Published online Nov 27, 2023. doi: 10.4240/wjgs.v15.i11.2413
Peer-review started: June 26, 2023
First decision: July 17, 2023
Revised: July 24, 2023
Accepted: August 15, 2023
Article in press: August 15, 2023
Published online: November 27, 2023
Processing time: 154 Days and 2.3 Hours
Gallbladder cancer (GC) is a common malignant tumor and one of the leading causes of cancer-related death worldwide. It is typically highly invasive, difficult to detect in the early stages, and has poor treatment outcomes, resulting in high mortality rates. The available treatment options for GC are relatively limited. One emerging treatment modality is hyperthermic intraperitoneal chemotherapy (HIPEC). HIPEC involves delivering heated chemotherapy directly into the abdominal cavity. It combines the strategies of surgical tumor resection and localized chemotherapy administration under hyperthermic conditions, aiming to enhance the concentration and effectiveness of drugs within the local tumor site while minimizing systemic toxicity.
To determine the effects of cytoreductive surgery (CRS) combined with HIPEC on the short-term prognosis of patients with advanced GC.
Data from 80 patients treated at the Punan Branch of Renji Hospital, Shanghai Jiao Tong University School of Medicine between January 2018 and January 2020 were retrospectively analyzed. The control group comprised 44 patients treated with CRS, and the research group comprised 36 patients treated with CRS combined with HIPEC. Then, the survival time and prognostic factors of the two groups were compared, as well as liver and kidney function indices before and six days after surgery. Adverse reactions and complications were recorded in both groups.
The baseline data of the research and control groups were similar (P > 0.05). Six days after surgery, the alanine aminotransferase, aspartate aminotransferase, total bilirubin, and direct bilirubin levels significantly decreased compared to the preoperative levels in both groups (P < 0.05). However, the values did not differ between the two groups six days postoperatively (P > 0.05). Similarly, the postoperative creatinine and blood urea nitrogen levels were significantly lower than the preoperative levels in both groups (P < 0.05), but they did not differ between the groups six days postoperatively (P > 0.05). Furthermore, the research group had fewer postoperative adverse reactions than the control group (P = 0.027). Finally, a multivariate Cox analysis identified the tumor stage, distant metastasis, and the treatment plan as independent factors affecting prognosis (P < 0.05). The three-year survival rate in the study group was higher than that in the control group (P = 0.002).
CRS combined with HIPEC lowers the incidence of adverse reactions and improves survival in patients with advanced GC.
Core Tip: This study explores the potential benefits of combining cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in treating gallbladder cancer (GC). Our results show that this combined approach may increase the median overall survival and three-year survival rates, and may decrease the incidence of postoperative complications. While the results are promising, this represents a step forward in GC treatment and suggests a possible new therapeutic strategy that warrants further investigation.
- Citation: Wu JX, Hua R, Luo XJ, Xie F, Yao L. Effects of cytoreductive surgery combined with hyperthermic perfusion chemotherapy on prognosis of patients with advanced gallbladder cancer. World J Gastrointest Surg 2023; 15(11): 2413-2422
- URL: https://www.wjgnet.com/1948-9366/full/v15/i11/2413.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i11.2413
The incidence of gallbladder cancer (GC) ranks first among all biliary tract tumors and is frequently observed in female patients. Additionally, cholecystolithiasis with chronic inflammation is a comorbidity considered a high-risk factor for GC[1]. With symptoms similar to those of biliary colic and chronic cholecystitis, GC can easily go undiagnosed or misdiagnosed. Moreover, this type of cancer is strongly invasive and develops rapidly; therefore, patients often have middle- or late-stage disease at diagnosis[2]. Approximately 30% of patients with GC in China complain of gallstones, inflammation, or polyps at the time of diagnosis, and some patients are unexpectedly diagnosed with GC on pathological examination after cholecystectomy in the hospital[3].
Surgical resection is the only curative treatment for GC. However, GC is highly malignant, can easily invade adjacent organs, and has a high lymph node metastasis rate and poor adjuvant treatment effect. Therefore, the prognosis is usually unfavorable, and the overall five-year survival rate is < 5%[4]. In cases of tumor recurrence, systemic therapy can be selected based on pathological characteristics to prolong survival time[5]. Therefore, close postoperative follow-up is crucial. Reoperation may not be ideal for patients with recurrent or metastatic GC. Traditional systemic chemotherapy has many side effects; therefore, it is crucial to choose postoperative adjuvant therapies with mild side effects and good curative effects[6,7].
Many years of research have resulted in hyperthermic intraperitoneal chemotherapy (HIPEC), a relatively new technology that has become crucial for treating intra-abdominal tumors[8]. After more than 40 years of clinical application and the continual development of perfusion technology, the consensus is that HIPEC significantly affects peritoneal cavity metastasis in patients with digestive tract tumors[9]. Existing level I evidence shows that HIPEC can treat and prevent peritoneal implantation of malignant peritoneal tumors and reduce the incidence of peritoneal metastasis[10]. Over time, HIPEC has been gradually applied to the gastrointestinal tract, gynecological tumors, and other fields, and its clinical efficacy and safety have been widely recognized[11]. Most studies on gynecological malignant tumors, especially advanced ovarian cancer[12], have reported good curative effects; thus, HIPEC has become the first-line treatment for gynecological malignant tumors. Since GC has a high degree of malignancy and metastasis rate, it requires further postoperative consolidation or adjuvant therapy to prolong survival and improve the patient’s quality of life[11]. Currently, postoperative adjuvant therapy is mainly systemic chemotherapy; however, some patients experience many intolerable side effects, compromising their quality of life. HIPEC for gastrointestinal and gynecological malignant tumors has delivered good results; therefore, we speculate that it is feasible to adopt HIPEC as an auxiliary treatment after GC surgery. The curative effects of HIPEC after GC surgery need to be clarified. Therefore, this study analyzed the short-term effects of cytoreductive surgery (CRS) combined with HIPEC in patients with advanced GC to provide a new, alternative treatment plan.
Data from 122 patients with advanced GC treated at the Punan Branch of Renji Hospital, Shanghai Jiao Tong University School of Medicine between January 2018 and January 2020 were analyzed retrospectively. Our hospital’s medical ethics committee approved this study.
The inclusion criteria were patients: (1) With confirmed GC through postoperative pathology; (2) With tumor, node, metastasis (TNM) stage III or IV disease; (3) Between 18 and 80 years old; (4) Who had not received radiotherapy, chemo
The exclusion criteria were patients: (1) With TNM stage I or II disease; (2) With abnormalities in the heart, lung, brain, liver, kidney, or other important organs; (3) With severe abdominal adhesion; (4) With other malignant tumors; (5) With blood system diseases or blood coagulation insufficiency; (6) With intestinal obstruction; (7) With cachexia; and (8) Who had received chemotherapy, radiotherapy or other adjuvant therapy after surgery.
Eighty patients who met these requirements were screened based on their electronic medical records. Among them, 44 patients treated with CRS were enrolled in the control group, and 36 patients treated with CRS combined with HIPEC were enrolled in the research group.
Clinical data of the patients were collected, including age, sex, tumor stage, degree of differentiation, comorbidities, tumor size, lymph node metastasis, nerve invasion, distal metastasis, and vascular invasion. In addition, changes in liver function indices before and after surgery were collected.
The primary outcome measures were survival time and prognosis. The secondary outcome measures were comparisons of the clinical data between the two groups, including indices related to liver and kidney function before surgery and six days after surgery. Adverse reactions and complications were also recorded in both groups.
Re check the patient in the outpatient department according to the doctor’s suggestion, or follow up the patient through telephone communication after discharge. The patients were followed up at 1, 2, 3, 6, 9, 12, 18, 24, 30 and 36 mo after operation. Patients who could not return to the hospital on time or regularly were followed up by telephone, and the circumstances of their recent situation were noted. This study followed each patient for three years or until death.
All statistical analyses were performed with SPSS version 26.0 (IBM SPSS Inc., Chicago, United States). Measurement data are presented as means ± SD. All data were subjected to normality tests and analyzed using independent-sample t-tests. Countable data were analyzed using χ2 tests. Survival time was analyzed based on the follow-up results, and the survival rates of the two groups were compared using the log-rank method. Survival curves of the two groups were plotted using the Kaplan-Meier method. Multivariate Cox regression analysis was performed to identify independent risk factors affecting patient prognosis. P-vales of < 0.05 were considered significant.
The research and control groups had similar baseline data (P > 0.05, Table 1).
| Factors | Control group (n = 44) | Research group (n = 36) | χ2 value | P value | |
| Age | < 60 yr | 17 | 19 | 1.600 | 0.205 |
| ≥ 60 yr | 27 | 17 | |||
| Gender | Male | 18 | 18 | 0.661 | 0.416 |
| Female | 26 | 18 | |||
| Tumor staging | Stage III | 21 | 15 | 0.293 | 0.587 |
| Stage IV | 23 | 21 | |||
| Differentiation degree | Low | 24 | 12 | 3.600 | 0.057 |
| Moderate | 20 | 24 | |||
| Comorbid stones | Yes | 39 | 29 | 1.014 | 0.313 |
| No | 5 | 7 | |||
| Tumor size | < 5 cm | 25 | 22 | 0.150 | 0.698 |
| ≥ 5 cm | 19 | 14 | |||
| Lymph node metastasis | Yes | 37 | 29 | 0.171 | 0.678 |
| No | 7 | 7 | |||
| Nerve invasion | Yes | 27 | 20 | 0.275 | 0.599 |
| No | 17 | 16 | |||
| Distant metastasis | Yes | 30 | 23 | 0.163 | 0.686 |
| No | 14 | 13 | |||
| Vascular invasion | Yes | 22 | 16 | 0.245 | 0.620 |
| No | 22 | 20 | |||
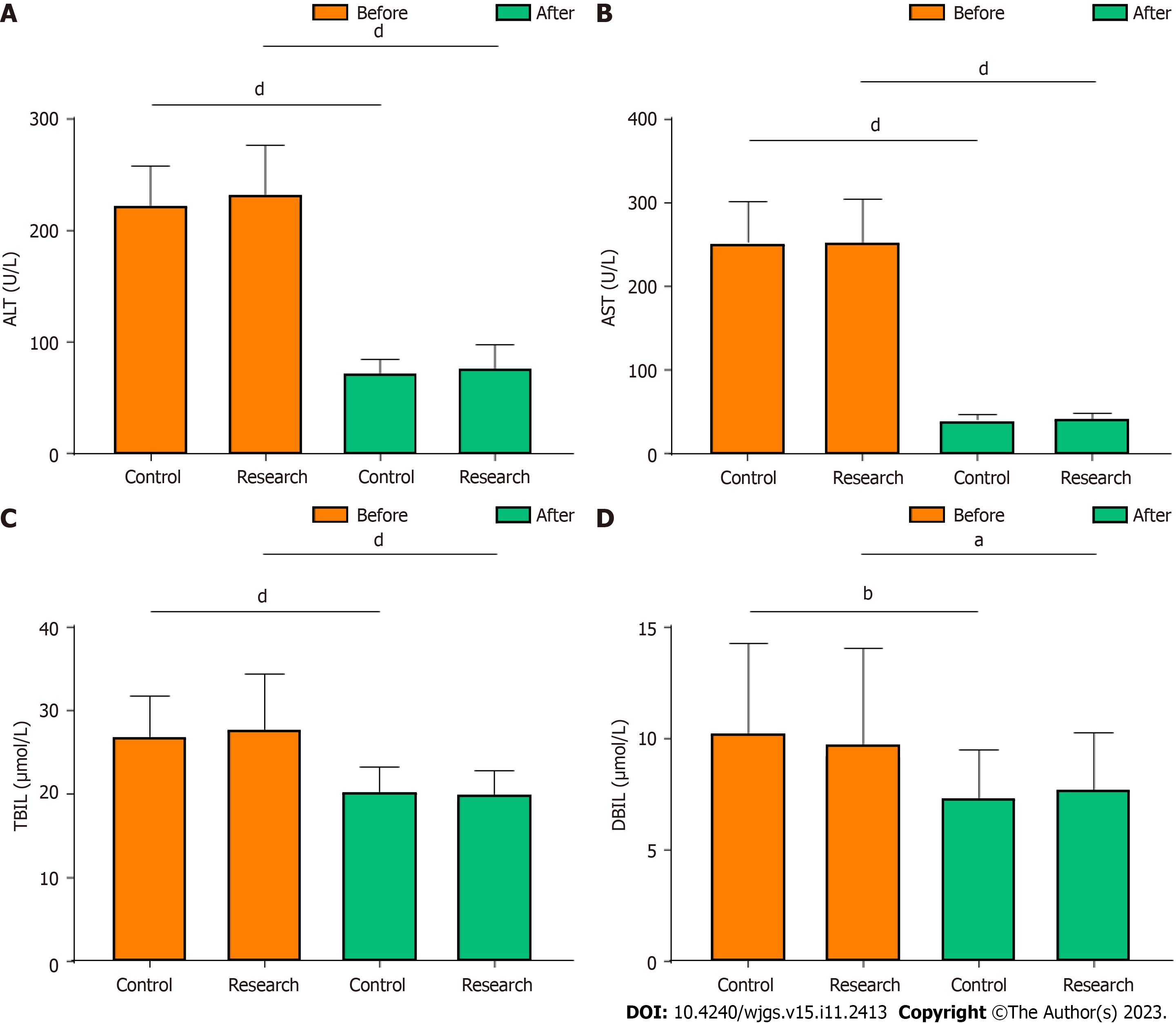
Liver function-related indices were compared between the two groups before and six days after surgery. The alanine aminotransferase, aspartate aminotransferase, total bilirubin, and direct bilirubin levels were significantly lower after surgery than before surgery in both groups (P < 0.05, Figure 1). However, after surgery, the levels did not differ between the two groups (P > 0.05, Figure 1).
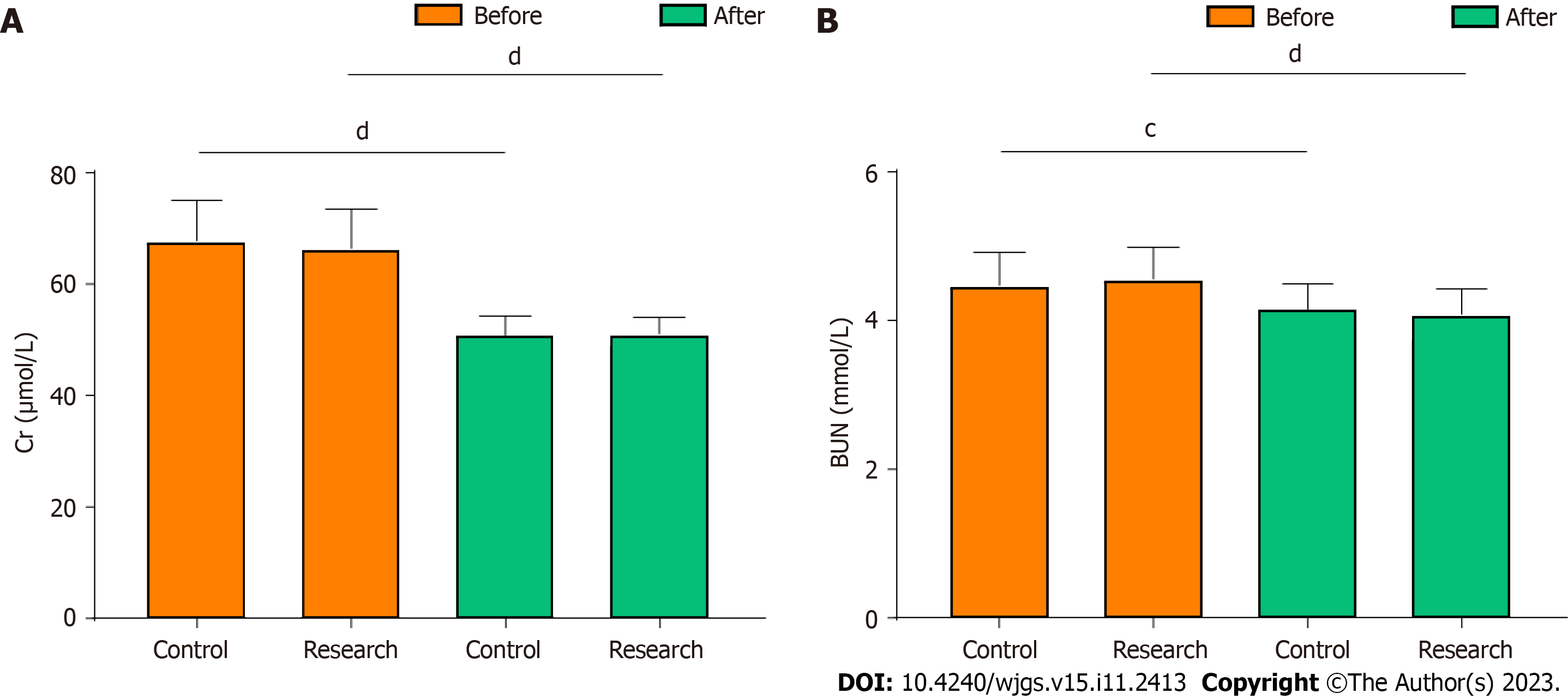
The renal function-related indices were compared between the two groups before and six days after surgery. The creatinine and blood urea nitrogen levels were significantly lower after surgery than before surgery in both groups (P < 0.05, Figure 2). However, after surgery, the levels did not differ between the two groups (P > 0.05, Figure 2).
The research group has notably fewer adverse reactions than the control group after surgery (P = 0.027, Table 2).
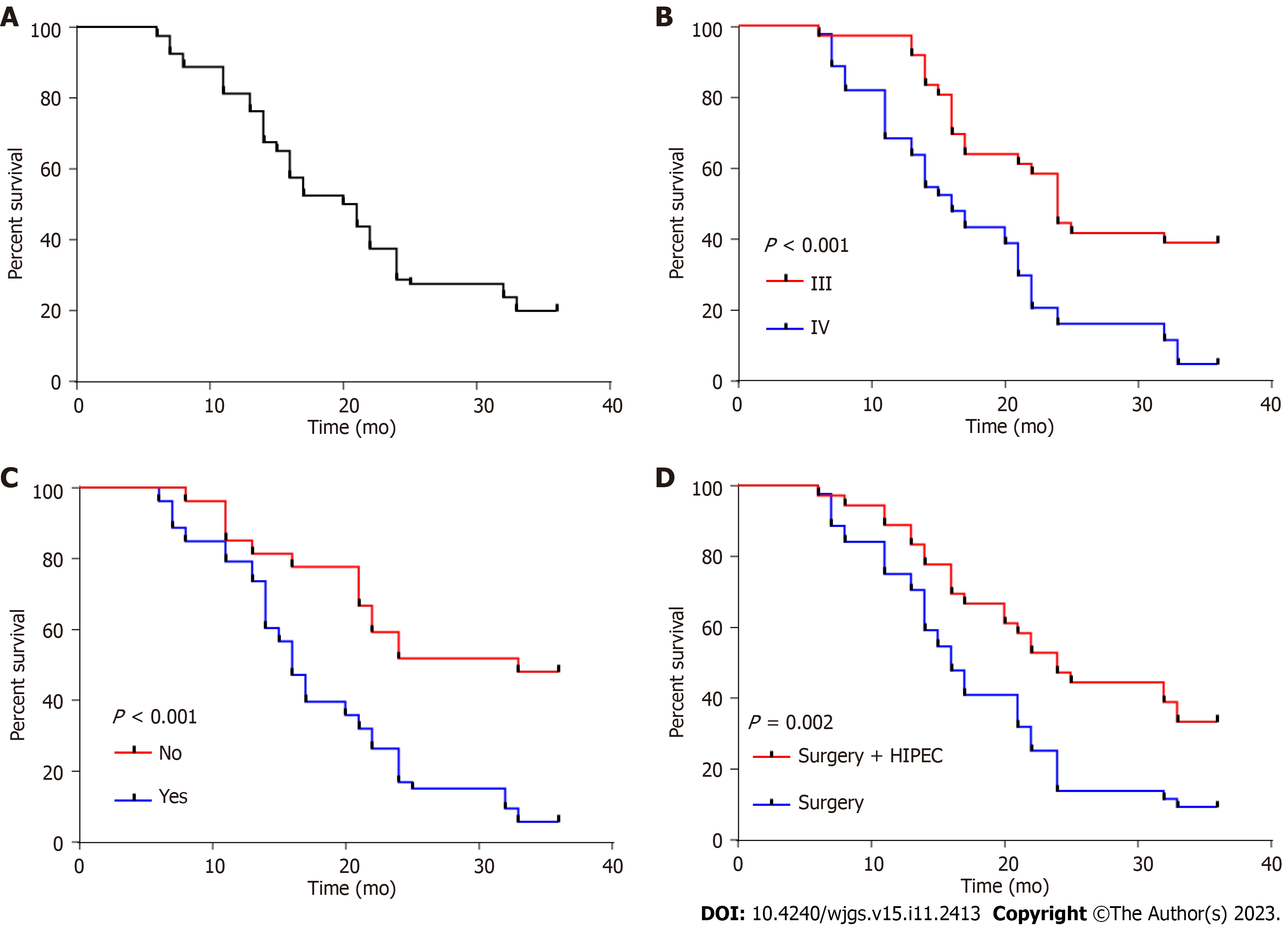
Patient survival was analyzed by Cox regression analysis; the univariate analysis identified the treatment plan, tumor stage, lymph node metastasis, distant metastasis, and vascular invasion as factors affecting prognosis (P < 0.05, Table 3). However, the multivariate analysis only identified the tumor stage, distant metastasis, and treatment plan as independent prognostic factors (P < 0.05, Table 4).
| Factors | β | SE | χ2 value | P value | HR | 95%CI | |
| Lower limit | Upper limit | ||||||
| Age | 0.111 | 0.253 | 0.195 | 0.659 | 1.118 | 0.681 | 1.834 |
| Sex | -0.308 | 0.255 | 1.460 | 0.227 | 0.735 | 0.446 | 1.211 |
| Tumor staging | 1.212 | 0.271 | 19.953 | < 0.001b | 3.360 | 1.974 | 5.719 |
| Differentiation degree | 0.255 | 0.251 | 1.026 | 0.311 | 1.290 | 0.788 | 2.111 |
| Comorbid stones | 0.126 | 0.360 | 0.122 | 0.727 | 1.134 | 0.560 | 2.296 |
| Tumor size | -0.218 | 0.254 | 0.739 | 0.390 | 0.804 | 0.489 | 1.322 |
| Lymph node metastasis | 1.380 | 0.469 | 8.650 | 0.003a | 3.976 | 1.585 | 9.977 |
| Nerve invasion | 0.345 | 0.257 | 1.794 | 0.180 | 1.411 | 0.853 | 2.336 |
| Distant metastasis | 1.143 | 0.308 | 13.73 | < 0.001b | 3.135 | 1.713 | 5.739 |
| Vascular invasion | 0.797 | 0.260 | 9.360 | 0.002a | 2.218 | 1.332 | 3.696 |
| Treatment plan | 0.740 | 0.262 | 7.960 | 0.005a | 2.095 | 1.253 | 3.503 |
| Factors | β | SE | χ2 value | P value | HR | 95% CI | |
| Lower limit | Upper limit | ||||||
| Distant metastasis | 0.802 | 0.319 | 6.309 | 0.012 | 2.230 | 1.193 | 4.171 |
| Lymph node metastasis | 0.449 | 0.509 | 0.777 | 0.378 | 1.566 | 0.578 | 4.247 |
| Distant metastasis | 0.900 | 0.312 | 8.289 | 0.004a | 2.459 | 1.333 | 4.536 |
| Vascular invasion | 0.440 | 0.273 | 2.599 | 0.107 | 1.552 | 0.909 | 2.65 |
| Treatment plan | 1.068 | 0.282 | 14.377 | < 0.001b | 2.908 | 1.675 | 5.05 |
In the final analysis of the study, Cox regression analysis was performed to examine prognostic indicators related to patient outcomes. The results indicated that patients with stage III tumors, no distal metastases and those who received CRS had significantly higher 3-year survival rates compared to their respective control groups (P < 0.01, Figure 3).
GC has an extremely low incidence (less than 4%); however, its five-year survival rate does not exceed 5%[14]. Most patients have middle- or late-stage disease at the first visit; thus, their survival time does not exceed one year[15]. Surgical resection is the only treatment option for GC. A simple cholecystectomy is feasible in the early stages without obvious metastasis[16]. However, surgery is more complicated for patients with metastasis regardless of the stage; if the imaging examination determines that the tumor is resectable and there are no absolute surgical contraindications, then organs with a possible correlation with tumor invasion should also be resected to ensure complete resection of the tumor[17]. In these cases, extended CRS of the GC, including the gallbladder, tumor, adjacent organs, lymph nodes, ligaments, and bile duct, is required. Additionally, the lymph nodes around the bile duct, portal vein, and duodenum should be resected as much as possible[4]. However, less than 10% of patients undergo tumor resection through surgery, and nearly 50% have lymph node metastasis[18].
During surgery, instruments may touch cancer cells at the margin, causing them to fall off and form an implantation metastasis[19]. Additionally, tumor dissection can sometimes increase intraperitoneal spread and growth of GC cells owing to their aggressive invasion and adhesion or growth variation. Systemic chemotherapy is frequently administered to patients with unresectable tumors or surgical contraindications. However, the effect of radiotherapy and chemo
HIPEC is a novel method that combines chemical and physical therapy[22]. This treatment takes advantage of the destructive effect of high temperature on tumor cells, killing them by adding heated chemotherapy drugs into the abdominal cavity[23]. Additionally, high temperatures promote the diffusion of chemotherapy drugs, so the drugs act on tumor cells more effectively. HIPEC treatment can also involve continuous washing through a power pump to increase the probability of contact between the chemotherapeutic drugs and tumor cells. As a result, the chemotherapeutic drugs are maintained at an effective concentration and produce stronger lethality. Compared with intravenous chemotherapy, HIPEC treatment alleviates adverse reactions and improves patient tolerance, delivering a more effective treatment for malignant abdominal tumors[24]. Therefore, HIPEC therapy is a safe and effective treatment suitable for malignant abdominal tumors. In the present study, the incidence of adverse reactions was notably lower in the research group than in the control group. However, liver and kidney function did not differ between the two groups after treatment. None
Finally, we analyzed the prognostic factors affecting three-year patient survival. The tumor stage, distant metastasis, and treatment plan were independent factors affecting prognosis. Many reports have suggested a correlation between tumor stage, distant metastasis, and prognosis in patients with advanced GC[19,25,26]. This study found that CRS com
Although this study confirmed that CRS combined with HIPEC improves the survival time of patients with advanced GC, there are some limitations. First, this was a single-center study with limited data, which may have resulted in bias in the analysis. Second, this study did not obtain long-term follow-up data from the patients. Therefore, whether the surgical plan affects the long-term prognosis requires further verification. We hope to conduct a prospective study with a longer follow-up period to improve our conclusions.
In summary, CRS combined with HIPEC decreases the incidence of adverse reactions and improves survival in patients with advanced GC. Although our study shows the encouraging results of CRS-HIPEC treatment for GC treatment, however, we understand that as a new therapeutic strategy, the long-term impact and efficacy of it need to be verified by further clinical studies.
Gallbladder cancer (GC) is one of the most deadly malignancies worldwide, with a high incidence of peritoneal meta
The primary research question was: Is CRS combined with HIPEC efficient and safe for managing GC? The key issues for resolution are the benefits and risks of this combined approach, including its effect on survival rates and postoperative complications compared with CRS. Addressing these questions is paramount to providing evidence-based guidance for clinicians for GC management; it could also potentially revolutionize the treatment paradigm and improve survival rates and quality of life for these patients. Moreover, it opens avenues for further research to optimize CRS-HIPEC protocols and identify patient subgroups that could benefit the most from this approach.
The main objective of this study was to evaluate the efficacy and safety of CRS combined with HIPEC for managing GC. We aimed to comprehensively understand the impact of CRS-HIPEC on survival rates and postoperative complications, which is crucial for future research as it informs clinical decision-making and establishes a foundation for refining trea
This study employed a retrospective analysis of the medical records of patients with GC treated with CRS-HIPEC. This method allows for a thorough examination of patient outcomes and treatment complications. Additionally, this study includes a comparative analysis of patients receiving CRS, highlighting the novel aspects of the CRS-HIPEC approach.
CRS-HIPEC significantly improved the survival rates of patients with GC. This study also highlighted an increased risk of certain postoperative complications. These results contribute to the field by providing empirical evidence for the efficacy and safety of CRS-HIPEC.
CRS-HIPEC significantly improves survival rates but with certain risks. Furthermore, the results of this study underscore the need for personalized patient selection to maximize benefits and minimize complications.
Future research should focus on optimizing CRS-HIPEC protocols and developing criteria for patient selection, which would enhance the benefits of this approach and mitigate the potential risks. Prospective, randomized controlled trials are also needed to corroborate these findings.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Inchingolo F, Italy; Lehrskov LL, Denmark S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Nakanuma Y, Sugino T, Nomura Y, Watanabe H, Terada T. Polypoid invasive carcinoma of the gallbladder-Another challenging polypoid neoplasm. J Hepatobiliary Pancreat Sci. 2022;29:531-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Roa JC, Basturk O, Adsay V. Dysplasia and carcinoma of the gallbladder: pathological evaluation, sampling, differential diagnosis and clinical implications. Histopathology. 2021;79:2-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 3. | Liu W, Chen W, Chen J, Hong T, Li B, Qu Q, He X. Neuroendocrine carcinoma of gallbladder: a case series and literature review. Eur J Med Res. 2019;24:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Cai XC, Wu SD. Gallbladder neuroendocrine carcinoma diagnosis, treatment and prognosis based on the SEER database: A literature review. World J Clin Cases. 2022;10:8212-8223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (2)] |
| 5. | Utsumi M, Aoki H, Nishimura S, Une Y, Kashima H, Kimura Y, Taniguchi F, Arata T, Katsuda K, Tanakaya K. Safety of Surgical Treatment for Elderly Patients with Gallbladder Carcinoma. Acta Med Okayama. 2019;73:241-246. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | You YH, Choi DW, Heo JS, Han IW, Choi SH, Jang KT, Han S. Can surgical treatment be justified for neuroendocrine carcinoma of the gallbladder? Medicine (Baltimore). 2019;98:e14886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Melillo A, Linden K, Spitz F, Atabek U, Gaughan J, Hong YK. Disparities in Treatment for Gallbladder Carcinoma: Does Treatment Site Matter? J Gastrointest Surg. 2020;24:1071-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Choi G, Jang S, Choi M, Yang S, Lee C, Kang CM. Curative intent radical cholecystectomy followed by hyperthermic intraperitoneal chemotherapy in ruptured intraductal papillary neoplasm of gallbladder with invasive carcinoma. Ann Hepatobiliary Pancreat Surg. 2022;26:113-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Randle RW, Levine EA, Clark CJ, Stewart JH, Shen P, Votanopoulos KI. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for gallbladder cancer: a retrospective review. Am Surg. 2014;80:710-713. [PubMed] |
| 10. | Leigh N, Solomon D, Pletcher E, Labow DM, Magge DR, Sarpel U, Golas BJ. Is cytoreductive surgery and hyperthermic intraperitoneal chemotherapy indicated in hepatobiliary malignancies? World J Surg Oncol. 2020;18:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Amblard I, Mercier F, Bartlett DL, Ahrendt SA, Lee KW, Zeh HJ, Levine EA, Baratti D, Deraco M, Piso P, Morris DL, Rau B, Tentes AAK, Tuech JJ, Quenet F, Akaishi E, Pocard M, Yonemura Y, Lorimier G, Delroeux D, Villeneuve L, Glehen O, Passot G; PSOGI and BIG RENAPE working groups. Cytoreductive surgery and HIPEC improve survival compared to palliative chemotherapy for biliary carcinoma with peritoneal metastasis: A multi-institutional cohort from PSOGI and BIG RENAPE groups. Eur J Surg Oncol. 2018;44:1378-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, de Hingh IHJT, van der Velden J, Arts HJ, Massuger LFAG, Aalbers AGJ, Verwaal VJ, Kieffer JM, Van de Vijver KK, van Tinteren H, Aaronson NK, Sonke GS. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N Engl J Med. 2018;378:230-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 986] [Article Influence: 140.9] [Reference Citation Analysis (0)] |
| 13. | Daoud AMO, Khalaf M, Nassar M. Limitations of the Karnofsky Performance Status Scale in kidney transplant recipients. Ann Med. 2022;54:1328-1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 14. | Vidal Panduro DA, Zegarra Buitron E, Cochella Tizon OJ, Morales Luna DA. Neuroendocrine Carcinoma of the Gallbladder. Cureus. 2022;14:e27022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 15. | Wang X, Liu C, Chen J, Chen L, Ren X, Hou M, Cui X, Jiang Y, Liu E, Zong Y, Duan A, Fu X, Yu W, Zhao X, Yang Z, Zhang Y, Fu J, Wang H. Single-cell dissection of remodeled inflammatory ecosystem in primary and metastatic gallbladder carcinoma. Cell Discov. 2022;8:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 16. | Rennie AT, Halbreich SL. Rare Case of Gallbladder Neuroendocrine Carcinoma. Cureus. 2022;14:e28531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Poddar E, Mainali P, Shrestha S, Gautam P, Twayana A, Pathak N, Tiwari A, Bhattarai A, Awale L, Kansakar PS. Xanthogranulomatous Cholecystitis Mimicking Carcinoma Gallbladder. Case Reports Hepatol. 2023;2023:2507130. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Xing J, Ding P, Wan X, Xu G, Mao Y, Sang X, Du S, Yang H. Application and Progress of Cultured Models of Gallbladder Carcinoma. J Clin Transl Hepatol. 2023;11:695-704. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Ramalhosa F, Amaral MJ, Serôdio M, Oliveira RC, Teixeira P, Cipriano MA, Tralhão JG. Clinicopathological prognostic factors for gallbladder carcinoma: a retrospective study. J Gastrointest Oncol. 2022;13:1997-2006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 20. | Shao J, Lu HC, Wu LQ, Lei J, Yuan RF, Shao JH. Simple cholecystectomy is an adequate treatment for grade I T1bN0M0 gallbladder carcinoma: Evidence from 528 patients. World J Gastroenterol. 2022;28:4431-4441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 21. | Guo L, Zhang J, Liu X, Liu H, Zhang Y, Liu J. Successful Treatment of Metastatic Gallbladder Carcinoma with PD-L1 Expression by the Combination of PD-1 Inhibitor Plus Bevacizumab with Chemotherapy: A Case Report. Onco Targets Ther. 2022;15:629-636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Filis P, Mauri D, Markozannes G, Tolia M, Filis N, Tsilidis K. Hyperthermic intraperitoneal chemotherapy (HIPEC) for the management of primary advanced and recurrent ovarian cancer: a systematic review and meta-analysis of randomized trials. ESMO Open. 2022;7:100586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 54] [Reference Citation Analysis (0)] |
| 23. | Kunte AR, Parray AM, Bhandare MS, Solanki SL. Role of prophylactic HIPEC in non-metastatic, serosa-invasive gastric cancer: a literature review. Pleura Peritoneum. 2022;7:103-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 24. | Zhang JF, Lv L, Zhao S, Zhou Q, Jiang CG. Hyperthermic Intraperitoneal Chemotherapy (HIPEC) Combined with Surgery: A 12-Year Meta-Analysis of this Promising Treatment Strategy for Advanced Gastric Cancer at Different Stages. Ann Surg Oncol. 2022;29:3170-3186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 25. | Feroz Z, Gautam P, Tiwari S, Shukla GC, Kumar M. Survival analysis and prognostic factors of the carcinoma of gallbladder. World J Surg Oncol. 2022;20:403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Xiang F, Liang X, Yang L, Liu X, Yan S. Contrast-enhanced CT radiomics for prediction of recurrence-free survival in gallbladder carcinoma after surgical resection. Eur Radiol. 2022;32:7087-7097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |