Published online Oct 27, 2023. doi: 10.4240/wjgs.v15.i10.2376
Peer-review started: July 1, 2023
First decision: August 4, 2023
Revised: August 17, 2023
Accepted: August 23, 2023
Article in press: August 23, 2023
Published online: October 27, 2023
Processing time: 117 Days and 12.5 Hours
Dedifferentiated liposarcoma (DDLS) has a worse prognosis and occurs most commonly in the retroperitoneal region and rarely in the intraperitoneal region. Histological diagnosis was revolutionized by the combined contributions of histo-immuno-chemistry and molecular biology. Aside from surgery, there is no con
A thirty-year-old black female presented with a large painful abdominal mass occupying nearly the entire abdomen and progressive weight loss was admitted for surgery. Abdominal computed tomography showed a large heterogeneous mass of the mesentery that was sized 18 cm × 16 cm in size and had heterogeneous contrast enhancement. During laparotomy, en bloc excision of the large and multilobulated gastrocolic ligament mass was performed. The initial post
Dedifferentiated liposarcomas are rare tumours that typically originate in the retroperitoneum but may arise in unexpected locations.
Core Tip: Dedifferentiated liposarcoma has a worse prognosis and occurs most commonly in a retroperitoneal location but rarely in an intraperitoneal location. Complete surgical excision with a negative microscopic margin (R0) remains the ideal treatment when the tumour is still localized. Here, we report the case of a young woman with disseminated giant dedifferentiated liposarcoma of the gastrocolic ligament. This case demonstrates the poor prognosis of dedifferentiated liposarcomas. To the best of our knowledge, a giant dedifferentiated liposarcoma of the gastrocolic ligament has not yet been reported.
- Citation: Kassi ABF, Yenon KS, Kassi FMH, Adjeme AJ, Diarra KM, Bombet-Kouame C, Kouassi M. Giant dedifferentiated liposarcoma of the gastrocolic ligament: A case report. World J Gastrointest Surg 2023; 15(10): 2376-2381
- URL: https://www.wjgnet.com/1948-9366/full/v15/i10/2376.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i10.2376
Liposarcoma is a rare malignant tumour of mesodermic origin that derives from adipose tissue[1,2]. Well-differentiated and dedifferentiated liposarcomas (DDLSs) are the most common retroperitoneal types[3]. Liposarcoma is one of the most common soft-tissue sarcomas that mostly affects adults in their 50s or 60s[4,5].
DDLS, a variant of malignant adipocytic tumours, has a worse prognosis and occurs most commonly in a retroperitoneal location but rarely in an intraperitoneal location[6,7,8,9]. Typically in these locations, the tumour is paucisymptomatic or even asymptomatic until it becomes large enough to compress the surrounding organs[10]. The challenges of histological diagnosis and the lack of consensus on the optimal therapy complicate the management of this cancer[8,11]. Histological diagnosis was revolutionized by the combined contributions of histo-immuno-chemistry and molecular biology[12]. When the tumour is still localized, complete surgical excision with a negative microscopic margin (R0) remains the ideal treatment[10,13]. Additional surgery, chemotherapy, radiotherapy and targeted therapies are also useful in the treatment of advanced or metastatic forms[11,14,15].
Herein, we report the case of a thirty-year-old black female with a giant DDLS of the gastrocolic ligament. To the best of our knowledge, DDLS originating from this unusual location has not been reported.
A thirty-year-old black female was admitted to our surgical unit with a large painful abdominal mass occupying nearly the entire abdomen.
This abdominal mass appeared eight months earlier. A gradual increase in its volume had been associated with the onset of abdominal pain, constipation and progressive weight loss.
The patient had a good health history.
No notable events were mentioned in her personal and family history.
The patient was visibly underweight. She presented with an irregular and painful hard abdominal mass with a diameter of approximately 28 cm.
Blood analysis revealed severe nutritional impairment and anaemia. The total protein level was 4.2 g/dL (normal range: 5.8-6.5 g/dL), the albumin level was 2.8 mg/dL (normal range: 3.5-5.5 mg/dL) and the haemoglobin level was 10.5 g/dL (normal range: 12-16 mg/dL).
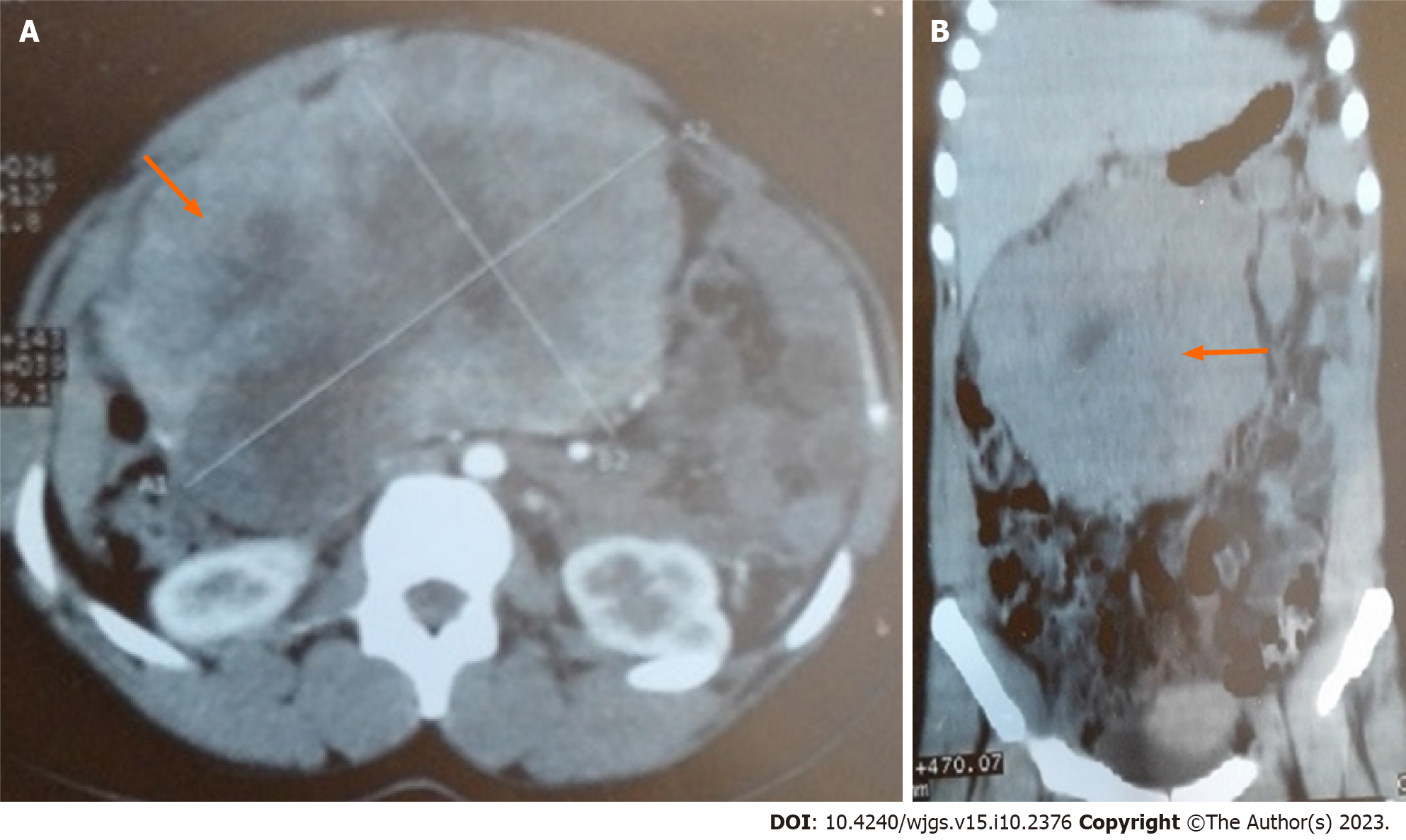
Abdominal computed tomography (CT) showed a large heterogeneous mesentery mass measuring 18 cm × 16 cm × 10.4 cm, and it was compressing the third part of the duodenum with heterogeneous contrast enhancement (Figure 1).
The final diagnosis of the presented case was DDLS of the gastrocolic ligament.
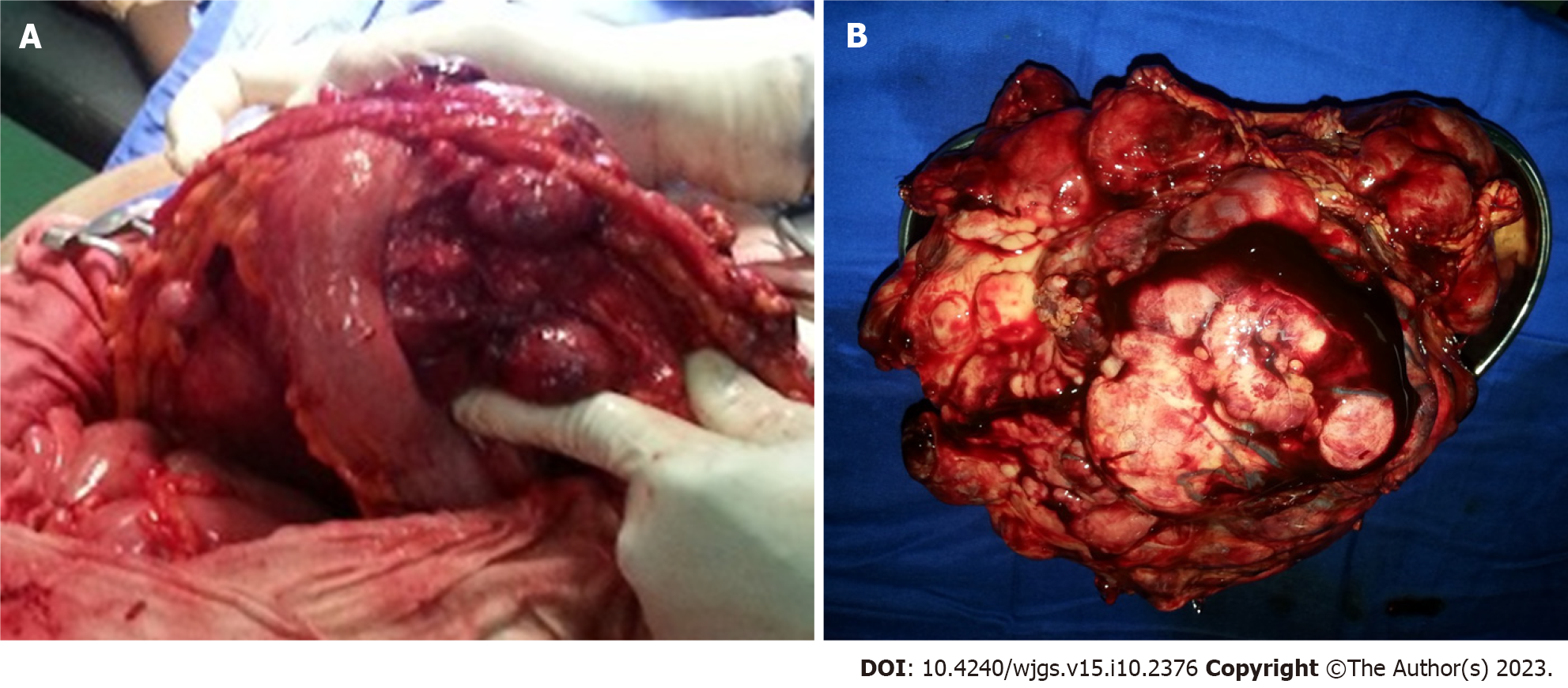
At laparotomy, a large multilobulated mass that was sized 30 cm × 20 cm × 12 cm and arose from the gastrocolic ligament was found (Figure 2A). In addition, many other smaller synchronous lesions were discovered in the liver, small bowel mesentery and peritoneum. An en bloc excision of the giant mass of the gastrocolic ligament was carried out, and associated biopsies of the small lesions were taken. The other smaller lesions virtually ruled out any possibility of curative excision.
Symptomatic treatment, such as nutritional support, was given postoperatively. After two days, the patient was gradually redirected to a normal diet. The patient was discharged 8 d after surgery without any complications. The patient did not undergo any adjuvant treatment for economic reasons. Three months later, follow-up abdominal CT studies revealed multiple peritoneal and vascular implants. The tumour followed an aggressive evolution with diffuse metastasis, causing the death of the patient less than 5 mo after the operation.
Macroscopic examination showed a large yellowish, nodular and myxoid mass with focal areas of haemorrhage and necrosis (Figure 2B).
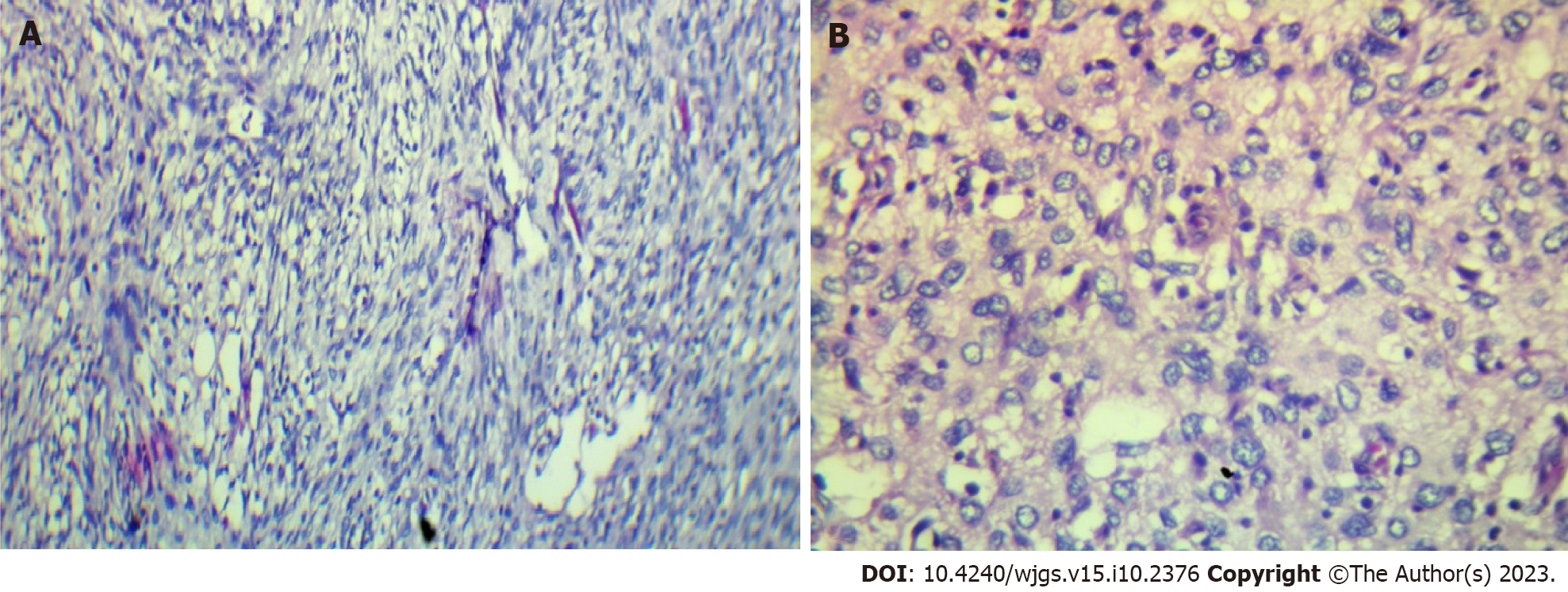
The histological examination revealed tumorous nodules that were poorly limited, often haemorrhagic, and located in fibrous or fibro-adipose tissue or in the peritoneum. The tumour proliferation was made up of masses with some fusiform cells (Figure 3A), which were sometimes more epithelioid (Figure 3B) and sometimes had pleomorphic nuclei. The mitotic rate was 10/10 per high power field. The tumour was not differentiated and was largely vascularized without necrosis. Immunohistochemical staining revealed that the tumour cells were negative for actin, desmin, calretinin, PS100, melana A, ERG, P63, AE1/AE3, EMA, chromogranin A and CDK4. BAP1 and P16 were partially positive in the neoplastic cells, while MDM2 was strongly expressed. The histo-pathological diagnosis was initially an undifferentiated sarcoma with fusiform and pleomorphic cells. The tumour was reclassified as DDLS according to the histopathological report and immu
The patient’s young age, unlike the cases usually reported and the rarity of its location at the gastrocolic ligament, makes our observation peculiar. Liposarcoma is the most common soft-tissue sarcoma in adults, with a peak incidence in the 5th-6th decade of life[4,5]. According to the World Health Organization, liposarcoma is classified into five main subtypes: Well-differentiated, dedifferentiated, myxoid, pleomorphic, and myxoid pleomorphic[6,14]. DDLS is a higher grade, often nonlipogenic, sarcoma with metastatic potential that is genetically similar to well-differentiated liposarcoma[16]. However, DDLS arises de novo in more than 90% of cases[17] and can exist without any well-differentiated cells[18]. Usually, DDLS are rare, located in the retroperitoneum and occasionally in the peritoneal cavity[6]. Localizations in the ascending colon, sigmoid colon, mesentery of the small bowel and oesophagus have been described, but no localization in the gastrocolic ligament has been reported to our knowledge[7,19,20,21]. Well-differentiated liposarcoma is typically associated with an adipose mass containing nonlipomatous components on abdominal CT and magnetic resonance imaging; the additional presence of a focal, nodular nonlipomatous region greater than 1 cm in size suggests DDLS[22]. Histo-pathological diagnosis is difficult; initially, this case was diagnosed as an undifferentiated sarcoma with fusiform and pleomorphic cells. In fact, tumours are generally diagnosed as an undifferentiated pleomorphous sarcoma on histology; the diagnosis is suspected in the presence of a well-differentiated liposarcoma[23]. It should be noted, however, that this well-differentiated component may be missing, as noted above. It has been reported that many cases of histological diagnosis of undifferentiated or poorly differentiated sarcoma located in the retroperitoneum were in fact DDLS[8]. The molecular features of DDLS overlap with well-differentiated liposarcoma[24,25]. In immunohistochemistry, both express MDM2 and CDK4 amplifications; MDM2 overexpression confirmed by fluorescence in situ hybridization helps distinguish it from pleomorphic liposarcoma and myxoid liposarcoma[16]. Radical surgery with R0 en bloc resection when possible, seems to offer longer survival and disease-free interval[14,18,26]. In all reported cases, radical surgery was performed as the first-line treatment. However, surgically, it is difficult to distinguish the well-differentiated components of healthy fat, which complicates complete excision surgery[16]. The most important prognostic factor for DDLS is the anatomic site, with retroperitoneal sites having an overall worse prognosis. In our observation, the numerous small lesions did not allow for curative excision, suggesting relapse, as seen three months later. Chemotherapy and radiotherapy remain poorly codified, and their therapeutic benefit has not yet been demonstrated[18]. DDLS is not very sensitive to chemotherapy, so new molecular targets are based on an understanding of disease biology, usually targeting a specific, aberrant genetic or molecular pathway[27,28]. Pazopanib, a tyrosine kinase inhibitor, may provide clinical benefit for patients with DDLS according to recent data[29].
The patient did not undergo any adjuvant treatment for economic reasons. Approximately 40% of DDLS will have local recurrence, 17% will metastasize, and 28% will have tumour-related mortality[6]. Three months later, follow-up abdominal CT studies revealed multiple peritoneal and vascular implants with necrotic areas. The patient died less than five months after surgery.
DDLSs are rare tumours that typically originate in the retroperitoneum but may arise in unexpected locations. The extremely rare primary gastrocolic liposarcoma presented here is an example. Immunohistochemistry and molecular biology are essential to confirm histological diagnosis. Surgical excision with oncologically appropriate margins is the gold standard of treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Cote d'Ivoire
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li S, China; Mostafavinia A, Iran S-Editor: Lin C L-Editor: A P-Editor: Wu RR
| 1. | Bock S, Hoffmann DG, Jiang Y, Chen H, Il'yasova D. Increasing Incidence of Liposarcoma: A Population-Based Study of National Surveillance Databases, 2001-2016. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Bachmann R, Eckert F, Gelfert D, Strohäker J, Beltzer C, Ladurner R. Perioperative strategy and outcome in giant retroperitoneal dedifferentiated liposarcoma-results of a retrospective cohort study. World J Surg Oncol. 2020;18:296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Mansfield SA, Pollock RE, Grignol VP. Surgery for Abdominal Well-Differentiated Liposarcoma. Curr Treat Options Oncol. 2018;19:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Amer KM, Congiusta DV, Thomson JE, Elsamna S, Chaudhry I, Bozzo A, Amer R, Siracuse B, Ghert M, Beebe KS. Epidemiology and survival of liposarcoma and its subtypes: A dual database analysis. J Clin Orthop Trauma. 2020;11:S479-S484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Matone J, Okazaki S, Maccapani GN, Amancio TT, Filippi RZ, Macedo AL. Giant gastric lipossarcoma: case report and review of the literature. Einstein (Sao Paulo). 2016;14:557-560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Sbaraglia M, Bellan E, Dei Tos AP. The 2020 WHO Classification of Soft Tissue Tumours: news and perspectives. Pathologica. 2021;113:70-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 541] [Article Influence: 108.2] [Reference Citation Analysis (0)] |
| 7. | Meher S, Mishra TS, Rath S, Sasmal PK, Mishra P, Patra S. Giant dedifferentiated liposarcoma of small bowel mesentery: a case report. World J Surg Oncol. 2016;14:250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 8. | Constantinoiu S, Achim IF, Cretu OE, Dumitru T, Constantin A, Enache S, Mates IN; -. Dedifferentiated Liposarcoma of Sigmoid Mesocolon - A Case Report. Chirurgia (Bucur). 2016;111:330-336. [PubMed] |
| 9. | Xiao J, Liu J, Chen M, Liu W, He X. Diagnosis and Prognosis of Retroperitoneal Liposarcoma: A Single Asian Center Cohort of 57 Cases. J Oncol. 2021;2021:7594027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Liao T, Du W, Li X, He S, Guan G, Zhu H, Wu J. Recurrent metastatic retroperitoneal dedifferentiated liposarcoma: a case report and literature review. BMC Urol. 2023;23:63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 11. | Kilpatrick SE. Dedifferentiated Liposarcoma: A Comprehensive Historical Review With Proposed Evidence-based Guidelines Regarding a Diagnosis in Need of Further Clarification. Adv Anat Pathol. 2021;28:426-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Zelger BG, Zelger B. Correspondence re: Guillou L, Gebhard S, Salmeron M, Coindre JM. Metastasizing fibrous histiocytoma of the skin: a clinicopathologic and immunohistochemical analysis of three cases. Mod Pathol 2000;13:654-60. Mod Pathol. 2001;14:534-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Varlas VN, Rhazi Y, Ionescu OM, Micu LG, Pop AL, Bacalbaşa N, Peneş NO. A dedifferentiated rare primary breast liposarcoma - case report and literature review. Rom J Morphol Embryol. 2021;62:301-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Reference Citation Analysis (0)] |
| 14. | Nishio J, Nakayama S, Nabeshima K, Yamamoto T. Biology and Management of Dedifferentiated Liposarcoma: State of the Art and Perspectives. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Horowitz J, Singhal M, Marrero D, Bashjawish F, Leto D, Winters M, Jeberaeel J. A Multi-Modality Treatment of Retroperitoneal De-Differentiated Liposarcoma. Am J Case Rep. 2020;21:e919245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Mack T, Purgina B. Updates in Pathology for Retroperitoneal Soft Tissue Sarcoma. Curr Oncol. 2022;29:6400-6418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 17. | Singer S, Antonescu CR, Riedel E, Brennan MF. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg. 2003;238:358-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 452] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 18. | Grifasi C, Calogero A, Carlomagno N, Campione S, D'Armiento FP, Renda A. Intraperitoneal dedifferentiated liposarcoma showing MDM2 amplification: case report. World J Surg Oncol. 2013;11:305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Brett CL, Miller DH, Jiang L, Wolfsen HC, Attia S, Hintenlang L, Jagadesh N, Miller RC. Dedifferentiated Liposarcoma of the Esophagus: A Case Report and Selected Review of the Literature. Rare Tumors. 2016;8:6791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Takeda K, Aimoto T, Yoshioka M, Nakamura Y, Yamahatsu K, Ishiwata T, Naito Z, Miyashita M, Uchida E. Dedifferentiated liposarcoma arising from the mesocolon ascendens: report of a case. J Nippon Med Sch. 2012;79:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Winn B, Gao J, Akbari H, Bhattacharya B. Dedifferentiated liposarcoma arising from the sigmoid mesocolon: a case report. World J Gastroenterol. 2007;13:4147-4148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Murphey MD, Arcara LK, Fanburg-Smith J. From the archives of the AFIP: imaging of musculoskeletal liposarcoma with radiologic-pathologic correlation. Radiographics. 2005;25:1371-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 226] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 23. | Weaver J, Downs-Kelly E, Goldblum JR, Turner S, Kulkarni S, Tubbs RR, Rubin BP, Skacel M. Fluorescence in situ hybridization for MDM2 gene amplification as a diagnostic tool in lipomatous neoplasms. Mod Pathol. 2008;21:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 202] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 24. | Lu J, Wood D, Ingley E, Koks S, Wong D. Update on genomic and molecular landscapes of well-differentiated liposarcoma and dedifferentiated liposarcoma. Mol Biol Rep. 2021;48:3637-3647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Watson S, Gruel N, Le Loarer F. New developments in the pathology and molecular biology of retroperitoneal sarcomas. Eur J Surg Oncol. 2023;49:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Vats M, Pandey D, Ahlawat H, Akhtar A, Singh N. Multiple Primary Dedifferentiated Liposarcoma of the Jejunal Mesentery: A Case Report and Review of Literature. J Clin Diagn Res. 2016;10:XD01-XD04. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Peng T, Zhang P, Liu J, Nguyen T, Bolshakov S, Belousov R, Young ED, Wang X, Brewer K, López-Terrada DH, Oliveira AM, Lazar AJ, Lev D. An experimental model for the study of well-differentiated and dedifferentiated liposarcoma; deregulation of targetable tyrosine kinase receptors. Lab Invest. 2011;91:392-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Saponara M, Stacchiotti S, Gronchi A. Pharmacological therapies for Liposarcoma. Expert Rev Clin Pharmacol. 2017;10:361-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Gahvari Z, Parkes A. Dedifferentiated Liposarcoma: Systemic Therapy Options. Curr Treat Options Oncol. 2020;21:15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |