Published online Nov 27, 2023. doi: 10.4240/wjgs.v15.i11.2646
Peer-review started: August 15, 2023
First decision: September 1, 2023
Revised: September 15, 2023
Accepted: October 17, 2023
Article in press: October 17, 2023
Published online: November 27, 2023
Processing time: 103 Days and 20.8 Hours
Cronkhite–Canada syndrome (CCS) is a rare sporadic polyposis syndrome that presents with gastrointestinal and ectodermal symptoms in addition to nutritional deficiencies. CCS combined with hypothyroidism is an even rarer condition, with no standard treatment guidelines.
The present study described 2 patients with CCS: A 67-year-old woman with concomitant hypothyroidism and 68-year-old man treated with endoscopic mucosal resection (EMR). Both patients had multiple gastrointestinal symptoms and ectodermal changes, along with multiple gastrointestinal polyps. Microscopic examination showed that the mucosa in both patients was hyperemic and edematous, with pathologic examination showing distorted, atrophic, and dilated glands. Patient 1 had concomitant hypothyroidism and was treated with levothyroxine. Due to her self-reduction of hormone dose, her disease relapsed. Patient 2 underwent EMR, but refused further hormonal or biological treatments. Subsequently, he was treated with an oral Chinese medical preparation.
Pharmacotherapy can induce and maintain remission in CCS patients, with adjuvant EMR, long-term follow-up, and endoscopic surveillance being necessary.
Core Tip: Cronkhite–Canada syndrome (CCS) is a rare, non-genetic syndrome characterized by ectodermal abnormalities and diffuse gastrointestinal polyps with protein loss. To date, 7 patients with CCS combined with hypothyroidism have been identified. We report two cases, one of which is combined with hypothyroidism. Through indexing and analyzing PubMed, Web of Science, and Embase databases, we summarized the clinical characteristics of CCS combined with hypothyroidism. Additionally, we concluded that pharmacotherapy can induce and maintain remission in CCS patients, with adjuvant endoscopic mucosal resection, long-term follow-up, and endoscopic surveillance being necessary.
- Citation: Lv YQ, Wang ML, Tang TY, Li YQ. Comprehensive treatment and a rare presentation of Cronkhite–Canada syndrome: Two case reports and review of literature. World J Gastrointest Surg 2023; 15(11): 2646-2656
- URL: https://www.wjgnet.com/1948-9366/full/v15/i11/2646.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i11.2646
Cronkhite–Canada syndrome (CCS), or polyposis hyperpigmentation alopecia nail dystrophy syndrome, is a rare, non-genetic syndrome characterized by ectodermal changes and gastrointestinal symptoms. Patients with CCS have a poor prognosis, with a 5-year mortality rate of 55%[1]. There are no standardized treatments, as the etiology and pathogenesis of CCS are unknown. At present, glucocorticoid is the primary treatment, either alone or combined with immunosuppressive or biological agents. Other treatments include nutritional support, anti-Helicobacter pylori treatment, tumor necrosis factor- (TNF-) inhibitors, and traditional Chinese medicines. This report describes the clinical characteristics of 2 patients diagnosed with CCS at the First Bethune Hospital of Jilin University and presents a review of the literature. Patient 1 was a 67-year-old woman diagnosed with CCS, with concomitant hypothyroidism, whereas Patient 2 was a 68-year-old man diagnosed with CCS, who underwent endoscopic mucosal resection (EMR). The aim of this study was to provide the basis for basic and clinical research on CCS.
Case 1: A 67-year-old woman was admitted to our hospital in August 2016, due to intermittent diarrhea for 2 mo, aggravated with bloody stool for 1 mo.
Case 2: A 68-year-old man was admitted to our hospital in May 2023, due to hypogeusia and diarrhea for 1 mo.
Case 1: Two months prior to admission, the patient had developed yellowish watery diarrhea (3–5 times per day). One month later, the frequency of defecation had increased to 5–8 times per day, accompanied by a small amount of dark red bloody stool, abdominal pain, and tenesmus. The patient experienced nausea and vomited her gastric contents occasionally. She also experienced fatigue, apathy, loss of appetite, hypogeusia, and alopecia, with a weight loss of 5 kg.
Case 2: One month prior to admission, the patient had developed hypogeusia, loss of appetite, abdominal pain, and yellowish watery diarrhea at a frequency of 4–10 times per day, after eating and without obvious inducement. One week prior to admission, colonoscopy at a local hospital showed colonic mucosal lesions and multiple polypoid mucosal bulges throughout the rectum. He also experienced alopecia, exfoliation of the skin on the hands and face, poor diet and sleep, and a weight loss of 15.5 kg during the previous month.
Case 1: The patient denied a specific previous medical history.
Case 2: The patient denied a specific previous medical history.
Case 1: The patient denied any personal history or family history related to the disease.
Case 2: The patient denied any personal history or family history related to the disease.
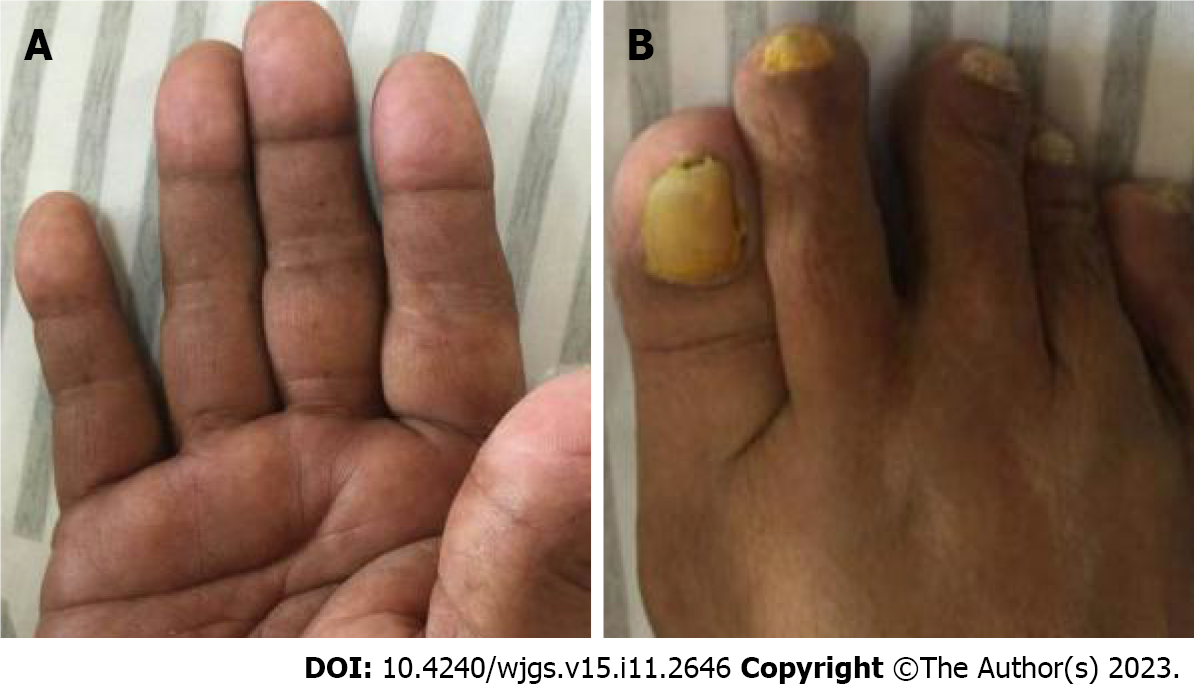
Case 1: On physical examination, the vital signs were as follows: Body temperature, 36.5 ºC; blood pressure, 107/63 mmHg; heart rate, 78 beats per min; respiratory rate, 16 breaths per min. Furthermore, examination showed malnutrition, hyperpigmentation of the skin on the hands and feet, alopecia, and nail dystrophy (Figure 1).
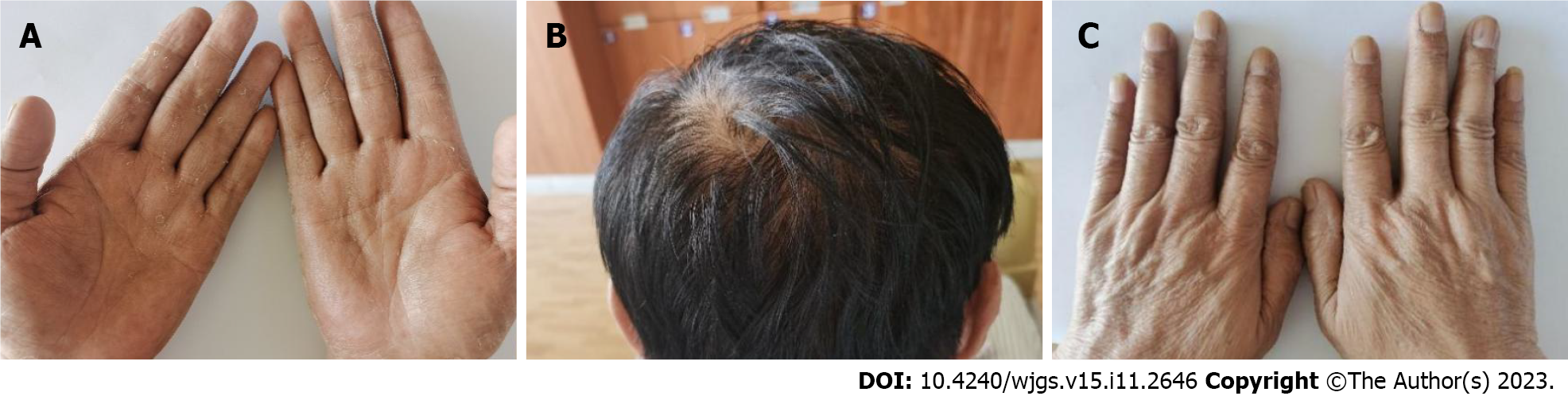
Case 2: The vital signs were as follows: Body temperature, 36.6 ºC; blood pressure, 96/61 mmHg; heart rate, 90 beats per min; respiratory rate, 18 breaths per min. Physical examination showed malnutrition, exfoliated skin on the face and hands, hyperpigmentation on the palms and backs of the hands (Figure 2A), alopecia (Figure 2B), and thickened and fragile nails on both hands and feet (Figure 2C). No edema was noted in either lower limb.
Case 1: Laboratory examination showed that the C-reactive protein level in this patient was 8.34 mg/L. Evaluation of thyroid function showed that her thyroid stimulating hormone (TSH) concentration was 26.080 µIU/mL (normal range: 0.27–4.2 µIU/mL) and her free thyroxine (FT4) concentration was 10.63 pmol/L (normal range: 12.0–22.0 pmol/L). Rheumatic and immune-related results were normal. Other laboratory results are summarized in Table 1.
| Parameter | Patient 1 | Patient 2 | Normal range |
| RBC as × 1012/L | 5.15 | 3.43 | 3.8-5.1/4.3–5.8 |
| Hb in g/L | 152 | 124 | 115–150/130–175 |
| K+ in mmol/L | 2.73 | 3.87 | 3.5–5.5 |
| Na+ in mmol/L | 135.6 | 136.3 | 137–147 |
| Ca2+ in mmol/L | 1.99 | 2.04 | 2.11–2.52 |
| TP in g/L | 45.9 | 63.5 | 65.0–85.0 |
| Albumin in g/L | 26.5 | 37.6 | 40.0–55.0 |
| Routine urine | Normal | Normal | |
| Fecal occult blood test | Positive | Positive | Negative |
| Fecal fat globule test | Negative | 0-1 | |
| Fecal culture | Negative | Negative | |
| ANA | Negative | Negative | |
| T-SPOT.TB | Negative | Negative |
Case 2: Laboratory examinations showed that the patient had a cytokeratin 19 concentration of 8.63 ng/mL, a carcinoembryonic antigen of 9.26 ng/mL, a carbohydrate antigen 242 concentration of 40.05 U/mL, and a carbohydrate antigen 199 concentration of 55.83 U/mL. Routine blood tests, liver and kidney function tests, and blood lipids showed no significant abnormalities, and he was negative for IgG4, IgG9, and anti-mitochondrial antibody M2. Other laboratory results are summarized in Table 1.
Case 1: The second phase of contrast-enhanced computed tomography (CT) colonography in this patient showed that the gastric wall of the antrum and angle was thickened, with nodular protrusions and partial enhancement. The partial small intestinal wall was also thickened and heterogeneously enhanced. Diffuse wall thickening and polypoid masses with heterogeneous enhancements were observed throughout the colon, especially in the left colon.
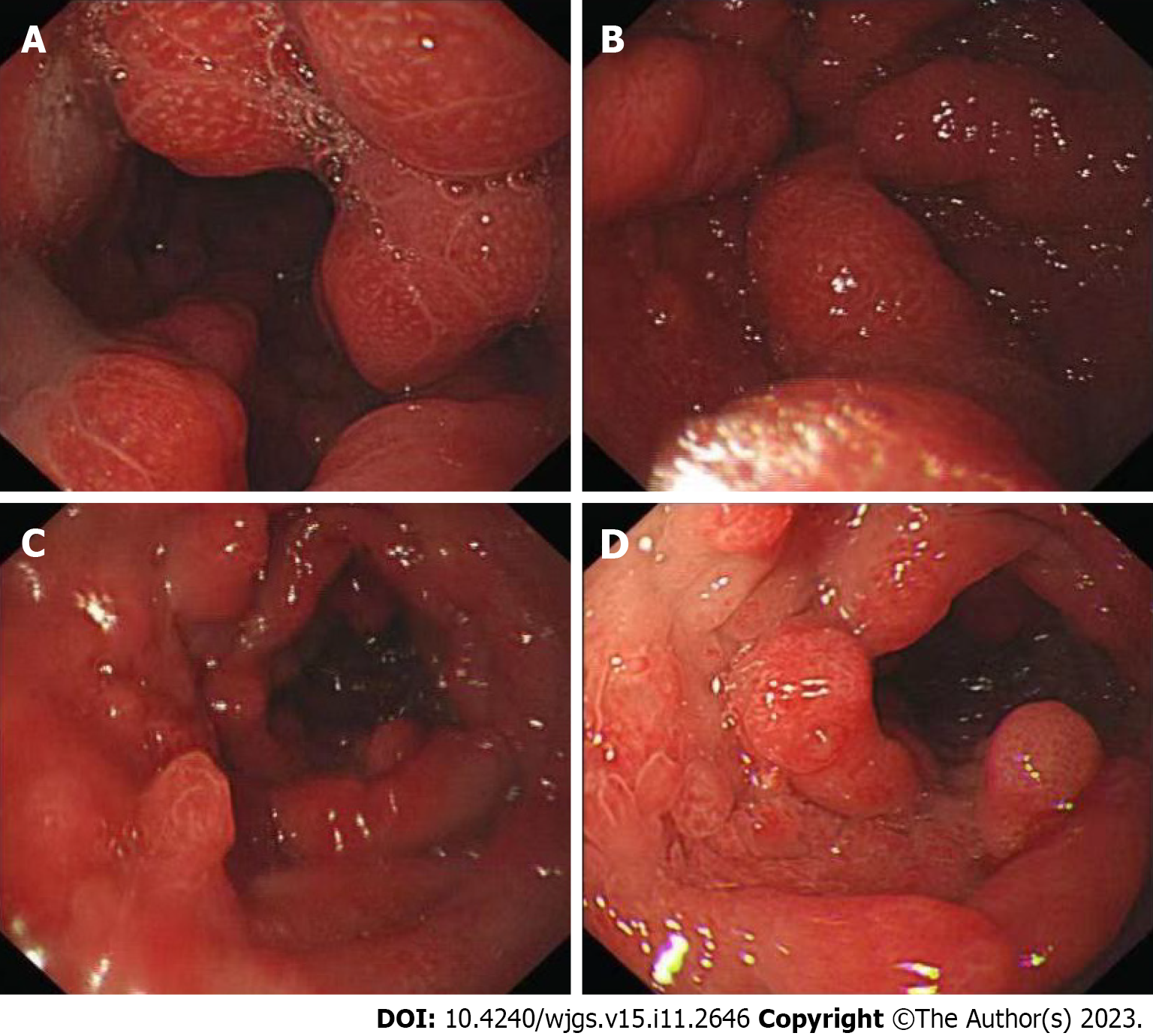
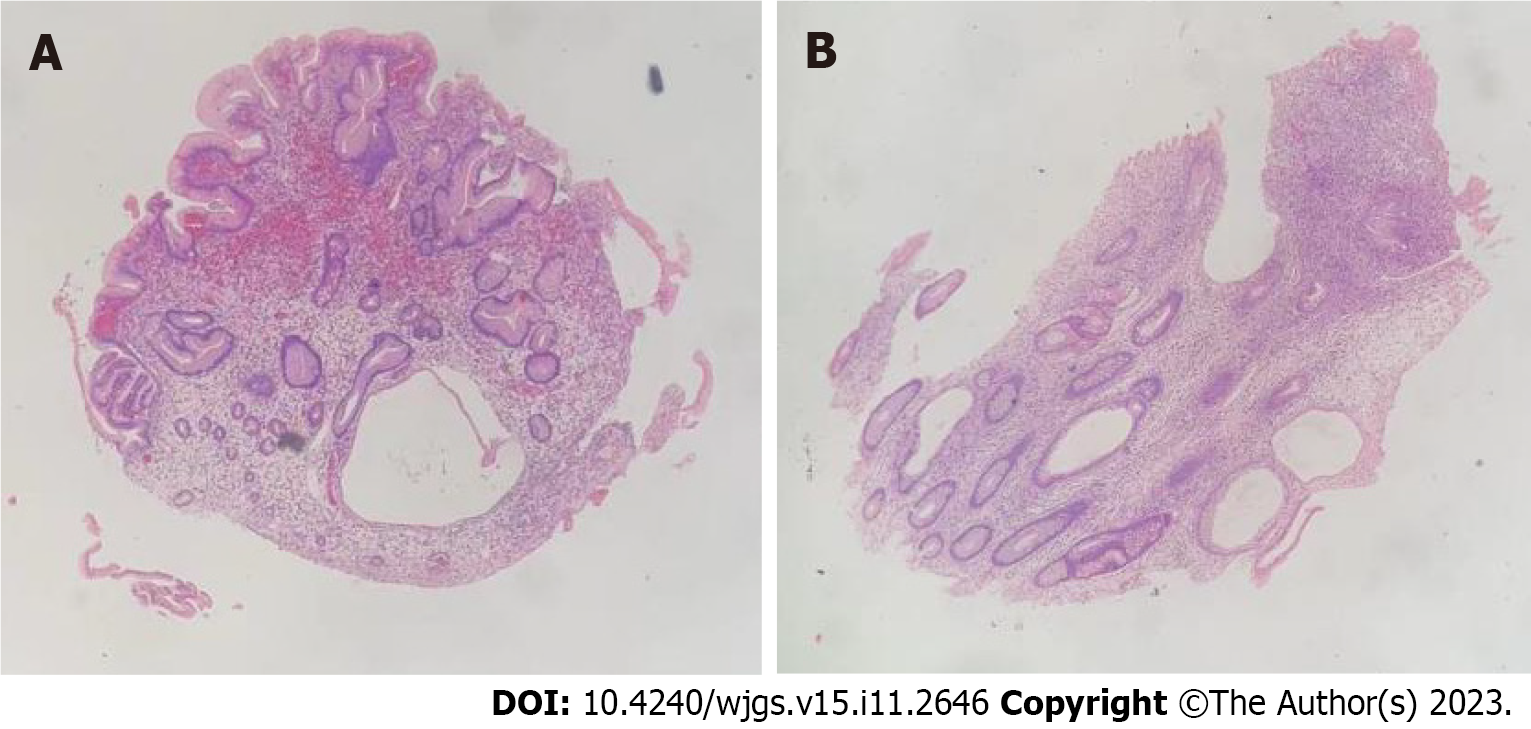
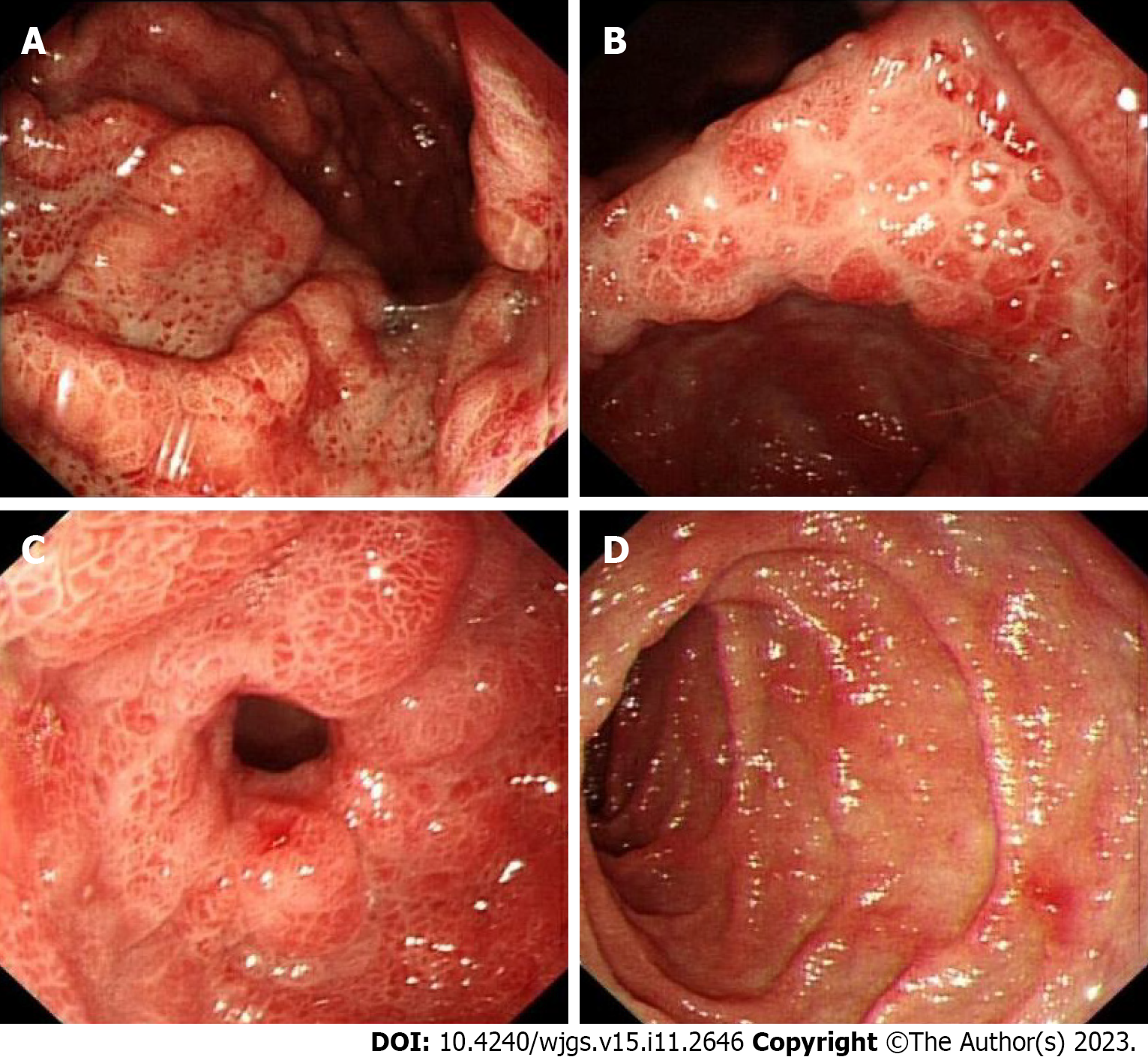
The patient also underwent endoscopic examinations. Gastroscopy showed a thickened edematous mucosa with extensive congestion. The gastric antrum showed scattered nodular-like mucosal uplift. The duodenal bulb and descending segment showed multiple mucosal protrusions of different shapes and sizes (Figure 3). Colonoscopy showed that the mucosa of the large intestine was rough, with nodular and polypoid protuberances of different shapes and sizes. While the mucosa at the protuberances was hyperemic and edematous, the vascular texture of the intervening intestinal wall disappeared and turned white (Figure 4). On pathological examination, multiple biopsies of the stomach and large intestine showed that the mucosal glands were atrophic and dilated. Proliferation of interstitial granulation tissue was observed, accompanied by the infiltration of lymphocytes and eosinophils, suggesting hamartomatous polyposis. A tubular adenoma was observed in the transverse colon, with moderate to severe epithelial dysplasia (Figure 5).
Case 2: Both plain and three-stage enhanced CT of the abdomen showed gastric wall thickening in the lesser curvature and antrum.
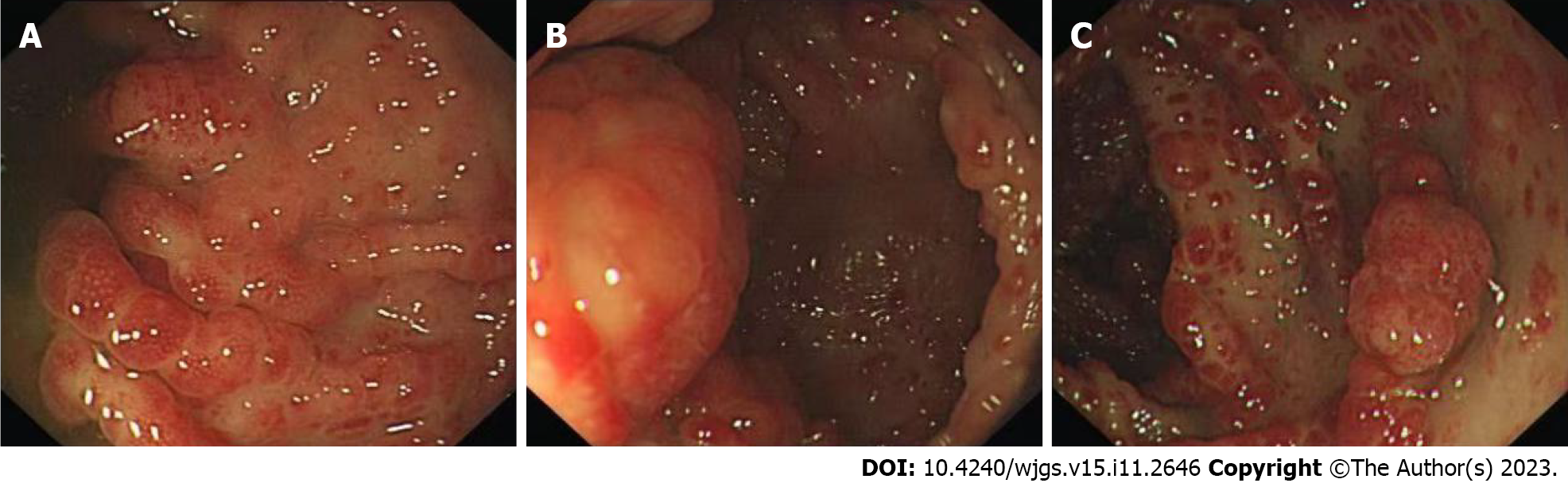
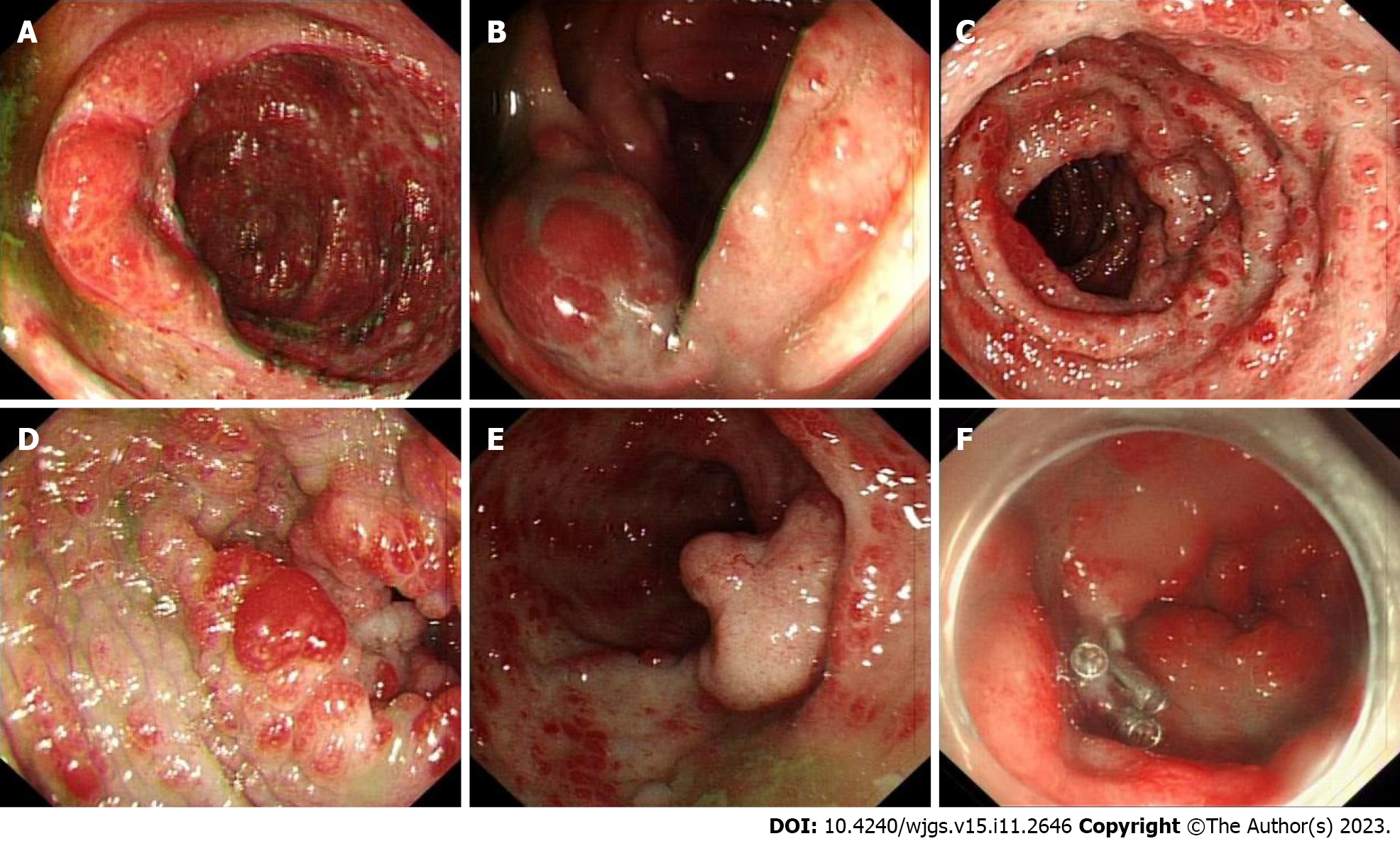
Gastroscopy showed hyperemic and edematous mucosa (with a hyperplastic and nodular appearance) in the fundus, angle, antrum, duodenal bulb, and descending segment; this was prominent between the lesions (Figure 6). Colonoscopy showed similar results in colonic mucosa. However, the nodules were of different sizes. A mucosal intumescent lesion measuring 2.5 cm × 2.5 cm was observed 10 cm from the anal verge (Figure 7). EMR was performed, with biopsy samples obtained at multiple sites.
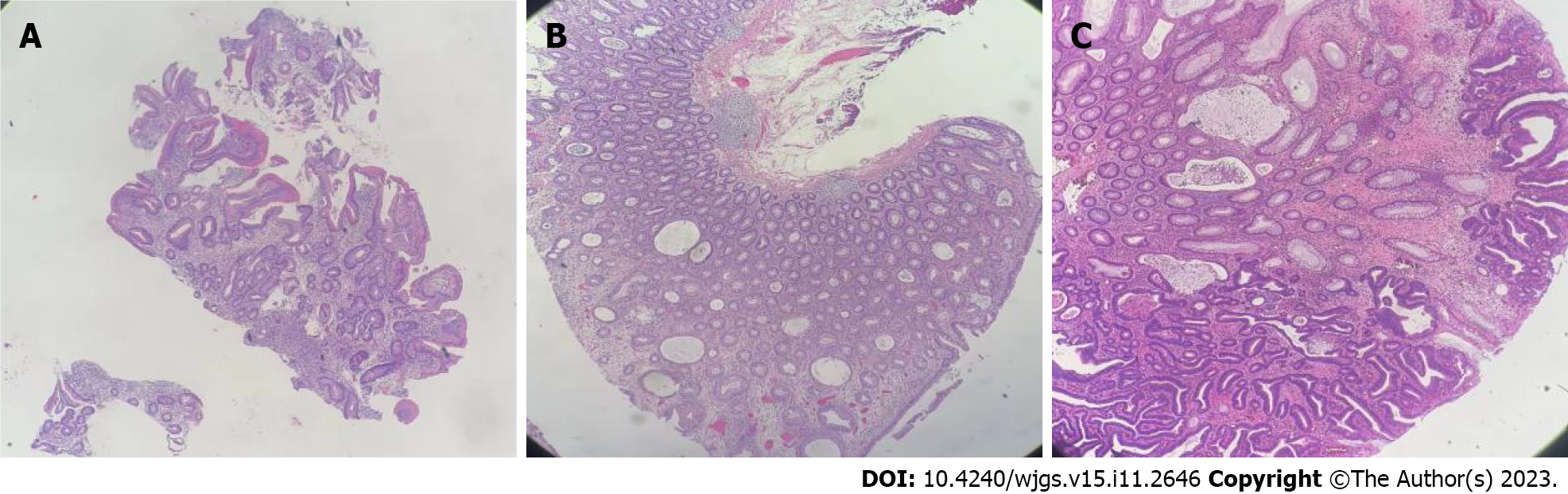
Gastroscopic pathology suggested that the lesions of the gastric body were consistent with hyperplastic polyps, acute and chronic mucosal inflammation. In the duodenum, distorted, branched, and hyperplastic glands were observed, indicating active chronic inflammation (Figure 8). Colonoscopy pathology showed active chronic enteritis and cryptitis in the terminal ileum, with round and blunt villi, and distorted glands, with infiltration of about 50 eosinophils per high-power field. Additionally, the pathology reports showed evidence of active chronic colitis, cryptitis, and crypt abscesses, with hyperplastic or atrophic, dilated, and distorted glands. This was accompanied by interstitial edema and eosinophil infiltration. The mucosal glands of the sigmoid colon and rectosigmoid junction were hyperplastic, with interstitial edema and crypt abscesses. CCS-related polyps could not be excluded (Figure 8). Examination of the rectal mass removed by EMR (Figure 7F) showed a villous tubular adenoma, consistent with high-grade intraepithelial neoplasia, with no apparent involvement of blood vessels or surgical margins. Immunohistochemical examination showed that about 60% of the cells were positive for Ki67, with high expression of MLH1 (+++), MSH2 (+++), MSH6 (+++), and PMS2 (+++), slight expression of P53 (+), and CK (+), and negative for CD31 and CD34 (Figure 8).
Case 1: Based on the aforementioned findings, Patient 1 was diagnosed with CCS and hypothyroidism.
Case 2: Based on the aforementioned findings, Patient 2 was diagnosed with CCS.
Case 1: Treatment of Patient 1 included nutritional support, thyroid hormone supplementation (12.5 ug/d for the 1st 3 d, 25 ug/d thereafter), administration of prednisone tablets (30 mg/d), and symptomatic treatment during hospitalization. After discharge, she was continued on prednisone tablets (30 mg/d).
Case 2: The patient was initially treated with acid suppression (proton pump inhibitors) and nutritional support, followed by EMR for the removal of colonic polypoid lesions and the rectal mass. After endoscopic surgery, however, the patient and his family refused further hormonal or biological treatments and asked to be discharged.
Case 1: After 9 d of comprehensive treatment, her symptoms, including diarrhea and bloody stool, significantly improved. Hair and nail loss, however, did not significantly improve. Two months after discharge, this patient elected to reduce her dose of prednisone to 10 mg/d, and 3 mo later to 5 mg/d. Four months after the diagnosis of CCS, the patient suddenly developed nausea, vomiting, abdominal distension, and other manifestations of intestinal obstruction. Unfortunately, we did not obtain the results of thyroid function reevaluation for this patient after discharge. Because histopathologic examination of her colon showed tubular adenoma and moderate to severe epithelial dysplasia, the possibility of malignant transformation was considered. The patient died of multiple organ failure after 1 wk of treatment in a local hospital.
Case 2: One month later, the patient was followed up by telephone. At present, the patient is receiving symptomatic and supportive treatment and has been treated with an oral Chinese medicine for more than 20 d. His hypogeusia and appetite have improved, his new nails are soft, and his skin pigmentation has improved. However, his alopecia has not changed, and he continues to have yellow watery diarrhea, 2–8 times per day. His body weight is 4.5 kg lower than his pre-discharge weight.
CCS is a rare, non-genetic syndrome characterized by ectodermal abnormalities and diffuse gastrointestinal polyps with protein loss. Since first described in 1955[2], more than 500 patients with CCS have been reported worldwide; most of these patients were Asian, with Japan accounting for more than 75%[3]. The average age at onset is 59 years, with more than 80% of these patients aged over 50 years at diagnosis[4]. The male to female ratio ranges from 1.5 to 2:1[5]. Patient prognosis is poor, with a 5-year mortality rate as high as 55%[1]. The 2 patients described in this study included one woman and one man, both aged over 50 years.
At present, the etiology of the disease is unclear. Autoimmune factors have been reported to be involved in its possible etiology and pathogenesis. Many patients with CCS show positive plasma antinuclear antibody (ANA) series, elevated IgG4 levels, or IgG4 (+) plasma cells infiltrating into the polyps. Elevated levels of plasma IgE have also been reported in 1 patient with CCS[5]. CCS was also found to be associated with hypothyroidism and other autoimmune diseases[6], such as membranous nephropathy[7], systemic lupus erythematosus, rheumatoid arthritis, and scleroderma. The involvement of autoimmune factors is supported by the overall good clinical response of CCS patients to immunosuppressive therapy. Both patients in the present study had normal ANA and plasma IgG4 levels, although Patient 1, who had CCS and hypothyroidism, had a significantly increased TSH level, accompanied by apathy and loss of appetite. To date, 7 patients with CCS have been diagnosed with associated hypothyroidism, making this condition extremely rare (Table 2). Furthermore, prior to this article, there was only one reported case of CCS with concomitant hypothyroidism in China. All patients were aged over 59 years at diagnosis, with the oldest patient being 82 years. All 7 patients had alopecia, nail dystrophy, diarrhea, and multiple polyps. After clinical treatments, the symptoms of 6 patients were relieved to varying degrees, whereas the 7th patient died of respiratory failure 1 year after treatment. This patient had hypothyroidism following surgery for Graves’ disease. The original text did not mention whether auto-antibodies were positive. However, because Graves’ disease is associated with autoimmune antibodies, we can be certain that the patient had autoimmune abnormalities. This further confirms the association of CCS with autoimmune factors[8]. Qiao et al[9] reported a CCS case in which the patient, however, had normal serum levels of antithyroglobulin and anti-thyroid peroxidase antibodies. Taken together, these findings indicate that the etiology of CCS and its relationship to autoimmunity are unclear, necessitating further research.
| Ref. | Age/sex | Country | Thyroid function | Ectodermal changes | Gastrointestinal symptoms | Treatment | Outcome |
| Størset et al[29], 1979 | 66/F | Sweden | T4 36 nmol/L, TSH 45.0 μU/mL, no thyroid antibodies detected | Alopecia, onychodystrophy | Diarrhea, gastrointestinal polyposis | Thyroxine-sodium | Improved symptoms, but no changes in neurological and electromyographic findings |
| Jones et al[30], 1984 | 82/F | United States | TT4 1.0 µg/dL, TSH 31.5 mIU/ml | Alopecia, onychodystrophy, hyperpigmentation | Diarrhea, gastrointestinal polyposis | Prednisone therapy was initiated at 20 mg/d orally, tapered to 10 mg/d within 2 wk, accompanied by nutritional support | Improved |
| Qiao et al[9], 2005 | 60/F | China | T3, TSH ↑, FT4 ↓ | Alopecia, nail dystrophy, hyperpigmentation | Diarrhea, intermittent abdominal pain, generalized gastrointestinal polyposis | Symptomatic treatments | Improved |
| Berzin et al[8], 2012 | 72/M | United States | TSH 1.72 mIU/L (after supplementing with levothyroxine) | Alopecia, onycholysis, and yellow nail discoloration | Diarrhea, hypogeusia, early satiety, generalized gastrointestinal polyposis | Prednisone 30 mg/d, symptomatic treatments | Died |
| No author[31], 2016 | 55/F | Korea | ND | Alopecia, onychodystrophy | Diarrhea with intermittent blood, nausea, abdominal pain, generalized gastrointestinal polyposis | Steroids and nutritional support | Improved |
| No author[32], 2018 | 56/M | United Kingdom | TSH 160 mIU/L, FT4 < 5 pmol/L | Alopecia, onychodystrophy, hyperpigmentation | Diarrhea, generalized gastrointestinal polyposis | Prednisolone | Improved |
| Dawra et al[33], 2018 | 60/M | India | TSH 94.65 µg/dL | Alopecia, onychodystrophy, hyperpigmentation | Diarrhea, anorexia, dysgeusia, multiple polyps | Cefixime, rifaximin, mesalamine, thyroid replacement, zinc supplementation (100 mg/d), along with micronutrient supplementation | Improved |
Infectious factors have also been associated with CCS. For example, Helicobacter pylori (H. pylori) infection has been associated with CCS, with symptoms being relieved after anti-H. pylori treatment[10]. Antibodies to Saccharomyces cerevisiae have also been detected in the plasma of CCS patients[11-13], and Clostridium difficile has been identified in the feces of patients with CCS[14]. These findings suggest that infection with several types of pathogenic bacteria is associated with CCS.
Although CCS is regarded as a non-genetic disease, genetic factors may be associated with its development. Although both patients in the present study denied that anyone in their families had a similar history, genome-wide association study revealed that mutations in the PRKDC gene, normally encoding the catalytic subunit of DNA-dependent protein kinase, may play a role in the pathogenesis of CCS[15]. Genetic analysis of a Chinese mother and child who were diagnosed with CCS revealed C.3921-3925delAAAAG (p.Ile1307fsX6) mutation in the APC gene[5].
Other factors associated with CCS included mental and physical stress[6], allergy[12], and gut microbiota[16]. Discontinuation of allergy-inducing agents, such as hair dyes and certain drugs, was found to reduce IgE concentrations and eosinophil infiltration, as well as to improve clinical symptoms in patients with CCS[12]. Moreover, the remission and regression of polyps after prednisolone (PSL) treatment of patients with CCS was accompanied by changes in the abundance and diversity of gut microbiota[16]. PSL can not only inhibit proinflammatory cytokines but can also mediate polyp regression by altering the composition of gut microbiota. Additional studies are needed to better understand the associations between proinflammatory cytokines and microecological dysbiosis, which might be involved in the pathogenesis of CCS.
CCS is characterized by ectodermal abnormalities, gastrointestinal symptoms, and protein loss. Ectodermal changes include alopecia, skin pigmentation, and nail dystrophy, including nail yellowing, atrophy, and loss. The main gastrointestinal symptoms are abdominal pain and diarrhea, frequently accompanied by nausea, acid regurgitation, anorexia, and abnormal taste. The occurrence of fractures has also been reported[17]. Based on the initial symptom, CCS can be divided into five types: Diarrhea (type 1), hypogeusia (type 2), dry or strange sensation in the mouth (type 3), abdominal pain (type 4), and alopecia (type 5)[18]. Patients 1 and 2 in the present study had types 1 and 2 CCS, respectively. Both patients had three main ectodermal changes, abdominal pain and nausea, with Patient 1 also having vomiting. Due to oral mucosal lesions, including inflammation and infection, and zinc and copper deficiency, patients may lose their taste[19]. Alopecia can be caused by malnutrition[20], whereas nail dystrophy has been associated with the bad nutritional status and inflammatory response[19]. Polyps in patients with CCS are distributed throughout the digestive tract, being common in the stomach and colon, less common in the small intestine and rectum, and almost nonexistent in the esophagus[10,21]. Polyps are usually diffusely distributed and nodular, with different shapes and sizes. Histologically observed glandular hyperplasia and cystic dilatation are accompanied by the infiltration of inflammatory cells, especially eosinophils. Both patients in the present study had both gastric and intestinal polyps, with findings on gastrointestinal endoscopy being consistent with CCS.
The pathology of CCS is not specific, with four histological types: hyperplastic, adenomatous, juvenile, and inflammatory polyps. The evolution of polyps may follow the mucosal hyperplasia (C-C polyps)-adenoma-carcinoma pathway[22]. About 12.5% of polyps are estimated to become cancerous, with this being a significant cause of death. Pathologic analysis of the polyps in Patient 1 showed tubular adenoma and moderate to severe epithelial dysplasia, complicated with an intestinal obstruction 4 mo after the diagnosis of CCS, suggesting malignant transformation of the adenoma. The rectal mass in Patient 2 was pathologically diagnosed as a high-grade intraepithelial neoplasia, necessitating immediate EMR. These findings emphasize the importance of close surveillance and prompt removal of polyps.
The average recovery times for diarrhea, taste abnormality, and ectodermal changes in patients with CCS are 51, 84, and 9 d, respectively, and the mean times to resolution of gastric and colonic polyps are 248 and 238 d, respectively[23]. Currently, there are no standard treatment guidelines, including duration of treatment, with most CCS patients receiving comprehensive empirical treatment based on glucocorticoids. Several studies have recommended treatment for 6–12 mo[24], suggesting that the steroid dose should be slowly tapered only after endoscopic confirmation of the regression of polyposis[25]. Although 30–49 mg/d oral prednisone was reported to have the optimal effect during the active stage of CCS[26], patients may relapse when glucocorticoid dose is gradually reduced. Additional studies in larger patient cohorts are needed to determine whether to use glucocorticoids, their duration and dose, regimens for reduction, and the need for maintenance therapy with other medications. Nutritional support is often combined with other treatments, making it difficult to accurately determine the effectiveness of nutritional support in patients with CCS[10]. Other treatments can include immunosuppressive agents, acid suppression, traditional Chinese medicines, salicylic acid preparations, TNF- inhibitors[27], and endoscopic or surgical treatment. Achieving a sustained endoscopic response is the therapeutic goal and associated with a reduced risk of cancer[4,25]. Kim et al[28] reported a successful case of fecal microbiota transplantation in the treatment of steroid-refractory CCS. The etiology and pathogenesis of this disease are not yet fully understood, and various other methods are still being explored.
In summary, CCS is a rare syndrome primarily affecting male patients with a relatively poor prognosis. Its etiology remains unclear, but current research suggests a strong association with autoimmunity. Based on the results of literature review in this article, it can be inferred that a clear diagnosis and treatment of hypothyroidism contribute to improving the prognosis. Clinical manifestations are diverse, including diarrhea, gastrointestinal polyps, skin hyperpigmentation, alopecia, and nail atrophy. Comprehensive treatment based on hormone therapy can lead to partial or complete remission of clinical symptoms. Polyps meeting the indications for endoscopic surgery should be actively treated surgically, which can prevent polyp malignancy and the occurrence of complications, such as intestinal obstruction and intussusception. Early diagnosis and treatment are crucial for inducing remission and improving disease prognosis. Long-term follow-up is necessary for subsequent treatment of this disease. In the future, this disease will still require further basic and clinical research, especially regarding its etiology and treatment approaches.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gluvic Z, Serbia; Hassan SA, United States; Osman Nuri Dilek, Turkey S-Editor: Lin C L-Editor: Filipodia P-Editor: Lin C
| 1. | Fan RY, Wang XW, Xue LJ, An R, Sheng JQ. Cronkhite-Canada syndrome polyps infiltrated with IgG4-positive plasma cells. World J Clin Cases. 2016;4:248-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Cronkhite LW Jr, Canada WJ. Generalized gastrointestinal polyposis; an unusual syndrome of polyposis, pigmentation, alopecia and onychotrophia. N Engl J Med. 1955;252:1011-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 247] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Schulte S, Kütting F, Mertens J, Kaufmann T, Drebber U, Nierhoff D, Töx U, Steffen HM. Case report of patient with a Cronkhite-Canada syndrome: sustained remission after treatment with corticosteroids and mesalazine. BMC Gastroenterol. 2019;19:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Watanabe C, Komoto S, Tomita K, Hokari R, Tanaka M, Hirata I, Hibi T, Kaunitz JD, Miura S. Endoscopic and clinical evaluation of treatment and prognosis of Cronkhite-Canada syndrome: a Japanese nationwide survey. J Gastroenterol. 2016;51:327-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 5. | Wu ZY, Sang LX, Chang B. Cronkhite-Canada syndrome: from clinical features to treatment. Gastroenterol Rep (Oxf). 2020;8:333-342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Kopáčová M, Urban O, Cyrany J, Laco J, Bureš J, Rejchrt S, Bártová J, Tachecí I. Cronkhite-Canada syndrome: review of the literature. Gastroenterol Res Pract. 2013;2013:856873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Onozato Y, Sasaki Y, Abe Y, Yaoita T, Yagi M, Mizumoto N, Shoji M, Kon T, Sakai T, Ueno Y. Cronkhite-Canada Syndrome Associated with Gastric Outlet Obstruction and Membranous Nephropathy: A Case Report and Review of the Literature. Intern Med. 2020;59:2871-2877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Berzin TM, Greenberger NJ, Levy BD, Loscalzo J. Clinical problem-solving. Worth a second look. N Engl J Med. 2012;366:463-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Qiao M, Lei Z, Nai-Zhong H, Jian-Ming X. Cronkhite-Canada syndrome with hypothyroidism. South Med J. 2005;98:575-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Okamoto K, Isomoto H, Shikuwa S, Nishiyama H, Ito M, Kohno S. A case of Cronkhite-Canada syndrome: remission after treatment with anti-Helicobacter pylori regimen. Digestion. 2008;78:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Murata I, Yoshikawa I, Endo M, Tai M, Toyoda C, Abe S, Hirano Y, Otsuki M. Cronkhite-Canada syndrome: report of two cases. J Gastroenterol. 2000;35:706-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Wen XH, Wang L, Wang YX, Qian JM. Cronkhite-Canada syndrome: report of six cases and review of literature. World J Gastroenterol. 2014;20:7518-7522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Takeuchi Y, Yoshikawa M, Tsukamoto N, Shiroi A, Hoshida Y, Enomoto Y, Kimura T, Yamamoto K, Shiiki H, Kikuchi E, Fukui H. Cronkhite-Canada syndrome with colon cancer, portal thrombosis, high titer of antinuclear antibodies, and membranous glomerulonephritis. J Gastroenterol. 2003;38:791-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Bandyopadhyay D, Hajra A, Ganesan V, Kar SS, Bhar D, Layek M, Mukhopadhyay S, Choudhury C, Choudhary V, Banerjee P. Cronkhite-Canada Syndrome: A Rare Cause of Chronic Diarrhoea in a Young Man. Case Rep Med. 2016;2016:4210397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Boland BS, Bagi P, Valasek MA, Chang JT, Bustamante R, Madlensky L, Sandborn WJ, Harismendy O, Gupta S. Cronkhite Canada Syndrome: Significant Response to Infliximab and a Possible Clue to Pathogenesis. Am J Gastroenterol. 2016;111:746-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Honjo H, Masuta Y, Otsuka Y, Masaki S, Minaga K, Kudo M, Watanabe T. Analyses of cytokine gene expression and fecal microbiota in a patient with Cronkhite-Canada syndrome successfully treated with prednisolone. DEN Open. 2024;4:e222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Dong J, Ma TS, Tu JF, Chen YW. Surgery for Cronkhite-Canada syndrome complicated with intussusception: A case report and review of literature. World J Gastrointest Surg. 2022;14:200-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 18. | Lu Y, Huang F, Wang Y, Zhou J, Zhao Q, Liu L. Clinical and Endoscopic Characteristics of Chinese Cronkhite-Canada Syndrome Patients: A Retrospective Study of 103 Cases. Dig Dis. 2021;39:488-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Chuamanochan M, Tovanabutra N, Mahanupab P, Kongkarnka S, Chiewchanvit S. Nail Matrix Pathology in Cronkhite-Canada Syndrome: The First Case Report. Am J Dermatopathol. 2017;39:860-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Watanabe-Okada E, Inazumi T, Matsukawa H, Ohyama M. Histopathological insights into hair loss in Cronkhite-Canada syndrome: diffuse anagen-telogen conversion precedes clinical hair loss progression. Australas J Dermatol. 2014;55:145-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Sweetser S, Ahlquist DA, Osborn NK, Sanderson SO, Smyrk TC, Chari ST; Boardman LA. Clinicopathologic features and treatment outcomes in Cronkhite-Canada syndrome: support for autoimmunity. Dig Dis Sci. 2012;57:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Nagata J, Kijima H, Hasumi K, Suzuki T, Shirai T, Mine T. Adenocarcinoma and multiple adenomas of the large intestine, associated with Cronkhite-Canada syndrome. Dig Liver Dis. 2003;35:434-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Hu H, Wu Y, Zhang Y, Zhang L, Zhang J, Zhang R. Comprehensive treatment of Cronkhite-Canada syndrome: A case report and literature review. Medicine (Baltimore). 2023;102:e32714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 24. | Sweetser S; Boardman LA. Cronkhite-Canada syndrome: an acquired condition of gastrointestinal polyposis and dermatologic abnormalities. Gastroenterol Hepatol (N Y). 2012;8:201-203. [PubMed] |
| 25. | Jiang D, Tang GD, Lai MY, Huang ZN, Liang ZH. Cronkhite-Canada syndrome with steroid dependency: A case report. World J Clin Cases. 2021;9:3466-3471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Yamakawa K, Yoshino T, Watanabe K, Kawano K, Kurita A, Matsuzaki N, Yuba Y, Yazumi S. Effectiveness of cyclosporine as a treatment for steroid-resistant Cronkhite-Canada syndrome; two case reports. BMC Gastroenterol. 2016;16:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Taylor SA, Kelly J, Loomes DE. Cronkhite-Canada Syndrome: Sustained Clinical Response with Anti-TNF Therapy. Case Rep Med. 2018;2018:9409732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Kim SY, Shin J, Park JS, Cha B, Seo Y, Park SH, Lee JH, Kim JS, Kwon G. The first report on effect of fecal microbiota transplantation as a complementary treatment in a patient with steroid-refractory Cronkhite-Canada syndrome: A case report. Medicine (Baltimore). 2022;101:e29135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Størset O, Todnem K, Waldum HL, Burhol PG, Kearney MS. A patient with Cronkhite-Canada syndrome, myxedema and muscle atrophy. Acta Med Scand. 1979;205:343-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Jones AF, Paone DB. Canada-Cronkhite syndrome in an 82-year-old woman. Am J Med. 1984;77:555-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Clinical Vignettes/Case Reports - Colon. Am J Gastroenterol. 2016;111:S592-S682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Irish Endocrine Society 42nd Annual Meeting, 19th and 20th October 2018. Ir J Med Sci. 2018;187:173-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Dawra S, Sharma V, Dutta U. Clinical and Endoscopic Remission in a Patient With Cronkhite-Canada Syndrome. Clin Gastroenterol Hepatol. 2018;16:e84-e85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |