Published online Jan 27, 2023. doi: 10.4240/wjgs.v15.i1.94
Peer-review started: November 14, 2022
First decision: December 1, 2022
Revised: December 6, 2022
Accepted: December 23, 2022
Article in press: December 23, 2022
Published online: January 27, 2023
Processing time: 65 Days and 5.4 Hours
Endoscopic submucosal dissection (ESD) is a treatment for early gastric cancer with the advantages of small invasion, fewer complications, and a low local recurrence rate. However, there is a high risk of complications such as bleeding and perforation, and the operation time is also longer. ESD operation time is closely related to bleeding and perforation.
To investigate the influencing factors associated with ESD operation time and postoperative delayed hemorrhage to provide a reference for early planning, early identification, and prevention of complications.
We conducted a retrospective study based on the clinical data of 520 patients with early gastric cancer in the Second Affiliated Hospital of Hainan Medical University from January 2019 to December 2021. The baseline data, clinical features, and endoscopic and pathological characteristics of patients were collected. The multivariate linear regression model was used to investigate the influencing factors of ESD operation time. Logistic regression analysis was carried out to evaluate the influencing factors of postoperative delayed hemorrhage.
The multivariate analysis of ESD operation time showed that the maximum lesion diameter could affect 8.815% of ESD operation time when other influencing factors remained unchanged. The operation time increased by 3.766% or 10.247% if the lesion was mixed or concave. The operation time increased by 4.417% if combined with an ulcer or scar. The operation time increased by 3.692% if combined with perforation. If infiltrated into the submucosa, it increased by 2.536%. Multivariate analysis of delayed hemorrhage after ESD showed that the maximum diameter of the lesion, lesion morphology, and ESD operation time were independent influencing factors for delayed hemorrhage after ESD. Patients with lesion ≥ 3.0 cm (OR = 3.785, 95%CI: 1.165-4.277), lesion morphology-concave (OR = 10.985, 95%CI: 2.133-35.381), and ESD operation time ≥ 60 min (OR = 2.958, 95%CI: 1.117-3.526) were prone to delayed hemorrhage after ESD.
If the maximum diameter of the lesion in patients with early gastric cancer is ≥ 3.0 cm, and the shape of the lesion is concave, or accompanied by an ulcer or scar, combined with perforation, and infiltrates into the submucosa, the ESD operation will take a longer time. When the maximum diameter of the lesion is ≥ 3.0 cm, the shape of the lesion is concave in patients and the operation time of ESD takes longer time, the risk of delayed hemorrhage after ESD is higher.
Core Tip: Gastric cancer is a common malignant tumor of the digestive system worldwide. Endoscopic submucosal dissection (ESD) is the first-line treatment for early gastric cancer. However, the long operation time of ESD and its postoperative delayed hemorrhage are the major complications, which can cause more severe cardiovascular complications, such as bradycardia and hypotension. In this retro
- Citation: Cai RS, Yang WZ, Cui GR. Associate factors for endoscopic submucosal dissection operation time and postoperative delayed hemorrhage of early gastric cancer. World J Gastrointest Surg 2023; 15(1): 94-104
- URL: https://www.wjgnet.com/1948-9366/full/v15/i1/94.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i1.94
Gastric cancer is a malignant tumor originating from the gastric epithelium. Early gastric cancer is generally defined as invasive gastric cancer that invades no more deeply than the submucosa, re
The patients with early gastric cancer who received ESD treatment in the Department Endoscopic Center of the Second Affiliated Hospital of Hainan Medical University from January 2019 to December 2021 were retrospectively analyzed.
Inclusion criteria: (1) Patients with early gastric cancer confirmed by endoscopic biopsy and pathological examination; (2) Patients that found no regional lymph nodes and distant metastasis in endoscopic ultrasonography and other imaging examinations; (3) All patients and their relatives were informed of the risks and benefits of ESD and signed a written informed consent; (4) The patient was informed of the study and agreed to participate; and (5) The patient’s information is complete.
Exclusion criteria: (1) Tumor infiltrating into muscular layer or serosa; (2) Patients with severe heart, brain, lung, and other important organ dysfunction; (3) Coagulation dysfunction; (4) Patients with a high risk of anesthesia or intolerance; (5) Suspicious lymph node metastasis; and (6) Two or more lesions appeared.
The baseline data, clinical features, and endoscopic and pathological characteristics of patients were collected, including gender, age, underlying diseases, medication history, lesion location, lesion shape, maximum lesion diameter, ulcer and scar, perforation, pathological diagnosis, depth of invasion, delayed hemorrhage and ESD operation time (min).
GIFQ260J gastroscope with an additional water supply function was used (Olympus, Japan). The front end of the gastroscope is provided with a soft transparent cap (Olympus, Japan). High-frequency electrical uses erbotomicc200 or vio200d (Erbe, Germany). Under general anesthesia, the submucosal injection during ESD operation consisted of glycerol, fructose solution, an appropriate amount of methylene blue, and epinephrine at a ratio of 1:10000. Sodium hyaluronate solution was diluted with normal saline (1:5) when necessary. The lesion was located under endoscopy, and the boundary between the lesion and the normal mucosa was determined by NBI amplification and 2.5% Lugol solution staining. Thermocoagulation markers were made around the lesion every 0.5 cm from 0.3 to 0.5 cm from the lesion border. The mucosa and submucosa were separated by submucosal injection along the lesion boundary. An incision was cut at about 0.5 cm outside the marker, and then the lesion was cut along the mucosa of the lesion edge with a dual knife until the submucosa was reached, and the lesion was gradually stripped along the submucosa. During the stripping, the hemostatic forceps were used intermittently until the tumor was completely stripped. Finally, thermal hemostatic forceps were used to stop the wound, and titanium clips were used to seal the wound for patients with deep dissection or cracks in the muscularis propria. The vital signs of patients were closely monitored after the operation. In this study, all ESD procedures were performed by a professional endoscopic physician with more than 15 years of technical experience using the same equipment.
The postoperative treatment and follow-up measures of ESD included postoperative placement of a gastric tube. According to the needs of the patient, the patient was fasted for 3-5D and received hemostasis, acid suppression, anti-infection, and intravenous nutritional support. Gastric tube drainage and patients with abdominal pain, abdominal distension, and other signs were closely observed. Patients continued to take gastric mucosal protective agents and proton pump acid inhibitors for 8 wk after discharge. The endoscopic review was performed once every 3, 6, and 12 mo after the operation, and then once a year. Postoperative chest and abdomen computed tomography examination was performed once a year.
ESD operation time is defined as the time (min) from the circumferential marking of the lesion to the complete resection of the lesion. Delayed hemorrhage was considered by the following situations within 24 h to 30 d after ESD: (1) Vomiting, dizziness, melena, and other symptoms; (2) Blood pressure drop > 20 mmHg or heart rate increase by 20 times/min; (3) Endoscopic examination confirmed surgical wound bleeding; and (4) Hemoglobin level decreased ≥ 2 g/dL after endoscopic treatment. At the time of discharge, researchers instructed patients on how to identify delayed bleeding, and collected patients’ delayed bleeding by phone or face-to-face after discharge.
This study is a retrospective study. All patient data obtained, recorded, and managed will be used for this study only, and all patient information will be kept strictly confidential and will not cause any harm to the patient. In addition, the research scheme was approved by the Ethics Committee of the Second Affiliated Hospital of Hainan Medical University.
SPSS26.0 statistical software was used for statistical analysis. The numerical variables that meet the normal distribution were described by mean ± SD, and the classification variables were described by frequency (percentage). The influencing factors of ESD operation time were analyzed by univariate analysis and multivariate analysis. In single-factor analysis, Pearson correlation analysis was used for age, independent sample t-test was used for two classification factors, variance analysis was used for multi-classification factors, and LSD test was used for pairwise comparison. The multivariate linear regression model was used for the multivariate analysis of ESD operation time. Also, a single-factor analysis of delayed hemorrhage after the operation was performed by χ2 test, and multivariate logistic regression analysis was used to analyze the influencing factors of delayed hemorrhage after ESD. Test level α = 0.05.
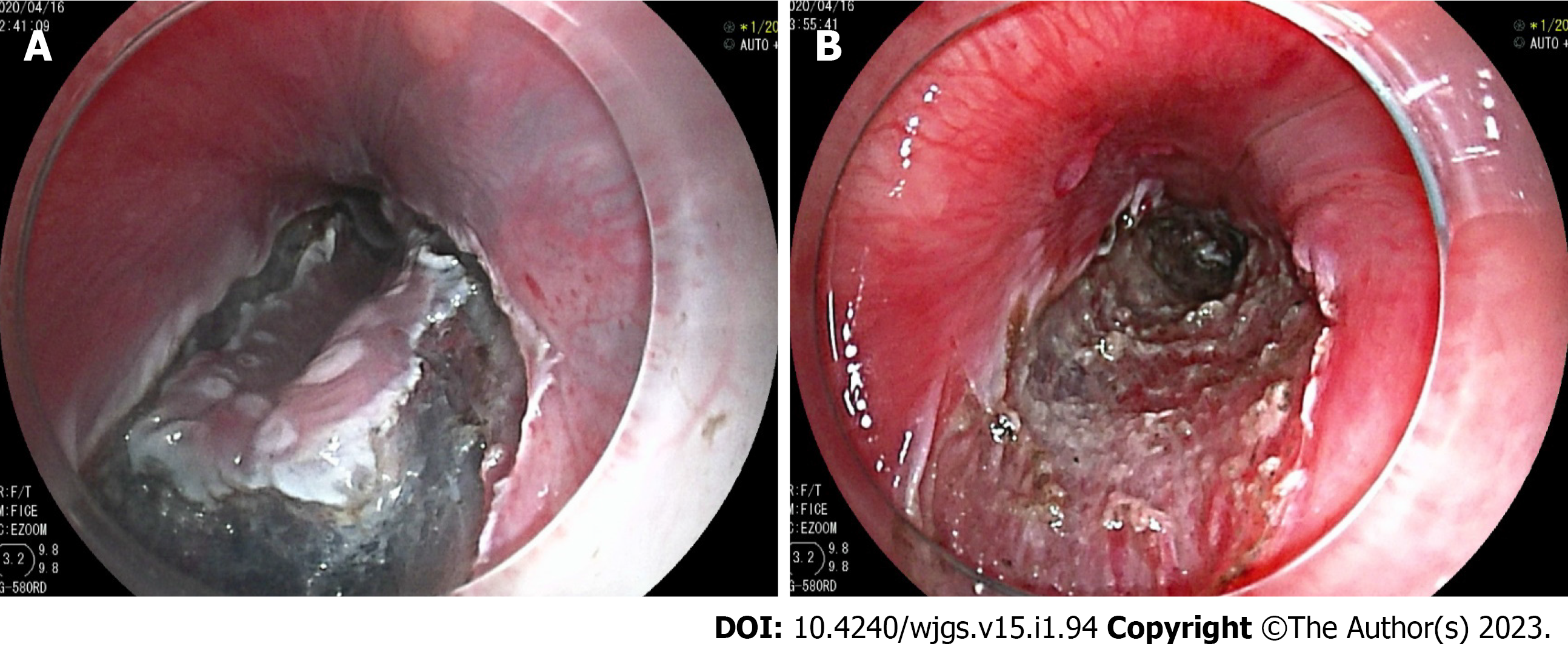
From January 2019 to December 2021, there were 551 patients with early gastric cancer received ESD treatment in total in our endoscopic center. As shown in the figure, endoscopy confirmed early gastric cancer (Figure 1A and B). Among them, 24 patients had incomplete data, and 7 patients did not meet the criteria for admission and discharge. Therefore, 520 patients with early gastric cancer were collected for this retrospective study. There were 367 males and 153 females, with a ratio of 2.40:1. The age of patients was between 31 and 84 years old and the average age was 57.81 ± 10.56 years old. The median maximum diameter of the lesion was 3.0 cm, ranging from 0.35 cm to 10.55 cm. The average operation time was 66.78 ± 40.89 min (10-160 min). In total, 508 (97.69%) lesions were completely resected, and 499 (95.96%) lesions met the standard of curative resection. There were 189 cases of upper gastric lesions, 112 cases of middle gastric lesions, and 219 cases of lower gastric lesions. Delayed hemorrhage occurred in 43 patients after ESD (8.27%). Hemorrhage patients underwent emergency endoscopic hemostasis and hemostasis was successful. 11 patients needed a blood transfusion.
The results of the single-factor analysis were shown in Table 1. Patients with maximum lesion diameter ≥ 3.00 cm had longer ESD operation time than patients with lesion diameter < 3.00 cm. The operation time of ESD in patients with different lesions was different, mixed type > concave type > flat type > uplift type. The operation time of patients with ulcers or scars was longer than those of patients without ulcers. Patients with perforation were longer than those without perforation. Patients with perforations had a longer duration of disease than those without. Lesions infiltrated into the submucosa more frequently than into the mucosa alone. And the differences were all statistically significant (P < 0.05).
| Factors | n | Operation time (min) (mean ± SD) | r/t/F | P value |
| Age (yr) | 520 | 66.78 ± 40.89 | 0. 191 | 0.812 |
| Sex | 0.311 | 0.764 | ||
| Male | 367 | 64.19 ± 41.21 | ||
| Female | 153 | 73.38 ± 35.82 | ||
| Underlying diseases | 0.921 | 0.146 | ||
| None | 379 | 63.11 ± 27.17 | ||
| Hypertension | 126 | 70.91 ± 32.19 | ||
| Diabetes | 12 | 67.24 ± 41.65 | ||
| Cirrhosis | 3 | 78.55 ± 30.52 | ||
| History of taking anticoagulant or antiplatelet drugs | 1.260 | 0.262 | ||
| Yes | 31 | 59.51 ± 48.44 | ||
| No | 489 | 66.12 ± 20.38 | ||
| Lesion location | 1.333 | 0.198 | ||
| Upper gastric body | 112 | 75.76 ± 31.76 | ||
| Middle gastric body | 70 | 63.36 ± 38.91 | ||
| Lower gastric body | 338 | 65.54 ± 39.18 | ||
| Maximum diameter of lesion | 8.691 | < 0.001 | ||
| < 3.00 cm | 351 | 52.22 ± 29.81 | ||
| ≥ 3.00 cm | 169 | 90.38 ± 40.21 | ||
| Lesion form | 11.123 | < 0.001 | ||
| Uplift type | 134 | 48.54 ± 28.11 | ||
| Flat type | 138 | 53.66 ± 31.81 | ||
| Concave type | 91 | 71.17 ± 33.71 | ||
| Mixed type | 157 | 109.33 ± 40.28 | ||
| Combined ulcer or scar | 7.288 | 0.001 | ||
| Yes | 64 | 93.17 ± 37.34 | ||
| No | 456 | 62.36 ± 31.97 | ||
| Combined perforation | 9.327 | < 0.001 | ||
| Yes | 21 | 121.33 ± 41.19 | ||
| No | 499 | 63.17 ± 36.85 | ||
| Infiltrative depth | 5.442 | 0.017 | ||
| Mucosa | 397 | 55.44 ± 36.18 | ||
| Submucosa | 123 | 87.23 ± 39.67 |
The ESD operation time (min) was taken as the dependent variable Y, and the variables with statistical significance in single factor analysis were taken as independent variables Xi. The multiple linear regression model was fitted by a stepwise method. The goodness of fit of the multiple linear regression model reached a large effect (r = 0.692). The model finally adjusts R2 was 0.563. The variance analysis results of the model showed that the F value was 54.866, and the P value was < 0.001. The regression equation was as follows: In = 21.674 + 8.815 × 1 + 3.766 × 2 + 10.247 × 3 + 4.417 × 4 + 3.692 × 5 + 2.536 × 6. When other factors remain unchanged, the maximum diameter of the lesion can affect the ESD operation time by 8.815%. If the lesion was concave or mixed, the operation time increased by 3.766% or 10.247%. If combined with an ulcer or scar, ESD operation time increased by 4.417%. The operation time increased by 3.692% if combined with perforation. If infiltrated into the submucosa, it increased by 2.536% (Table 2).
| Factors | Unstandardized β value | Sx value | Standardized β value | t value | P value |
| Intercept | 21.674 | 0.433 | - | 36.188 | < 0.001 |
| Maximum diameter of lesion | 8.815 | 0.684 | 0.732 | 3.812 | < 0.001 |
| Lesion form - flat | 1.277 | 1.475 | 0.032 | 0.333 | 0.493 |
| Lesion form - concave type | 3.766 | 0.872 | 0.383 | 4.456 | < 0.001 |
| Lesion form - mixed | 10.247 | 0.929 | 0.633 | 9.580 | < 0.001 |
| Combined ulcer or scar | 4.417 | 0.305 | 0.180 | 3.154 | 0.007 |
| Combined perforation | 3.692 | 2.303 | 0.153 | 2.340 | 0.020 |
| Infiltrative depth - submucosa | 2.536 | 0.569 | 0.077 | 2.652 | 0.008 |
The subjects were divided into a bleeding group and a non-bleeding group according to whether postoperative delayed hemorrhage occurred. The results of the single-factor analysis were shown in Table 3. Patients with hypertension, a history of taking anticoagulant or antiplatelet drugs, maximum lesion diameter, lesion morphology, and ESD operation time were associated with postoperative delayed hemorrhage (P < 0.05). Gender, age, other underlying diseases except for hypertension, location of lesion, ulcer or scar, perforation, and depth of infiltration were not associated with delayed hemorrhage after the operation (P > 0.05).
| Factors | Bleeding group (n = 43) | Non-bleeding (n = 477) | χ2 value | P value |
| Age (yr) | 0.120 | 0.729 | ||
| < 60 | 23 | 242 | ||
| ≥ 60 | 20 | 235 | ||
| Sex | 0.015 | 0.903 | ||
| Male | 30 | 337 | ||
| Female | 13 | 140 | ||
| Underlying diseases | ||||
| Hypertension | 16 | 110 | 4.301 | 0.038 |
| Diabetes | 2 | 10 | 1.142 | 0.285 |
| Cirrhosis | 0 | 3 | - | - |
| History of taking anticoagulant or antiplatelet drugs | 59.148 | < 0.001 | ||
| Yes | 14 | 17 | ||
| No | 29 | 460 | ||
| Lesion location | 0.489 | 0.783 | ||
| Upper gastric body | 10 | 102 | ||
| Middle gastric body | 7 | 63 | ||
| Lower gastric body | 26 | 312 | ||
| Maximum diameter of lesion | 29.677 | < 0.001 | ||
| < 3.00 cm | 13 | 338 | ||
| ≥ 3.00 cm | 30 | 139 | ||
| Lesion form | 11.098 | 0.011 | ||
| Uplift type | 7 | 127 | ||
| Flat type | 12 | 126 | ||
| Concave type | 15 | 76 | ||
| Mixed type | 9 | 148 | ||
| Combined ulcer or scar | 0.020 | 0.887 | ||
| Yes | 5 | 59 | ||
| No | 38 | 418 | ||
| Combined perforation | 1.044 | 0.307 | ||
| Yes | 3 | 18 | ||
| No | 40 | 459 | ||
| Infiltrative depth | 3.274 | 0.070 | ||
| Mucosa | 28 | 369 | ||
| Submucosa | 15 | 108 | ||
| ESD operation time | 6.979 | 0.008 | ||
| < 60 min | 16 | 277 | ||
| ≥ 60 min | 27 | 200 |
Taking the occurrence or not of delayed hemorrhage after ESD as the dependent variable, and the factors with statistical significance in single factor analysis as independent variables, the multivariate logistic regression model was fitted. As shown in Table 4. The maximum diameter of lesions, lesion morphology, and ESD operation time were independent factors for delayed hemorrhage after ESD. Patients with lesions ≥ 3.0 cm (OR = 3.785, 95%CI: 1.165-4.277), lesion morphology-concave type (OR = 10.985, 95%CI: 2.133-35.381), and ESD operation time ≥ 60 min (OR = 2.958, 95%CI: 1.117-3.526) were prone to delayed hemorrhage after ESD.
| Factors | β | Sx | Wald value | OR (95%CI) | P value |
| Hypertension | |||||
| No | 1 | ||||
| Yes | 0.776 | 0.522 | 2.175 | 2.137 (0.912-2.643) | 0.136 |
| Taking anticoagulants | |||||
| No | |||||
| Yes | 1.841 | 1.062 | 2.851 | 4.377 (0.657-37.912) | 0.078 |
| Maximum diameter of lesion | |||||
| < 3.00 cm | 1 | ||||
| ≥ 3.00 cm | 0.941 | 0.347 | 7.399 | 3.785 (1.165-4.277) | 0.011 |
| Lesion form | |||||
| Uplift type | 1 | ||||
| Flat type | 0.701 | 1.031 | 0.452 | 2.011 (0.251-15.664) | 0.072 |
| Concave type | 2.378 | 1.679 | 4.917 | 10.585 (2.133-35.381) | 0.007 |
| Mixed type | 1.327 | 1.720 | 0.873 | 2.816 (0.463-19.832) | 0.254 |
| ESD operation time | |||||
| < 60 min | 1 | ||||
| ≥ 60 min | 1.446 | 1.271 | 3.541 | 2.958 (1.117-3.526) | 0.011 |
In the past, surgical resection is the standard treatment for early gastric cancer. However, conventional surgery has the disadvantages of large invasion, more postoperative complications, and longer recovery time[8]. Compared to surgical resection, ESD has the advantage of small invasion, more tolerable for patients. And it also can be used as multiple surgical treatments for the same patient or multiple parts of treatment at the same time. Moreover, it has been recognized by experts all over the world and has been used as the first-line treatment for early gastric cancer[9,10]. The amount of time for an ESD is one of the best indicators to measure the difficulty of surgical operation. And IT is very beneficial for the operation plan arrangement and complications prevention if the difficulty and operation time could be predicted in advance. In previous studies, the multivariate analysis suggested that tumor location and size were important predictors of operation time[11,12]. The operation difficulty of different positions of the gastric cavity varies greatly, which will affect the operation time of ESD[13]. In this study, the same equipment and the ESD operation method were used by the same surgeon, which means the influence of different equipment on ESD operation time was excluded. Single-factor and multi-factor analyses were conducted after excluding the above-mixed factors. We found that the maximum diameter of the lesion, lesion morphology, ulcer or scar, perforation, and depth of invasion were independent factors affecting the ESD operation time. Previous studies found that intraoperative perforation was an independent predictor of prolonged ESD operation time[14,15]. Our research also has similar conclusions. In ESD, small perforations can be treated under endoscopy, and the operation time will be prolonged due to the need for metal clips to seal the wound[16]. Longer operation time can increase the risks of complications[17]. Therefore, shortening ESD operation time can reduce intraoperative and postoperative complications of ESD[18]. Thus, the prediction of operative time is crucial for both patients and surgeons. First of all, if we can predict if it will be a longer operation time, we can arrange for senior experts to complete difficult and long-term surgery, and shorten the operation time. Secondly, according to the length of the operation time, anesthesiologists can also use different anesthesia methods. Finally, the prediction of operation time can help operators to take corresponding measures to prevent complications in time, such as venous thrombosis, intraoperative aspiration, or postoperative pneumonia. However, the ESD operation technique is difficult, and the incidence of complications such as bleeding and perforation is high[19]. Usually, intraoperative bleeding and perforation can be treated immediately. But delayed bleeding can lead to severe consequences such as hemorrhagic shock if it is found and treated not timely[20]. Generally, artificial ulcers formed by ESD turn into fibrosis and thicken the gastric wall around 2 wk after surgery, and the healing takes about 8 wk[21]. Some studies have shown that about 1/4 of the artificial ulcers appear as visible blood vessels on the 3rd day after ESD, and these broken blood vessels may be one of the main reasons for postoperative delayed bleeding[22].
Among the 520 patients in this study, there were 43 (8.27%) patients with postoperative delayed bleeding, which was aligned with other literature. Takeuchi et al[23] retrospectively analyzed the data of 833 patients with early gastric cancer and precancerous lesions treated with ESD. and found that the longer duration of ESD in gastric cancer patients was an important risk factor for postoperative bleeding. Previous studies have shown that the lesion size after ESD is the only risk factor for delayed bleeding[24,25]. Resection of large lesions can cause more damage to gastric wall blood vessels, and the risk of postoperative bleeding is higher. The results of this study further confirmed the conclusion that a lesion ≥ 3 cm was more likely to postoperative bleeding. This suggests that endoscopic surgeons need to control the resection area as much as possible during the operation to avoid more gastric mucosal damage. We used magnifying and narrow-band imaging electronic chromoendoscopy to accurately determine the lesion boundary before surgery, and then accurately remove the lesion, which well controlled the operational area of surgical resection.
Previous studies have shown that flat lesions and concave lesions are associated with delayed bleeding after ESD. The results of this study suggested that concave lesions were more likely to have postoperative delayed bleeding (P = 0.007). This result can be explained by the following reasons. Firstly, compared with the uplift lesions, the concave lesions were closer to the muscular layer, and inappropriate biopsy can cause submucosa fibrosis easily, which leads to the increased probability of intraoperative bleeding. Furthermore, the submucosal vessels of flat lesions were richer than those of uplift lesions, thus, the risk of postoperative bleeding was higher. Long operation time is usually associated with frequent dissection and unskilled operation. Unskilled operation and repeated dissection often lead to vascular injury in the lower gastric mucosa and muscularis propria, which is easy to cause early postoperative delayed bleeding. Large lesions and deep infiltration, combined with perforation, can increase the difficulty of mucosal dissection and operation time and therefore easily damage blood vessels. Taking anticoagulant or antiplatelet drugs can inhabit ulcer-induced proliferation of gastric epithelial cells, thereby inhibiting angiogenesis during gastric ulcer healing, resulting in delayed bleeding more likely after ESD. It has been shown to be an independent risk factor for delayed bleeding after early and late ESD[26,27]. Studies have found that antithrombotic drugs are independent risk factors for bleeding after ES[25]. However, there was no significant difference in the distribution of aspirin administration history between the bleeding group and the non-bleeding group in this study. This may be the following reasons: Patients were required to discontinue anticoagulant drugs for one week before ESD or replace other drugs under the guidance of cardiovascular physicians. Patients with severe cardiovascular diseases were not treated in the department, so there was a selection bias in this study. It may also be related to the small sample size. This study was a single-center retrospective analysis with limited sample size and possible bias that was difficult to eliminate.
In summary, patients with early gastric cancer with a maximum lesion diameter ≥ 3.0 cm, concave morphology, associated ulceration or scarring, combined perforation, and infiltration into the submucosa had a longer ESD operation time. Most importantly, the risk of delayed bleeding after ESD is higher when the maximum diameter of the lesion is ≥ 3.0 cm, the lesion morphology is concave, and the ESD operation time is longer. Therefore, we suggest that such patients should be treated with caution. Before the operation, the risk should be fully assessed. During the operation, the bleeding should be strictly controlled and the wound should be properly handled. The operation should also be carried out by experienced physicians. Finally, close observation also should be performed after the operation.
Endoscopic submucosal dissection (ESD) has become a new development trend in the treatment of early gastric cancer due to its special minimally invasive advantages. Although it is minimally invasive surgery, it also has some risks such as bleeding and perforation.
The time of ESD operation is closely related to bleeding and perforation.
This study aims to investigate the operation time of endoscopic subspecific section and the influencing factors of delayed bleeding after operation.
The baseline data, clinical features, and endoscopic and pathological characteristics of patients were collected. The multivariate linear regression model was used to investigate the influencing factors of ESD operation time. Logistic regression analysis was carried out to evaluate the influencing factors of postoperative delayed hemorrhage.
The maximum diameter of the lesion, lesion morphology, and ESD operation time were independent influencing factors for delayed hemorrhage after ESD. Patients with lesion ≥ 3.0 cm (OR = 3.785, 95%CI: 1.165-4.277), lesion morphology-concave (OR = 10.985, 95%CI: 2.133-35.381), and ESD operation time ≥ 60 min (OR = 2.958, 95%CI: 1.117-3.526) were prone to delayed hemorrhage after ESD.
The risk of delayed bleeding after ESD is higher when the maximum diameter of the lesion is ≥ 3.0 cm, the lesion morphology is concave, and the ESD operation time is longer.
Further research should be made on other factors related to delayed bleeding after ESD operation, such as factors during operation and individual related factors. Strict control of surgical indications and adherence to individualized treatment can help reduce the occurrence of complications.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Armour M, Australia; Darai E, France; Eisenberg VH, Israel S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55699] [Article Influence: 7957.0] [Reference Citation Analysis (132)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64129] [Article Influence: 16032.3] [Reference Citation Analysis (174)] |
| 3. | Takeuchi M, Kawakubo H, Shimada A, Hoshino S, Matsuda S, Mayanagi S, Irino T, Fukuda K, Nakamura R, Wada N, Takeuchi H, Kitagawa Y. The Results of Sentinel Node Mapping for Patients with Clinically Early Staged Gastric Cancer Diagnosed with pT2/deeper Tumors. World J Surg. 2021;45:3350-3358. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1323] [Article Influence: 330.8] [Reference Citation Analysis (2)] |
| 5. | Higuchi K, Kaise M, Noda H, Kirita K, Koizumi E, Umeda T, Akimoto T, Omori J, Akimoto N, Goto O, Tatsuguchi A, Iwakiri K. Three-dimensional flexible endoscopy enables more accurate endoscopic recognition and endoscopic submucosal dissection marking for superficial gastric neoplasia: a pilot study to compare two- and three-dimensional imaging. Surg Endosc. 2021;35:6244-6250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Takada J, Araki H, Mizutani T, Ozawa N, Sugiyama T, Kubota M, Ibuka T, Shimizu M. Safety of Carbon Dioxide Insufflation during Endoscopic Submucosal Dissection for Esophageal Squamous Cell Carcinoma. Dig Dis. 2019;37:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Yang LS, Taylor ACF, Thompson AJV, Desmond PV, Holt BA. Quantifying early gastric cancer in Australia: What is the opportunity for gastric endoscopic submucosal dissection? J Gastroenterol Hepatol. 2021;36:2813-2818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Wang S, Zhang Z, Liu M, Li S, Jiang C. Endoscopic Resection Compared with Gastrectomy to Treat Early Gastric Cancer: A Systematic Review and Meta-Analysis. PLoS One. 2015;10:e0144774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 9. | Kim SJ, Choi CW. Common Locations of Gastric Cancer: Review of Research from the Endoscopic Submucosal Dissection Era. J Korean Med Sci. 2019;34:e231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Nishizawa T, Yahagi N. Long-Term Outcomes of Using Endoscopic Submucosal Dissection to Treat Early Gastric Cancer. Gut Liver. 2018;12:119-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 11. | Ribeiro-Mourão F, Veloso N, Dinis-Ribeiro M, Pimentel-Nunes P. Endoscopic Submucosal Dissection of Gastric Superficial Lesions: Predictors for Time of Procedure in a Portuguese Center. GE Port J Gastroenterol. 2015;22:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Ahn JY, Choi KD, Choi JY, Kim MY, Lee JH, Choi KS, Kim DH, Song HJ, Lee GH, Jung HY, Kim JH. Procedure time of endoscopic submucosal dissection according to the size and location of early gastric cancers: analysis of 916 dissections performed by 4 experts. Gastrointest Endosc. 2011;73:911-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Fuccio L, Bhandari P, Maselli R, Frazzoni L, Ponchon T, Bazzoli F, Repici A. Ten quality indicators for endoscopic submucosal dissection: what should be monitored and reported to improve quality. Ann Transl Med. 2018;6:262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Jeong JY, Oh YH, Yu YH, Park HS, Lee HL, Eun CS, Han DS. Does submucosal fibrosis affect the results of endoscopic submucosal dissection of early gastric tumors? Gastrointest Endosc. 2012;76:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Nagata S, Jin YF, Tomoeda M, Kitamura M, Yuki M, Yoshizawa H, Kubo C, Ito Y, Uedo N, Ishihara R, Iishi H, Tomita Y. Influential factors in procedure time of endoscopic submucosal dissection for gastric cancer with fibrotic change. Dig Endosc. 2011;23:296-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Mangiavillano B, Viaggi P, Masci E. Endoscopic closure of acute iatrogenic perforations during diagnostic and therapeutic endoscopy in the gastrointestinal tract using metallic clips: a literature review. J Dig Dis. 2010;11:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Isomoto H, Ohnita K, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Akiyama M, Ozawa E, Nakao K, Kohno S, Shikuwa S. Clinical outcomes of endoscopic submucosal dissection in elderly patients with early gastric cancer. Eur J Gastroenterol Hepatol. 2010;22:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Mannen K, Tsunada S, Hara M, Yamaguchi K, Sakata Y, Fujise T, Noda T, Shimoda R, Sakata H, Ogata S, Iwakiri R, Fujimoto K. Risk factors for complications of endoscopic submucosal dissection in gastric tumors: analysis of 478 lesions. J Gastroenterol. 2010;45:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 19. | Lian J, Chen S, Zhang Y, Qiu F. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc. 2012;76:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 220] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 20. | Saito I, Tsuji Y, Sakaguchi Y, Niimi K, Ono S, Kodashima S, Yamamichi N, Fujishiro M, Koike K. Complications related to gastric endoscopic submucosal dissection and their managements. Clin Endosc. 2014;47:398-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Kakushima N, Fujishiro M, Kodashima S, Kobayashi K, Tateishi A, Iguchi M, Imagawa A, Motoi T, Yahagi N, Omata M. Histopathologic characteristics of gastric ulcers created by endoscopic submucosal dissection. Endoscopy. 2006;38:412-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Goto O, Fujishiro M, Kodashima S, Minatsuki C, Niimi K, Ono S, Yamamichi N, Koike K. Short-term healing process of artificial ulcers after gastric endoscopic submucosal dissection. Gut Liver. 2011;5:293-297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Takeuchi T, Ota K, Harada S, Edogawa S, Kojima Y, Tokioka S, Umegaki E, Higuchi K. The postoperative bleeding rate and its risk factors in patients on antithrombotic therapy who undergo gastric endoscopic submucosal dissection. BMC Gastroenterol. 2013;13:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Matsumura T, Arai M, Maruoka D, Okimoto K, Minemura S, Ishigami H, Saito K, Nakagawa T, Katsuno T, Yokosuka O. Risk factors for early and delayed post-operative bleeding after endoscopic submucosal dissection of gastric neoplasms, including patients with continued use of antithrombotic agents. BMC Gastroenterol. 2014;14:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Okamoto K, Watanabe T, Komeda Y, Kono T, Takashima K, Okamoto A, Kono M, Yamada M, Arizumi T, Kamata K, Minaga K, Yamao K, Nagai T, Asakuma Y, Takenaka M, Sakurai T, Matsui S, Nishida N, Chikugo T, Kashida H, Kudo M. Risk Factors for Postoperative Bleeding in Endoscopic Submucosal Dissection of Colorectal Tumors. Oncology. 2017;93 Suppl 1:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Nagata M. Internal traction method using a spring-and-loop with clip (S-O clip) allows countertraction in gastric endoscopic submucosal dissection. Surg Endosc. 2020;34:3722-3733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Luo JC, Peng YL, Chen TS, Huo TI, Hou MC, Huang HC, Lin HC, Lee FY. Clopidogrel inhibits angiogenesis of gastric ulcer healing via downregulation of vascular endothelial growth factor receptor 2. J Formos Med Assoc. 2016;115:764-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |