Published online Jan 27, 2023. doi: 10.4240/wjgs.v15.i1.49
Peer-review started: September 20, 2022
First decision: October 5, 2022
Revised: October 18, 2022
Accepted: December 21, 2022
Article in press: December 21, 2022
Published online: January 27, 2023
Processing time: 120 Days and 0.1 Hours
Nearly 66% of occurrences of gastric cancer (GC), which has the second-highest death rate of all cancers, arise in developing countries. In several cancers, the predictive significance of inflammatory markers has been established.
To identify clinical characteristics and develop a specific nomogram to determine overall survival for GC patients.
Nine hundred and four GC patients treated at the First Affiliated Hospital of Anhui Medical University between January 2010 and January 2013 were recruited. Prognostic risk variables were screened for Cox analysis. The C index, receiver operator characteristic (ROC) curve, and decision curve analysis were used to evaluate the nomogram.
Tumor node metastasis stage, carcinoembryonic antigen, systemic immune-inflammation index, and age were identified as independent predictive variables by multivariate analysis. Systemic immune-inflammation index value was superior to that of other inflammatory indicators. The ROC indicated the nom
We created a novel nomogram to forecast the prognosis of GC patients following curative gastrectomy based on blood markers and other characteristics. Both surgeons and patients can benefit significantly from this new scoring system.
Core Tip: According to our study, the prognosis of patients with gastric cancer (GC) was significantly influenced by the systemic immune-inflammation index, carcinoembryonic antigen, tumor node metastasis stage, and age. We created a novel nomogram to predict the prognosis of GC patients following curative gastrectomy based on blood markers and other characteristics. Both surgeons and patients can benefit significantly from this new scoring system.
- Citation: Luo PQ, Song ED, Liu F, Rankine AN, Zhang LX, Wei ZJ, Han WX, Xu AM. Development and validation of a novel nomogram for predicting overall survival in gastric cancer based on inflammatory markers. World J Gastrointest Surg 2023; 15(1): 49-59
- URL: https://www.wjgnet.com/1948-9366/full/v15/i1/49.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i1.49
Nearly 66% of gastric cancer (GC) diagnoses, which has the second-highest death rate of all cancers[1], occur in developing countries[1,2]. The only curative treatment for patients is radical surgery, which increases the likelihood of a successful cure and lengthens patient survival. The high likelihood of cancer recurrence, however, means that the 5-year overall survival (OS) is still poor even after surgery[3]. The tumor node metastasis (TNM) stage is correlated with the prognosis of GC patients, but it is difficult to determine prior to surgery. Carcinoembryonic antigen (CEA) is one of the most utilized serum indicators in relation to stomach cancer according to recent research[4-6]. In order to diagnose cancer and predict recurrence following surgery, CEA has been employed[4]. The neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) are two additional blood indices that can be used to assess the prognosis of malignancy[5-7]. Additionally, the level of hemoglobin is related to a patient’s prognosis[8]. The purpose of this study was to identify additional clinical blood indicators that may be used to evaluate GC patient prognosis and create a trustworthy scoring system.
There has been much research on the connection between cancers and inflammation. Cancer caused by inflammation has been shown to damage DNA and create microscopic metastases[9]. The body’s immune system may become less effective, and tumor growth may be accelerated by the systemic inflammatory response. According to a related study, lymphocytes (LY), platelets (PLT), and neu
From January 2010 to January 2013, 904 GC patients were admitted to the First Affiliated Hospital of Anhui Medical University and provided blood samples and clinical data. All chosen participants were randomly divided into training (n = 543) and validation (n = 361) cohorts for the study’s final analysis. Our hospital’s Institutional Review Board and Ethical Committee approved this study.
The criteria for admission included: (1) A histological diagnosis indicated GC; (2) The malignancy was definitively and entirely removed after surgery; (3) All of the patients’ peripheral blood tests were completed within 2 d after the operation; and (4) Multiple organ failure was not present. Patients were excluded if they met any of the following criteria: (1) They had other primary tumors; (2) They had undergone radiotherapy and chemotherapy prior to surgery; (3) They had any diseases that could interfere with peripheral blood cells, such as infections; and (4) They had passed away within 1 mo after operation. Finally, a cohort of 904 GC patients was examined.
Through the medical records department, information on the patient’s age, sex, differentiation grade, tumor size, and other characteristics as well as clinical pathology was acquired. NE, LY, and PLT, etc. were obtained 3 d before the operation, and peripheral blood was analyzed. The CEA and hemoglobin cutoff values were obtained based on normal levels, and the median was used to determine the NE, LY, and PLT cutoff values. SII was calculated by PLT count × NE count/LY count. According to the ideal cutoff values, which were determined using the Youden index [maximum (sensitivity + specificity - 1)][16], patients were divided into low and high groups. Patients were assigned to groups based on NLR–PLR as follows: (high NLR) + (high PLR) = 2; (only one high group) = 1; (low NLR) + (low PLR) = 0. The assignment of NLR-PLT was similar.
The categorical values were analyzed by the χ2 test or the Fisher exact test, and continuous variables were analyzed by the Student’s t test. The Cox appropriate hazard model was used to perform both multivariate and univariate survival analyses. In order to assess the accuracy of the prognostic model, the C-index and receiver operator characteristic (ROC) were utilized. R Studio and the SPSS program (version 19.0) were used for the full data analysis process.
By using the ROC curve of the greatest Youden index, we calculated the preoperative NLR, PLR, and SII value. Based on the Youden index, the optimal cutoff value of NLR, PLR, SII was calculated to be 2.0, 160.0, and 475.6, respectively.
Table 1 showed the clinical data of the 904 GC patients (training group = 543, validation group = 361). The training group and validation group had no statistically significant differences (P > 0.05).
| Variables | Training cohort (n = 543) | Validation cohort (n = 361) | P value |
| Pathological types | 0.369 | ||
| Adenocarcinoma | 96 (91.3) | 336 (93.1) | |
| Signet-ring cell | 25 (4.6) | 14 (3.9) | |
| Adenosquamous | 1 (0.2) | 2 (0.6) | |
| Squamous carcinoma | 4 (0.7) | 4 (1.1) | |
| Mucinous cell | 17 (3.1) | 5 (1.4) | |
| Macroscopic type | 0.932 | ||
| Borrmann I | 22 (4.1) | 15 (4.2) | |
| Borrmann II | 391 (72.0) | 260 (72.0) | |
| Borrmann III | 113 (20.8) | 72 (19.9) | |
| Borrmann IV | 17 (3.1) | 14 (3.9) | |
| Tumor location | 0.415 | ||
| Upper | 250 (46.1) | 156 (43.3) | |
| Middle | 123 (22.7) | 85 (23.6) | |
| Lower | 169 (31.2) | 119 (33.1) | |
| Surgery selection | 0.344 | ||
| Distal gastrectomy | 117 (21.5) | 88 (24.4) | |
| Total gastrectomy | 401 (73.8) | 261 (72.6) | |
| Proximal gastric resection | 25 (4.6) | 11 (3.0) | |
| Differentiated grade | 0.662 | ||
| High | 25 (4.6) | 16 (4.4) | |
| Middle | 268 (49.5) | 181 (50.2) | |
| Poor | 248 (45.9.3) | 164 (45.4) | |
| Sex | 0.669 | ||
| Male | |||
| Female | 142 (26.2) | 89 (24.7) | |
| Age | 0.925 | ||
| < 60 | |||
| ≥ 60 | 334 (61.5) | 224 (62) | |
| Neutrophil count | 3.17 ± 4.49 | 2.89 ± 4.06 | 0.326 |
| Platelet count | 196.5 ± 71.70 | 205.48 ± 84.35 | 0.086 |
| Lymphocyte count | 1.64 ± 1.68 | 1.58 ± 0.59 | 0.491 |
Prognostic factors identified by univariate analysis were sex, hemoglobin, age, TNM, NLR, tumor size, PLR, SII, and CEA (Table 2). Multivariate analysis revealed that age, CEA, SII, and TNM were independent predictive factors for GC patients (Table 3).
| Characteristics | β | HR (95%CI) | P value |
| Sex (male/female) | -0.305 | 0.737 (0.546, 0.995) | 0.046 |
| Age (< 60 / ≥ 60 yr) | 0.333 | 1.395 (1.071, 1.818) | 0.014 |
| NLR (< 2 / ≥ 2) | 0.406 | 1.502 (1.163, 1.940) | 0.001 |
| Tumor size (< 5 / ≥ 5 cm) | 0.810 | 2.248 (1.746, 2.894) | < 0.001 |
| TNM stage | 1.062 | 2.892 (1.897, 4.409) | < 0.001 |
| Histologic type | -0.788 | 0.455 (0.140, 1.478) | 0.190 |
| Neutrophil count | 0.007 | 0.992 (0.963, 1.023) | 0.622 |
| Platelet count | 0.001 | 0.999 (0.997, 1.000) | 0.247 |
| Lymphocyte count | 0.048 | 1.049 (0.966, 1.139) | 0.259 |
| PLR (< 120/> 120) | 0.482 | 1.619 (1.251, 2.096) | < 0.001 |
| Pathological types | -0.614 | 0.541 (0.255, 1.148) | 1.148 |
| Macroscopic type | -0.114 | 0.893 (0.486, 1.640) | 0.714 |
| Tumor location | -0.102 | 0.903 (0.543, 1.502) | 0.695 |
| Surgery selection | 0.098 | 0.903 (0.806, 1.508) | 0.540 |
| CEA (5 g/L) | 1.238 | 3.449 (2.679, 4.440) | < 0.001 |
| SII (< 475.6/ > 475.6) | 0.632 | 1.881 (1.464, 2.417) | < 0.001 |
| NLR-PLR | 0.286 | 1.331 (1.194, 1.483) | < 0.001 |
| NLR-PLT | 0.269 | 1.308 (1.158, 1.477) | < 0.001 |
| Hemoglobin | -0.350 | 0.705 (0.549, 0.905) | 0.006 |
| Characteristic | Beta | HR (95%CI) | P value |
| TNM | 0.888 | 2.429 (1.588, 3.716) | < 0.001 |
| CEA | 0.839 | 2.313 (1.774, 3.015) | < 0.001 |
| SII | 0.405 | 1.499 (1.165, 1.930) | 0.002 |
| Age | 0.303 | 1.354 (1.034, 1.771) | 0.028 |
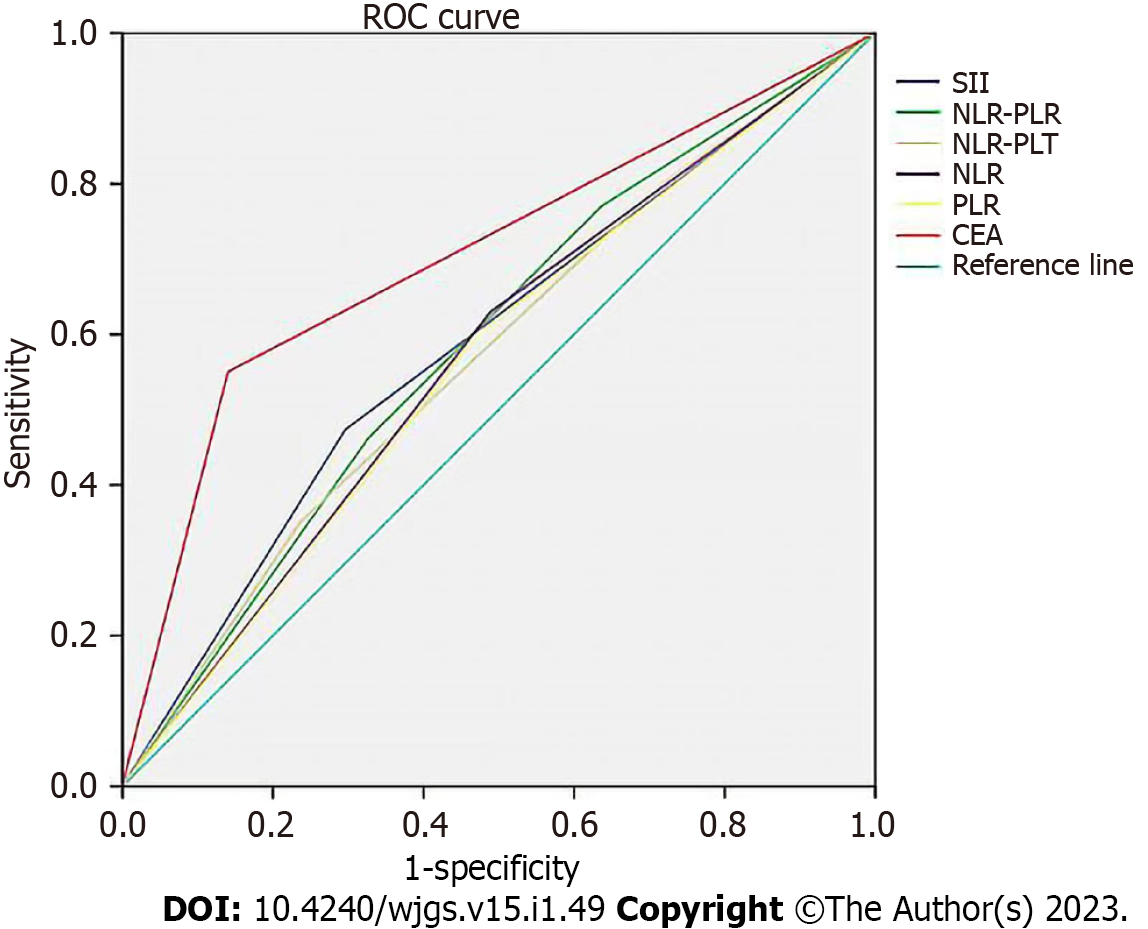
We used the ROC curve to compare the utility of all the inflammatory indicators in GC patients (Figure 1). The area under the curve (AUC) for SII was bigger than that of NLR, NLR-PLT, PLR, and NLR-PLR.
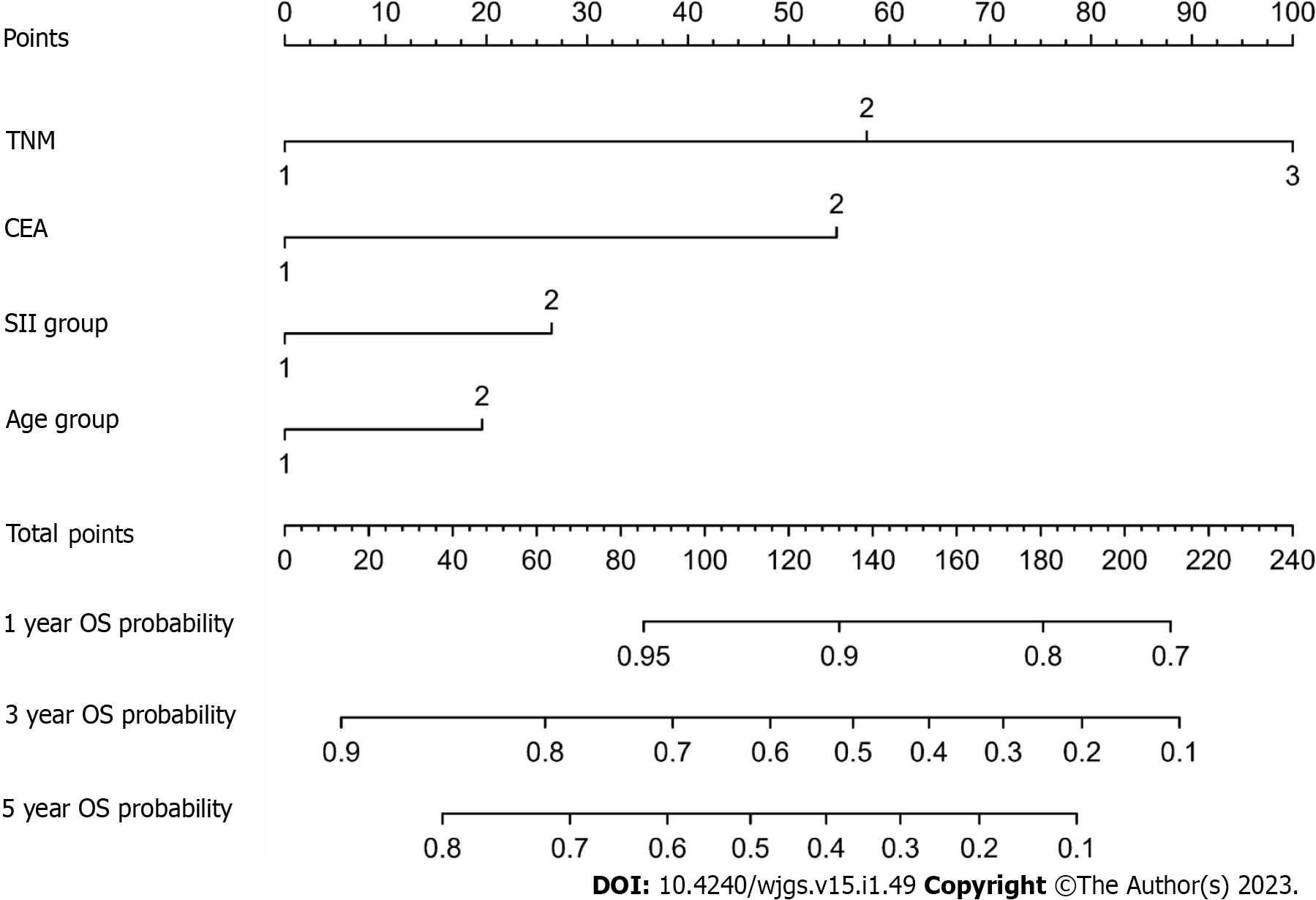
A novel nomogram was created to predict the OS of GC based on the multivariate analysis result (Figure 2). Table 4 revealed the nomogram scoring method.
| SII | Points | Age | Points | CEA | Points | TNM | Points |
| 1 | 0 | 1 | 0 | 1 | 0 | I | 0 |
| 2 | 26 | 2 | 20 | 2 | 55 | II | 58 |
| III | 100 |
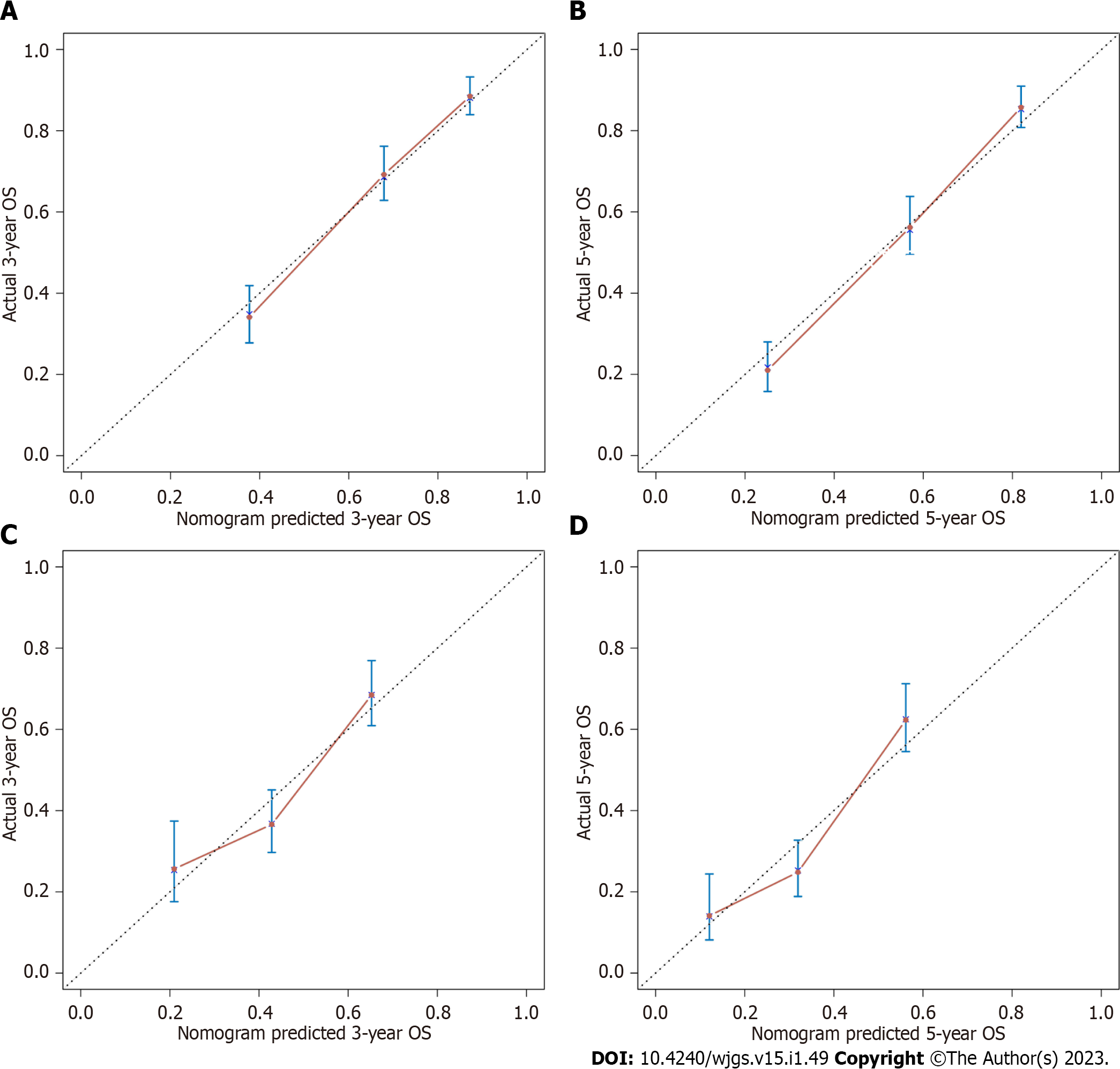
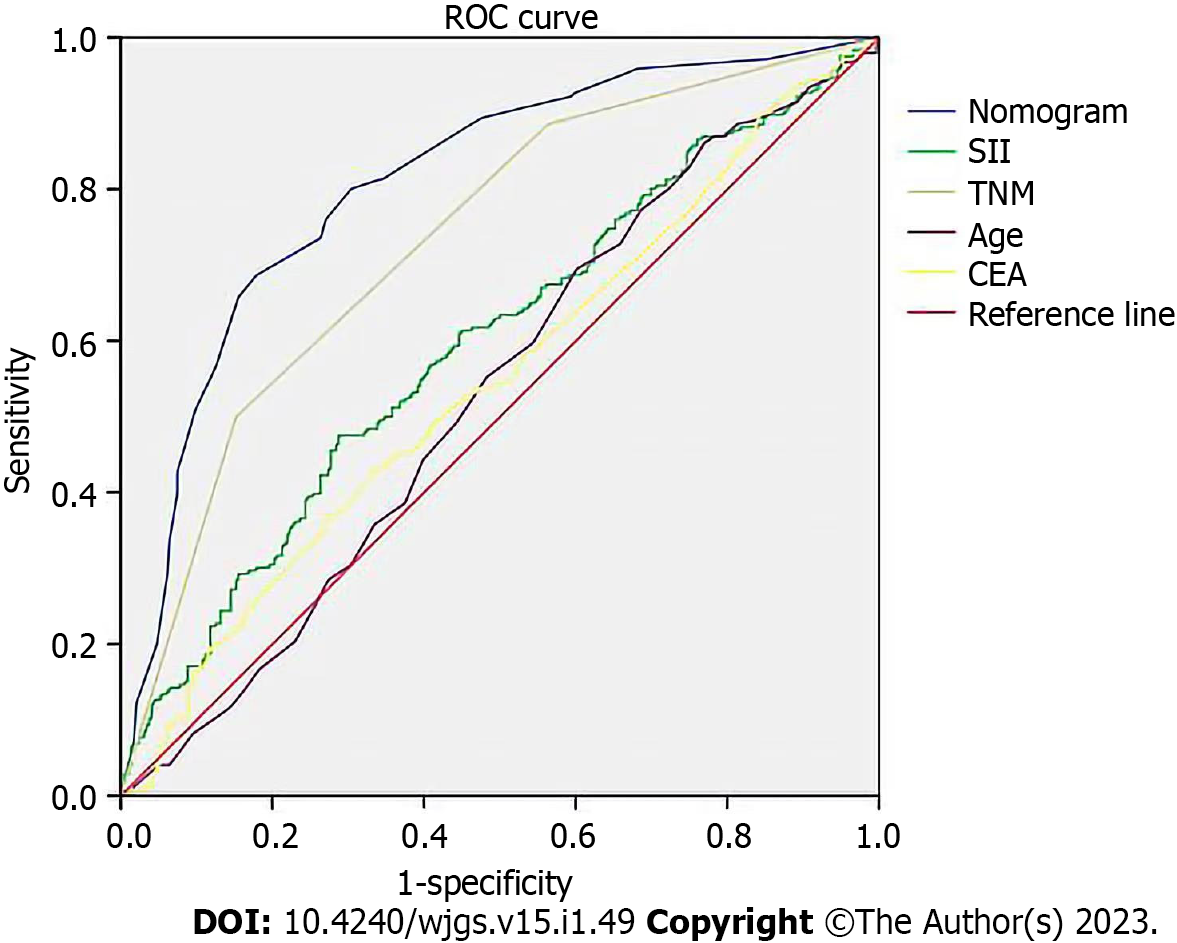
We applied calibration curves to verify the model in the training and validation groups (Figure 3). In the training group, the nomogram’s C-index was 0.736, whereas in the validation group, it was 0.651. In order to further demonstrate the nomogram performance, we displayed the ROC of the nomogram (Figure 4). In addition, the AUC of the nomogram was large, showing that nomogram is dependable.
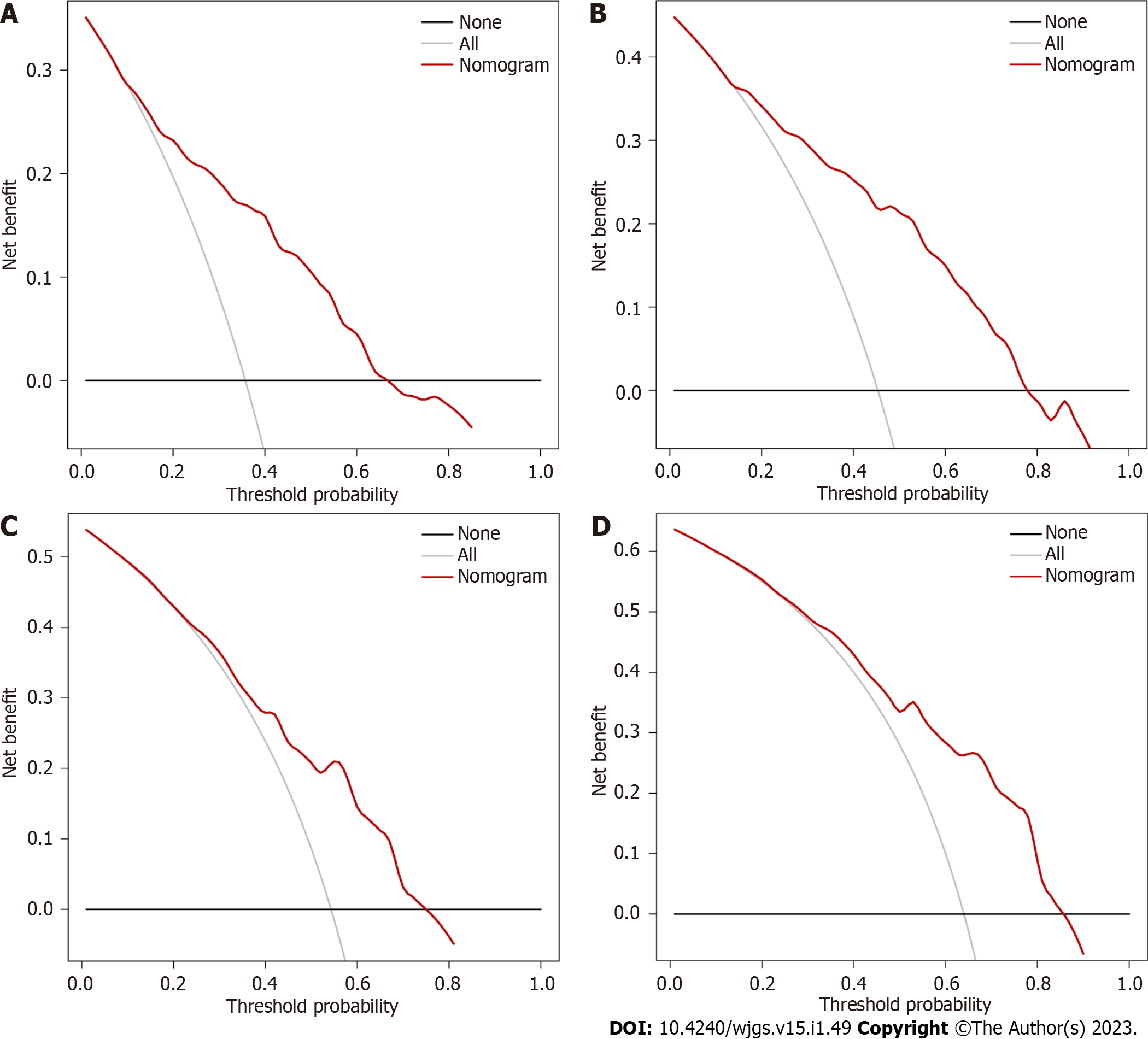
Decision curve analysis results indicated the clinical use of the novel model for estimating 3-year and 5-year survival in GC patients in the training group and validation group (Figure 5).
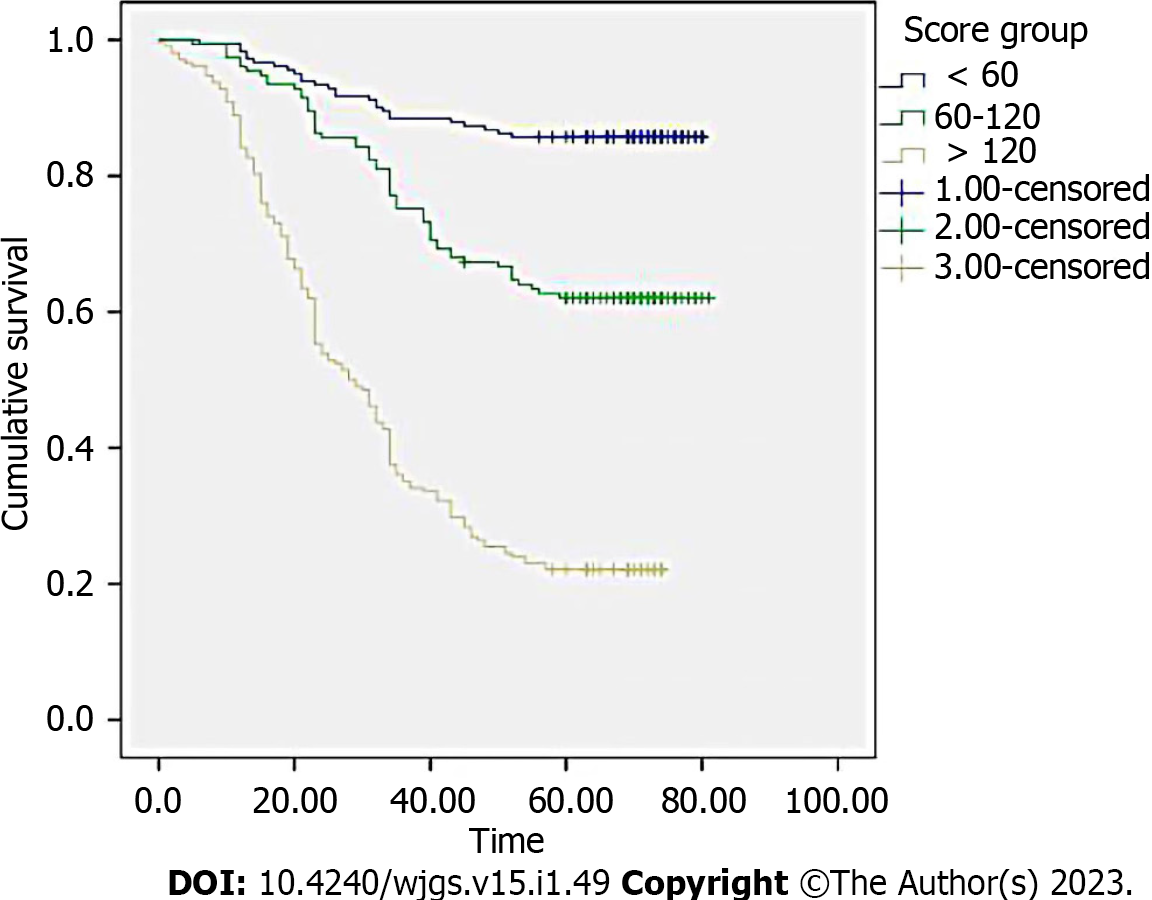
The training group was then separated into three subgroups depending on the cutoff value (< 60 was low risk; 60–120 was medium risk; > 120 was high risk). The Kaplan-Meier curve demonstrated the good outcomes (Figure 6).
The only curative form of treatment for GC is generally believed to be surgery. Early GC is typically difficult to diagnose due to the limitations of available procedures. The 5-year survival rate at the moment is quite poor. As a result, several researchers have worked to enhance the prognosis for GC patients. TNM stage and lymph node metastases were identified as important independent risk factors. However, because it is challenging to evaluate these prognostic factors prior to surgery, substantial research has been done recently on serum markers. This study, to the best of our knowledge, is the first to compare the serum score system and then create a novel nomogram that combines peripheral blood markers and clinical factors to predict OS at 1 year, 3 years, and 5 years.
The results demonstrated that age, SII, TNM stage, and CEA were each independent predictors of survival in GC patients. SII was a more effective indicator to predict OS based on the fact that its AUC was higher than that of NLR-PLR and NLR-PLT. The C-index of our newly constructed nomogram, which was based on independent prognostic variables, was 0.736, indicating that it is quite accurate in predicting GC patients’ prognoses. This nomogram is an accurate score system because the decision curve analysis and calibration curve both supported its clinical use. Nomograms are more valuable than TNM stages for predicting prognosis in several cancers[17,18]. Since the nomogram’s AUC in this study was higher than other elements, surgeons may use this scoring system to accurately assess a patient’s prognosis and choose the most beneficial course of action in the clinic.
Four factors in our nomogram were significantly influenced by SII. According to recent studies, inflammation may have an impact on the development of cancer and long-term survival of patients[19]. SII, which may include NE count, PLT count, and LY count, was among them but was less frequently reported. NLR-PLT and NLR-PLR were associated with GC patient prognosis, whereas SII was an independent prognostic factor and had higher value. Our study proved that CEA was a reliable prognostic factor and that it may be used to screen for cancer recurrence. As a result, we need to pay more attention to patients who have elevated levels of CEA. Age was another important prognostic factor, and this result was consistent with earlier research[20]. With the increase of age, the immunity of elderly patients decreases significantly, which leads to recurrence and metastasis of cancer, thus elderly patients with GC typically have a worse outcome. As a result, these important factors need to be given more emphasis in order to improve patient outcomes, and the nomogram may be used more frequently in clinics.
In conclusion, our research showed that age, SII, TNM stage, and CEA were major predictive factors of the prognosis of GC patients, and the new nomogram was a valid prognostic tool for them.
Nearly 66% of instances of gastric cancer (GC), which has the second-highest death rate of all cancers, occur in developing countries. The only curative treatment for patients is considered to be a radical surgery, which increases the likelihood of a successful cure and lengthens patient survival.
The high likelihood of cancer recurrence means that the 5-year overall survival (OS) is still poor even after surgery. The tumor node metastasis (TNM) stage is connected with the prognosis of GC patients, but it is difficult to determine prior to surgery.
To investigate more clinical characteristics and develop a specific nomogram to forecast OS for GC patients.
Nine hundred and four GC patients treated at the First Affiliated Hospital of Anhui Medical University between January 2010 and January 2013 were recruited. Prognostic risk variables were screened using the Cox analysis. The C-index and receiver operator characteristic (ROC) curve were used to construct and evaluate the nomogram.
TNM stage, carcinoembryonic antigen, systemic immune-inflammation index, and age were identified as independent predictive variables by multivariate analysis. The systemic immune-inflammation index value was superior to that of other inflammatory indicators. The ROC indicated the nomogram had a higher area under the curve than other factors, and its C-index for assessing the validation and training groups of GC patients was extremely reliable.
We created a novel nomogram to predict the prognosis of GC patients following curative gastrectomy based on the blood markers and other characteristics.
Both surgeons and patients can benefit significantly from this new scoring system. The nomogram may be used more frequently in clinics.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chisthi MM, India; Rojas A, Chile S-Editor: Fan JR L-Editor: Filipodia P-Editor: Zhao S
| 1. | Rumgay H, Shield K, Charvat H, Ferrari P, Sornpaisarn B, Obot I, Islami F, Lemmens VEPP, Rehm J, Soerjomataram I. Global burden of cancer in 2020 attributable to alcohol consumption: a population-based study. Lancet Oncol. 2021;22: 1071-1080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 362] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 2. | Liu Y, Zhang Q, Ren C, Ding Y, Jin G, Hu Z, Xu Y, Shen H. A germline variant N375S in MET and gastric cancer susceptibility in a Chinese population. J Biomed Res. 2012;26: 315-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Wang W, Li YF, Sun XW, Chen YB, Li W, Xu DZ, Guan XX, Huang CY, Zhan YQ, Zhou ZW. Prognosis of 980 patients with gastric cancer after surgical resection. Chin J Cancer. 2010;29:923-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Cai Q, Zhou W, Li J, Ou X, Chen C, Cai S, He W, Xu J, He Y. Association of Preoperative Serum Carcinoembryonic Antigen and Gastric Cancer Recurrence: A Large Cohort Study. J Cancer. 2021;12:397-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5762] [Article Influence: 240.1] [Reference Citation Analysis (0)] |
| 6. | Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med. 2019;18:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 831] [Article Influence: 166.2] [Reference Citation Analysis (0)] |
| 7. | Proctor MJ, Talwar D, Balmar SM, O'Reilly DS, Foulis AK, Horgan PG, Morrison DS, McMillan DC. The relationship between the presence and site of cancer, an inflammation-based prognostic score and biochemical parameters. Initial results of the Glasgow Inflammation Outcome Study. Br J Cancer. 2010;103:870-876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 8. | Huang XZ, Yang YC, Chen Y, Wu CC, Lin RF, Wang ZN, Zhang X. Preoperative Anemia or Low Hemoglobin Predicts Poor Prognosis in Gastric Cancer Patients: A Meta-Analysis. Dis Markers. 2019;2019:7606128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4947] [Cited by in RCA: 4535] [Article Influence: 323.9] [Reference Citation Analysis (0)] |
| 10. | Carruthers R, Tho LM, Brown J, Kakumanu S, McCartney E, McDonald AC. Systemic inflammatory response is a predictor of outcome in patients undergoing preoperative chemoradiation for locally advanced rectal cancer. Colorectal Dis. 2012;14:e701-e707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 11. | Kemal Y, Demirağ G, Ekiz K, Yücel I. Mean platelet volume could be a useful biomarker for monitoring epithelial ovarian cancer. J Obstet Gynaecol. 2014;34:515-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Hu W, Yu J, Huang Y, Hu F, Zhang X, Wang Y. Lymphocyte-Related Inflammation and Immune-Based Scores Predict Prognosis of Chordoma Patients After Radical Resection. Transl Oncol. 2018;11:444-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Ishizuka M, Oyama Y, Abe A, Kubota K. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients undergoing surgery for gastric cancer. J Surg Oncol. 2014;110:935-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Wang BL, Tian L, Gao XH, Ma XL, Wu J, Zhang CY, Zhou Y, Guo W, Yang XR. Dynamic change of the systemic immune inflammation index predicts the prognosis of patients with hepatocellular carcinoma after curative resection. Clin Chem Lab Med. 2016;54:1963-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Geng Y, Shao Y, Zhu D, Zheng X, Zhou Q, Zhou W, Ni X, Wu C, Jiang J. Systemic Immune-Inflammation Index Predicts Prognosis of Patients with Esophageal Squamous Cell Carcinoma: A Propensity Score-matched Analysis. Sci Rep. 2016;6:39482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 178] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 16. | YOUDEN WJ. Index for rating diagnostic tests. Cancer. 1950;3:32-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 126] [Reference Citation Analysis (0)] |
| 17. | Zivanovic O, Jacks LM, Iasonos A, Leitao MM Jr, Soslow RA, Veras E, Chi DS, Abu-Rustum NR, Barakat RR, Brennan MF, Hensley ML. A nomogram to predict postresection 5-year overall survival for patients with uterine leiomyosarcoma. Cancer. 2012;118:660-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26: 1364-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1306] [Cited by in RCA: 2303] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 19. | Roxburgh CS, Salmond JM, Horgan PG, Oien KA, McMillan DC. The relationship between the local and systemic inflammatory responses and survival in patients undergoing curative surgery for colon and rectal cancers. J Gastrointest Surg. 2009;13:2011-8; discussion 2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Wang SB, Qi WX, Chen JY, Xu C, Kirova YM, Cao WG, Cai R, Cao L, Yan M, Cai G. Competing risk nomogram predicting initial loco-regional recurrence in gastric cancer patients after D2 gastrectomy. Radiat Oncol. 2019;14:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |