Published online Jan 27, 2023. doi: 10.4240/wjgs.v15.i1.82
Peer-review started: July 25, 2022
First decision: September 26, 2022
Revised: October 11, 2022
Accepted: November 7, 2022
Article in press: November 7, 2022
Published online: January 27, 2023
Processing time: 176 Days and 16.7 Hours
Endoscopic variceal treatment (EVT) is recommended as the mainstay choice for the management of high-risk gastroesophageal varices and acute variceal bleed
To evaluate the effects of postoperative use of PPIs on post-EVT complications in patients with liver cirrhosis during hospitalization.
Patients with a diagnosis of liver cirrhosis who were admitted to the Department of Gastroenterology of the General Hospital of Northern Theater Command, treated by an attending physician between January 2016 and June 2020 and underwent EVT during their hospitalization were included. Logistic regression analyses were performed to explore the effects of postoperative use of PPIs on the development of post-EVT complications during hospitalization. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated.
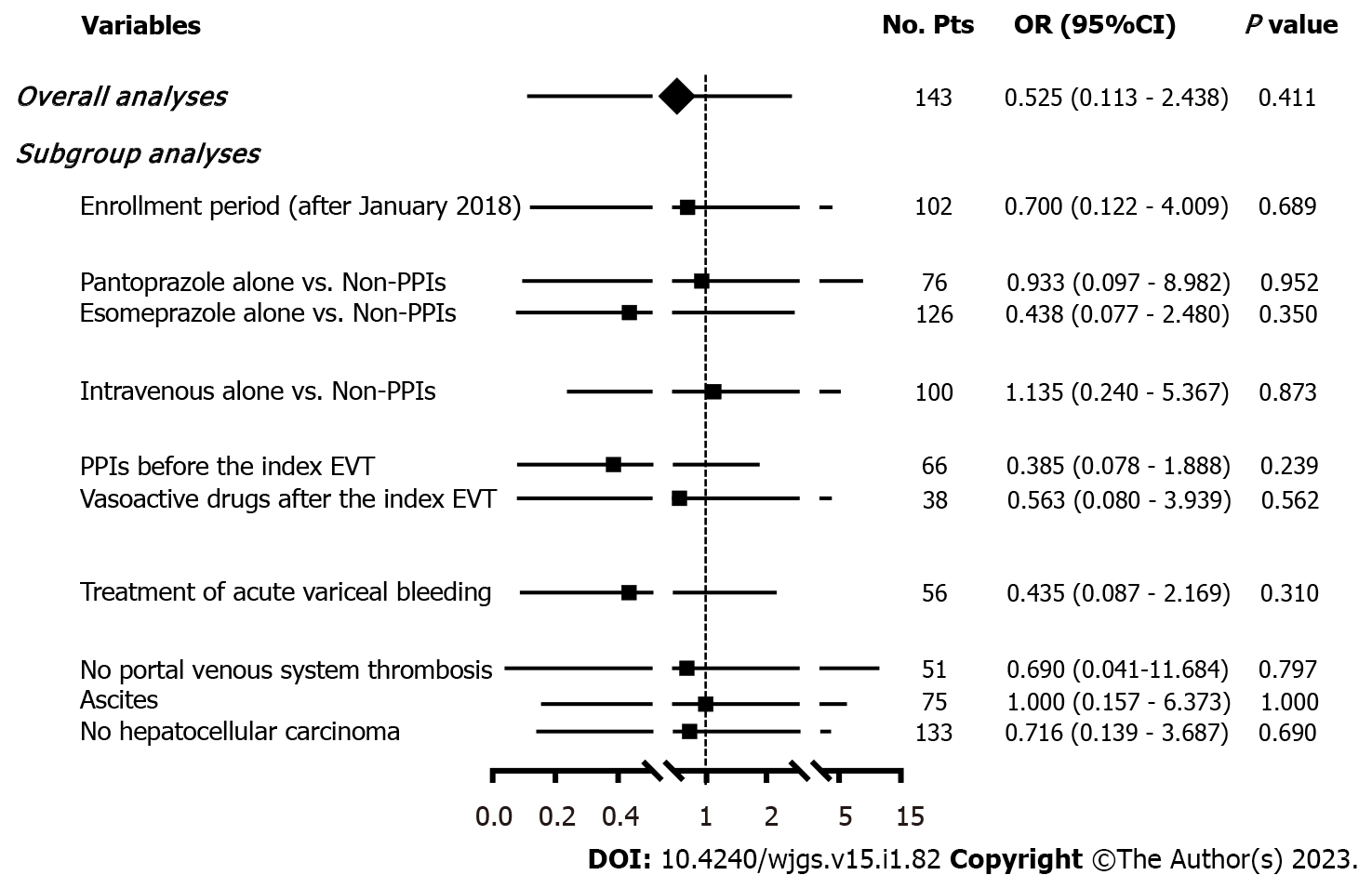
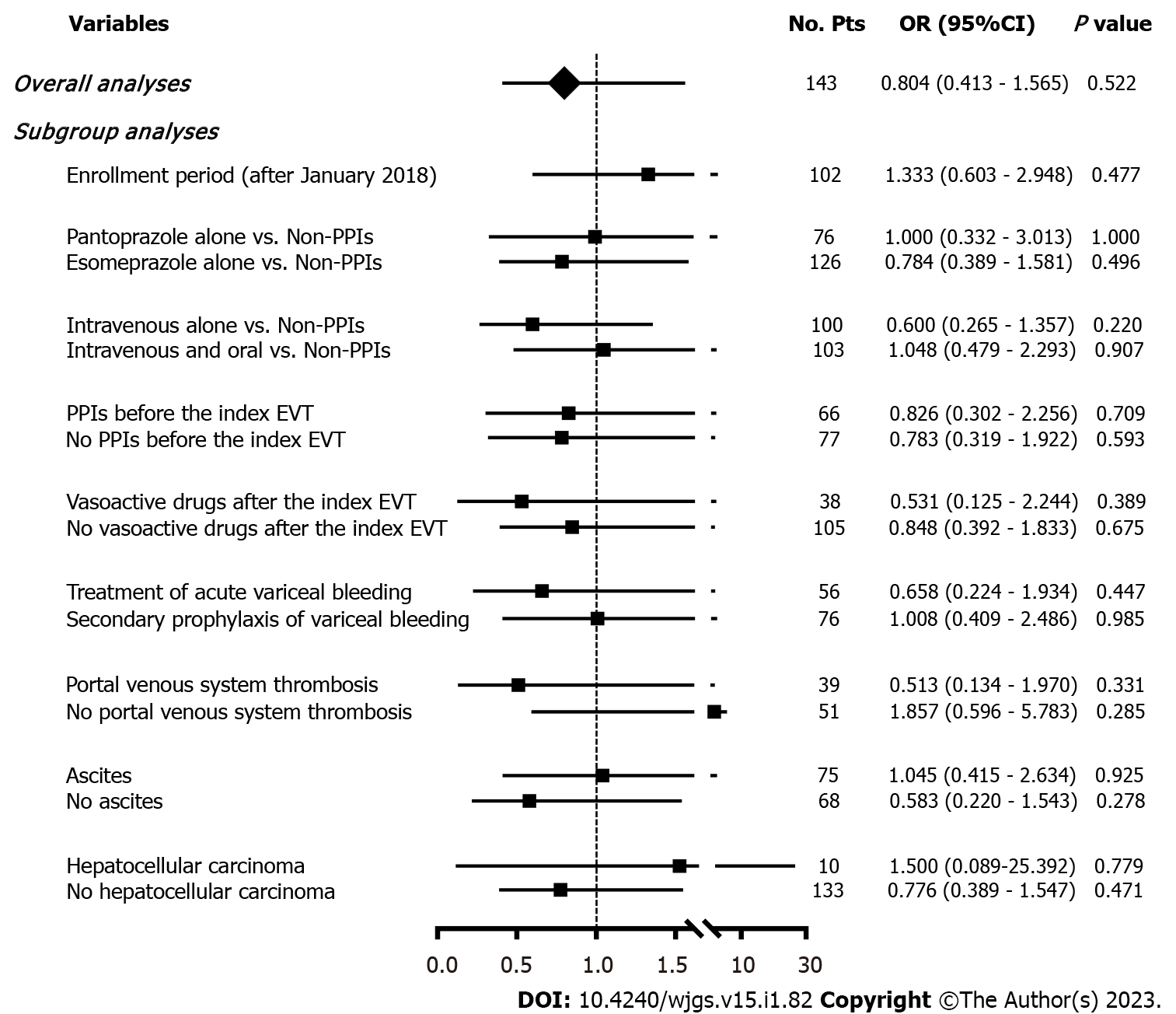
A total of 143 patients were included. The incidence of post-EVT GIB and other post-EVT complications was 4.90% and 46.85%, respectively. In the overall analyses, postoperative use of PPIs did not significantly reduce the risk of post-EVT GIB (OR = 0.525, 95%CI = 0.113-2.438, P = 0.411) or other post-EVT complications (OR = 0.804, 95%CI = 0.413-1.565, P = 0.522). In the subgroup analyses according to the enrollment period, type and route of PPIs after the index EVT, use of PPIs before the index EVT, use of vasoactive drugs after the index EVT, indication of EVT (prophylactic and therapeutic), and presence of portal venous system thrombosis, ascites, and hepatocellular carcinoma, the effects of postoperative use of PPIs on the risk of post-EVT GIB or other post-EVT complications remain not statistically significant.
Routine use of PPIs after EVT should not be recommended in patients with liver cirrhosis for the prevention of post-EVT complications during hospitalization.
Core Tip: The role of proton pump inhibitors (PPIs) in the management of post-endoscopic variceal treatment (EVT) complications remains controversial. We conducted a retrospective study to explore the effects of postoperative use of PPIs on post-EVT gastrointestinal bleeding (GIB) and other post-EVT complications in patients with liver cirrhosis during hospitalization. We found that postoperative use of PPIs was not beneficial for reducing the development of post-EVT GIB and other post-EVT complications during hospitalization. Collectively, routine use of PPIs after EVT during hospitalization may not be recommended, and their indications should be carefully evaluated.
- Citation: Zhang YY, Wang L, Shao XD, Zhang YG, Ma SZ, Peng MY, Xu SX, Yin Y, Guo XZ, Qi XS. Effects of postoperative use of proton pump inhibitors on gastrointestinal bleeding after endoscopic variceal treatment during hospitalization. World J Gastrointest Surg 2023; 15(1): 82-93
- URL: https://www.wjgnet.com/1948-9366/full/v15/i1/82.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i1.82
Acute variceal bleeding (AVB) is a serious complication of liver cirrhosis, indicating the disease progression and development of hepatic decompensation[1,2]. Endoscopic variceal treatment (EVT) is recommended as the major choice for the prevention and treatment of AVB[3,4]. However, the incidence of post-EVT gastrointestinal bleeding (GIB) ranges from 8% to 25%[5,6], which is mainly due to recurrent varices and post-EVT ulcers[7]. In detail, about 4% of patients develop recurrent variceal bleeding after EVT, and 3-25% of patients develop post-EVT ulcer-related GIB[2,8]. Notably, the mortality of GIB secondary to post-EVT ulcer is as high as 52%[2].
Considering the benefits of proton pump inhibitors (PPIs) on the prevention of post-EVT GIB[9,10], the American Society for Gastrointestinal Endoscopy recommends the use of PPIs after endoscopic variceal ligation (EVL) to decrease the rate of ligation-induced ulcer[11] and the Chinese Medical Association also recommends the postoperative use of PPIs to improve the hemostasis success and reduce the rates of ulcer and recent post-EVT GIB[12]. Indeed, the clinicians often use PPIs after EVT in clinical practice[13]. However, the British Society of Gastroenterology states that PPIs are only recommended in the presence of peptic ulcers[14]. Additionally, the Baveno VII consensus also states that patients who used PPIs before EVT should discontinue their use immediately after EVT unless they are strictly indicated[3]. Recent evidence also suggests that the use of PPIs in patients with liver cirrhosis may increase the risk of hepatic encephalopathy and spontaneous bacterial peritonitis[15]. Therefore, whether the routine use of PPIs after EVT is beneficial remains controversial. For this reason, we conducted a retrospective study to evaluate the effects of postoperative use of PPIs on post-EVT GIB and other post-EVT complications in patients with liver cirrhosis during hospitalization.
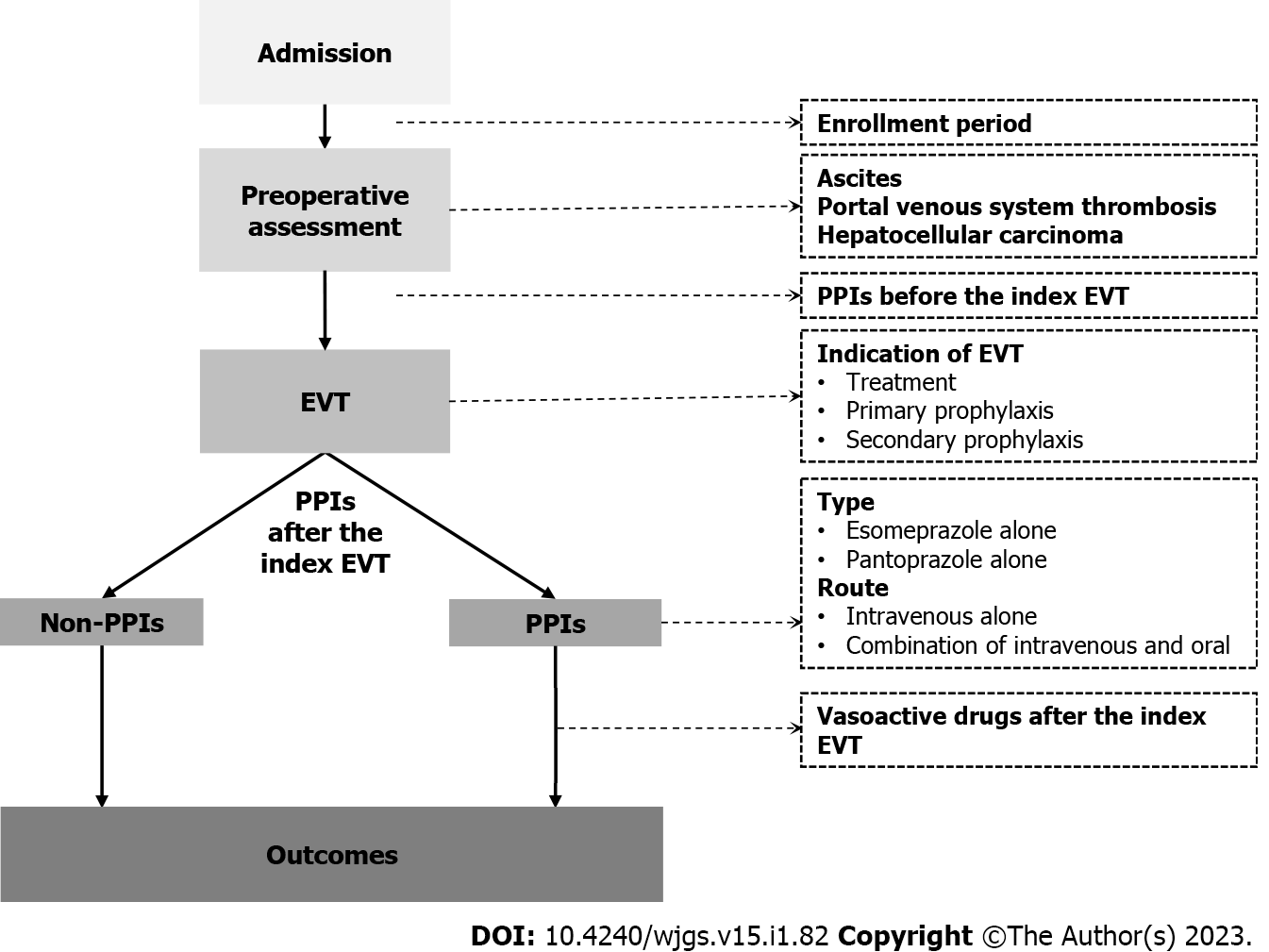
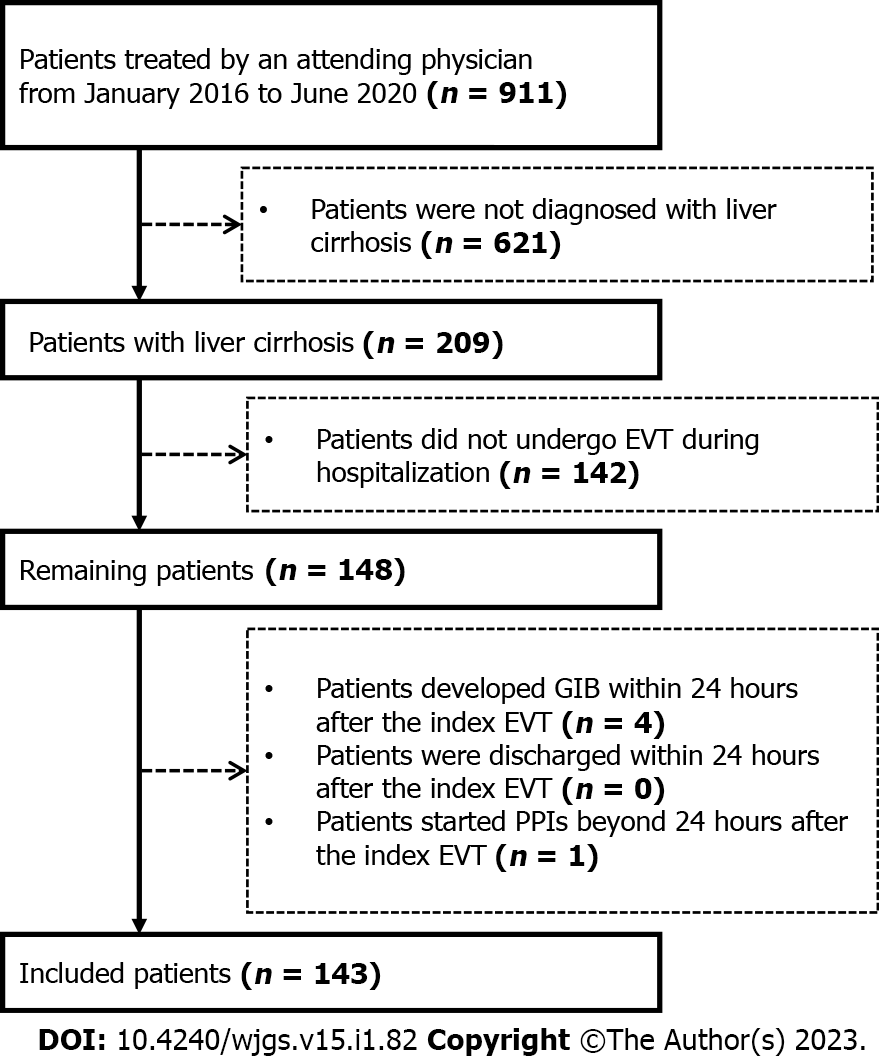
This study was approved by the Medical Ethical Committee of the General Hospital of Northern Theater Command with an approval number [Y (2022) 072] and was performed according to the principles of Declaration of Helsinki. The requirement for patients’ informed consent for this study was waived due to its retrospective nature. In this study, we retrospectively reviewed the medical records of 911 patients who were consecutively admitted to the Department of Gastroenterology of the General Hospital of Northern Theater Command between January 2016 and June 2020 and treated by an attending physician (XQ)[16-20]. We further selected patients who were diagnosed with liver cirrhosis and underwent EVT during their hospitalization. Exclusion criteria were: 1) patients who developed GIB or were discharged within 24 h after the index EVT; and 2) patients who started the use of PPIs beyond 24 h after the index EVT. Repeated admissions, malignancies, and other comorbidities were not excluded.
By reviewing electronical medical records, demographic data (i.e., age and gender), etiologies of liver cirrhosis, laboratory tests (i.e., white blood cell, hemoglobin, platelet count, total bilirubin, albumin, alanine aminotransferase, serum creatinine, sodium, and international normalized ratio), and other complications of liver cirrhosis [i.e., ascites, jaundice, hepatic encephalopathy, portal venous system thrombosis (PVST)[17], and hepatocellular carcinoma (HCC)] at admission were collected. Model for end-stage liver disease (MELD) score, Child-Pugh score, and Child-Pugh class at admission were calculated[21].
All EVT procedures were performed by the same experienced endoscopist (XS) at our department[22,23]. EVL and endoscopic cyanoacrylate glue injection (ECGI) were the first-line choices for the management of esophageal and gastric varices, respectively. Endoscopic injection sclerotherapy (EIS) was performed, if EVL was technically difficult, where active massive bleeding impaired visualization or local scar tissue prevented esophageal varices from being aspirated into the cap to achieve ligation. Indication (i.e., treatment of AVB and primary and secondary prophylaxis of variceal bleeding) and type (i.e., EVL, ECGI and EIS) of EVT and endoscopic findings [i.e., grade of esophageal varices (EVs), red sign of EVs, and active bleeding under endoscopy] were reviewed. The use of PPIs before the index EVT and vasoactive drugs (i.e., octreotide, somatostatin, and terlipressin) after the index EVT were also reviewed. If a patient underwent two or more EVT procedures during the same hospitalization, only the data before the second EVT procedure would be collected.
Postoperative PPIs were routinely used in all patients who underwent EVT before January 2018. Since then, this attending physician has systematically reviewed the evidence and questioned the clinical significance of use of PPIs following EVT[10]. Thus, postoperative PPIs would be given on demand if a patient was diagnosed with peptic ulcers, esophageal, gastric, and/or duodenal mucosal erosions, or white nipple signs on endoscopy, developed active variceal bleeding during EVT procedures, or complained of acid-related upper gastrointestinal symptoms (i.e., heartburn and acid regurgitation). Enrollment period, type (i.e., esomeprazole and pantoprazole), route (i.e., intravenous and oral), dosage (i.e., 40 mg once daily, 40 mg twice daily, and 80 mg twice daily), date of starting and discontinuation, and duration of PPIs after the index EVT were reviewed. These data were extracted until post-EVT GIB, the second EVT procedure, or discharge, whichever came first.
Patients were divided into PPIs and non-PPIs groups. The PPIs group was defined as patients who had started on PPIs within 24 h after the index EVT for at least one day before post-EVT GIB, the second EVT procedure, or discharge, whichever came first. The non-PPIs group was defined as patients who had not received PPIs after the index EVT until post-EVT GIB, the second EVT procedure, or discharge, whichever came first (Figure 1).
The primary outcome was the development of post-EVT GIB during hospitalization. Post-EVT GIB was defined as the presence of hematemesis, and/or melena, and/or hematochezia, and/or firm clinical or laboratory evidence of acute blood loss from the gastrointestinal tract after the index EVT[24]. Other post-EVT complications included retrosternal pain/discomfort, nausea/vomiting, heartburn/acid regurgitation, fever, diarrhea, and abdominal pain.
All statistical analyses were performed using the IBM SPSS 20.0 (IBM Corp, Armonk, NY, USA). Continuous variables were expressed as median (range) and mean ± standard deviation, and categorical variables were expressed as frequency (percentage). The non-parametric Mann-Whitney U test was used to compare continuous variables between PPIs and non-PPIs groups, and the Chi-square test and Fisher's exact test were used to compare categorical variables between the two groups. Logistic regression analyses were performed to explore the impact of postoperative PPIs on post-EVT GIB and other post-EVT complications during hospitalization. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. Subgroup analyses were performed according to the enrollment period, type and route of PPIs after the index EVT, use of PPIs before the index EVT, use of vasoactive drugs after the index EVT, indication of EVT, and presence of PVST, ascites, and HCC (Figure 1). A two-sided P < 0.05 was considered statistically significant.
A total of 148 patients with cirrhosis underwent EVT during their hospitalization. Finally, 143 patients were included (Figure 2). Of them, 83 were in the PPIs group and 60 in the non-PPIs group. The median duration of PPIs administration was 6 (1-13) d. The median hospital stay after EVT was 6 (2-16) d. Patient characteristics are shown in Table 1. Hepatitis B virus infection alone (36.36%) was the most common etiology of liver cirrhosis followed by alcohol abuse alone (23.08%). The median MELD score and Child-Pugh score were 10.24 and 6.00, respectively. Eighty (55.94%), 14 (9.79%), 6 (4.20%), 41 (28.67%), 1 (0.70%), and 1 (0.70%) patient were treated with EVL alone, ECGI alone, EIS alone, EVL combined with ECGI, EIS combined with ECGI, and EVL combined with ECGI and EIS, respectively (Table 2).
| Variables | Overall | PPIs | Non-PPIs | P value | |||
| No.Pts | Median (range), mean ± SD, or frequency (percentage) | No.Pts | Median (range), mean ± SD, or frequency (percentage) | No.Pts | Median (range), mean ± SD, or frequency (percentage) | ||
| Age (yr) | 143 | 56.00 (28.00-88.00) | 83 | 58.00 (30.00-88.00) | 60 | 54.50 (28.00-79.00) | 0.089 |
| 55.88 ± 11.77 | 57.40 ± 11.86 | 53.78 ± 11.41 | |||||
| Male | 143 | 104 (72.73%) | 83 | 61 (73.49%) | 60 | 43 (71.67%) | 0.809 |
| HBV infection alone | 143 | 52 (36.36%) | 83 | 31 (37.35%) | 60 | 21 (35.00%) | 0.773 |
| HCV infection alone | 143 | 11 (7.69%) | 83 | 4 (4.82%) | 60 | 7 (11.67%) | 0.202 |
| Alcohol abuse alone | 143 | 33 (23.08%) | 83 | 22 (26.51%) | 60 | 11 (18.33%) | 0.252 |
| White blood cell (109/L) | 141 | 3.50 (0.80-19.60) | 81 | 3.60 (0.80-19.60) | 60 | 3.45 (1.00-17.40) | 0.381 |
| 3.99 ± 2.60 | 4.15 ± 2.74 | 3.78 ± 2.40 | |||||
| Hemoglobin (g/L) | 141 | 89.00 (48.00-155.00) | 81 | 83.00 (48.00-155.00) | 60 | 97.50 (57.00-149.00) | 0.081 |
| 93.21 ± 26.69 | 90.15 ± 27.48 | 97.35 ± 25.23 | |||||
| Platelet count (109/L) | 141 | 75.00 (15.00-470.00) | 81 | 76.00 (22.00-268.00) | 60 | 71.00 (15.00-470.00) | 0.970 |
| 92.58 ± 66.85 | 87.80 ± 52.28 | 99.03 ± 82.61 | |||||
| Total bilirubin (μmol/L) | 132 | 20.40 (5.60-106.10) | 74 | 24.35 (7.00-106.10) | 58 | 16.30 (5.60-96.60) | 0.006 |
| 25.67 ± 18.53 | 28.72 ± 19.92 | 21.78 ± 15.91 | |||||
| Albumin (g/L) | 133 | 33.40 (20.50-48.70) | 75 | 31.80 (20.50-45.70) | 58 | 35.30 (21.80-48.70) | 0.048 |
| 33.21 ± 5.91 | 32.41 ± 5.48 | 34.26 ± 6.32 | |||||
| Alanine aminotransferase (U/L) | 132 | 20.92 (4.47-1465.50) | 74 | 19.40 (7.57-1465.50) | 58 | 23.62 (4.47-185.02) | 0.228 |
| 38.52 ± 127.21 | 43.85 ± 168.05 | 31.72 ± 30.61 | |||||
| Serum creatinine (μmol/L) | 135 | 64.93 (34.51-501.52) | 76 | 64.52 (34.51-117.66) | 59 | 65.21 (36.39-501.52) | 0.591 |
| 70.77 ± 42.31 | 66.97 ± 18.75 | 75.68 ± 60.31 | |||||
| Sodium (mmol/L) | 134 | 138.85 (124.00-151.00) | 75 | 138.70 (124.00-151.00) | 59 | 139.00 (133.10-147.70) | 0.798 |
| 138.55 ± 3.38 | 138.49 ± 3.65 | 138.64 ± 3.04 | |||||
| International normalized ratio | 135 | 1.29 (0.90-2.55) | 75 | 1.29 (0.90-2.55) | 60 | 1.31 (0.92-2.04) | 0.685 |
| 1.36 ± 0.26 | 1.37 ± 0.27 | 1.35 ± 0.25 | |||||
| MELD score | 129 | 10.24 (6.65-30.03) | 71 | 10.51 (6.65-30.03) | 58 | 9.93 (7.14-22.06) | 0.214 |
| 11.44 ± 3.78 | 11.75 ± 4.03 | 11.05 ± 3.45 | |||||
| Child-Pugh score | 130 | 6.00 (5.00-12.00) | 72 | 7.00 (5.00-12.00) | 58 | 6.00 (5.00-10.00) | 0.388 |
| 6.76 ± 1.59 | 6.89 ± 1.68 | 6.60 ± 1.47 | |||||
| Child-Pugh class | |||||||
| A | 66 (50.77%) | 35 (48.61%) | 31 (53.45%) | ||||
| B | 130 | 57 (43.85%) | 72 | 32 (44.44%) | 58 | 25 (43.10%) | 0.709 |
| C | 7 (5.38%) | 5 (6.94%) | 2 (3.45%) | ||||
| Ascites | 143 | 75 (52.45%) | 83 | 45 (54.22%) | 60 | 30 (50.00%) | 0.618 |
| Jaundice | 132 | 12 (9.09%) | 74 | 9 (12.16%) | 58 | 3 (5.17%) | 0.166 |
| Hepatic encephalopathy | 143 | 1 (0.70%) | 83 | 0 (0.00%) | 60 | 1 (1.67%) | 0.420 |
| Portal venous system thrombosis | 90 | 39 (43.33%) | 55 | 25 (45.45%) | 35 | 14 (40.00%) | 0.611 |
| Hepatocellular carcinoma | 143 | 10 (6.99%) | 83 | 6 (7.23%) | 60 | 4 (6.67%) | 1.000 |
| Variables | Overall | PPIs | Non-PPIs | P value | |||
| No.Pts | Frequency (percentage) | No.Pts | Frequency (percentage) | No.Pts | Frequency (percentage) | ||
| Grade of esophageal varices | |||||||
| Mild | 27 (19.01%) | 14 (17.07%) | 13 (21.67%) | ||||
| Moderate | 142 | 35 (24.65%) | 82 | 21 (25.61%) | 60 | 14 (23.33%) | 0.783 |
| Severe | 80 (56.34%) | 47 (57.32%) | 33 (55.00%) | ||||
| Red sign of esophageal varices | 143 | 98 (68.53%) | 83 | 57 (68.67%) | 60 | 41 (68.33%) | 0.965 |
| Active bleeding | 143 | 5 (3.50%) | 83 | 5 (6.02%) | 60 | 0 (0.00%) | 0.074 |
| Indication of EVT | |||||||
| Treatment | 56 (39.16%) | 34 (40.96%) | 22 (36.67%) | ||||
| Primary prophylaxis | 143 | 11 (7.69%) | 83 | 10 (12.05%) | 60 | 1 (1.67%) | 0.035 |
| Secondary prophylaxis | 76 (53.15%) | 39 (46.99%) | 37 (61.67%) | ||||
| Type of EVT | |||||||
| EVL | 80 (55.94%) | 46 (55.42%) | 34 (56.67%) | ||||
| ECGI | 14 (9.79%) | 6 (7.23%) | 8 (13.33%) | ||||
| EIS | 143 | 6 (4.20%) | 83 | 4 (4.82%) | 60 | 2 (3.33%) | 0.799 |
| EVL+ECGI | 41 (28.67%) | 25 (30.12%) | 16 (26.67%) | ||||
| EIS+ECGI | 1 (0.70%) | 1 (1.20%) | 0 (0.00%) | ||||
| EVL+ECGI+EIS | 1 (0.70%) | 1 (1.20%) | 0 (0.00%) | ||||
| PPIs before the index EVT | 143 | 66 (46.15%) | 83 | 42 (50.60%) | 60 | 24 (40.00%) | 0.209 |
| Vasoactive drugs after the index EVT | 143 | 38 (26.57%) | 83 | 27 (32.53%) | 60 | 11 (18.33%) | 0.058 |
| Post-EVT GIB | 143 | 7 (4.90%) | 83 | 3 (3.61%) | 60 | 4 (6.67%) | 0.453 |
| Other post-EVT complications | 143 | 67 (46.85%) | 83 | 37 (44.58%) | 60 | 30 (50.00%) | 0.521 |
Seven (4.90%) patients developed post-EVT GIB, including three in the PPIs group and four in the non-PPIs group. The median interval between the index EVT and post-EVT GIB was 4 (2-7) d. Only one of them underwent endoscopy and it was found that the source of post-EVT GIB was a post-EVT ulcer. All of them were administered immediately with intravenous vasoactive drugs for the management of post-EVT GIB and two received blood transfusions. Other post-EVT complications were recorded in 67 (46.85%) patients. Logistic regression analyses showed that postoperative use of PPIs was not significantly associated with the risk of post-EVT GIB (OR = 0.525, 95%CI = 0.113-2.438, P = 0.411) (Figure 3) or other post-EVT complications (OR = 0.804, 95%CI = 0.413-1.565, P = 0.522) (Figure 4).
In all subgroup analyses according to the enrollment period, type and route of PPIs after the index EVT, use of PPIs before the index EVT, use of vasoactive drugs after the index EVT, indication of EVT, and presence of PVST, ascites, and HCC, logistic regression analyses showed that postoperative use of PPIs was not significantly associated with the risk of post-EVT GIB (Figure 3) or other post-EVT complications (Figure 4).
PPIs are one of the most commonly used drugs in the world[25]. Increasing evidence suggests that the use of PPIs may reduce the abundance and diversity of gut microbiota, leading to the growth of pathogens and the overgrowth of unhealthy species, and that it may be associated with bone fracture, clostridium difficile infection, spontaneous bacterial peritonitis, and hepatic encephalopathy[25,26]. These harms have raised serious concerns about the rational use of PPIs worldwide[27]. Therefore, clinicians should carefully consider the postoperative use of PPIs during hospitalization, and assess the optimal effective dosage and duration of PPIs to avoid their related side effects.
Our study found that postoperative use of PPIs had no significant effect on post-EVT GIB and other post-EVT complications. Our study has several advantages in terms of study design. First, all patients were diagnosed and treated by the same attending physician and all EVT procedures were also performed by the same endoscopist, which avoids heterogeneity in the management of patients. Second, patients who underwent prophylactic and therapeutic EVT procedures were both included. Third, subgroup analyses were comprehensively performed according to the enrollment period, type and route of PPIs after the index EVT, use of PPIs before the index EVT, use of vasoactive drugs after the index EVT, indication of EVT (prophylactic and therapeutic EVT), and presence of PVST, ascites, and HCC, which minimizes the impact of confounding factors on statistical results. Fourth, all included patients had been evaluated for at least 24 h since the index EVT, which potentially rules out the effect of technical failure on patients’ outcomes.
Post-EVT ulcer, which is one of the main causes of post-EVT GIB, is primarily due to early slippage of rubber bands, sclerosant-induced inflammatory necrosis, and tissue glue-induced caseous necrosis[7,28-31]. It has been traditionally believed that the presence of gastric acid delays ulcer healing[32]. Esophageal motility may be temporarily impaired due to nerve plexus injury after EVT, which delays gastric acid clearance and aggravates the progression of ulcers[33,34]. PPIs are potent acid inhibitors widely used for various acid-related diseases and may promote early healing of post-EVT ulcers by reducing gastric acid secretion, thereby probably decreasing the risk of post-EVT GIB[26,32,35]. In contrast, our study did not demonstrate the benefits of postoperative PPIs in reducing the development of post-EVT GIB. There are some explanations for this unexpected phenomenon. First, post-EVT ulcers are more prone to develop bleeding primarily due to persistent portal hypertension, but not gastric acid[4,31]. Second, the use of PPIs can only reduce the size of ulcers, but not the number of ulcers[36]. Notably, the size of ulcers is not associated with the risk of bleeding[36]. Third, we only observed the impact of short-term use of PPIs on the development of post-EVT GIB during hospitalization. However, post-EVT ulcer healing often requires a duration of about 2 wk[37,38].
Our previous meta-analysis showed a significant benefit of PPIs on post-EVT GIB in patients who underwent prophylactic EVL, but not therapeutic EVT[10]. However, the present study could not confirm the protective effect of postoperative use of PPIs on GIB after prophylactic EVT, because none of the patients who underwent EVT for primary or secondary prophylaxis of variceal bleeding developed post-EVT GIB. Nevertheless, it has been proposed that post-EVL ulcers are usually shallower with only superficial mucosal damage, which may heal more easily with the use of PPIs[37]. Patients who need EVT for the treatment of AVB often have a white nipple, red nipple, or mucosal erosion on endoscopy. Undoubtedly, their conditions are more severe, where the anti-acid effect of PPIs may be insufficient for the improvement of ulcer healing[4].
Except for post-EVT GIB, EVT can also cause other procedure-related complications, which are mild and reversible[3,7]. We did not find any significant effect of PPIs on the development of other post-EVT complications. This can be explained by the fact that only a fraction of post-EVT complications, such as acid regurgitation and heartburn, are related to gastric acid[39]. By comparison, retrosternal dis
Our study has some limitations. First, the total number of the patients included was small in this study. Second, there were a few cases of post-EVT GIB, which made our statistical analyses under
Our study suggested that postoperative use of PPIs could not reduce the development of post-EVT GIB and other post-EVT complications during hospitalization. Therefore, PPIs after EVT should not be routinely used during hospitalization, and their indications should be carefully evaluated.
Endoscopic variceal treatment (EVT) is frequently used in cirrhosis with high-risk gastroesophageal varices and acute variceal bleeding. However, it is often associated with a high risk of post-EVT complications, especially postoperative gastrointestinal bleeding (GIB).
The role of proton pump inhibitors (PPIs) after EVT remains controversial.
To evaluate the impact of postoperative use of PPIs on post-EVT GIB and other post-EVT complications in patients with liver cirrhosis during hospitalization.
We retrospectively reviewed 911 patients who were consecutively admitted to the Department of Gastroenterology of the General Hospital of Northern Theater Command between January 2016 and June 2020 and treated by an attending physician. Logistic regression analyses were performed to explore the impact of postoperative PPIs on post-EVT GIB and other post-EVT complications during hospitalization.
A total of 143 patients were included. The incidence of post-EVT GIB and other post-EVT complications was 4.90% and 46.85%, respectively. In either overall or subgroup analyses, postoperative use of PPIs did not significantly reduce the risk of post-EVT GIB or other post-EVT complications.
Postoperative use of PPIs was not beneficial for reducing the development of post-EVT GIB and other post-EVT complications during hospitalization.
PPIs after EVT should not be routinely used during hospitalization, and their indications should be carefully evaluated. Prospective studies are required to further validate the conclusions of this study.
We are indebted to our study team, including Wen-Chun Bao, Fei-Fei Hou, Ze-Qi Guo, Jing-Qiao Zhang, Xin-Miao Zhou, Miao-Miao Li, Yang An, Rui-Rui Feng, Cen Hong, Yang-Lan He, Hai-Juan Yao, and Le Wang, for establishing and updating the database which prospectively recorded the patients treated by Dr. Xing-Shun Qi.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Delsa H, Morocco; Wu R, China S-Editor: Liu GL L-Editor: Ma JY - MedE A P-Editor: Liu GL
| 1. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 850] [Article Influence: 212.5] [Reference Citation Analysis (1)] |
| 2. | Vanbiervliet G, Giudicelli-Bornard S, Piche T, Berthier F, Gelsi E, Filippi J, Anty R, Arab K, Huet PM, Hebuterne X, Tran A. Predictive factors of bleeding related to post-banding ulcer following endoscopic variceal ligation in cirrhotic patients: a case-control study. Aliment Pharmacol Ther. 2010;32:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1537] [Cited by in RCA: 1477] [Article Influence: 492.3] [Reference Citation Analysis (2)] |
| 4. | Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1438] [Article Influence: 179.8] [Reference Citation Analysis (3)] |
| 5. | Petrasch F, Grothaus J, Mössner J, Schiefke I, Hoffmeister A. Differences in bleeding behavior after endoscopic band ligation: a retrospective analysis. BMC Gastroenterol. 2010;10:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Schepke M, Kleber G, Nürnberg D, Willert J, Koch L, Veltzke-Schlieker W, Hellerbrand C, Kuth J, Schanz S, Kahl S, Fleig WE, Sauerbruch T; German Study Group for the Primary Prophylaxis of Variceal Bleeding. Ligation versus propranolol for the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology. 2004;40:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 132] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Zuckerman MJ, Elhanafi S, Mendoza Ladd A. Endoscopic Treatment of Esophageal Varices. Clin Liver Dis. 2022;26:21-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Schmitz RJ, Sharma P, Badr AS, Qamar MT, Weston AP. Incidence and management of esophageal stricture formation, ulcer bleeding, perforation, and massive hematoma formation from sclerotherapy versus band ligation. Am J Gastroenterol. 2001;96:437-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Lin L, Cui B, Deng Y, Jiang X, Liu W, Sun C. The Efficacy of Proton Pump Inhibitor in Cirrhotics with Variceal Bleeding: A Systemic Review and Meta-Analysis. Digestion. 2021;102:117-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Zhu J, Qi X, Yu H, Su C, Guo X. Acid suppression in patients treated with endoscopic therapy for the management of gastroesophageal varices: a systematic review and meta-analysis. Expert Rev Gastroenterol Hepatol. 2018;12:617-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Hwang JH, Shergill AK, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Evans JA, Fanelli RD, Fisher DA, Foley KQ, Fonkalsrud L, Jue T, Khashab MA, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Sharaf R, Cash BD; American Society for Gastrointestinal Endoscopy. The role of endoscopy in the management of variceal hemorrhage. Gastrointest Endosc. 2014;80:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (1)] |
| 12. | Chinese Society of Hepatology Chinese Medical Association; Chinese Society of Gastroenterology Chinese Medical Association; Chinese Society of Endoscopy Chinese Medical Association. Guidelines for the diagnosis and treatment of esophageal and gastric variceal bleeding in cirrhotic portal hypertension. J Clin Hepatol. 2016;32:203-219. [DOI] [Full Text] |
| 13. | Blasi A, Machlab S, Risco R, Costa-Freixas JP, Hernández-Cely G, Horta D, Bofill A, Ruiz-Ramirez P, Profitos J, Sanahuja JM, Fernandez-Simon A, Gómez MV, Sánchez-Delgado J, Cardenas A. A multicenter analysis of the role of prophylactic transfusion of blood products in patients with cirrhosis and esophageal varices undergoing endoscopic band ligation. JHEP Rep. 2021;3:100363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Tripathi D, Stanley AJ, Hayes PC, Patch D, Millson C, Mehrzad C, Austin A, Ferguson JW, Olliff SP, Hudson M, Christie JM; Clinical Services and Standards Committee of the British Society of Gastroenterology. U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. 2015;1680-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 454] [Cited by in RCA: 414] [Article Influence: 41.4] [Reference Citation Analysis (2)] |
| 15. | Li DK, Chung RT. Use of proton pump inhibitors in chronic liver diseases. Clin Liver Dis (Hoboken). 2017;10:148-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Hong C, Xu X, Feng R, Romeiro FG, Zhang D, Bai Z, Guo X, Qi X. Use of iron sucrose injection in anemia patients with reduced serum iron concentration during hospitalizations of digestive and liver diseases. Ann Palliat Med. 2021;10:1145-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Wang L, Guo X, Xu X, Xu S, Han J, Wang R, Guo Z, Yi F, Qi X. No Association of Homocysteine, Anticardiolipin Antibody, and Anti-β2 Glycoprotein I Antibody With Portal Venous System Thrombosis in Liver Cirrhosis. Clin Appl Thromb Hemost. 2021;27:10760296211010969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Li M, Guo Z, Zhang D, Xu X, Romeiro FG, Mancuso A, Zhang J, Feng R, Zhou X, Hong C, Qi X. Correlation of Serum Cardiac Markers with Acute Decompensating Events in Liver Cirrhosis. Gastroenterol Res Pract. 2020;2020:4019289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Feng R, Guo X, Kou Y, Xu X, Hong C, Zhang W, An Y, Philips CA, Mancuso A, Qi X. Association of lipid profile with decompensation, liver dysfunction, and mortality in patients with liver cirrhosis. Postgrad Med. 2021;133:626-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | An Y, Xu X, Ren T, Tong Z, Romeiro FG, Mancuso A, Guo X, Qi X. Adherence to Non-Selective Beta Blockers for Prevention of Variceal Bleeding in Cirrhotic Patients. Int J Gen Med. 2021;14:6713-6724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Peng Y, Qi X, Guo X. Child-Pugh Versus MELD Score for the Assessment of Prognosis in Liver Cirrhosis: A Systematic Review and Meta-Analysis of Observational Studies. Medicine (Baltimore). 2016;95:e2877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 340] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 22. | Wang L, Guo X, Shao X, Xu X, Zheng K, Wang R, Chawla S, Basaranoglu M, Qi X. Association of endoscopic variceal treatment with portal venous system thrombosis in liver cirrhosis: a case-control study. herap Adv Gastroenterol. 2022;15:17562848221087536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Li Q, Wang R, Guo X, Li H, Shao X, Zheng K, Qi X, Li Y. Contrast-Enhanced CT May Be a Diagnostic Alternative for Gastroesophageal Varices in Cirrhosis with and without Previous Endoscopic Variceal Therapy. Gastroenterol Res Pract. 2019;2019:6704673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Drolz A, Schramm C, Seiz O, Groth S, Vettorazzi E, Horvatits T, Wehmeyer MH, Goeser T, Roesch T, Lohse AW, Kluwe J. Risk factors associated with bleeding after prophylactic endoscopic variceal ligation in cirrhosis. Endoscopy. 2021;53:226-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L, Tigchelaar EF, Jankipersadsing SA, Cenit MC, Harmsen HJ, Dijkstra G, Franke L, Xavier RJ, Jonkers D, Wijmenga C, Weersma RK, Zhernakova A. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740-748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 780] [Cited by in RCA: 931] [Article Influence: 103.4] [Reference Citation Analysis (0)] |
| 26. | Zhu J, Yu H, Mancuso A, Qi X. Proton pump inhibitors in liver cirrhosis: a review of benefits and harms. AME Medical Journal. 2017;2:36-36. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Savarino V, Tosetti C, Benedetto E, Compare D, Nardone G. Appropriateness in prescribing PPIs: A position paper of the Italian Society of Gastroenterology (SIGE) - Study section "Digestive Diseases in Primary Care". Dig Liver Dis. 2018;50:894-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Garg PK, Sidhu SS, Bhargava DK. Role of omeprazole in prevention and treatment of postendoscopic variceal sclerotherapy esophageal complications. Double-blind randomized study. Dig Dis Sci. 1995;40:1569-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Woodward SC, Herrmann JB, Cameron JL, Brandes G, Pulaski EJ, Leonard F. Histotoxicity of cyanoacrylate tissue adhesive in the rat. Ann Surg. 1965;162:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 135] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Kunstlinger F, Brunelle F, Chaumont P, Doyon D. Vascular occlusive agents. AJR Am J Roentgenol. 1981;136:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 73] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Cho E, Jun CH, Cho SB, Park CH, Kim HS, Choi SK, Rew JS. Endoscopic variceal ligation-induced ulcer bleeding: What are the risk factors and treatment strategies? Medicine (Baltimore). 2017;96:e7157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Johlin FC, Labrecque DR, Neil GA. Omeprazole heals mucosal ulcers associated with endoscopic injection sclerotherapy. Dig Dis Sci. 1992;37:1373-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Snady H, Korsten MA. Esophageal acid-clearance and motility after endoscopic sclerotherapy of esophageal varices. Am J Gastroenterol. 1986;81:419-422. [PubMed] |
| 34. | Grande L, Planas R, Lacima G, Boix J, Ros E, Esteve M, Morillas R, Gasull MA. Sequential esophageal motility studies after endoscopic injection sclerotherapy: a prospective investigation. Am J Gastroenterol. 1991;86:36-40. [PubMed] |
| 35. | Gimson A, Polson R, Westaby D, Williams R. Omeprazole in the management of intractable esophageal ulceration following injection sclerotherapy. Gastroenterology. 1990;99:1829-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Shaheen NJ, Stuart E, Schmitz SM, Mitchell KL, Fried MW, Zacks S, Russo MW, Galanko J, Shrestha R. Pantoprazole reduces the size of postbanding ulcers after variceal band ligation: a randomized, controlled trial. Hepatology. 2005;41:588-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 37. | Young MF, Sanowski RA, Rasche R. Comparison and characterization of ulcerations induced by endoscopic ligation of esophageal varices versus endoscopic sclerotherapy. Gastrointest Endosc. 1993;39:119-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 73] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Nijhawan S, Rai RR, Nepalia S, Pokharana DS, Bharagava N. Natural history of postligation ulcers. Am J Gastroenterol. 1994;89:2281-2282. [PubMed] |
| 39. | Boparai V, Rajagopalan J, Triadafilopoulos G. Guide to the use of proton pump inhibitors in adult patients. Drugs. 2008;68:925-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Zubarik R, Eisen G, Mastropietro C, Lopez J, Carroll J, Benjamin S, Fleischer DE. Prospective analysis of complications 30 days after outpatient upper endoscopy. Am J Gastroenterol. 1999;94:1539-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Kapoor A, Dharel N, Sanyal AJ. Endoscopic Diagnosis and Therapy in Gastroesophageal Variceal Bleeding. Gastrointest Endosc Clin N Am. 2015;25:491-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 42. | Lo GH, Perng DS, Chang CY, Tai CM, Wang HM, Lin HC. Controlled trial of ligation plus vasoconstrictor versus proton pump inhibitor in the control of acute esophageal variceal bleeding. J Gastroenterol Hepatol. 2013;28:684-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |