remains questionable and should be evaluated individually.
Core Tip: A systematic literature review on hypophosphatemia and its value as a prognostic factor for post-hepatectomy liver failure and overall morbidity after liver surgery was performed. In three of nine included studies hypophosphatemia was associated with better postoperative outcomes, while six studies revealed hypophosphatemia as a predictive factor for worse patient outcomes. Data show that postoperative hypophosphatemia and changes of postoperative serum phosphorus might be used as a predictor after liver surgery. However, to be implemented in clinical practice as routine measurement more studies and data are needed.
- Citation: Riauka R, Ignatavicius P, Barauskas G. Hypophosphatemia as a prognostic tool for post-hepatectomy liver failure: A systematic review. World J Gastrointest Surg 2023; 15(2): 249-257
- URL: https://www.wjgnet.com/1948-9366/full/v15/i2/249.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i2.249
Post-hepatectomy liver failure (PHLF) is a severe and lethal complication occurring in patients after partial liver resection and defined by derangement of liver function, coagulopathy, high lactate and encephalopathy[1]. Incidence of PHLF varies from 1% to 32% with reported perioperative mortality up to 60%[2,3]. PHLF is a significant complication and one of the main causes of mortality in patients after liver resection even with advancing surgical techniques and perioperative management[4,5]. A wide variety of preoperative predictive factors, such as patient-related (male sex, older age, obesity), hepatic parenchyma-related (cirrhosis, fibrosis, steatosis, preoperative chemotherapy, cholestasis), surgery-related (high blood loss, extended liver resection, prolonged inflow occlusion and operating time) and postoperative course-related (hemorrhage, infections), might contribute to PHLF[4,6,7]. Despite various scoring systems for standardizing PHLF and predicting postoperative morbidity and mortality, early identification of patients, who will develop PHLF remains challenging[8].
Regenerative potential of hepatocytes and the compensatory capacity of the functional liver remnant allow resection of up to 80% of the healthy liver[9,10]. Patients at risk of PHLF do not exhibit a normal regenerative response and may present with early disorders in normal metabolic responses, such as failure to utilize serum phosphorus postoperatively[11,12]. Consequently, absence of a decrease in postoperative serum phosphorus might be an early predictive factor of PHLF. Organic phosphate is part of several important biological processes such as signal transduction, energy transfer and formation of high-energy bonds and is critical to multiple metabolic processes[13]. Blood phosphate levels are regulated by various organs such as bone, parathyroid glands, small intestine, kidneys and liver, thus the pathophysiology of postoperative hypophosphatemia is multifactorial[14,15].
According to Woodard et al[16] liver tissue contains approximately 0.3% phosphorus by weight. Low serum phosphate levels after major liver resection might be associated with removal of liver mass containing phosphorus, resulting in blood phosphate movement into the hepatocytes and, subsequently, better liver regeneration[17,18]. Some studies suggested that postoperative serum hypophosphatemia might predict better outcomes in patients with acute liver failure[19,20]. Absence of hypophosphatemia after major liver resections might help identify patients with an increased chance of PHLF.
Several studies present contradictory results. Postoperative hypophosphatemia was reported to be associated with a higher risk of postoperative complications, thus refuting the previous statements[21-23]. It is hypothesized that liver regeneration after major hepatectomy results in serum phosphorus replenishing intracellular phosphorus levels needed for ATP synthesis and further regeneration processes. Therefore, low serum phosphorus levels impair liver regeneration, resulting in liver dysfunction and failure[24,25]. Therefore, data on postoperative hypophosphatemia as a prognostic factor for PHLF are yet to be systemically analyzed. Our aim was to perform a systematic literature review of hypophosphatemia as a prognostic tool for PHLF and overall morbidity.
This systematic review was performed according to the preferred reporting items for systematic reviews and meta-analyses statement[26]. A study protocol for the review was registered in the International Prospective Register of Systematic Reviews database: CRD42020197717.
PubMed, Cochrane, Lippincott Williams & Wilkins and Reference Citation Analysis databases were searched up to March 31, 2022. Our search terms included: (hypophosphatemia OR phosphorus) AND (hepatectomy OR liver resection) AND (post-operative hepatic insufficiency OR mortality OR complications OR liver failure OR liver insufficiency). After checking titles and abstracts, inappropriate studies were excluded. The remaining full-text articles were reviewed carefully. Additionally, reference lists of selected articles were reviewed for eligible studies.
Inclusion criteria for selected studies were: (1) Studies written in English language; and (2) Studies analyzing postoperative hypophosphatemia as a prognostic factor for PHLF, overall postoperative morbidity and liver regeneration (patients after different types of liver resections, including living-donor liver donation). Exclusion criteria were as follows: (1) Abstracts, case reports, editorials, letters, systematic reviews and meta-analyses; (2) Studies with incomplete data for further analysis (studies with no reported postoperative complications or phosphorus, studies analyzing postoperative hypophosphatemia in liver transplant recipients); (3) Duplicate studies; and (4) Studies in languages other than English.
Selected studies were evaluated by two investigators independently, and necessary data was extracted including name of the first author, year of publication, type of study, number of patients included in the study, study population (type of surgery performed), postoperative phosphorus, main and secondary outcomes (PHLF, overall postoperative morbidity and liver regeneration) and their correlation with postoperative hypophosphatemia. In cases of disagreement, differences in opinion were resolved by a third author.
The quality assessment of the included cohort studies was performed according to the Newcastle-Ottawa Scale[27]. Evaluation ranged from 0 to 9 points, and studies with a Newcastle-Ottawa Scale score of ≥ 6 were considered as high quality. Due to heterogeneity of included studies and analyzed populations meta-analysis and subgroup analysis was not conducted.
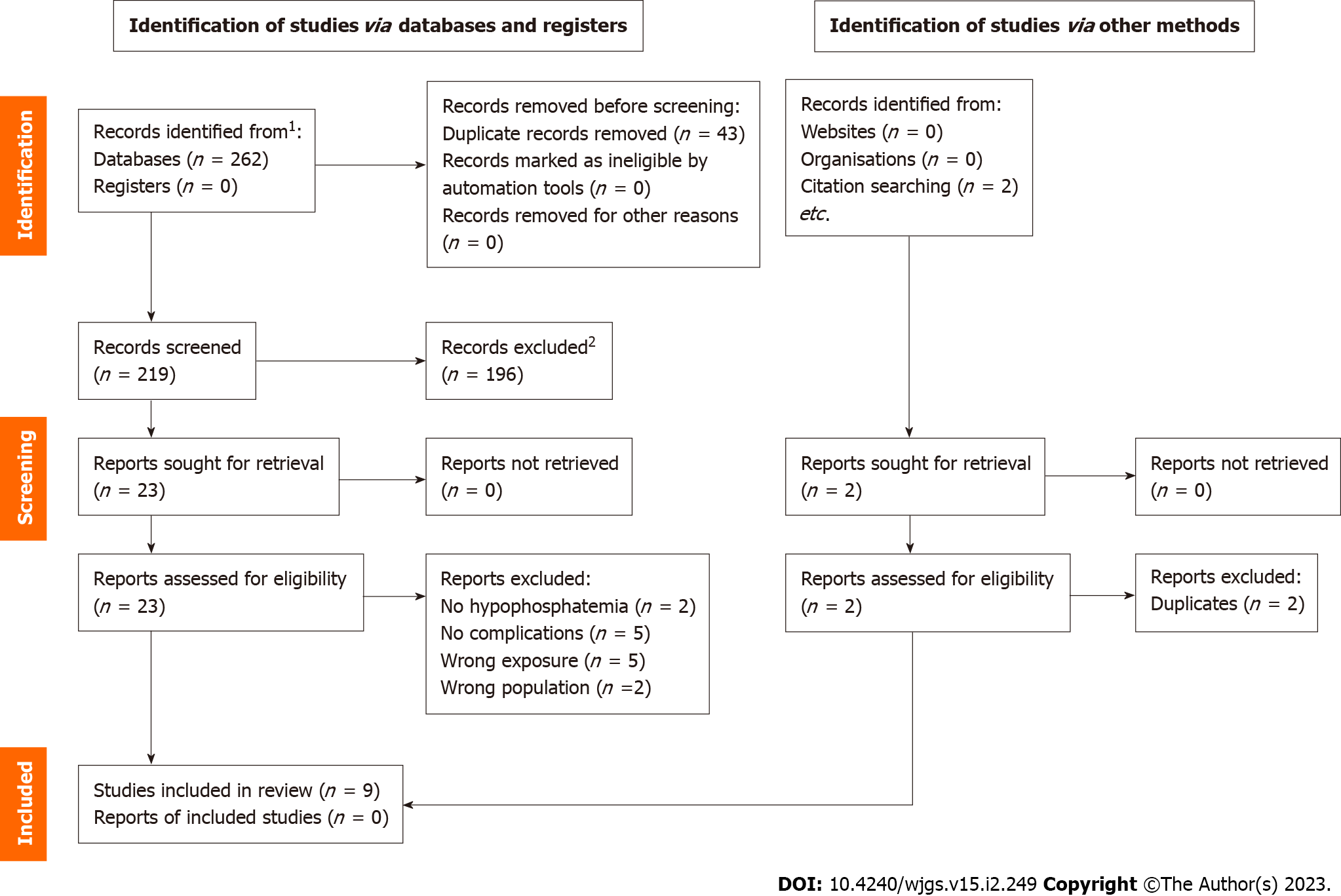
PubMed, Cochrane, and Lippincott Williams & Wilkins databases were searched, and 264 articles were initially retrieved. After removing 45 duplicates, 219 articles were left for screening. One hundred ninety-six articles were removed after screening titles and abstracts due to inappropriate topics, leaving 23 full-text articles for further assessment. After final assessment, nine studies (eight retrospective and one prospective cohort study) with 1677 patients were included in the systematic review (Table 1). The selection process is summarized by preferred reporting items for systematic reviews and meta-analyses flow diagram (Figure 1).
| Ref. | Study type | NOS | N | Study population | Cutoff phosphorus level | Main outcomes | Results | Conclusions |
| George et al[28], 1992 | Retrospective | 6 | 44 | Liver resections | ≤ 2.5 mg/dL | Postoperative complications | Profound HP group had higher frequency rate of postoperative complications (P < 0.005) | Hypophosphatemia increased risk of postoperative complications |
| Buell et al[21], 1998 | Retrospective | 6 | 35 | Liver resections and cryosurgery | < 2.5 mg/dL | Postoperative complications | More complications in HP group (80% vs 28%; P < 0.05) | Hypophosphatemia increased risk of postoperative complications |
| Giovannini et al[22], 2002 | Retrospective | 7 | 59 | Liver resections | ≤ 2.5 mg/dL | Postoperative complications | HP (< 1.5 mg/dL) associated with increase in rate of complications (P < 0.001) | Hypophosphatemia increased risk of postoperative complications |
| Smyrniotis et al[23], 2003 | Prospective | 7 | 30 | Liver resections | < 1.5 mg/dL | Postoperative complications | Patients with HP (< 1.5 mg/dL) had more complications | Hypophosphatemia increased risk of postoperative complications |
| Yuan et al[24], 2011 | Retrospective | 6 | 132 | LDLT | < 1 mg/dL | Liver insufficiency | MV binary logistic regression: Postoperative nadir serum phosphorus (P = 0.01) was independently related to hepatic functional impairment (ß = -5.927, odds ratio 0.003; 95%CI: 0.000-0.239). Postoperative nadir of serum phosphorus < 1 mg/dL (P = 0.006, AUC = 0.731) led to more severe hepatic dysfunction | Hypophosphatemia increased risk of postoperative liver insufficiency |
| Squires et al[11], 2014 | Retrospective | 7 | 719 | Liver resections | < 2.4 mg/dL | Liver insufficiency | UV: Patients with POD2 phosphorus > 2.4 demonstrated a significantly increased risk of PHLF (P = 0.020). MV: POD2 phosphorus > 2.4 mg/dL remained independently associated with a significantly increased risk of PHLF (HR = 1.78; 95%CI: 1.02-3.17; P = 0.048) | Absence of postoperative hypophosphatemia increased risk of postoperative complications and liver insufficiency |
| Hallet et al[29], 2016 | Retrospective | 7 | 402 | Liver resections | ≤ 2.01 mg/dL | Liver insufficiency | More patients with HP recovered from LI compared to those with NP (90.9% vs 65.0%, P = 0.03) | Postoperative hypophosphatemia associated with better recovery from PHLF |
| Margonis et al[30], 2016 | Retrospective | 7 | 95 | Liver resections | ≤ 2.4 mg/dL | Liver insufficiency | LI was lower in patients with HP (P = 0.01). MV analysis: Normal/high serum phosphorus on POD2 (HR = 3.24, 95%CI: 1.23-8.56; P = 0.02) remained independently associated with a worse OS | Postoperative hypophosphatemia associated with better OS, better liver regeneration and lower rate of liver insufficiency |
| Serrano et al[31], 2019 | Retrospective | 7 | 161 | LDLT | ≤ 2.5 mg/dL | Liver insufficiency | LI 1.77 mg/dL vs no LI 2.01 mg/dL for no LI cohort at a median of 1.6 d (38 h) postoperatively (P = 0.003). ROC postoperative phosphate levels through the first 38 h best predicted LI (sensitivity, 90%; specificity, 55.6%; positive predictive value, 11.8%; negative predictive value, 98.8%; AUC, 0.731) | Hypophosphatemia increased risk of postoperative liver insufficiency |
The majority of included studies were characterized by wide variation (inconsistency) of the extent of performed liver resections[11,21-23,28-30]. Indications for liver surgery [colorectal cancer liver metastases, metastatic neuroendocrine tumors, cholangiocarcinoma, hepatocellular carcinoma, sarcoma, metastases of other primary tumors and benign diseases (hemangiomas, cysts, primary sclerosing cholangitis)] were also different. Patients with local ablation of liver tumors were included in one study[21]. Hypophosphatemia after living-donor liver donation was analyzed in 2 studies[24,31]. Quality of the selected studies was evaluated by two investigators independently according to the Newcastle-Ottawa Scale. All selected studies scored ≥ 6 points with the of the studies (6) scoring 7 points, making them eligible to be included in further analysis (Table 1).
PHLF as the main outcome associated with postoperative hypophosphatemia was analyzed in five out of nine included studies[11,24,29-31]. Liver failure in selected studies was defined using 50-50 criteria introduced by Balzan et al[8] [prothrombin time < 50% and serum bilirubin > 50 μmol/L on postoperative day 5 (the 50-50 criteria)] or Mullen et al[32] (peak postoperative serum bilirubin > 7.0 mg/dL). In the four remaining studies, general postoperative complications (intraabdominal or gastrointestinal bleeding, intraabdominal abscess, pneumonia, pleural effusion, pancreatitis, biliary fistula, neurological disorders, etc) with no or inadequate data on liver failure were analyzed[21-23,28]. Only two of the included studies analyzed serum phosphorus levels in relation to postoperative liver regeneration[29,30]. The extensive analysis of the included studies is presented in Table 1.
Cutoff values of hypophosphatemia varied from < 1 mg/dL to ≤ 2.5 mg/dL in selected studies with ≤ 2.5 mg/dL being the most used defining value (3 studies[22,28,31]). Only four of the included studies utilized hypophosphatemia values based on previous studies[11,28-30]. Phosphate concentration in the majority of the selected studies was measured daily starting with postoperative day 1 and was measured up to 10 d postoperatively with days 1, 2 and 3 after the operation being the most popular. In two studies the timing of serum phosphorus measurements was not reported[24,29].
This is the first systematic review including 1677 patients and analyzing postoperative serum phosphorus levels in correlation with PHLF and general surgical complications. In our systematic review we found that changes in postoperative serum phosphorus concentration may be useful for predicting outcomes of patients after extensive liver resections. However, each case and result need to be analyzed individually.
One of the first published studies on the topic reported that patients with more severe hypophosphatemia experienced a significantly higher rate of postoperative complications compared to patients with milder levels of phosphorus decrease[28]. Similar results were reported by Buell et al[21] analyzing clinical implications of hypophosphatemia following major hepatic resection or cryosurgery for liver tumors. The incidence of surgery-related complications was greater in patients with hypophosphatemia compared to patients without phosphorus decrease. Interestingly, hypophosphatemia did not increase complication rates or intensive care and hospital stay in patients undergoing cryotherapy. Two other studies by Giovannini et al[22] and Smyrniotis et al[23] reported similar outcomes. Patients with severe hypophosphatemia developed more complications and experienced a longer intensive care stay compared with patients with milder hypophosphatemia levels.
Similarly, Yuan et al[24] reported postoperative serum phosphorus nadir was independently associated with severe hepatic dysfunction. Authors of the study hypothesized that low serum phosphorus levels may be responsible for impaired intracellular regeneration processes resulting in further hepatic dysfunction. Hypophosphatemia as a predictive factor for PHLF was reported in a recent study by Serrano et al[31]. Patients with liver failure had significantly lower serum phosphorus levels at a median 38 h after operation.
In contrast, Squires et al[11], Hallet et al[29] and Margonis et al[30] revealed that patients with an absence of postoperative hypophosphatemia were more likely to experience higher rates of complications, liver insufficiency and even worse overall survival. Additionally, Hallet et al[29] and Margonis et al[30] reported better recovery from PHLF in patients with hypophosphatemia compared to patients with normophosphatemia. Only two of the selected studies (Hallet et al[29] and Margonis et al[30]) analyzed liver regeneration and reported better postoperative liver regeneration in cases of postoperative hypophosphatemia. The association between liver regeneration and postoperative serum hypophosphatemia relates to high energy consumption during the hepatocyte regeneration processes. Serum phosphorus is primarily used to foster liver recovery processes, such as DNA synthesis, reaching its maximum during the first 72 h after liver resection; however, it takes about 7 d for bone phosphorus to be mobilized into the blood[13,29,30,33].
Moreover, according to Margonis et al[30], patients who developed hyperphosphatemia after surgery had a worse overall survival, a higher risk of death and a worse liver regeneration index reaching up to 7 mo after liver surgery with exact mechanisms still uncertain. It is of interest to mention, that the latter three studies advocating the idea of a positive influence of hypophosphatemia on liver regeneration were published in the last 5 years and analyzed more than 70% (n = 1216) of patients from all included studies (n = 1677).
By analyzing the proposed mechanisms of phosphorus influence, it becomes evident that both hypotheses are based on the fact that phosphorus is needed to foster regenerative response. The difference is in the details. The first theory emphasizes the failure of cells to utilize phosphorus, and the second theory proposes that there is a lack of phosphorus to be utilized. Data on the dynamics of phosphorus concentrations in the pre- and postoperative periods could help understand the meaning of hypophosphatemia. Unfortunately, only two of the included studies provided data on preoperative phosphorus levels[22,24].
Phosphate is an essential element, necessary in a number of physiological processes such as skeletal mineralization and development, nucleotide structuring, membrane composition, etc[34,35]. Most phosphorus (85%) in the human body is found in the skeleton and maintained through the bone-kidney-intestine homeostatic network[36]. The outcome of this homeostatic network is a dynamic balance between urinary phosphate losses, intestinal phosphate absorption and reabsorption from bones, regulated by parathyroid hormone, fibroblast growth factor 23 and vitamin D.
The main reasons of non-surgery related hypophosphatemia are redistribution of phosphorus from extracellular fluids into cells, decreased intestinal absorption, high renal phosphate excretion and decreased proximal reabsorption with reduced activation of vitamin D[37]. For many years, increased liver regeneration and associated metabolic processes were thought to be the main reason of surgery-related hypophosphatemia. However, some authors have suggested that the severity of postoperative hypophosphatemia may not depend on just the extent of serum phosphorus uptake by the regenerating liver[13]. Studies by Salem et al[38] and Nafidi et al[39] revealed that phosphate renal loss was a more credible cause of postoperative hypophosphatemia than phosphorus consumption by the regenerating liver in their patients. Nomura et al[40] reported that liver surgery-related hypophosphatemia and hyperphosphaturia were associated with abnormal urinary nicotinamide metabolism in the liver and kidneys. The results were drawn from in vitro studies with opossum kidney cells and an animal model. However, exact mechanisms and factors of renal phosphaturia are yet to be investigated, analyzed and adapted for clinical use. Further studies are needed to better understand homeostasis of phosphorus to optimize patient outcomes.
The present systematic review has several limitations. First, only nine studies with a relatively small number of patients were eligible for inclusion in this systematic review. Second, eight out of nine included studies were of a retrospective design with a potential source of bias, while only one was a prospective cohort study. Differences between size of the investigated groups, different phosphorus cutoff values, the extent of liver resection not clearly defined, varying primary and secondary outcomes, varying statistical methods and phosphorus measurement days and intervals were other factors further contributing to increased heterogeneity. Finally, due to high heterogeneity between included studies there were not enough data to perform the appropriate meta-analysis.
We present the first systematic review analyzing postoperative serum phosphorus correlation with PHLF and general surgical complications. Changes in postoperative serum phosphorus concentrations may be useful for predicting outcomes of patients after extensive liver resections. However, it is inconclusive whether the incidence or absence of post-hepatectomy hypophosphatemia is related to a better postoperative outcome. In our opinion, routine measurement of perioperative serum phosphorus levels remains questionable, and results should be evaluated individually to prevent PHLF and reduce overall liver surgery-related patient morbidity.
Post-hepatectomy liver failure (PHLF) is a severe and serious complication occurring after high-volume liver resections and presenting with high perioperative mortality rates. There are contradictory results regarding serum phosphorus association with postoperative outcomes. Changes in serum phosphorus levels might predict development of PHLF and improve its treatment results.
Data of serum phosphorus level changes as a prognostic tool for PHLF is scarce and needs to be systemically analyzed.
To perform the first systematic review analyzing hypophosphatemia as a prognostic tool for PHLF and general complications.
Study protocol for the review was registered in the International Prospective Register of Systematic Reviews database (D42020197717). This systematic review was conducted according to the preferred reporting items for systematic reviews and meta-analyses guidelines. PubMed, Cochrane and Lippincott Williams & Wilkins databases were searched up to March 31, 2022 using relevant search terms.
After thorough research, nine studies with 1677 patients were included in the systematic review. The majority of the included studies were retrospective. However, due to high heterogeneity between included studies there were not enough data to perform appropriate the meta-analysis.
Changes of postoperative serum phosphorus concentration may be useful for predicting outcomes of patients after extensive liver resections. However, the decision to measure and interpret results needs to be considered individually with routine phosphorus level measurements, and its benefits remain questionable.
Further high volume, non-randomized studies are needed to better analyze postoperative hypophosphatemia as a predictive factor for PHLF and general surgical outcomes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Lithuania
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kovacevic T, Bosnia and Herzegovina; Qiu YD, China S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | Søreide JA, Deshpande R. Post hepatectomy liver failure (PHLF) – Recent advances in prevention and clinical management. Eur J Surg Oncol. 2021;47:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (1)] |
| 2. | Sultana A, Brooke-Smith M, Ullah S, Figueras J, Rees M, Vauthey JN, Conrad C, Hugh TJ, Garden OJ, Fan ST, Crawford M, Makuuchi M, Yokoyama Y, Büchler M, Padbury R. Prospective evaluation of the International Study Group for Liver Surgery definition of post hepatectomy liver failure after liver resection: an international multicentre study. HPB (Oxford). 2018;20:462-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Gilg S, Sandström P, Rizell M, Lindell G, Ardnor B, Strömberg C, Isaksson B. The impact of post-hepatectomy liver failure on mortality: a population-based study. Scand J Gastroenterol. 2018;53:1335-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 4. | Hammond JS, Guha IN, Beckingham IJ, Lobo DN. Prediction, prevention and management of postresection liver failure. Br J Surg. 2011;98:1188-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 5. | Helling TS. Liver failure following partial hepatectomy. HPB (Oxford). 2006;8:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Lee EC, Park SJ, Han SS, Shim JR, Park HM, Lee SD, Kim SH. Risk prediction of post-hepatectomy liver failure in patients with perihilar cholangiocarcinoma. J Gastroenterol Hepatol. 2018;33:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Truant S, Oberlin O, Sergent G, Lebuffe G, Gambiez L, Ernst O, Pruvot FR. Remnant liver volume to body weight ratio > or =0.5%: A new cut-off to estimate postoperative risks after extended resection in noncirrhotic liver. J Am Coll Surg. 2007;204:22-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 213] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 8. | Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824-828, discussion 828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 823] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 9. | Lee SG, Hwang S. How I do it: assessment of hepatic functional reserve for indication of hepatic resection. J Hepatobiliary Pancreat Surg. 2005;12:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Schneider PD. Preoperative assessment of liver function. Surg Clin North Am. 2004;84:355-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Squires MH 3rd, Dann GC, Lad NL, Fisher SB, Martin BM, Kooby DA, Sarmiento JM, Russell MC, Cardona K, Staley CA 3rd, Maithel SK. Hypophosphataemia after major hepatectomy and the risk of post-operative hepatic insufficiency and mortality: an analysis of 719 patients. HPB (Oxford). 2014;16:884-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Lock JF, Malinowski M, Seehofer D, Hoppe S, Röhl RI, Niehues SM, Neuhaus P, Stockmann M. Function and volume recovery after partial hepatectomy: influence of preoperative liver function, residual liver volume, and obesity. Langenbecks Arch Surg. 2012;397:1297-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Datta HK, Malik M, Neely RD. Hepatic surgery-related hypophosphatemia. Clin Chim Acta. 2007;380:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Zheng J, Glezerman IG, Sadot E, McNeil A, Zarama C, Gönen M, Creasy J, Pak LM, Balachandran VP, D’Angelica MI, Allen PJ, DeMatteo RP, Kingham TP, Jarnagin WR, Jaimes EA. Hypophosphatemia after Hepatectomy or Pancreatectomy: Role of the Nicotinamide Phosphoribosyltransferase. J Am Coll Surg. 2017;225:488-497.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Tatsumi S, Miyagawa A, Kaneko I, Shiozaki Y, Segawa H, Miyamoto K. Regulation of renal phosphate handling: inter-organ communication in health and disease. J Bone Miner Metab. 2016;34:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Woodard HQ, White DR. The composition of body tissues. Br J Radiol. 1986;59:1209-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 461] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 17. | Tan HP, Madeb R, Kovach SJ, Orloff M, Mieles L, Johnson LA, Bozorgzadeh A, Marcos A. Hypophosphatemia after 95 right-lobe living-donor hepatectomies for liver transplantation is not a significant source of morbidity. Transplantation. 2003;76:1085-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Filik L, Karakayali H, Dalgiç A, Emiroğlu R, Haberal M. Hypophosphatemia in living liver donors. Transplant Proc. 2006;38:559-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Chung PY, Sitrin MD, Te HS. Serum phosphorus levels predict clinical outcome in fulminant hepatic failure. Liver Transpl. 2003;9:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Baquerizo A, Anselmo D, Shackleton C, Chen TW, Cao C, Weaver M, Gornbein J, Geevarghese S, Nissen N, Farmer D, Demetriou A, Busuttil RW. Phosphorus ans an early predictive factor in patients with acute liver failure. Transplantation. 2003;75:2007-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Buell JF, Berger AC, Plotkin JS, Kuo PC, Johnson LB. The clinical implications of hypophosphatemia following major hepatic resection or cryosurgery. Arch Surg. 1998;133:757-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 22. | Giovannini I, Chiarla C, Nuzzo G. Pathophysiologic and clinical correlates of hypophosphatemia and the relationship with sepsis and outcome in postoperative patients after hepatectomy. Shock. 2002;18:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Smyrniotis V, Kostopanagiotou G, Katsarelias D, Theodoraki K, Hondros K, Kouskouni E. Changes of serum phosphorus levels in hepatic resections and implications on patients’ outcomes. Int Surg. 2003;88:100-104. [PubMed] |
| 24. | Yuan D, Wei YG, Li B, Yan LN, Wen TF, Zhao JC, Zeng Y, Chen KF. Evaluation outcomes of donors in living donor liver transplantation: a single-center analysis of 132 donors. Hepatobiliary Pancreat Dis Int. 2011;10:480-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Lee HW, Suh KS, Kim J, Shin WY, Cho EH, Yi NJ, Lee KU. Hypophosphatemia after live donor right hepatectomy. Surgery. 2008;144:448-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40322] [Article Influence: 10080.5] [Reference Citation Analysis (2)] |
| 27. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12634] [Article Influence: 842.3] [Reference Citation Analysis (0)] |
| 28. | George R, Shiu MH. Hypophosphatemia after major hepatic resection. Surgery. 1992;111:281-286. [PubMed] |
| 29. | Hallet J, Karanicolas PJ, Zih FS, Cheng E, Wong J, Hanna S, Coburn NG, Law CH. Hypophosphatemia and recovery of post-hepatectomy liver insufficiency. Hepatobiliary Surg Nutr. 2016;5:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Margonis GA, Amini N, Buettner S, Ghasebeh MA, Besharati S, Kim Y, Gani F, Sobhani F, Samaha M, Kamel IR, Pawlik TM. Impact of Perioperative Phosphorus and Glucose Levels on Liver Regeneration and Long-term Outcomes after Major Liver Resection. J Gastrointest Surg. 2016;20:1305-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Serrano OK, Mongin SJ, Berglund D, Goduguchinta V, Reddy A, Vock DM, Kirchner V, Kandaswamy R, Pruett TL, Chinnakotla S. Clinical utility of postoperative phosphate recovery profiles to predict liver insufficiency after living donor hepatectomy. Am J Surg. 2019;218:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, Abdalla EK, Curley SA, Capussotti L, Clary BM, Vauthey JN. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854-62; discussion 862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 517] [Article Influence: 28.7] [Reference Citation Analysis (1)] |
| 33. | Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1091] [Cited by in RCA: 1175] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 34. | Bugg NC, Jones JA. Hypophosphataemia. Pathophysiology, effects and management on the intensive care unit. Anaesthesia. 1998;53:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 79] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Land C, Schoenau E. Fetal and postnatal bone development: reviewing the role of mechanical stimuli and nutrition. Best Pract Res Clin Endocrinol Metab. 2008;22:107-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Florenzano P, Cipriani C, Roszko KL, Fukumoto S, Collins MT, Minisola S, Pepe J. Approach to patients with hypophosphataemia. Lancet Diabetes Endocrinol. 2020;8:163-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 37. | Christov M, Jüppner H. Phosphate homeostasis disorders. Best Pract Res Clin Endocrinol Metab. 2018;32:685-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 38. | Salem RR, Tray K. Hepatic resection-related hypophosphatemia is of renal origin as manifested by isolated hyperphosphaturia. Ann Surg. 2005;241:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Nafidi O, Lapointe RW, Lepage R, Kumar R, D’Amour P. Mechanisms of renal phosphate loss in liver resection-associated hypophosphatemia. Ann Surg. 2009;249:824-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Nomura K, Tatsumi S, Miyagawa A, Shiozaki Y, Sasaki S, Kaneko I, Ito M, Kido S, Segawa H, Sano M, Fukuwatari T, Shibata K, Miyamoto K. Hepatectomy-related hypophosphatemia: a novel phosphaturic factor in the liver-kidney axis. J Am Soc Nephrol. 2014;25:761-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |