Published online Oct 27, 2023. doi: 10.4240/wjgs.v15.i10.2357
Peer-review started: June 16, 2023
First decision: July 23, 2023
Revised: July 29, 2023
Accepted: August 18, 2023
Article in press: August 18, 2023
Published online: October 27, 2023
Processing time: 133 Days and 6.2 Hours
Gastric adenosquamous carcinoma (ASC) is rare and characterized by coexisting of adenocarcinoma andsquamous carcinoma within the same tumor. We present a female patient with gastric ASC who had an elevated serum level of alpha-fetopro
A 50-year-old woman presented to our department with a chief complain of a 6-mo history of bloating. She had no basic diseases including heart diseases and respiratory diseases, and she also denied any prior history of dysphagia, hemate
We presented a rare case of gastric ASC with elevated serum AFP level, which may be new subtype of AFP-pro
Core Tip: Most patients diagnosed with gastric cancer (GC) have a pathological phenotype of adenocarcinoma, and gastric adenosquamous carcinoma (ASC) is rare. We presented a rare case of gastric ASC with elevated serum alpha-fetoprotein (AFP) level, which may be new subtype of AFP-producing GC. AFP-GC is an aggressive cancer with high incidence of liver or lymph node metastasis. Follow-up detection of serum AFP might be a useful tool to predict prognosis.
- Citation: Sun L, Wei JJ, An R, Cai HY, Lv Y, Li T, Shen XF, Du JF, Chen G. Gastric adenosquamous carcinoma with an elevated serum level of alpha-fetoprotein: A case report. World J Gastrointest Surg 2023; 15(10): 2357-2361
- URL: https://www.wjgnet.com/1948-9366/full/v15/i10/2357.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i10.2357
It is well known that gastric cancer (GC) is the fourth most common cancer worldwide with a third highest incidence and mortality in China[1]. With a change in population structure and population growth, it is also suggested that the incidence of GC has increased by 25% between 2007 and 2017[2]. 1 in 78 women and 1 in 33 men developed GC over a lifetime[2]. Most patients diagnosed with gastric carcinoma had a pathological phenotype of adenocarcinoma which has been studied well over the last decades, and National Comprehensive Cancer Network guidelines also present a detailed management strategy for GC with a adenocarcinoma phenotype. However, there also exist other histological types of gastric carcinoma including primary gastric squamous cell carcinoma, carcinoid, and primary adenosquamous carcinoma (ASC) which is characterized by coexisting of adenocarcinoma and squamous carcinoma within the same tumor. Gastric ASC is rare and clinical features of ASC were described largely in case reports or case series.
A 50-year-old woman presented to our department with a chief complain of a 6-mo history of bloating.
Symptoms started 6-mo before presentation.
She had no basic diseases including heart diseases, respiratory diseases, active or chronic hepatits, liver cirrhosis, and she also denied any prior history of dysphagia, hematemesis, melena, rectal bleeding, hematochezia, or unintentional weight loss.
The patient denied any family history of malignant tumors.
On physical examination, the vital signs were as follows: Body temperature, 36.7 °C; blood pressure, 125/76 mmHg; heart rate, 78 beats per min; respiratory rate, 18 breaths per min. Furthermore, the abdomen is flat without touching any lumps, without tenderness, rebound pain, or muscle tension. Digital anal examination was not performed.
Serum tumor markers including carcinoembryonic antigen (CEA), cancer antigen 199 (CA199), CA724, CA125, and CA242 were all normal, while the level of serum alpha-fetoprotein (AFP) increased to 172 ng/mL. Liver function indi
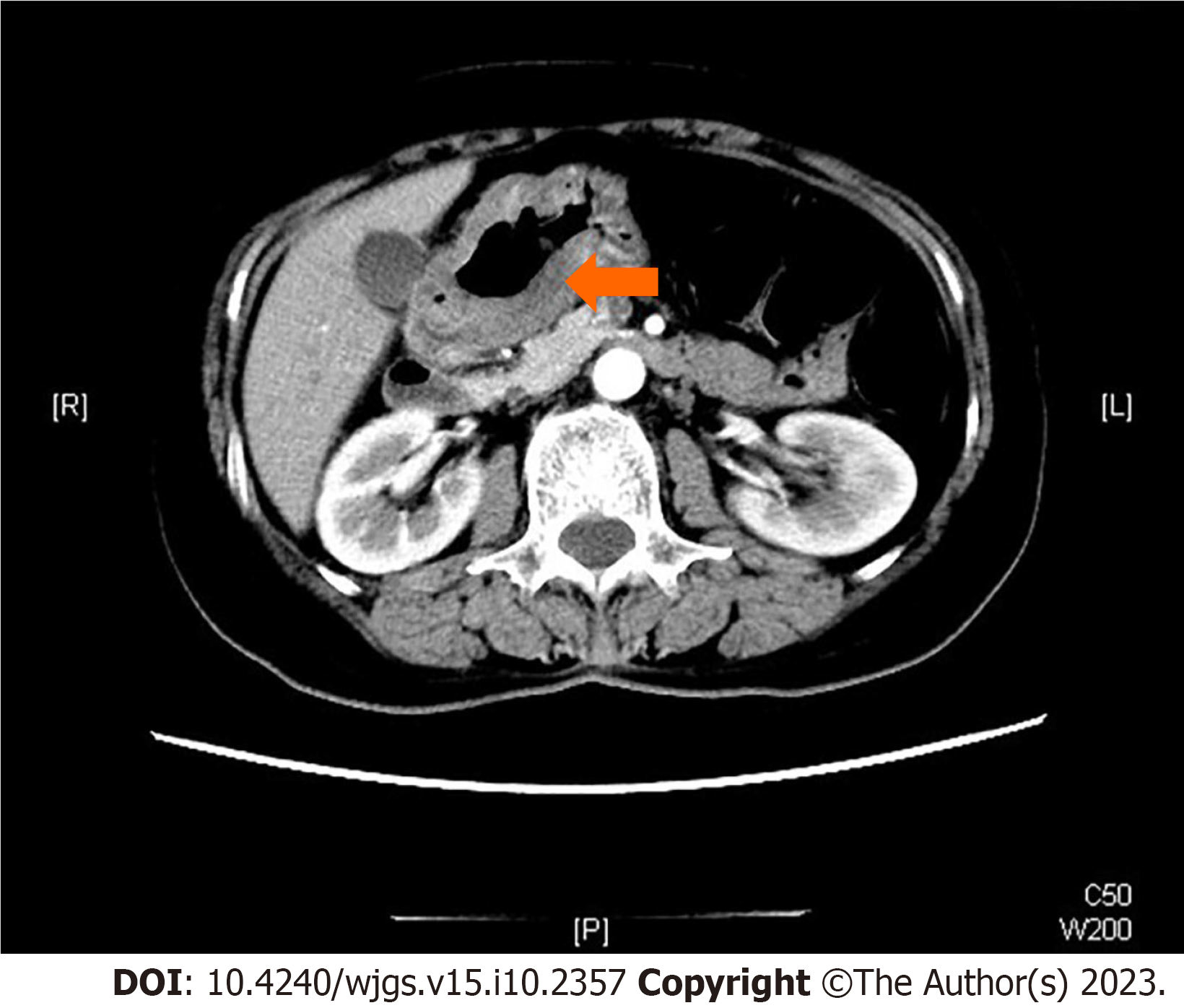
The patient further received an enhanced computed tomography scan which demonstrated an invasive lesion close to the pylorus with a still clear margin of the tumor to peripheral organs such as the pancreas and liver (Figure 1). Scattered lymph nodes were visible around, whereas no sign of liver metastasis was discovered.
Combined with the patient’s medical history, the final diagnosis was ASC.
A laparoscopic distant radical gastrectomy was performed after exclusion of surgical contraindications.
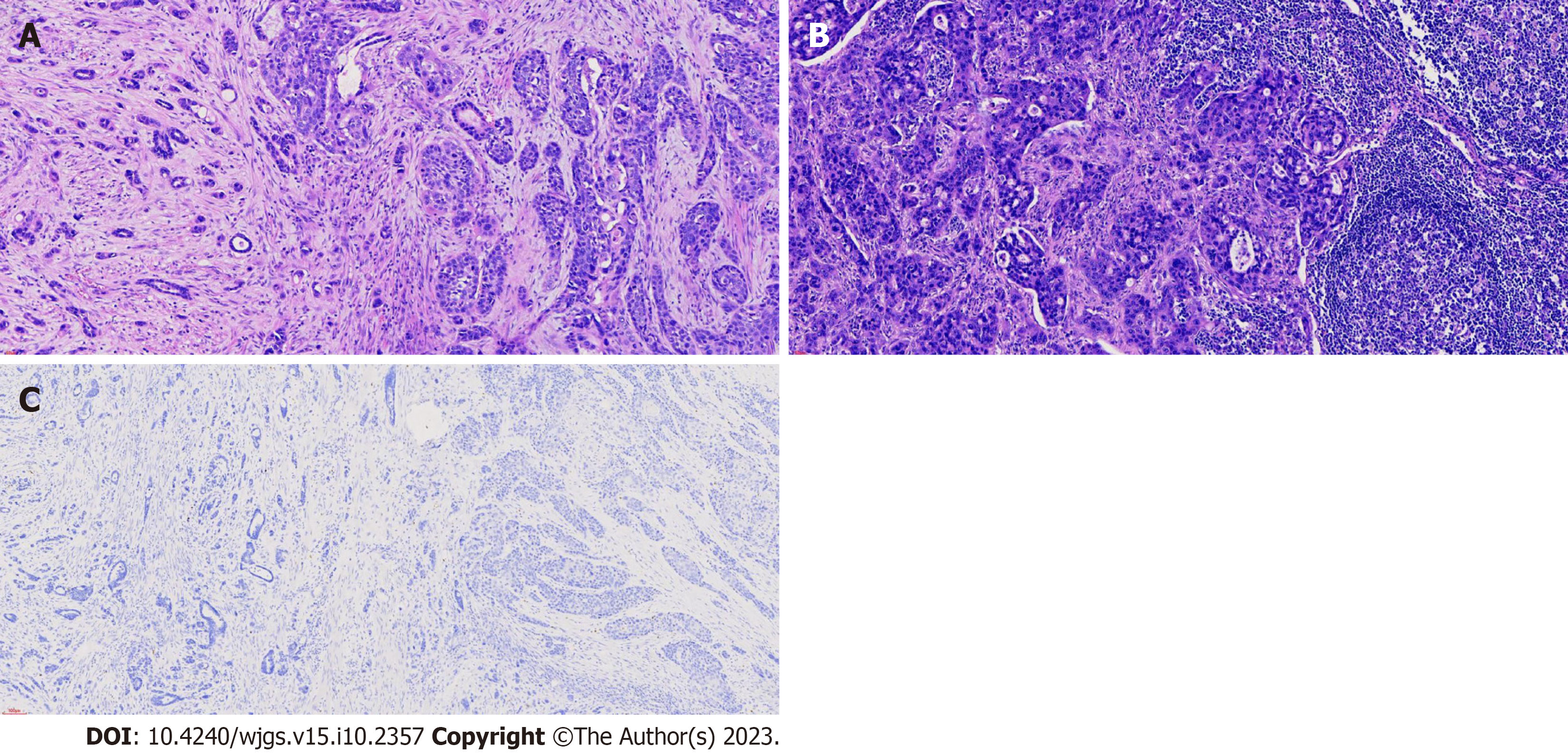
Postoperative pathology results showed that the tumor displayed an ulcerated ASC phenotype, 90% of medium to highly-differentiated squamous cell carcinoma, 10% of poorly differentiated adenocarcinoma (Figure 2A) and metastatic lymph nodes (Figure 2B). Surprisingly, the serum level of AFP decreased to normal level on post operation day 5. The tumor cells were positive for CK5/6, p63, and CEA, and negative for AFP (Figure 2C) and Epstein-Barr encoding region.
In this case report, we presented a female patient with gastric ASC who had an elevated serum level of AFP. Although immunohistochemistry staining results for AFP protein in tumor tissues were negative, serum AFP level decreased to normal level after a laparoscopic distant radical gastrectomy in a short period. Therefore, it is possible that gastric ASC in our patient may be accompanied with or even produces soluble AFP. As far as we know, this is the first case of gastric ASC with elevated AFP level partly due to a very low incidence of gastric ASC, which was suggested to account for less than 1% of all gastric malignancies[3]. The whole story of gastric ASC has not been fully elucidated and only two case series with a total of 287 cases summarized clinicopathological features of gastric ASC[4]. The diagnosis of gastric ASC is supported by the presence of both actinic cheilitis (AC) and ASC components with squamous cell carcinoma (SCC) accounting for at least 25% of tumors. In our case, SCC component accounted for approximately 90% of tumor. In addition, the location of tumor in our case was close to pylorus instead of cardia or esophagus, which was in consistent with previous studies showing the most common location of lower third for gastric ASC[4]. No evidence of other AC or SCC was found elsewhere in the body which further confirmed the diagnosis of gastric ASC for our case.
It was suggested that gastric ASC was an extremely aggressive cancer and distant metastasis was usually found, with liver being the most common location for distant metastasis[3,4]. Detailed analysis showed that both AC and SCC com
Radical resection of tumor remains the optimal treatment for patients without distant metastasis, and the following adjuvant therapy has not been established. Whether to choose chemotherapy or radiotherapy or a combination largely depends on the predominant component presented in gastric ASC. Due to personal reason, this patient refused to receive to any further chemotherapy or radiotherapy. The prognosis of gastric ASC was worse than typical gastric AC with a low 3-year overall survival ranging from 15.4%-32.4%[3-5]. The patient in this report died one year after operation.
Based on the change of serum AFP in this patient before and after operation, we speculated that gastric ASC in this patient may be a new subtype of AFP-producing GC (AFPGC). Commonly, increased serum level of AFP could be seen in AFPGC or in hepatoid adenocarcinoma of the stomach (HAS). AFPGC is defined as primary GC with serum AFP level more than 20 ng/mL or positive immunohistochemistry staining of AFP in the tumor. The diagnosis of HAS is mainly dependent on the pathological character of hepatocellular carcinoma-like differentiation of GC. We didn’t find any proof of HAS in our case and the biological behavior in our case also partly matched those found in AFPGC. The most common location of AFPGC was gastric antrumand corpus[6], and the serum level of AFP predicted the 5-year overall survival[7]. AFPGC is also an aggressive cancer with high incidence of liver or lymph node metastasis[7]. We identified an elevation of serum AFP 6 mo after surgery in this patient, and the level of AFP remained high 3 mo before his death. No evidence of liver metastasis was identified during the follow-up period.
In conclusion, we presented a rare case of gastric ASC with elevated serum AFP level, which may be new subtype of AFP-producing GC. Follow-up detection of serum AFP might be a useful tool to predict patient prognosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: koganti SB, United States; Kotelevets SM, Russia S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21354] [Article Influence: 2135.4] [Reference Citation Analysis (3)] |
| 2. | Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L, Fleming T, Forouzanfar MH, Hancock J, Hay RJ, Hunter-Merrill R, Huynh C, Hosgood HD, Johnson CO, Jonas JB, Khubchandani J, Kumar GA, Kutz M, Lan Q, Larson HJ, Liang X, Lim SS, Lopez AD, MacIntyre MF, Marczak L, Marquez N, Mokdad AH, Pinho C, Pourmalek F, Salomon JA, Sanabria JR, Sandar L, Sartorius B, Schwartz SM, Shackelford KA, Shibuya K, Stanaway J, Steiner C, Sun J, Takahashi K, Vollset SE, Vos T, Wagner JA, Wang H, Westerman R, Zeeb H, Zoeckler L, Abd-Allah F, Ahmed MB, Alabed S, Alam NK, Aldhahri SF, Alem G, Alemayohu MA, Ali R, Al-Raddadi R, Amare A, Amoako Y, Artaman A, Asayesh H, Atnafu N, Awasthi A, Saleem HB, Barac A, Bedi N, Bensenor I, Berhane A, Bernabé E, Betsu B, Binagwaho A, Boneya D, Campos-Nonato I, Castañeda-Orjuela C, Catalá-López F, Chiang P, Chibueze C, Chitheer A, Choi JY, Cowie B, Damtew S, das Neves J, Dey S, Dharmaratne S, Dhillon P, Ding E, Driscoll T, Ekwueme D, Endries AY, Farvid M, Farzadfar F, Fernandes J, Fischer F, G/Hiwot TT, Gebru A, Gopalani S, Hailu A, Horino M, Horita N, Husseini A, Huybrechts I, Inoue M, Islami F, Jakovljevic M, James S, Javanbakht M, Jee SH, Kasaeian A, Kedir MS, Khader YS, Khang YH, Kim D, Leigh J, Linn S, Lunevicius R, El Razek HMA, Malekzadeh R, Malta DC, Marcenes W, Markos D, Melaku YA, Meles KG, Mendoza W, Mengiste DT, Meretoja TJ, Miller TR, Mohammad KA, Mohammadi A, Mohammed S, Moradi-Lakeh M, Nagel G, Nand D, Le Nguyen Q, Nolte S, Ogbo FA, Oladimeji KE, Oren E, Pa M, Park EK, Pereira DM, Plass D, Qorbani M, Radfar A, Rafay A, Rahman M, Rana SM, Søreide K, Satpathy M, Sawhney M, Sepanlou SG, Shaikh MA, She J, Shiue I, Shore HR, Shrime MG, So S, Soneji S, Stathopoulou V, Stroumpoulis K, Sufiyan MB, Sykes BL, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tessema GA, Thakur JS, Tran BX, Ukwaja KN, Uzochukwu BSC, Vlassov VV, Weiderpass E, Wubshet Terefe M, Yebyo HG, Yimam HH, Yonemoto N, Younis MZ, Yu C, Zaidi Z, Zaki MES, Zenebe ZM, Murray CJL, Naghavi M. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2838] [Cited by in RCA: 2948] [Article Influence: 368.5] [Reference Citation Analysis (0)] |
| 3. | Chen H, Shen C, Yin R, Yin Y, Chen J, Han L, Zhang B, Chen Z. Clinicopathological characteristics, diagnosis, treatment, and outcomes of primary gastric adenosquamous carcinoma. World J Surg Oncol. 2015;13:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Feng F, Zheng G, Qi J, Xu G, Wang F, Wang Q, Guo M, Lian X, Zhang H. Clinicopathological features and prognosis of gastric adenosquamous carcinoma. Sci Rep. 2017;7:4597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Quan J, Zhang R, Liang H, Li F, Liu H. The clinicopathologic and prognostic analysis of adenosquamous and squamous cell carcinoma of the stomach. Am Surg. 2013;79:E206-E208. [PubMed] |
| 6. | Lin HJ, Hsieh YH, Fang WL, Huang KH, Li AF. Clinical manifestations in patients with alpha-fetoprotein-producing gastric cancer. Curr Oncol. 2014;21:e394-e399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Liu X, Cheng Y, Sheng W, Lu H, Xu Y, Long Z, Zhu H, Wang Y. Clinicopathologic features and prognostic factors in alpha-fetoprotein-producing gastric cancers: analysis of 104 cases. J Surg Oncol. 2010;102:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |