Published online Jan 27, 2023. doi: 10.4240/wjgs.v15.i1.60
Peer-review started: July 11, 2022
First decision: November 18, 2022
Revised: November 23, 2022
Accepted: December 23, 2022
Article in press: December 23, 2022
Published online: January 27, 2023
Processing time: 190 Days and 13 Hours
Pancreatic ductal adenocarcinoma is a common malignancy. Despite all advancements, the prognosis remains, poor with an overall 5-year survival of only 10.8%. Recently, a robotic platform has become an attractive tool for treating pancreatic cancer (PC). While recent studies indicated improved lymph node (LN) harvest during robotic pancreaticoduodenectomy (PD), data on long-term outcomes are insufficient.
To evaluate absolute LN harvest during PD. Secondary outcomes included evaluating the association between LN harvest and short- and long-term oncological outcomes for three different surgical approaches.
We conducted an analysis of the National Cancer Database, including patients diagnosed with PC who underwent open, laparoscopic, or robotic PD in 2010-2018. One-way analysis of variance was used to compare continuous variables, chi-square test - for categorical. Overall survival was defined as the time between surgery and death. Median survival time was estimated with the Kaplan-Meier method, and groups were compared with the Wilcoxon test. A Cox proportional hazards model was used to assess the association of covariates with survival after controlling for patient characteristics and procedure type.
17169 patients were included, 8859 (52%) males; mean age 65; 14509 (85%) white. 13816 (80.5%) patients had an open PD, 2677 (15.6%) and 676 (3.9%) - laparoscopic and robotic PD respectively. Mean comorbidity index (Charlson-Deyo Score) 0.50. On average, 18.84 LNs were harvested. Mean LN harvest during open, laparoscopic and robotic PD was 18.59, 19.65 and 20.70 respectively (P < 0.001). On average 2.49 LNs were positive for cancer and did not differ by the procedure type (P = 0.26). Vascular invasion was noted in 42.6% of LNs and did differ by the approach: 42.1% for open, 44.0% for laparoscopic and 47.2% for robotic PD (P = 0.015). Median survival for open PD was 26.1 mo, laparoscopic - 27.2 mo, robotic - 29.1 mo (P = 0.064). Survival was associated with higher LN harvest, while higher number of positive LNs was associated with higher mortality.
Our study suggests that robotic PD is associated with increased intraoperative LN harvest and has comparable short-term oncological outcomes and survival compared to open and laparoscopic approaches.
Core Tip: This retrospective study evaluated absolute lymph node (LN) harvest during pancreaticoduodenectomy (PD) for analyzed over 17000 patients who underwent PD from 2010 to 2018. The number of LN harvested differed by the procedure type (open, laparoscopic, robotic), with the highest harvest obtained with the robotic approach. Procedure type was not associated with mortality or readmission rate within 30 d of hospital discharge. However, an increasing number of LN harvested was associated with survival, while a higher number of LN that were positive for cancer was associated with earlier mortality on multivariate analysis. Our study suggests that robotic PD has better LN harvest and is comparable to open and laparoscopic approaches for short-term oncological outcomes and survival.
- Citation: Kalabin A, Mani VR, Kruse RL, Schlesselman C, Li KY, Staveley-O'Carroll KF, Kimchi ET. New perspectives on robotic pancreaticoduodenectomy: An analysis of the National Cancer Database. World J Gastrointest Surg 2023; 15(1): 60-71
- URL: https://www.wjgnet.com/1948-9366/full/v15/i1/60.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i1.60
Pancreatic ductal adenocarcinoma (PDAC) is the 11th most common malignancy diagnosed in the United States (US)[1]. The incidence of PDAC has increased over the past several decades; in 2022, it is estimated that there will be 62210 cases and 49830 deaths[2]. Late detection, early metastases, and resistance to therapy all contribute to its poor prognosis. Despite advancements in detection, surgical techniques, and widely adopted multidisciplinary care approaches, the prognosis remains poor with an overall 5-year survival of only 10.8%[1].
Surgery is the only potentially curative therapy for pancreatic cancer (PC), and pancreaticoduodenectomy (PD) is usually required to remove tumors in the head and neck of the pancreas. The very first resection of a periampullary tumor was performed in 1909, and the original technique of PD was described by Dr. Allen Oldfather Whipple in 1935[3]. The first laparoscopically assisted PD was done in 1994, and minimally invasive techniques evolved significantly in early 2000s, when Khachfe et al[4] performed the first robotic PD in 2001. Currently, it remains one of the most complex and technically challenging surgeries of the gastrointestinal system/alimentary tract. According to current literature, no major differences in outcomes result from different modifications of the PD procedure, including conventional, pylorus-preserving, or minimally invasive approaches. In addition, more extensive surgery including retroperitoneal lymphadenectomy, was studied in a prospective, single institution, randomized clinical trial, with comparable outcomes[5]. However, with the emergence of minimally invasive surgery the paradigm began to shift, and the utilization of laparoscopic and robotic PD approaches has recently increased and continues to gain in popularity.
Although the relatively new robotic PD approach offers equivalent or even slightly improved short-term perioperative outcomes with comparable rates of complications (pancreatic fistula and delayed gastric emptying), length of stay, and short-term oncologic outcomes (resection margins and mortality rates), the data regarding long-term oncologic outcomes are limited, as robotic PD gained ground only in the 2000s and is not universally accepted[6,7]. However, lymph node status is an important predictor of recurrence and survival in surgically treatment of PC, and recent reports clearly demonstrated superior lymph node harvest using the robotic approach[8-10]. It is unclear if better lymph node harvest with robotic PD translates into improved outcomes in patients with PC.
We undertook the current study to compare open, laparoscopic, and robotic PD in terms of the absolute number of lymph nodes harvested. Secondary objectives included short-term oncological outcomes (e.g., duration of hospital stay) as well as the association of lymph node yield with long-term oncologic outcomes.
Because we used only publicly available, anonymized data that preclude reidentifying of participants, our study was exempt from Institutional Review Board Review.
We requested records from the National Cancer Database (NCDB) for patients with pancreatic adenocarcinoma diagnosed between 2004 and 2018. The NCDB is a joint project of the American Cancer Society and the Commission on Cancer of the American College of Surgeons. It includes more than 1500 cancer programs in the United States and Puerto Rico. Approximately 70% of newly diagnosed cancer cases in the United States are reported to the NCDB.
Patients with adenocarcinoma were identified with the International Classification of Diseases for Oncology, Third Edition (ICD-O-3), using codes (C25. C25.0, C25.1, C25.3, C25.4, C25.7, C25.8, and C25.9).
Histological codes indicating adenocarcinoma (814: 8140/2 adenocarcinoma in situ; 8140/3 adenocarcinoma, not otherwise specified), duct carcinoma (850: 8500/2 intraductal adenocarcinoma noninfiltrating, not otherwise specified; 8500/3 invasive carcinoma of no special type) and other tumors of the head and neck of the pancreas that were treated with PD were also included. Tumors were classified as clinical stage I, II or III by the American Joint Committee on Cancer (AJCC, eighth edition).
We included all adult (age ≥ 18) patients who underwent PD based on site-specific coding in the database as well as type of procedure.
We excluded procedures performed before 2010 because surgical approach was not consistently reported. Patients lacking documentation on surgical approach or diagnostic confirmation were similarly excluded. We did not include cases with the ICD-O-3 code C25.2 (Malignant neoplasm of tail of pancreas), tumors classified as clinical stage IV using the AJCC, 8th edition) cancer staging scale, and patients who had pancreatic surgery other than PD.
Covariates included patient characteristics (age, sex, race, comorbidities), tumor characteristics (grade, tumor size, clinical T classification, tumor location), treatment details (receipt and timing of chemotherapy, radiotherapy, hormone therapy, immunotherapy, and or type of surgery), and histopathology (pathologic T, pathologic N, nodal yield, lymph node ratio, margin status, lymph node vascular invasion). Secondary outcomes included length of stay, 30-d and 90-d mortality, 30-d readmission, and time to death. Patients who died in the hospital were excluded from analysis of length of stay and readmission.
Descriptive statistics were calculated for all covariates and outcomes. Continuous variables were compared across procedure type with one-way analysis of variance and categorical variables were compared with the chi-square test. Surgeries that started as laparoscopic or robotic and were converted to open were assigned to their original category.
Overall survival (OS) was defined as the time between surgery and death. Median survival time was estimated with the Kaplan-Meier method, and groups were compared with the Wilcoxon test. A Cox proportional hazards model was used to assess the association of covariates with survival after controlling for patient characteristics and procedure type. Observations were censored at the last follow-up if death was not observed. Variables that were significantly related to survival in bivariable analysis were candidates for the Cox model. The small number of tumors recorded as larger than 200 mm (n = 21, 0.12%) were recoded to 200 mm both to avoid undue influence in the multivariable model and because tumors of this size are rare and raise questions about the accuracy of reporting. Statistical significance was defined as P < 0.05. All statistical analysis was performed with Statistical Analysis Software (SAS) for Windows version 9.4 (SAS Institute, Inc., Cary, NC).
The statistical methods of this study were reviewed by Robin L Kruse and Chase Schlesselman.
We included 17169 patients who underwent PD from 2010 to 2018 (Table 1). Most patients (13816, 80.5%) had an open procedure, 2677 (15.6%) had a laparoscopic procedure, and 676 (3.9%) underwent robotic surgery. Mean age at the time of surgery was 64.9 years [95%confidence interval (CI): 64.7-65.0], 8310 (48.4%) were females and 8859 were males (51.6%). Most (14509, 84.5%) patients identified themselves as white and 1739 (10.1%) as African American, with several groups too small to analyze separately that were included as “Other” (766, 4.5%). A smaller number (155, 0.90%) did not specify their racial identity. Hispanic ethnicity was indicated by 981 patients (5.7%). Mean comorbidity index (Charlson-Deyo Score) for the total cohort was 0.50 (95%CI: 0.49-0.51). Most patients (63.9%) had a score of 0, while 26.0% had a score of 1 and 10% of patients scored 2 or more (scores were capped at 3 in the database).
| Characteristic | Total (n = 17169) | Type of procedure | P value | ||
| Open (n = 13816) | Laparoscopic1 (n = 2677) | Robotic2 (n = 676) | |||
| Age, mean (95%CI)3 | 64.9 (64.7, 65.0) | 64.81 (64.62, 64.99) | 64.97 (64.55, 65.39) | 65.36 (64.47, 66.25) | 0.38 |
| Sex | 0.93 | ||||
| Female | 8310 (48.4) | 6694 (48.45) | 1287 (48.08) | 329 (48.67) | |
| Male | 8859 (51.6) | 7122 (51.55) | 1390 (51.92) | 347 (52.33) | |
| Race4 | 0.18 | ||||
| White | 14509 (84.5) | 11658 (84.38) | 2284 (85.32) | 567 (83.88) | |
| Black | 1739 (10.1) | 1435 (10.39) | 237 (8.85) | 67 (9.91) | |
| Other | 766 (4.5) | 597 (4.32) | 133 (4.97) | 36 (5.33) | |
| Unknown | 155 (0.9) | 126 (0.91) | 23 (0.86) | 6 (0.89) | |
| Hispanic ethnicity | 0.009 | ||||
| Yes | 981 (5.7) | 809 (5.86) | 145 (5.42) | 27 (3.99) | |
| No | 16188 (94.29) | 13007 (94.14) | 2532 (95.58) | 649 (96.01) | |
| Charlson-Deyo score | 0.52 | ||||
| 0 | 10977 (63.9) | 8867 (64.18) | 1692 (63.21) | 418 (61.83) | |
| 1 | 4471 (26.0) | 3578 (25.90) | 710 (26.52) | 183 (27.07) | |
| 2 | 1134 (6.6) | 904 (6.54) | 175 (6.54) | 55 (8.14) | |
| 3 or more | 587 (3.4) | 467 (3.38) | 100 (3.74) | 20 (2.96) | |
| Surgical procedure | 0.07 | ||||
| With partial gastrectomy | 14068 (81.94) | 11357 (82.20) | 2152 (80.39) | 559 (82.69) | |
| Without partial gastrectomy | 3101 (18.06) | 2459 (17.80) | 525 (19.61) | 117 (17.31) | |
Tumor characteristics are presented in Table 2. Adenocarcinoma was histologically confirmed in 7085 patients (41.3%), and in 6775 (39.5%) patients the final pathology was coded as ductal carcinoma, with both groups representing more than 80% of the cohort. The remainder (3309, 19.3%) had other malignant and benign histology codes. The overwhelming majority of the patients had pancreatic head lesions (15196, 88.5%) and the mean tumor size was 33.2mm (95%CI: 32.9-33.5). In the open PD group, 80.4% of patients were coded as AJCC clinical stage 1 or 2, compared with 78.7% and 68.5% in the laparoscopic and robotic groups, respectively.
| Total (n = 17169) | Type of procedure | P value | |||
| Open (n = 13816) | Laparoscopic1 (n = 2677) | Robotic2 (n = 676) | |||
| Year of diagnosis | < 0.0001 | ||||
| 2010 | 1374 (8.0) | 1212 (8.77) | 148 (5.53) | 14 (2.07) | |
| 2011 | 1514 (8.82) | 1238 (8.96) | 250 (9.34) | 26 (3.85) | |
| 2012 | 1601 (9.32) | 1347 (9.75) | 225 (8.40) | 29 (4.29) | |
| 2013 | 1738 (10.12) | 1466 (10.61) | 244 (9.11) | 28 (4.14) | |
| 2014 | 1816 (10.58) | 1469 (10.63) | 286 (10.68) | 61 (9.02) | |
| 2015 | 1986 (11.57) | 1587 (11.49) | 314 (11.73) | 85 (12.57) | |
| 2016 | 2154 (12.55) | 1665 (12.05) | 374 (13.97) | 115 (17.01) | |
| 2017 | 2099 (12.23) | 1625 (11.67) | 361 (13.49) | 113 (16.72) | |
| 2018 | 2887 (16.82) | 2207 (15.97) | 475 (17.74) | 205 (30.33) | |
| Histology | < 0.0001 | ||||
| Adenocarcinoma | 7085 (41.27) | 5688 (41.17) | 1177 (43.97) | 220 (32.54) | |
| Duct carcinoma | 6775 (39.46) | 5482 (39.68) | 1005 (37.54) | 288 (42.60) | |
| Other | 3309 (19.27) | 2646 (19.15) | 495 (18.49) | 168 (24.85) | |
| Primary Site (C25.2 excluded) | < 0.0001 | ||||
| Head of pancreas | 15196 (88.51) | 12365 (89.50) | 2253 (84.16) | 578 (85.50) | |
| Body of pancreas | 671 (3.91) | 446 (3.23) | 174 (6.50) | 51 (7.54) | |
| Pancreatic duct | 83 (0.48) | 62 (0.45) | 18 (0.67) | 3 (0.44) | |
| Islet of Langerhans or endocrine pancreas | 37 (0.22) | 26 (0.19) | 11 (0.41) | 0 | |
| Other/unspecified | 11182 (6.88) | 917 (6.64) | 221 (8.26) | 44 (6.51) | |
| AJCC Clinical Stage | 0.0002 | ||||
| 0 | 321 (1.87) | 261 (1.89) | 48 (1.79) | 12 (1.78) | |
| 1 | 230 (1.34) | 202 (1.46) | 21 (0.78) | 7 (1.04) | |
| 1A | 1979 (11.53) | 1593 (11.53) | 297 (11.09) | 89 (13.17) | |
| 1B | 4539 (26.44) | 3715 (26.89) | 703 (26.26) | 121 (17.90) | |
| 2 | 135 (0.79) | 122 (0.88) | 11 (0.41) | 2 (0.30) | |
| 2A | 3320 (19.34) | 2686 (19.44) | 511 (19.09) | 123 (18.20) | |
| 2B | 3154 (18.37) | 2522 (18.25) | 514 (19.20) | 109 (16.12) | |
| 3 | 612 (3.56) | 507 (3.67) | 97 (4.62) | 8 (1.18) | |
| Unknown | 2888 (16.82) | 2208 (15.98) | 475 (17.74) | 205 (30.33) | |
| Grade | < 0.0001 | ||||
| Well differentiated | 1993 (13.95) | 1627 (14.01) | 287 (13.03) | 79 (16.77) | |
| 2 – Moderately differentiated | 6093 (42.66) | 4903 (42.23) | 990 (44.96) | 200 (42.46) | |
| 3 – Poorly differentiated | 3976 (27.84) | 3256 (28.05) | 614 (27.88) | 106 (22.51) | |
| 4 - Undifferentiated | 190 (1.33) | 158 (1.36) | 23 (1.04) | 9 (1.91) | |
| Not determined | 2030 (14.21) | 1665 (14.34) | 288 (13.08) | 77 (16.35) | |
| Tumor size in mm, mean (95%CI) | 33.21 (32.95, 33.48) | 31.95 (30.69, 33.21) | 33.13 (32.44, 33.82) | 33.29 (33.00, 33.58) | 0.015 |
Overall, the frequency of PD in the database increased from 1374 in 2010 to 2887 in 2018, with laparoscopic and robotic procedures representing a greater proportion of the total over time. While the majority of PD over the study period and in 2018 (76.4%) were still performed with an open approach, the increasing trend of minimally invasive techniques is readily apparent. The proportion of laparoscopic PD increased from 10.8% in 2010 to 16.5% in 2018 (Table 2). During the same period, the proportion of robotic-assisted PD increased from 1.0% to 7.1%. Even though the overall number of Whipple procedures more than doubled over this time, laparoscopic, and robotic PD in particular, remained rare operations at most facilities.
Overall, an average of 18.8 (95%CI: 18.7-19.0) lymph nodes were harvested (Table 3). The number of lymph nodes harvested differed by surgical approach (P < 0.0001). Mean intraoperative lymph node harvest was 18.6 during open PD, 19.6 during laparoscopic procedures, and 20.7 with a robotic approach. Lymph nodes that were pathologically confirmed to have cancer cells averaged 2.49 for the entire cohort (95%CI: 2.44-2.55) and did not differ by procedure type (P = 0.26). Vascular invasion was noted in 42.6% (7313 patients) of pathologically examined lymph nodes. Vascular invasion differed by surgical approach, with 42.1% for open procedures, 44.0% for laparoscopic procedures, and 47.2% for robotic surgeries (P = 0.015).
| Characteristic | Total (n = 17169) | Type of procedure | P value | ||
| Open (n = 13816) | Laparoscopic1 (n = 2677) | Robotic2 (n = 676) | |||
| Mean Lymph nodes harvested (95%CI) | 18.84 (18.69, 18.98) | 18.59 (18.43, 18.75) | 19.65 (19.29, 20.02) | 20.70 (19.89, 21.51) | < 0.0001 |
| Mean Lymph nodes positive (95%CI) | 2.49 (2.44, 2.55) | 2.48 (2.48, 2.54) | 2.58 (2.45, 2.72) | 2.37 (2.11, 2.64) | 0.26 |
| Vascular invasion | 0.0115 | ||||
| Yes | 7313 (42.6) | 5816 (42.1) | 1178 (44.0) | 319 (47.2) | |
| No | 7764 (45.2) | 6259 (45.3) | 1208 (45.1) | 297 (43.9) | |
| Unknown | 2092 (12.2) | 1741 (12.6) | 291 (10.9) | 60 (8.9) | |
| AJCC Pathological Stage | 0.02 | ||||
| 0 | 341 (1.99) | 281 (2.03) | 45 (1.68) | 15 (2.22) | |
| 1 | 79 (0.46) | 68 (0.49) | 9 (0.34) | 2 (0.30) | |
| 1A | 995 (5.80) | 778 (5.63) | 169 (6.31) | 48 (7.10) | |
| 1B | 1102 (6.42) | 918 (6.64) | 148 (5.53) | 36 (5.33) | |
| 2 | 45 (0.26) | 44 (0.32) | 1 (0.04) | 0 | |
| 2A | 2849 (16.59) | 2322 (16.81) | 435 (16.25) | 92 (13.61) | |
| 2B | 8430 (49.10) | 6826 (49.41) | 1335 (49.87) | 269 (39.79) | |
| 3 | 317 (1.85) | 262 (1.90) | 47 (1.76) | 8 (1.18) | |
| Unknown | 3011 (17.54) | 2317 (16.77) | 488 (18.23) | 206 (30.47) | |
| Surgical margins | 0.75 | ||||
| No residual tumor (R0) | 13728 (79.96) | 11042 (79.92) | 2150 (80.31) | 536 (79.29) | |
| Microscopic residual tumor (R1) | 3232 (18.82) | 2601 (18.83) | 495 (18.49) | 136 (20.12) | |
| Macroscopic residual tumor (R2) | 87 (0.51) | 73 (0.53) | 13 (0.49) | 1 (0.15) | |
| Cannot be accessed | 122 (0.71) | 100 (0.72) | 19 (0.71) | 3 (0.44) | |
| Length of stay (95%CI) | 10.77 (10.63, 10.90) | 10.92 (10.77, 11.07) | 10.29 (9.92, 10.66) | 9.61 (8.97, 10.25) | < 0.0001 |
| Readmission 30 d (readmitted) | 1398 (8.14) | 1113 (8.06) | 227 (8.48) | 58 (8.58) | 0.71 |
| Mortality 30 d (dead) | 381 (2.67) | 312 (2.69) | 55 (2.50) | 14 (2.99) | 0.80 |
| Mortality 90 d (dead) | 752 (5.30) | 634 (5.50) | 97 (4.42) | 21 (4.48) | 0.09 |
Patients were characterized according to the pathological stage (Table 3), with 80.7% assigned to stages 0, 1, or 2. Overall, 13728 patients (80.0%) had R0 resection. In the open PD group, 79.9% of patients had R0 resection, compared with 80.3% and 79.3% with laparoscopic and robotic approaches, respectively (P = 0.75). There was no difference in the proportion of microscopic and macroscopic positive margins between groups. Patients spent an average of 10.7 d in the hospital. Robotic PD was associated with reduced length of stay after surgery (9.6 d) compared to open and laparoscopic approaches respectively (10.9 and 10.3 d, respectively; P < 0.0001). Prolonged hospital stay (≥ 10 d) was observed for 38.7% of patients in the open group, 33.6% of patients in the laparoscopic group, and 28.4% of those in the robotic group (P < 0.0001). Overall, 8.1% of patients had an unplanned readmission within 30 d of discharge; this did not differ between groups (P = 0.71). Following surgery, 30-d mortality was 2.7% and 90-d mortality was 5.3%. Mortality did not differ significantly between the groups.
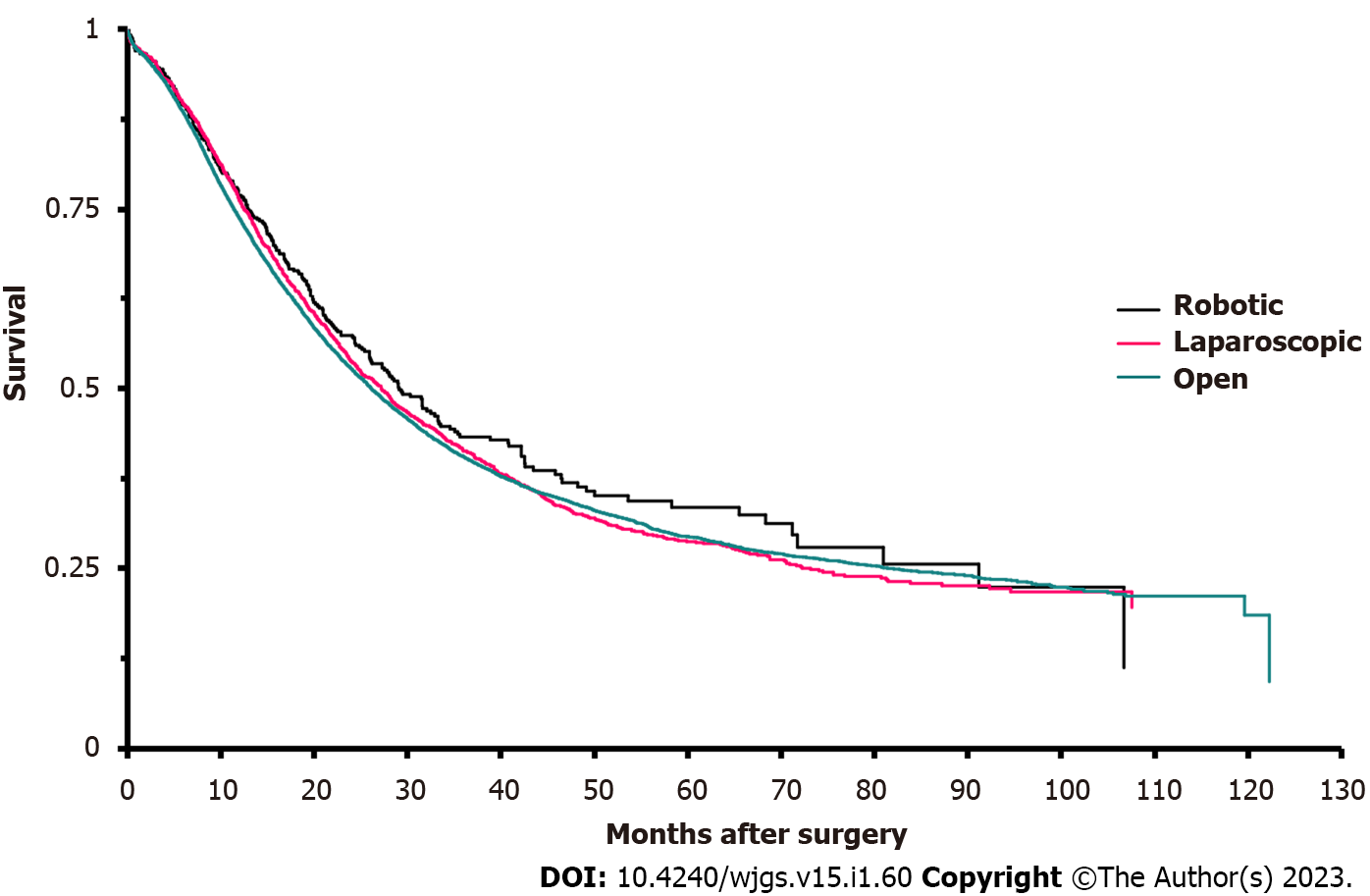
Median survival for patients who received open surgery was 26.1 mo (95%CI: 25.4-26.9). Patients who had laparoscopic surgery had a median survival of 27.2 mo (95%CI: 25.1-28.7), while those who had robotic procedures had a median survival of 29.1 mo (95%CI: 25.9-33.4). Survival did not differ by surgical approach (P = 0.064) (Figure 1). Several variables were associated with survival after surgery (Table 4). Greater age, tumor grades above 1, residual tumor at the surgical margins, pathological stages above 0, lower income quartiles, Charlson-Deyo scores above 0, larger tumor size, and longer times between diagnosis and surgery were all associated with earlier mortality. Compared with adenocarcinoma, duct carcinoma and other cancers were associated with delayed mortality, as was increasing year of diagnosis. Gender and surgical approach were not associated with survival. Of note, greater number of lymph nodes examined was associated with prolong survival while greater number of lymph nodes positive for cancer was associated with earlier mortality.
| Characteristic | Parameter estimate | Hazard ratio | 95%CI | P value |
| Age (yr) | 0.01621 | 1.02 | 1.01-1.02 | < 0.0001 |
| Male sex | 0.02903 | 1.03 | 0.98-1.08 | 0.20 |
| Race: White | ref | |||
| Black | -0.0599 | 0.94 | 0.87-1.02 | 0.13 |
| Other | -0.15749 | 0.85 | 0.76-0.96 | 0.009 |
| Unknown | -0.16688 | 0.85 | 0.65-1.10 | 0.21 |
| Hispanic ethnicity: No | ref | |||
| Yes | -0.15238 | 0.86 | 0.78-0.95 | 0.0037 |
| Unknown | -0.03096 | 0.97 | 0.82-1.15 | 0.72 |
| Tumor grade: 1 | ref | |||
| 2 | 0.45571 | 1.58 | 1.45-1.72 | < 0.0001 |
| 3 | 0.70413 | 2.02 | 1.85-2.21 | < 0.0001 |
| 4 | 0.80073 | 2.23 | 1.82-2.73 | < 0.0001 |
| Not determined, unknown | 0.35723 | 1.43 | 1.28-1.60 | < 0.0001 |
| Surgical approach: Open | ref | |||
| MIS, MIS to open | -0.0402 | 0.96 | 0.90-1.02 | 0.19 |
| Robotic, robotic to open | 0.00838 | 1.01 | 0.88-1.15 | 0.90 |
| Surgical margins: No residual tumor | ref | |||
| Macroscopic residual tumor | 0.44741 | 1.56 | 1.19-2.05 | 0.0013 |
| Microscopic residual tumor | 0.34752 | 1.42 | 1.34-1.49 | < 0.0001 |
| Unknown, indeterminate | 0.40122 | 1.49 | 1.15-1.94 | 0.0026 |
| AJCC Pathological stage: 0 | ref | |||
| 1/1A/1B | 0.49238 | 1.64 | 1.22-2.18 | 0.0008 |
| 2/2A/2B | 0.90708 | 2.48 | 1.86-3.29 | < 0.0001 |
| 3 | 1.10653 | 3.02 | 2.21-4.14 | < 0.0001 |
| Census block median income quartile: > 63332 | ||||
| $50354-$63332 | 0.06511 | 1.07 | 1.01-1.13 | 0.027 |
| $40227-$50353 | 0.17171 | 1.19 | 1.12-1.26 | < 0.0001 |
| < $40227 | 0.19323 | 1.21 | 1.14-1.30 | < 0.0001 |
| Unknown | 0.12115 | 1.13 | 0.61-2.10 | 0.70 |
| Histology: Adenocarcinoma | ref | |||
| Duct carcinoma | -0.05251 | 0.95 | 0.91-0.99 | 0.027 |
| All others | -0.72939 | 0.48 | 0.44-0.52 | < 0.0001 |
| Charlson-Deyo score: 0 | ||||
| 1 | 0.10936 | 1.12 | 1.06-1.17 | < 0.0001 |
| 2 | 0.18942 | 1.21 | 1.11-1.32 | < 0.0001 |
| 3 or more | 0.35643 | 1.43 | 1.26-1.62 | < 0.0001 |
| Lymph nodes examined | -0.01026 | 0.99 | 0.99-0.99 | < 0.0001 |
| Lymph nodes positive for cancer | 0.05025 | 1.05 | 1.04-1.06 | < 0.0001 |
| Tumor size (mm)1 | 0.00479 | 1.01 | 1.00-1.01 | < 0.0001 |
| Year of diagnosis | -0.03434 | 0.97 | 0.96-0.98 | < 0.0001 |
| Weeks between diagnosis and surgery | 0.00702 | 1.01 | 1.01-1.01 | < 0.0001 |
In our study of over 17000 patients who underwent PD from 2010 to 2018, we found that the number of lymph nodes harvested differed by procedure type (open, laparoscopic, robotic), but the number of lymph nodes that tested positive for cancer was not associated with type of procedure. After controlling for patient and tumor characteristics in a multivariable model, increasing number of lymph nodes harvested was associated with survival, while increasing number of lymph nodes that were positive for cancer was associated with earlier mortality. Procedure type was not associated with mortality or readmission within 30 d of hospital discharge.
Pancreatic surgery remains one of the most complicated and technically challenging surgical procedures due to the retroperitoneal location of the organ and its proximity to major vascular structures. With the known advantages of minimally invasive techniques and the potential of performing complex surgeries with enhanced precision and accuracy using robotic techniques, robotic PD has the potential to be a safe and feasible alternative to open and laparoscopic approaches. Data regarding long-term outcomes of robotic PD are lacking, however, as the technique is still developing and has not been universally integrated into routine surgical training and practice. In our work, we aimed to analyze PC data from the NCDB, because it represents a significant portion of newly diagnosed cancer cases nationwide and is considered one of the most comprehensive sources of cancer information in US[11].
In our study, most (80.5%) of the surgeries were done using the open approach. Robotic PD was performed only in 3.9% of all PD cases. This highlights that robotic surgery has not been widely adopted; furthermore, the recently published Miami International Guideline on Minimally Invasive Pancreas Resection did not recommend a minimally invasive approach over open PD[12]. This is likely due to the limited number of training programs that have incorporated comprehensive training protocols for robotic pancreatic surgery in their curricula and the time needed to retrain established pancreatic surgeons on the robotic platform. Nonetheless, robotic outcomes continue to improve; recent data regarding outcomes of robotic PD have shown a significant decrease in postoperative mortality (from 6.7% to 1.8%) and comparable short-term outcomes with laparoscopic and open approaches[13-16]. Our study confirmed the overall trend of increased utilization of the robotic approach for PD, with an increase in prevalence from 1.0% to 7.1% over the study period.
Lymph node status is an important indicator of survival in patients with PC, allows for proper staging, and aids in choosing the treatment strategies. Schwarz et al[17] postulated that both the lymph node ratio and the number of lymph nodes examined are important prognostic factors. They suggested that examining 15 total lymph nodes with curative-intent PD would optimize operative benefits. We report an average of 18.8 Lymph nodes examined overall, which is consistent with this guideline. Interestingly, a significantly higher percentage of lymph nodes had vascular invasion in the robotic group compared to the laparoscopic and open groups. The possibility that pathologists are more diligent at centers where robotic procedures are performed is raised by the increased presence of vascular invasion in the lymph nodes with metastatic disease found in robotic cases despite no difference in positive lymph nodes found between operative groups. If this were true, this may also explain the increased number of lymph nodes counted in robotic cases. On the other hand, the robotic approach is recognized to have more efficient retroperitoneal dissection of the celiac axis and superior mesenteric artery lymph nodes[9].
Short-term oncological outcomes including R0 resection, unplanned 30-d readmission, and 30- and 90-d mortality were comparable between the groups and are consistent with current literature[18,19]. Our study demonstrated that robotic PD is associated with reduced length of stay compared to open and laparoscopic approaches. This may affect psychological and psychosocial well-being for patients and should not be ignored.
Although survival analysis suggested that robotic PD is associated with a relatively longer median survival that than laparoscopic and open approaches, the difference was not statistically significant. However, our study provides new evidence on the comparable OS of patients undergoing robotic PD and warrants attention. This further supports the application of robotic techniques in the treatment of PC. However, additional prospective studies directly comparing minimally invasive and open PD approaches are needed to validate our findings and to further endorse utilization of the robotic surgical platform.
There are several potential limitations to this study. First, because surgical approach was not randomly assigned, there is potential for confounding. We used multivariable analysis to control for differences between groups, but it is possible that an important variable was not available to us. For example, the NCDB does not adequately characterize type of neoadjuvant therapy (chemotherapy vs chemoradiation) and it was excluded from the final analysis to avoid dropping too many cases. Secondly, the small number of institutions performing robotic PD may have unduly influenced the pathologic interpretations and tumor registry reporting. Third, NCDB does not include detailed operative reports, or types and rate of postoperative complications, precluding analysis of technical aspects or post operative complications. In addition, large national databases always carry inherent risk of coding errors and variation by staff at participating institutions. Moreover, AJCC clinical staging does not contain an assessment for resectability using consensus guidelines, and surgical approach could have been chosen by radiographic staging of the tumor.
Our retrospective analysis of the NCBD demonstrated that robotic PD was both associated with increased number of lymph nodes harvested during surgery and equivalent to open and laparoscopic approaches with respect to rate of cancer positive lymph nodes, short-term oncological outcomes, and OS. This supports the continued incorporation of robotic PD into the surgical treatment of pancreatic neoplasms.
Despite all advancements pancreatic ductal adenocarcinoma is still considered one of the deadliest types of cancer with an overall 5-year survival of only 10.8%. Pancreaticoduodenectomy (PD) is the only potentially curative approach for resectable pancreatic cancer (PC) and robotic PD has gain popularity in recent years.
Recent literature suggests that relatively new robotic PD approach offers comparable or even slightly improved short-term outcomes and equivalent rates of postoperative complications, however the data regarding long-term oncologic outcomes are limited. On the other hand, new studies demonstrated superior lymph node (LN) harvest using the robotic PD platform that could be an important predictor of recurrence and survival. Hence, we decided to analyze the National Cancer Database (NCDB) and compare open, laparoscopic and robotic PD in terms of absolute number of LN harvest and association of lymph node yield with long-term oncological outcomes.
The primary outcome was to evaluate absolute LN harvest during open, laparoscopic and robotic PD. Secondary outcomes included evaluating the association between LN harvest and short- and long-term oncological outcomes for three different surgical approaches, and more specifically - the association of LN harvest with overall survival (OS).
Retrospective analysis of NCDB patients diagnosed with PC who underwent PD in 2010-2018. One-way analysis of variance was used for continuous variables, chi-square test - for categorical. OS was defined as the time between surgery and death. Median survival time was estimated with the Kaplan-Meier method, and groups were compared with the Wilcoxon test. A Cox proportional hazard model was used to access the association of covariates with survival after controlling for patient characteristics and procedure type.
17169 patients were included in the final analysis. 13816 (80.5%) patients had an open PD, 2677 (15.6%) and 676 (3.9%) - laparoscopic and robotic PD respectively. On average 18.84 LNs were harvested during PD. Mean LN harvest during open, laparoscopic and robotic PD was 18.59, 19.65 and 20.70 LNs respectively (P < 0.001). On average, 2.49 LNs were positive for cancer and did not differ by the procedure type (P = 0.26). Median survival for open PD was 26.1 mo, laparoscopic - 27.2 mo, robotic - 29.1 mo (P = 0.064). Survival was associated with higher number of positive LN harvest, while higher number of positive LNs was associated with higher mortality.
Our study demonstrated that robotic PD was associated with increased number of lymph nodes harvested during surgery and equivalent to open and laparoscopic approaches with respect to short-term oncological outcomes and overall survival. This supports the continued incorporation of robotic PD into the surgical treatment of pancreatic neoplasms.
Our study provides new evidence on superior LN harvest and comparable overall survival of patients undergoing robotic PD and warrants attention. Additional prospective studies directly comparing robotic and open approaches are needed to validate our findings and to further endorse utilization of the robotic surgical platform.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hendi M, China; Pan YZ, China; Shah OJ, India S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | National Institutes of Health. Cancer Stat Facts: Pancreatic Cancer. [Internet] [accessed 30 March 2022]. Available from: https://seer.cancer.gov/statfacts/html/pancreas.html. |
| 2. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11406] [Article Influence: 3802.0] [Reference Citation Analysis (4)] |
| 3. | Griffin JF, Poruk KE, Wolfgang CL. Pancreatic cancer surgery: past, present, and future. Chin J Cancer Res. 2015;27:332-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 63] [Reference Citation Analysis (0)] |
| 4. | Khachfe HH, Habib JR, Harthi SA, Suhool A, Hallal AH, Jamali FR. Robotic pancreas surgery: an overview of history and update on technique, outcomes, and financials. J Robot Surg 2022. 16:483-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Yeo CJ, Cameron JL, Sohn TA, Coleman J, Sauter PK, Hruban RH, Pitt HA, Lillemoe KD. Pancreaticoduodenectomy with or without extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma: comparison of morbidity and mortality and short-term outcome. Ann Surg. 1999;229:613-22; discussion 622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 276] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 6. | Shi Y, Jin J, Qiu W, Weng Y, Wang J, Zhao S, Huo Z, Qin K, Wang Y, Chen H, Deng X, Peng C, Shen B. Short-term Outcomes After Robot-Assisted vs Open Pancreaticoduodenectomy After the Learning Curve. JAMA Surg. 2020;155:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 7. | Liu Q, Zhao Z, Zhang X, Wang W, Han B, Chen X, Tan X, Xu S, Zhao G, Gao Y, Gan Q, Yuan J, Ma Y, Dong Y, Liu Z, Wang H, Fan F, Liu J, Lau WY, Liu R. Perioperative and Oncological Outcomes of Robotic Versus Open Pancreaticoduodenectomy in Low-Risk Surgical Candidates: A Multicenter Propensity Score-Matched Study. Ann Surg. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 43] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 8. | You MS, Lee SH, Choi YH, Shin BS, Paik WH, Ryu JK, Kim YT, Jang DK, Lee JK, Kwon W, Jang JY, Kim SW. Lymph node ratio as valuable predictor in pancreatic cancer treated with R0 resection and adjuvant treatment. BMC Cancer. 2019;19:952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Da Dong X, Felsenreich DM, Gogna S, Rojas A, Zhang E, Dong M, Azim A, Gachabayov M. Robotic pancreaticoduodenectomy provides better histopathological outcomes as compared to its open counterpart: a meta-analysis. Sci Rep. 2021;11:3774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Napoli N, Kauffmann EF, Vistoli F, Amorese G, Boggi U. State of the art of robotic pancreatoduodenectomy. Updates Surg. 2021;73:873-880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Boffa DJ, Rosen JE, Mallin K, Loomis A, Gay G, Palis B, Thoburn K, Gress D, McKellar DP, Shulman LN, Facktor MA, Winchester DP. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol. 2017;3:1722-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 895] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 12. | Asbun HJ, Moekotte AL, Vissers FL, Kunzler F, Cipriani F, Alseidi A, D'Angelica MI, Balduzzi A, Bassi C, Björnsson B, Boggi U, Callery MP, Del Chiaro M, Coimbra FJ, Conrad C, Cook A, Coppola A, Dervenis C, Dokmak S, Edil BH, Edwin B, Giulianotti PC, Han HS, Hansen PD, van der Heijde N, van Hilst J, Hester CA, Hogg ME, Jarufe N, Jeyarajah DR, Keck T, Kim SC, Khatkov IE, Kokudo N, Kooby DA, Korrel M, de Leon FJ, Lluis N, Lof S, Machado MA, Demartines N, Martinie JB, Merchant NB, Molenaar IQ, Moravek C, Mou YP, Nakamura M, Nealon WH, Palanivelu C, Pessaux P, Pitt HA, Polanco PM, Primrose JN, Rawashdeh A, Sanford DE, Senthilnathan P, Shrikhande SV, Stauffer JA, Takaori K, Talamonti MS, Tang CN, Vollmer CM, Wakabayashi G, Walsh RM, Wang SE, Zinner MJ, Wolfgang CL, Zureikat AH, Zwart MJ, Conlon KC, Kendrick ML, Zeh HJ, Hilal MA, Besselink MG; International Study Group on Minimally Invasive Pancreas Surgery (I-MIPS). The Miami International Evidence-based Guidelines on Minimally Invasive Pancreas Resection. Ann Surg. 2020;271:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 319] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 13. | Hoehn RS, Nassour I, Adam MA, Winters S, Paniccia A, Zureikat AH. National Trends in Robotic Pancreas Surgery. J Gastrointest Surg. 2021;25:983-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 14. | Yan Q, Xu LB, Ren ZF, Liu C. Robotic vs open pancreaticoduodenectomy: a meta-analysis of short-term outcomes. Surg Endosc. 2020;34:501-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Kamarajah SK, Bundred J, Marc OS, Jiao LR, Manas D, Abu Hilal M, White SA. Robotic vs conventional laparoscopic pancreaticoduodenectomy a systematic review and meta-analysis. Eur J Surg Oncol. 2020;46:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 16. | Zureikat AH, Beane JD, Zenati MS, Al Abbas AI, Boone BA, Moser AJ, Bartlett DL, Hogg ME, Zeh HJ 3rd. 500 Minimally Invasive Robotic Pancreatoduodenectomies: One Decade of Optimizing Performance. Ann Surg. 2021;273:966-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 17. | Schwarz RE, Smith DD. Extent of lymph node retrieval and pancreatic cancer survival: information from a large US population database. Ann Surg Oncol. 2006;13:1189-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 190] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Torphy RJ, Friedman C, Halpern A, Chapman BC, Ahrendt SS, McCarter MM, Edil BH, Schulick RD, Gleisner A. Comparing Short-term and Oncologic Outcomes of Minimally Invasive Versus Open Pancreaticoduodenectomy Across Low and High Volume Centers. Ann Surg. 2019;270:1147-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 19. | Baker EH, Ross SW, Seshadri R, Swan RZ, Iannitti DA, Vrochides D, Martinie JB. Robotic pancreaticoduodenectomy for pancreatic adenocarcinoma: role in 2014 and beyond. J Gastrointest Oncol. 2015;6:396-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |