Published online Jan 27, 2023. doi: 10.4240/wjgs.v15.i1.32
Peer-review started: September 18, 2022
First decision: October 20, 2022
Revised: November 4, 2022
Accepted: December 13, 2022
Article in press: December 13, 2022
Published online: January 27, 2023
Processing time: 121 Days and 20 Hours
According to relevant investigation and analysis, there are few research studies on the effect of excessive chemotherapy cycles after D2 gastrectomy on the survival of patients with gastric cancer.
To determine whether excessive chemotherapy cycles provide extra survival benefits, reduce recurrence rate, and improve survival rate in patients with stage II or III gastric cancer.
We analyzed and summarized 412 patients with stage II gastric cancer and 902 patients with stage III gastric cancer who received D2 gastrectomy plus adjuvant chemotherapy or neoadjuvant chemotherapy. Analysis and comparison at a ratio of 1:1 is aimed at reducing realistic baseline differences (n = 97 in each group of stage II, n = 242 in each group of stage III). Progression-free survival, overall survival and recurrence were the main outcome indicators.
When the propensity score was matched, the baseline features of stage II and III gastric cancer patients were similar between the two groups. After a series of investigations, Kaplan-Meier found that the progression-free survival and overall survival of stage II and III gastric cancer patients were consistent between the two groups. The local metastasis rate (P = 0.002), total recurrence rate (P < 0.001) and distant metastasis rate (P = 0.001) in the ≥ 9 cycle group of stage III gastric cancer were statistically lower than those in the < 9 cycle group. The interaction analysis by Cox proportional hazard regression model showed that intestinal type, proximal gastrectomy, and ≥ 6 cm maximum diameter of tumor had a higher risk of total mortality in the < 9 cycles group.
Overall, ≥ 9 chemotherapy cycles is not recommended for patients with stage II and stage III gastric cancer because it has an insignificant role in the prognosis of gastric cancer. However, for patients with stage III gastric cancer, ≥ 9 cycles of chemotherapy was shown to significantly decrease recurrence.
Core Tip: This retrospective study determined the survival benefit of excess chemotherapy cycles for gastric cancer after D2 gastrectomy. No difference in progression-free survival and overall survival was observed between patients receiving ≥ 9 or < 9 cycles of chemotherapy. Stage III gastric cancer patients receiving ≥ 9 cycles of chemotherapy had significantly lower overall recurrence, local-regional metastasis, and distant metastasis. The Cox proportional risk regression model was used in the exploration and analysis that intestinal type, proximal gastrectomy, and ≥ 6 cm maximum tumor diameter had a higher risk of total mortality in the < 9 cycles of chemotherapy group.
- Citation: Li YF, Zhang WB, Gao YY. Prognostic effect of excessive chemotherapy cycles for stage II and III gastric cancer patients after D2 + gastrectomy. World J Gastrointest Surg 2023; 15(1): 32-48
- URL: https://www.wjgnet.com/1948-9366/full/v15/i1/32.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v15.i1.32
Common cancer cases in the world include gastric cancer which is also the main cause of human cancer death[1]. Specifically, gastric cancer, one of the most common cancers in the world, has a survival rate of only 20%. Stomach cancer is a malignant tumor in which cancer cells attach to the gastric mucosa and gradually spread throughout the body. At the beginning, there are no special symptoms of gastric cancer and the patient is unaware of the problem. However, with the extension of time and the deterioration of the disease, patient's stomachs are gradually unable to digest, causing discomfort. In the final stages, gastric cancer patients will vomit, experience pain, and in severe cases will cough up blood. They present with abdominal swelling, lymph node metastasis and so on. According to the relevant data, the production of gastric cancer is affected by different factors. The initial symptom may be chronic gastritis or bacterial infection, or the gastrointestinal discomfort caused by genes and adverse environment, which may turn into gastric cancer. Among them, the most important factor affecting the occurrence of gastric cancer is the bad environment. If the soil or water contains excessive nitrate and other chemical elements, it is very likely to lead to the occurrence of gastric cancer, and people will inevitably ingest these elements during the diet. In daily life, salty food can also lead to stomach cancer. Moldy food can also cause stomach cancer if consumed for a long time. Generally speaking, the incidence of gastric cancer in women is much lower than that in men, and the most important type of gastric cancer is adenocarcinoma, including diffuse gastric cancer and intestinal gastric cancer. The older people are, the more likely they are to develop stomach cancer, ranging in age from 50 to 80. The incidence of stomach cancer of our people is not low in the global scope, and is far higher than the world average level. Stomach cancer accounts for nearly a quarter of cancer deaths. In the early stages of gastric cancer, when there is no lymphatic metastasis, endoscopic treatment is recommended. In the middle stage of gastric cancer, when the cancer cells are not yet spreading throughout the body, it can be treated by D2 gastrectomy. After the tumor is removed, adjuvant therapy is given postoperatively to reduce the likelihood of bacterial infection and avoid the risk of death.
Although D2 gastrectomy and postoperative adjuvant therapy are the only radical methods for the treatment of gastric cancer at this stage, patients with stage Ⅱ and Ⅲ gastric cancer have a higher recurrence rate after surgery and do not have a higher long-term survival rate. In academia, experts and scholars have discussed the value and effect of postoperative adjuvant chemotherapy for gastric cancer. With the development of the times and the progress of society, more and more people analyze the influence of postoperative adjuvant therapy on gastric cancer patients through experiments and research contents. In this context, people increasingly affirm the value of postoperative adjuvant chemotherapy for gastric cancer. According to relevant data, postoperative adjuvant chemotherapy reduced the mortality rate by more than 20% compared with surgery alone. Therefore, the comprehensive treatment mode of surgery combined with adjuvant chemotherapy has been used more frequently in the treatment of gastric cancer. However, at present, the duration of adjuvant therapy after radical gastrectomy has not been determined, and the correlation between the length of chemotherapy cycles and the effect of chemotherapy is not clear. In other words, with the development of the times, D2 gastrectomy[2] and subsequent adjuvant chemotherapy are constantly improved, and the overall survival period (OS) of gastric cancer patients has been well transformed, but from the perspective of long-term survival, there are still limitations[3,4].
Excessive chemotherapy cycles to treat gastric cancer has been proposed, but the survival benefit has not been determined. According to the Chinese Society of Clinical Oncology clinical guidelines[5] for the diagnosis and treatment of gastric cancer, preoperative neoadjuvant chemotherapy is recommended for 2-4 cycles, and perioperative neoadjuvant chemotherapy is recommended for 2-4 cycles before surgery and 6-8 cycles after surgery. Therefore, ≥ 9 cycles of chemotherapy would be considered as excessive chemotherapy cycles.
In our previous study[6] of patients with stage II and stage III gastric cancer, the mean of chemotherapy cycles was 9.65 ± 3.86 and 9.87 ± 3.84, respectively. In addition, we analyzed 1-, 3- and 5-year survival rates for patients receiving < 9 cycles of chemotherapy, and found them to be 92.4% (257/278), 66.9% (186/278), and 46.4% (129/278), respectively. The 1-, 3-, and 5-year survival rates for patients receiving ≥ 9 cycles of chemotherapy were found to be 92.5% (577/624), 62.9% (393/624), and 46.3% (289/624), respectively.
In essence, chemotherapy can be both good and bad for patients. Excessive chemotherapy cycles (≥ 9) give rise to unpleasant side effects and harmful effects on physical function. However, the appropriate number of chemotherapy cycles may eliminate any residual cancer cells. The ultimate aim of our research was to determine whether excessive chemotherapy cycles (≥ 9) increase survival and decrease recurrence in patients with stage II and III gastric cancer.
We summarized the relevant data from 2002 to 2020 of more than 400 patients with stage II gastric cancer and 900 patients with stage III gastric cancer who underwent gastrectomy for the treatment of gastric cancer. According to the data, lymph node dissection was higher than D2 (complete removal of group 1 and group 2 Lymph nodes). The clinicopathological characteristics included age at surgery, sex, nerve invasion, vascular invasion, number of positive lymph nodes, depth of tumor invasion, number of chemotherapy cycles, TNM stage (according to the 8th edition of the American Joint Board on Cancer), maximum tumor diameter, Lauren classification, retinal metastasis, type of gastrectomy, chemotherapy administration, surgical margin, multi-organ resection, chemotherapy protocol, and group Clavien-Dindo grading of texture, multiple metastases, OS, complications, and progression-free survival (PFS). The number of postoperative chemotherapy cycles, the number of neoadjuvant chemotherapy cycles, medical records, surgical records and follow-up data were analyzed retrospectively.
The inclusion criteria consisted of: (1) Neoadjuvant chemotherapy or adjuvant chemotherapy before radical gastrectomy; (2) Histologically proven gastric cancer; (3) There was no serious damage to the organs after the operation; (4) Complete clinicopathological and follow-up data; and (5) Except for gastric cancer, there were no other malignancies or causes of death. Exclusion criteria are classified as follows: (1) There is no complete clinical data; (2) Other systemic tumors; (3) Non gastric cancer was confirmed by pathological classification; and (4) Bypass surgery and palliative surgery.
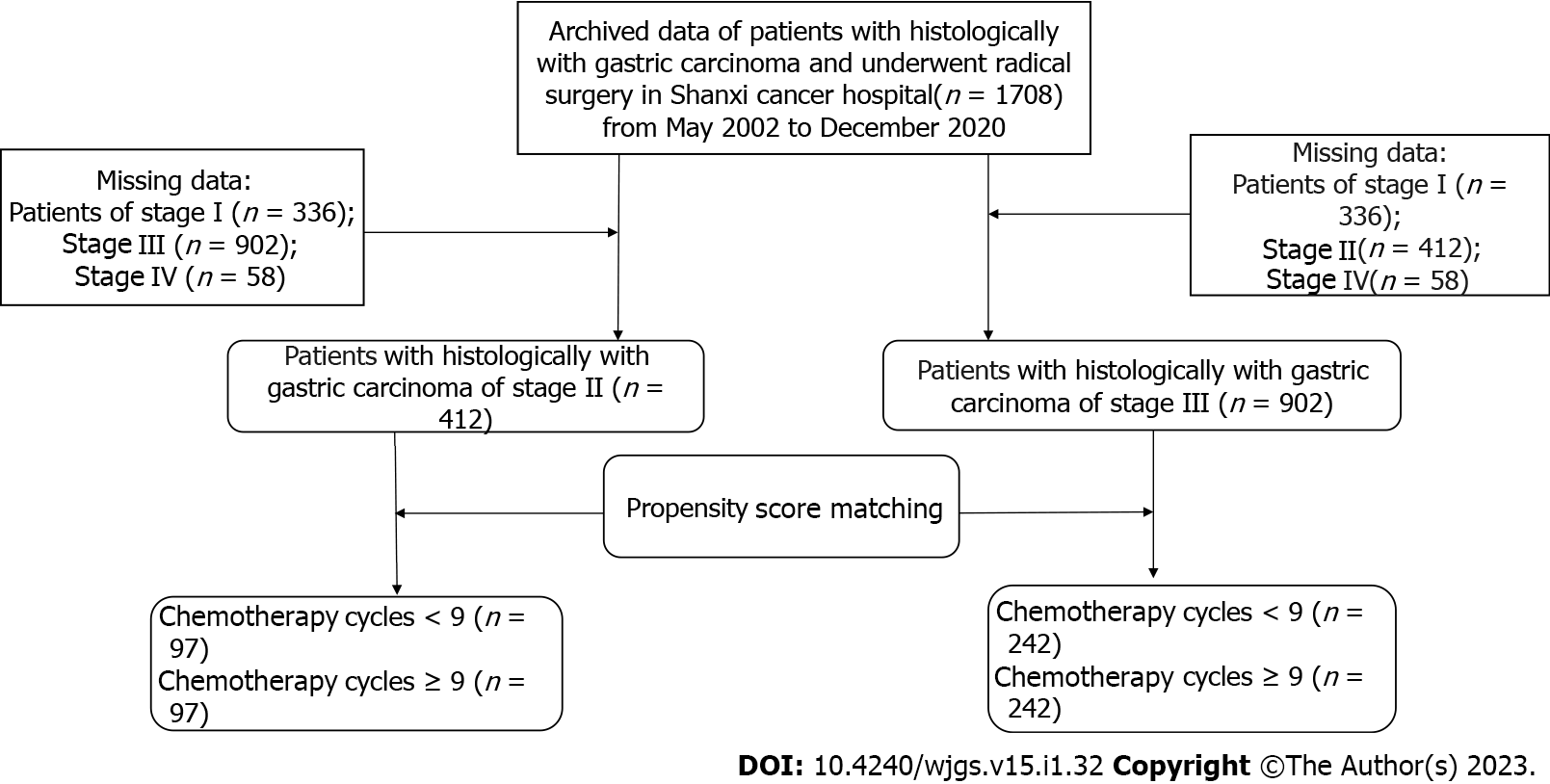
The American Joint Board on Cancer's 8 TNM grade reclassified tumor stages. Because this study is retrospective, consent is not required. After a series of reviews, the Ethics Committee of Shanxi Cancer Hospital finally approved the study. This study was consistent with the standards of the Declaration of Helsinki, so patient anonymity was adopted and patient data and information were not disclosed to the public. The specific research content and process are shown in Figure 1.
Patients received individualized chemotherapy regimens. This paper summarized the dose ranges and other details of several common regimens: (1) Oxaliplatin (130 mg/m2), S-1 and oxaliplatin (SOX), S-1 (40-60 mg), the above-mentioned drugs twice a day, the 1st to 14th d, rest for 7 d; (2) S-1, the aforementioned drug twice daily, with the specific dose schedule determined by the patient's area. From day 1 to day 14, 40-60 mg, then rest for 7 d; (3) S-1 + apatinib, apatinib (500 mg) administered once daily continuously and S-1 (40-60 mg) administered twice daily on day 1 to day 14, then rest for 7 d; (4) Folinic acid, fluorouracil, and oxaliplatin (FOLFOX), folinic acid (200 mg/m2), fluorouracil (2800 mg/m2), and oxaliplatin (85 mg/m2) administered every 3 wk; (5) Oxaliplatin and capecitabine (also known as XELOX) were given intravenous oxaliplatin (150 mg/m2) on the 1st day of every three cycles and orally capecitabine (1000 mg/m2) twice a day from day 1 to day 14, followed by a rest for 7 d; (6) Capecitabine was taken orally twice a day (1000 mg/m2), with a rest of 7 days from day 1 to day 14; (7) Cisplatin and fluorouracil (also known as DCF), S-1 + docetaxel, cisplatin (75 mg/m2), docetaxel (75 mg/m2) on day 1 to day 5, fluorouracil (750 mg/m2) on day 1 to day 5, S-1 (40-60 mg) on day 1 to day 14, orally twice a day, then rest for 7 d; and (8) Oral administration of defluoruridine (1000 mg/m2) twice a day from day 1 to day 28, followed by rest for 14 d.
All excised specimens were examined to determine the histological response to neoadjuvant chemotherapy and pathological staging. The number of surviving tumor cells in the tumor determines the grade of tumor regression. According to Ryan criteria[6]: Grade 0 (complete response), no residual tumor cells. Grade 1 (primary remission), with scattered tumor cells; Grade 2 (moderate remission), tumor cell aggregation with fibrosis; Grade 3 (mild remission), with substantial tumor cell retention. The toxicity associated with neoadjuvant chemotherapy was evaluated according to Standard 5.0, a common term for adverse events[7].
Patients were followed up until December 2020. The second-stage follow-up was 41.51 ± 21.18 mo, and the third-stage follow-up was 43.56 ± 24.45 mo. Follow-up was conducted every 3 mo for 1 year after surgery, every 6 mo for 2 years to 5 years, and annually thereafter. Routine follow-up included laboratory tests, physical examinations, pelvic ultrasound, chest radiographs, magnetic resonance imaging, and computed tomography.
Sex, age at surgery, vascular invasion, nerve invasion, depth of tumor invasion, number of positive lymph nodes, Lauren classification, maximum tumor diameter, type of gastrectomy, and human epidermal growth factor receptor-2 (HER2) status were used for propensity score matching (PSM) using 1:1 nearest neighborhood with no replacement and calipers adjusted for sample size and matching success. If a patient is a match, a correlation analysis of primary and secondary endpoints will be performed. The main contents are PFS and OS. The secondary endpoints were tumor recurrence and metastasis, multiple metastases, and recurrence patterns.
Each group generated a Kaplan-Meier survival curve using a log-rank comparison. The category variable analysis was tested using appropriate tests. The P values on both sides were 0.05, which had statistical value. The date of return visit is calculated from the date of surgery to the time of last contact. OS is the time between surgery and death or the last follow-up. PFS refers to the time between surgery and the first recorded death or recurrence.
All data were analyzed and explored using SPSS v25.0 software (IBM Corp., Armonk, NY, United States). The classification variable was expressed as percentage, and the test methods used in the analysis were Fisher's exact test and chi-square test. Continuous data were expressed as mean ± standard deviation, and t-test was used for analysis. Survival analysis of PFS and OS was performed using Kaplan-Meier method, which was compared with the log-rank test method. Median was used for the non-normal distribution parameters, and the analysis method was Mann-Whitney test. Subgroup analyses were performed by the Cox hazard regression model. P < 0.05 was considered statistically significant. PSM was performed with the Hansen and Bowers overall balance test. Relative multivariate imbalance L1 test was used to determine standardized mean difference < 0.25. The χ2 test was used to compare the differences in recurrence, local-regional recurrence, peritoneal metastasis, and distant metastasis between the two groups.
Nonetheless, the interaction effect between chemotherapy cycles and Lauren classification, types of gastrectomy, and maximum diameter of the tumor on OS were determined for the first time.
Patients in the < 9 cycles group received the following chemotherapy regimens: (1) 1 patient received S-1; (2) 28 patients received SOX; (3) 4 patients received S-1 + docetaxel; (4) 10 patients received deofuridine; (5) 3 patients received XELOX; (6) 32 patients received FOLFOX; and (7) 18 patients received multiple regimen combinations. Patients in the ≥ 9 cycles group received the following chemotherapy regimens: (1) 61 patients received S-1; (2) 6 patients received SOX; (3) 9 patients received S-1 + apatinib; (4) 15 patients received capecitabine; (5) 2 patients received FOLFOX; and (6) 5 patients received multiple regimen combinations. Three patients in the < 9 cycles group and 21 patients in the ≥ 9 cycles group received neoadjuvant chemotherapy plus adjuvant chemotherapy. Ninety-four patients in the < 9 cycles group and 76 patients in the ≥ 9 cycles group received only postoperative adjuvant chemotherapy.
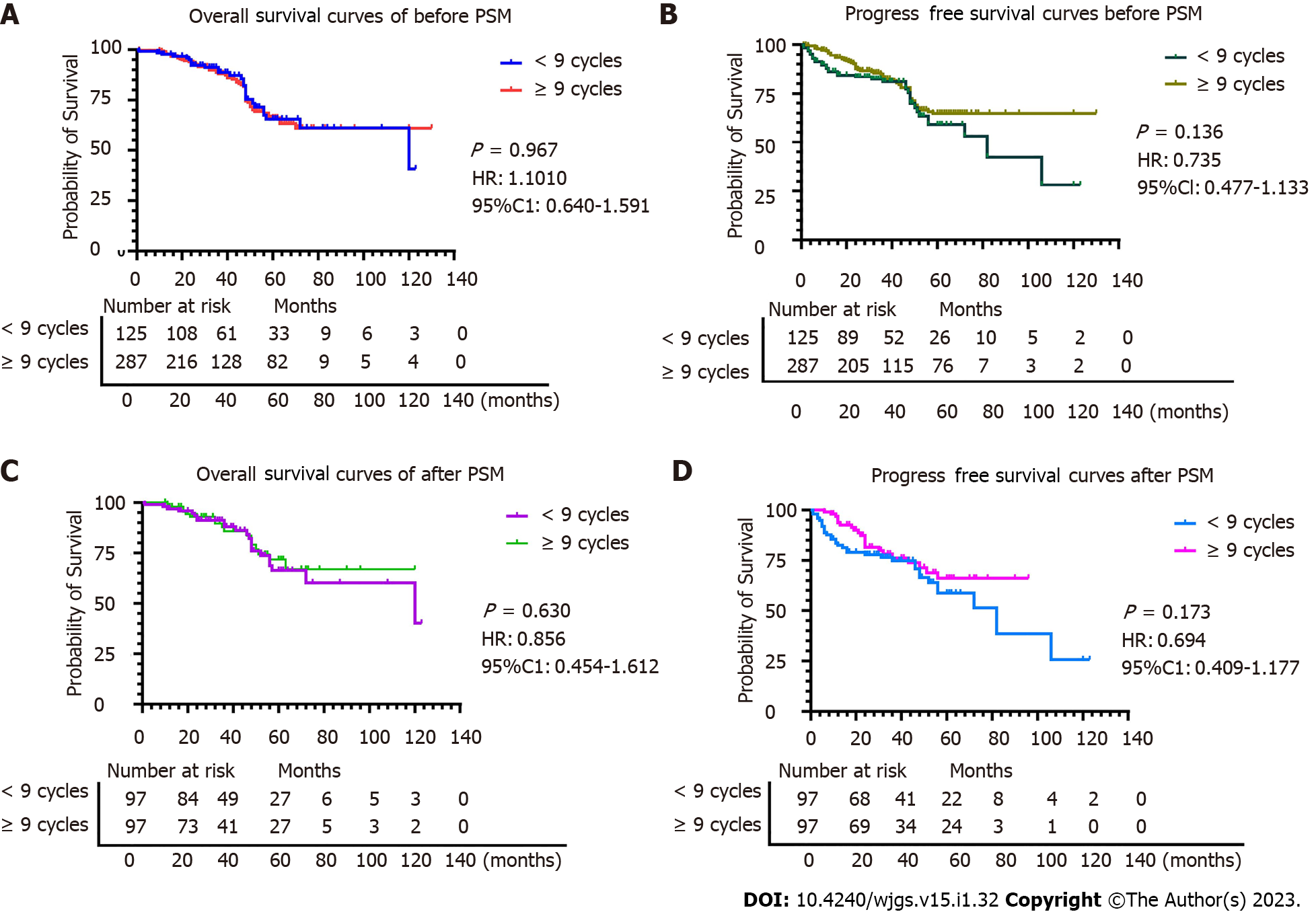
All patients with TNM stage II (n = 412) were grouped based on nine variables (sex, vascular invasion, nerve invasion, number of positive lymph nodes, depth of tumor invasion, maximum tumor diameter, Lauren classification, type of gastrectomy, and HER2 status) according to the cycles of chemotherapy received (< 9 cycles vs ≥ 9 cycles) (Table 1). Significant differences in sex (P = 0.022) and age (P < 0.001) were observed between the < 9 cycles group vs the ≥ 9 cycles group before PSM. However, after PSM, in which 194 patients were included (97 patients in the <9 cycles group and 97 patients in ≥ 9 cycles group), no significant differences were observed between the two groups (P > 0.05). The Hansen and Bowers overall balance test indicated that the distribution between the two groups was well balanced after PSM (Figures 2 and 3).
| Variables | Before PSM | P value | After PSM | P value | ||
| < 9 cycles, n = 125 | ≥ 9 cycles, n = 287 | < 9 cycles, n = 97 | ≥ 9 cycles, n = 97 | |||
| Sex | 0.022 | 0.718 | ||||
| Male | 95 | 245 | 77 | 79 | ||
| Female | 30 | 42 | 20 | 18 | ||
| Age in yr | 55.38 ± 10.91 | 60.44 ± 9.85 | < 0.001 | 56.46 ± 10.10 | 56.46 ± 10.10 | 0.215 |
| Depth of tumor invasion | 0.248 | 0.998 | ||||
| T1 | 7 | 6 | 5 | 4 | ||
| T2 | 11 | 18 | 10 | 10 | ||
| T3 | 79 | 195 | 60 | 62 | ||
| T4 | 28 | 68 | 22 | 21 | ||
| Number of positive lymph nodes | 0.064 | 0.740 | ||||
| 0 | 64 | 172 | 52 | 49 | ||
| 1-2 | 53 | 107 | 39 | 43 | ||
| 3-6 | 4 | 5 | 4 | 3 | ||
| ≥ 7 | 4 | 3 | 2 | 2 | ||
| Type of gastrectomy | 0.448 | 0.249 | ||||
| Proximal | 14 | 24 | 10 | 8 | ||
| Distal | 41 | 93 | 32 | 26 | ||
| Total | 0 | 170 | 55 | 63 | ||
| Vascular invasion | 0.561 | 0.468 | ||||
| Negative | 77 | 168 | 54 | 59 | ||
| Positive | 48 | 119 | 42 | 38 | ||
| Neural invasion | 0.719 | 1.000 | ||||
| Negative | 79 | 176 | 64 | 64 | ||
| Positive | 46 | 111 | 33 | 33 | ||
| Lauren classification | 0.793 | 0.493 | ||||
| Intestinal | 60 | 143 | 47 | 52 | ||
| Diffuse | 27 | 58 | 21 | 19 | ||
| Mixed | 38 | 86 | 29 | 26 | ||
| Maximum diameter of tumor in cm | 0.603 | 0.410 | ||||
| < 6 | 87 | 207 | 70 | 75 | ||
| ≥ 6 | 38 | 80 | 27 | 22 | ||
| Surgical margin | 0.740 | 0.562 | ||||
| Negative | 123 | 281 | 95 | 96 | ||
| Positive | 2 | 6 | 2 | 2 | ||
| HER2 | 0.337 | 0.911 | ||||
| Negative | 70 | 171 | 55 | 54 | ||
| Positive | 55 | 116 | 42 | 43 | ||
OS and PFS were similar in both groups before and after PSM (P > 0.05), indicating that in patients with stage II gastric cancer ≥ 9 chemotherapy cycles does not impart a survival benefit (Figures 2 and 3). In detail, the 1-year OS rate (96.9% vs 97.9%, log-rank P = 0.650), 3-year OS rate (89.7% vs 89.7%, log-rank P = 1.000), and 5-year OS rate (79.4% vs 83.5%, log-rank P = 0.460) were not statistically different. The 1-year PFS rate was statistically different between the ≥ 9 cycles group and the < 9 cycles group (93.8% vs 82.4%, log-rank P = 0.015, respectively). However, that benefit was not observed in the 3-year PFS rate (76.3% vs 81.4%, log-rank P = 0.379) and in the 5-year PFS rate (69.1% vs 77.3%, log-rank P = 0.195). No differences were observed between the < 9 cycles group and the ≥ 9 cycles group for recurrence (22.7% vs 12.4%, respectively, P = 0.059), local-regional metastasis (11.3% vs 11.3%, respectively, P = 0.117), and distant metastasis (5.2% vs 6.2%, respectively, P = 0.204).
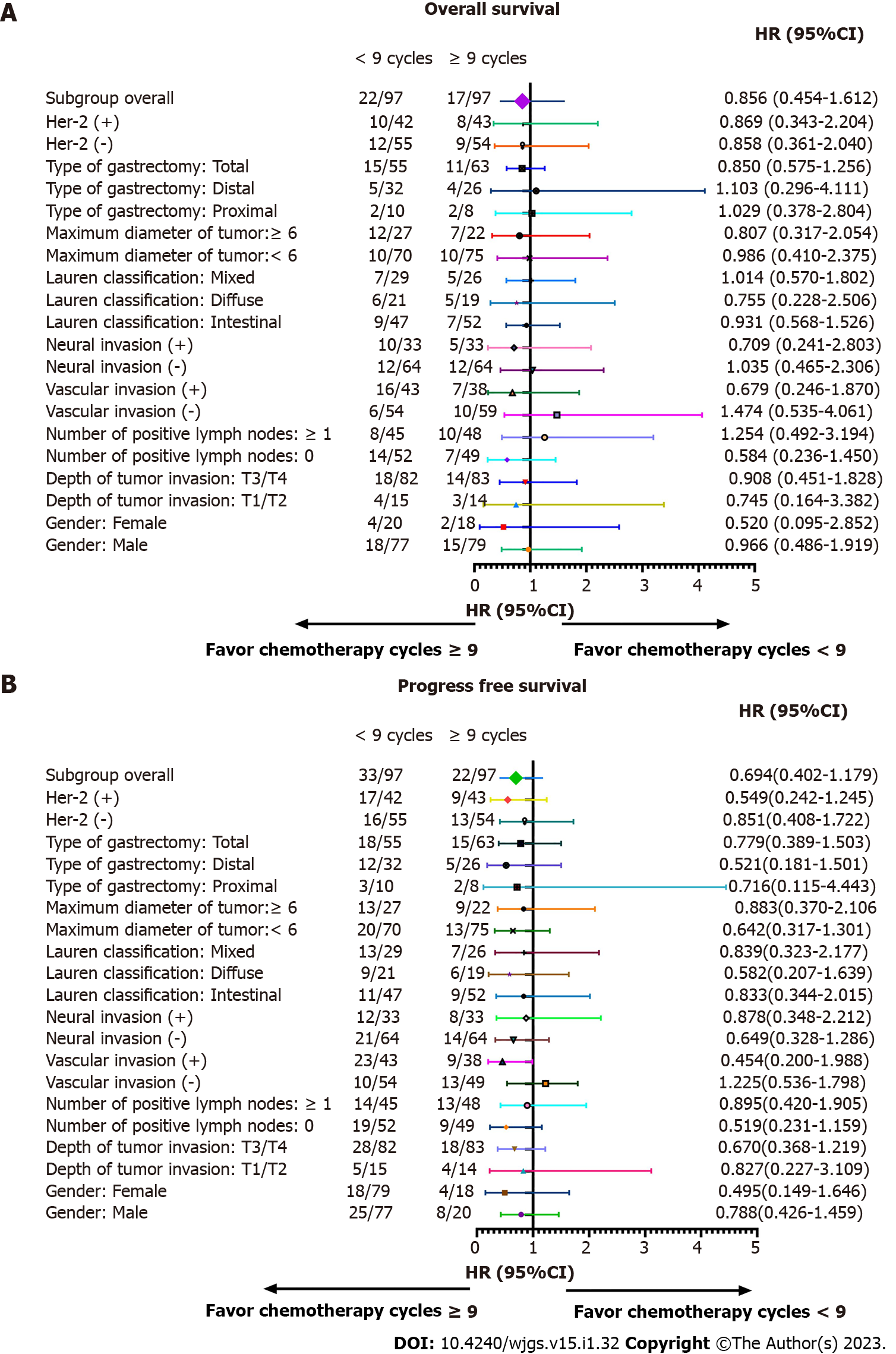
We performed subgroup analyses according to depth of tumor invasion, sex, vascular invasion, number of positive lymph nodes, Lauren classification, neural invasion, types of gastrectomy, maximum diameter of tumor, and HER2 in order to determine if a survival benefit of ≥ 9 cycles was evident in specific patient populations. After subgroup analysis, the differences in OS and PFS between the two groups were not statistically significant (Tables 2 and 3, and Figure 3).
| Variables | Death | Total | HR | 95%CI | P value | P for interaction |
| Sex | 0.219 | |||||
| Male | 33 | 156 | 0.966 | 0.486-1.919 | 0.922 | |
| Female | 6 | 38 | 0.520 | 0.095-2.852 | 0.452 | |
| Depth of tumor invasion | 0.749 | |||||
| T1/T2 | 7 | 29 | 0.745 | 0.164-3.382 | 0.702 | |
| T3/T4 | 32 | 165 | 0.908 | 0.451-1.828 | 0.786 | |
| Number of positive lymph nodes | 0.458 | |||||
| 0 | 21 | 101 | 0.584 | 0.236-1.450 | 0.247 | |
| ≥ 1 | 18 | 93 | 1.254 | 0.492-3.194 | 0.635 | |
| Vascular invasion | 0.729 | |||||
| Negative | 24 | 128 | 1.474 | 0.535-4.061 | 0.431 | |
| Positive | 15 | 66 | 0.679 | 0.246-1.870 | 0.453 | |
| Neural invasion | 0.937 | |||||
| Negative | 36 | 163 | 1.035 | 0.465-2.306 | 0.932 | |
| Positive | 22 | 93 | 0.709 | 0.241-2.083 | 0.531 | |
| Lauren classification | 0.553 | |||||
| Intestinal | 16 | 99 | 0.931 | 0.568-1.526 | 0.766 | |
| Diffuse | 11 | 40 | 0.755 | 0.228-2.506 | 0.647 | |
| Mixed | 12 | 55 | 1.014 | 0.570-1.802 | 0.963 | |
| Maximum diameter of tumor in cm | 0.167 | |||||
| < 6 | 20 | 145 | 0.986 | 0.410-2.375 | 0.976 | |
| ≥ 6 | 19 | 49 | 0.807 | 0.317-2.054 | 0.653 | |
| Type of gastrectomy | 0.664 | |||||
| Proximal | 4 | 18 | 1.029 | 0.378-2.804 | ||
| Distal | 9 | 58 | 1.103 | 0.296-4.111 | ||
| Total | 26 | 118 | 0.850 | 0.575-1.256 | ||
| HER2 | 0.656 | |||||
| Negative | 21 | 109 | 0.858 | 0.361-2.040 | ||
| Positive | 18 | 85 | 0.869 | 0.343-2.204 |
| Variables | Death or recurrence | Total | HR | 95%CI | P value | P for interaction |
| Sex | 0.385 | |||||
| Male | 33 | 156 | 0.788 | 0.426-1.459 | 0.449 | |
| Female | 22 | 38 | 0.495 | 0.149-1.646 | 0.252 | |
| Depth of tumor invasion | 0.226 | |||||
| T1/T2 | 9 | 29 | 0.827 | 0.227-3.109 | 0.779 | |
| T3/T4 | 46 | 165 | 0.670 | 0.368-1.219 | 0.190 | |
| Number of positive lymph nodes | 0.842 | |||||
| 0 | 28 | 101 | 0.519 | 0.232-1.159 | 0.110 | |
| ≥ 1 | 27 | 93 | 0.895 | 0.420-1.905 | 0.773 | |
| Vascular invasion | 0.743 | |||||
| Negative | 23 | 128 | 1.225 | 0.536-1.798 | 0.630 | |
| Positive | 32 | 66 | 0.454 | 0.200-0.988 | 0.047 | |
| Neural invasion | 0.732 | |||||
| Negative | 35 | 163 | 0.649 | 0.328-1.286 | 0.216 | |
| Positive | 20 | 93 | 0.878 | 0.348-2.212 | 0.782 | |
| Lauren classification | 0.622 | |||||
| Intestinal | 20 | 99 | 0.833 | 0.344-2.015 | 0.685 | |
| Diffuse | 15 | 40 | 0.582 | 0.207-1.639 | 0.306 | |
| Mixed | 20 | 55 | 0.839 | 0.323-2.177 | 0.718 | |
| Maximum diameter of tumor in (cm) | 0.128 | |||||
| < 6 | 33 | 145 | 0.642 | 0.317-1.301 | 0.219 | |
| ≥ 6 | 22 | 49 | 0.883 | 0.370-2.106 | 0.779 | |
| Type of gastrectomy | 0.356 | |||||
| Proximal | 5 | 18 | 0.716 | 0.115-4.443 | 0.720 | |
| Distal | 17 | 58 | 0.521 | 0.181-1.501 | 0.227 | |
| Total | 35 | 118 | 0.779 | 0.389-1.503 | 0.483 | |
| HER2 | 0.200 | |||||
| Negative | 29 | 109 | 0.851 | 0.408-1.772 | 0.665 | |
| Positive | 26 | 85 | 0.549 | 0.242-1.245 | 0.151 |
Patients in the < 9 cycles group received the following chemotherapy regimens: (1) 5 patients received S-1 + apatinib; (2) 4 patients received S-1 + DCF; (3) 10 patients received SOX + FOLFOX; (4) 4 patients received S-1 + FOLFOX; (5) 8 patients received XELOX; (6) 98 patients received FOLFOX; and (7) 18 patients received multiple regimen combinations. Patients in the ≥ 9 cycles group received the following chemotherapy regimens: (1) 142 patients received S-1; (2) 2 patients received SOX; (3) 2 patients received S-1 + DCF; (4) 29 patients received capecitabine; (5) 9 patients received doxifluridine; (6) 6 patients received SOX + FOLFOX; (7) 2 patients received FOLFOX; and (8) 50 patients received multiple regimen combinations. Twenty-four patients in the < 9 cycles group and forty-one patients in the ≥ 9 cycles group received neoadjuvant chemotherapy plus adjuvant chemotherapy. Two hundred eighteen patients in the < 9 cycles group and two hundred and one patients in the ≥ 9 cycles group received only postoperative adjuvant chemotherapy.
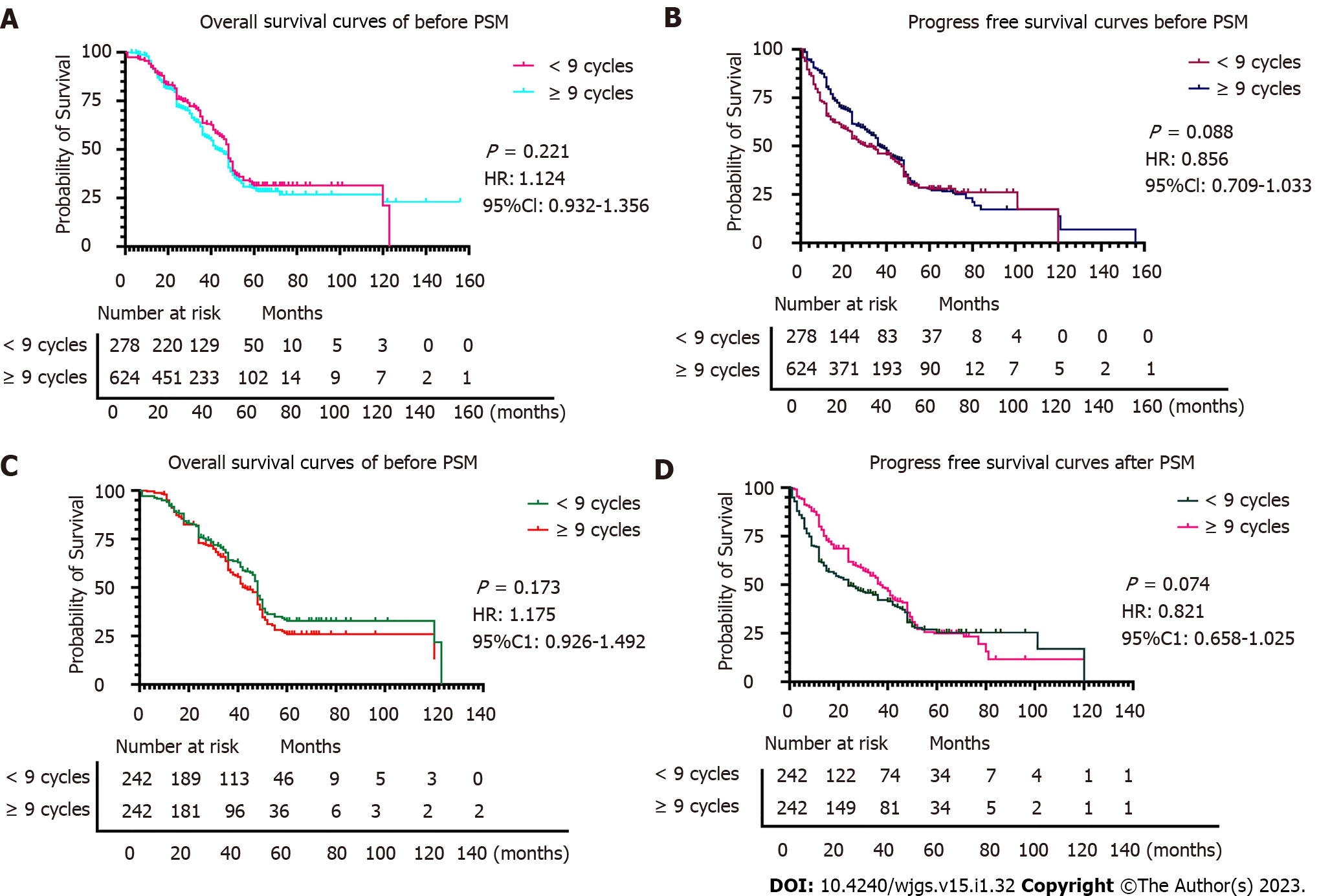
All patients with TNM stage III (n = 902) were grouped based on nine variables (sex, vascular invasion, nerve invasion, number of positive lymph nodes, depth of tumor invasion, maximum tumor diameter, Lauren classification, type of gastrectomy, and HER2 status) according to the cycles of chemotherapy received (< 9 cycles vs ≥ 9 cycles). Significant differences in age (P < 0.001) and type of gastrectomy (P = 0.044) were observed between the < 9 cycles group and the ≥ 9 cycles group before PSM. After PSM, in which 484 patients were included (there were 242 patients in the ≥ 9 cycle group and 242 patients in the < 9 cycle group), differences were observed between variables in the two groups (P > 0.05). The Hansen and Bowers overall balance test indicated that the distribution between the two groups was well balanced after PSM (Table 4, Figures 4 and 5).
| Variables | Before PSM | P value | After PSM | P value | ||
| < 9 cycles, n = 278 | ≥ 9 cycles, n = 624 | < 9 cycles, n = 242 | ≥ 9 cycles, n = 242 | |||
| Sex | 0.082 | 0.298 | ||||
| Male | 207 | 497 | 185 | 175 | ||
| Female | 71 | 127 | 57 | 67 | ||
| Age in yr | 56.97 ± 9.85 | 59.91 ± 10.03 | < 0.001 | 57.35 ± 9.31 | 58.01 ± 9.17 | 0.418 |
| Depth of tumor invasion | 0.568 | 0.754 | ||||
| T2 | 1 | 1 | 0 | 0 | ||
| T3 | 72 | 152 | 60 | 63 | ||
| T4 | 205 | 471 | 182 | 179 | ||
| Number of positive lymph nodes | 0.110 | 0.756 | ||||
| 0 | 0 | 4 | 0 | 0 | ||
| 1-2 | 39 | 111 | 36 | 34 | ||
| 3-6 | 68 | 155 | 60 | 67 | ||
| ≥ 7 | 171 | 354 | 146 | 141 | ||
| Type of gastrectomy | 0.044 | 0.903 | ||||
| Proximal | 16 | 36 | 13 | 12 | ||
| Distal | 89 | 154 | 71 | 71 | ||
| Total | 173 | 454 | 158 | 159 | ||
| Vascular invasion | 0.852 | 0.916 | ||||
| Negative | 67 | 154 | 59 | 60 | ||
| Positive | 211 | 470 | 183 | 182 | ||
| Neural invasion | 0.156 | 0.288 | ||||
| Negative | 85 | 211 | 74 | 85 | ||
| Positive | 193 | 403 | 168 | 157 | ||
| Lauren classification | 0.664 | 0.597 | ||||
| Intestinal | 52 | 125 | 46 | 42 | ||
| Diffuse | 153 | 315 | 127 | 127 | ||
| Mixed | 73 | 184 | 69 | 73 | ||
| Maximum diameter of tumor in cm | 0.346 | 0.467 | ||||
| < 6 | 134 | 322 | 119 | 111 | ||
| ≥ 6 | 144 | 302 | 123 | 131 | ||
| Surgical margin | 0.571 | 0.254 | ||||
| Negative | 260 | 577 | 225 | 218 | ||
| Positive | 18 | 47 | 17 | 24 | ||
OS and PFS were similar in both groups before and after PSM (P > 0.05), indicating that in patients with stage III gastric cancer ≥ 9 chemotherapy cycles does not impart a survival benefit (Figures 4 and 5). In detail, the 1-year OS rate (91.7% vs 92.5%, log-rank P = 0.735), 3-year OS rate (67.4% vs 63.6%, log-rank P = 0.389), and 5-year OS rate (47.1% vs 42.5%, log-rank P = 0.315) were not statistically different. The 1-year and 3-year PFS rates in the ≥ 9 period group and the <9 period group were statistically significant (80.1% vs 62.0%, log-rank P < 0.001, respectively, and 44.2% vs 54.5%, log-rank P = 0.023, respectively). However, that benefit was not observed in the 5-year PFS rate (38.4% vs 33.9%, log-rank P = 0.298). We observed that ≥ 9 chemotherapy cycles can significantly reduce the probability of recurrence compared to < 9 chemotherapy cycles (24.4% vs 48.8%, respectively, P < 0.001), local-regional metastasis (10.7% vs 21.1%, respectively, P = 0.002), and distant metastasis (12.4% vs 24.0%, respectively, P = 0.001) but not peritoneal metastasis (1.2% vs 3.7%, respectively, P = 0.090).
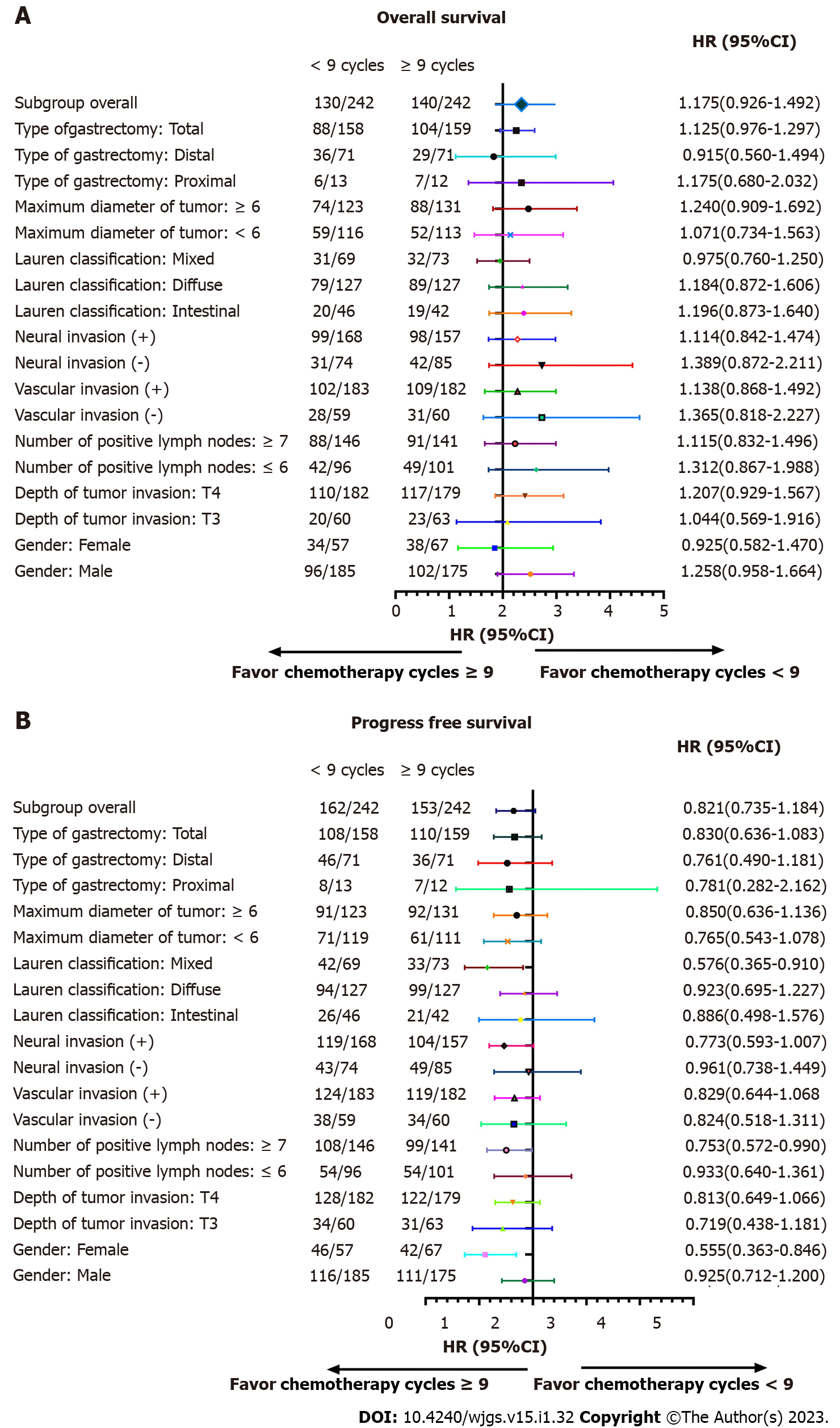
We performed subgroup analyses according to depth of tumor invasion, sex, vascular invasion, neural invasion, number of positive lymph nodes, maximum diameter of tumor, Lauren classification, types of gastrectomy, and HER2 in order to determine if a survival benefit of ≥ 9 cycles was evident in specific patient populations. The analyses demonstrated that the ≥ 9 chemotherapy cycles group had increased OS compared to the < 9 chemotherapy cycles for most subgroups (Table 5). Significant interactions were observed between chemotherapy cycles and the number of positive lymph nodes (P for interaction = 0.007), Lauren classification (P for interaction = 0.002), type of gastrectomy (P for interaction = 0.004), and maximum tumor diameter (P for interaction < 0.001).
| Variables | Death | Total | HR | 95%CI | P value | P for interaction |
| Sex | 0.639 | |||||
| Male | 198 | 360 | 1.258 | 0.951-1.664 | 0.108 | |
| Female | 72 | 124 | 0.925 | 0.582-1.470 | 0.741 | |
| Depth of tumor invasion | 0.127 | |||||
| T3 | 43 | 123 | 1.044 | 0.569-1.916 | 0.888 | |
| T4 | 227 | 361 | 1.207 | 0.929-1.567 | 0.158 | |
| Number of positive lymph nodes | 0.007 | |||||
| ≤ 6 | 91 | 197 | 1.312 | 0.867-1.988 | 0.199 | |
| ≥ 7 | 179 | 287 | 1.115 | 0.832-1.496 | 0.466 | |
| Vascular invasion | 0.099 | |||||
| Negative | 58 | 119 | 1.365 | 0.818-2.277 | 0.233 | |
| Positive | 211 | 365 | 1.138 | 0.868-1.492 | 0.350 | |
| Neural invasion | 0.059 | |||||
| Negative | 73 | 159 | 1.389 | 0.872-2.211 | 0.166 | |
| Positive | 197 | 325 | 1.114 | 0.842-1.474 | 0.451 | |
| Lauren classification | 0.002 | |||||
| Intestinal | 39 | 88 | 1.196 | 0.873-1.640 | 0.264 | |
| Diffuse | 168 | 254 | 1.184 | 0.872-1.606 | 0.280 | |
| Mixed | 63 | 142 | 0.975 | 0.760-1.250 | 0.840 | |
| Maximum diameter of tumor in cm | < 0.001 | |||||
| < 6 | 108 | 230 | 1.071 | 0.734-1.563 | 0.722 | |
| ≥ 6 | 162 | 254 | 1.240 | 0.909-1.692 | 0.174 | |
| Type of gastrectomy | 0.004 | |||||
| Proximal | 13 | 25 | 1.175 | 0.680-2.032 | 0.564 | |
| Distal | 65 | 142 | 0.915 | 0.560-1.494 | 0.722 | |
| Total | 192 | 317 | 1.125 | 0.976-1.297 | 0.105 |
After further interaction subgroup analyses, patients with ≤ 6 positive lymph nodes [hazard ratio (HR): 1.312, 95% confidence interval (CI): 0.867-1.988], with intestinal type (HR: 1.196, 95%CI: 0.873-1.640), receiving proximal gastrectomy (HR: 1.175, 95%CI: 0.680-2.032), with ≥ 6 cm maximum diameter of tumor (HR: 1.240, 95%CI: 0.909-1.692) showing a higher risk of total mortality in the < 9 cycles group compared with the ≥ 9 cycles group (Table 6).
| Variables | Death or recurrence | Total | HR | 95%CI | P value | P for interaction |
| Sex | 0.418 | |||||
| Male | 227 | 360 | 0.925 | 0.712-1.200 | 0.555 | |
| Female | 88 | 124 | 0.555 | 0.363-0.846 | 0.006 | |
| Depth of tumor invasion | 0.266 | |||||
| T3 | 65 | 123 | 0.719 | 0.438-1.181 | 0.193 | |
| T4 | 250 | 361 | 0.813 | 0.649-1.066 | 0.145 | |
| Number of positive lymph nodes | 0.170 | |||||
| ≤ 6 | 108 | 197 | 0.933 | 0.640-1.361 | 0.719 | |
| ≥ 7 | 207 | 287 | 0.753 | 0.572-0.990 | 0.042 | |
| Vascular invasion | 0.382 | |||||
| Negative | 72 | 119 | 0.824 | 0.518-1.311 | 0.414 | |
| Positive | 243 | 365 | 0.829 | 0.644-1.068 | 0.147 | |
| Neural invasion | 0.469 | |||||
| Negative | 92 | 159 | 0.961 | 0.638-1.449 | 0.851 | |
| Positive | 223 | 325 | 0.773 | 0.593-1.007 | 0.056 | |
| Lauren classification | 0.083 | |||||
| Intestinal | 47 | 88 | 0.886 | 0.498-1.576 | 0.632 | |
| Diffuse | 193 | 254 | 0.923 | 0.695-1.227 | 0.042 | |
| Mixed | 75 | 142 | 0.576 | 0.365-0.910 | 0.406 | |
| Maximum diameter of tumor in cm | 0.236 | |||||
| < 6 | 132 | 230 | 0.765 | 0.543-1.078 | 0.126 | |
| ≥ 6 | 183 | 254 | 0.850 | 0.636-1.136 | 0.271 | |
| Type of gastrectomy | 0.605 | |||||
| Proximal | 15 | 25 | 0.781 | 0.282-2.162 | 0.635 | |
| Distal | 82 | 142 | 0.761 | 0.490-1.181 | 0.223 | |
| Total | 218 | 317 | 0.830 | 0.636-1.083 | 0.169 |
According to relevant data, the literature shows that the number of chemotherapy cycles received by patients is associated with the prognosis. A study conducted in China showed that patients with triple-negative breast cancer who received at least four chemotherapy cycles had a significantly better survival rate[8]. Another study in China focused on the link between the number of chemotherapy cycles and the survival rate of patients with bone-only metastasis[9]. Survival factors and prognostic factors of nasopharyngeal carcinoma patients were explored and analyzed, and the conclusion was drawn that the influencing factors of OS included the number of chemotherapy cycles and the number of metastatic sites. An investigation in Australia showed that the survival rate and pathological response rates of patients with muscle invasive bladder cancer were better in patients receiving 4 cycles of neoadjuvant chemotherapy compared to patients receiving 3 cycles of neoadjuvant chemotherapy[10]. A study in China observed that the optimal number of adjuvant chemotherapy cycles for colon cancer patients is often less than 5[11].
While some studies have shown that more cycles of chemotherapy lead to a better prognosis, other studies have demonstrated no effect or a worsened effect. For example, patients with ovarian cancer receiving ≥ 5 chemotherapy cycles had a poorer prognosis than patients receiving 3-4 cycles[12]. Another study found that chemotherapy does not reduce survival in patients with inoperable stage III NSCLC. However, increased cycles (3 or more) led to more grade 3 toxicities[13]. In addition, a different study conducted on patients with ovarian cancer demonstrated that additional cycles did not affect the recurrence or complete pathologic response[14]. The 5-year survival rate of locally advanced rectal cancer treated with chemotherapy was higher than that of untreated patients[15]. Finally, patients with colorectal cancer who received adjuvant chemotherapy had a better 3-year survival rate than those who received shorter courses of chemotherapy[16].
Although it seems that increased chemotherapy cycles tend to achieve an oncologic benefit, the data is lacking for gastric cancer. Through a series of studies and analyses, the minimum number of cycles should be completed in gastric cancer patients to reduce the rate of tumor growth. During this process, the researchers found that patients who completed less than four cycles did not have a higher survival rate[17]. By analyzing the contents of previous studies, we can see that there is a certain correlation between gastric cancer recurrence and chemotherapy cycle. It was proved that > 9 cycles of che
In the current study, the data demonstrated that ≥ 9 chemotherapy cycles did not confer any oncological benefit compared to < 9 chemotherapy cycles, indicating that ≥ 9 cycles may be considered overtreatment in stage II gastric cancer patients. Excessive chemotherapy may cause unpleasant side effects and impact the immune system, hepatic function, renal function, etc. However, ≥ 9 chemotherapy cycles did significantly reduce the probability of overall recurrence, local-regional metastasis, and distant metastasis rates in stage III gastric cancer patients but did not affect OS or PFS. Excessive chemotherapy cycles may have a psychological effect for patients (i.e. a patient may have less anxiety while being treated despite any side effects).
At present, there are some urgent problems in the research process. First of all, this study mainly conducted retrospective analysis and focused on a single factor. Although PSM was used to reduce the bias, it was still not accurate enough. The purpose of using PSM is to conduct a simulated randomized experiment. Secondly, the chemotherapy regimen is not standardized and complete; therefore, the effects of different chemotherapy regimens were not analyzed. Nonetheless, the interaction effect between chemotherapy cycles and Lauren classification, types of gastrectomy, and maximum diameter of the tumor on OS were determined for the first time.
Overall, patients with stage II and III gastric cancer with chemotherapy cycles ≥ 9 have no significant effect on the prognosis of gastric cancer, so ≥ 9 cycles of chemotherapy are not adopted. However, in essence, ≥ 9 cycles of chemotherapy has a certain benefit in reducing the recurrence rate of stage III gastric cancer patients. Due to the lack of relevant data on gastric cancer and chemotherapy cycles at the present stage, it is necessary to complete the chemotherapy regimen in a more standardized way, so as to deepen the research and finally clarify the correlation between the prognosis of gastric cancer and chemotherapy cycles.
Several studies have shown an oncological benefit with increased cycles of chemotherapy in different cancer types. However, some studies have shown no effect or a worsened effect.
According to a series of exploration and analysis, it is found that there is no abundant data to prove the correlation between the prognosis of gastric cancer and the duration of chemotherapy.
The main purpose of this study is to analyze and explore whether there is a correlation between survival rate and chemotherapy cycle in patients with stage II gastric cancer and stage III gastric cancer.
A 1:1 ratio was used in the propensity score matching analysis to reduce the differences between groups with different chemotherapy cycles. Progression-free survival, overall survival and recurrence were components of outcome indicators.
There was no statistically significant difference in progression-free survival and overall survival between the two groups of stage II and III patients. However, overall recurrence (P < 0.001), local-regional metastasis (P = 0.002), and distant metastasis (P = 0.001) in the ≥ 9 chemotherapy cycles group were significantly lower than those in the < 9 chemotherapy cycles group for stage III gastric cancer patients.
For stage II and III gastric cancer patients, ≥ 9 cycles of chemotherapy should not be considered as far as possible, because ≥ 9 cycles of chemotherapy cannot effectively reduce the recurrence rate.
After a series of studies, it is found that the relationship between the prognosis of gastric cancer and the chemotherapy cycle needs to be further explored to make a more abundant and standardized chemotherapy regimen.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Casella C, Italy; Endo S, Japan; Senchukova M, Russia S-Editor: Liu GL L-Editor: Filipodia P-Editor: Liu GL
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64442] [Article Influence: 16110.5] [Reference Citation Analysis (176)] |
| 2. | Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1140] [Cited by in RCA: 1306] [Article Influence: 87.1] [Reference Citation Analysis (1)] |
| 3. | Tsuburaya A, Guan J, Yoshida K, Kobayashi M, Yoshino S, Tanabe K, Yoshikawa T, Oshima T, Miyashita Y, Sakamoto J, Tanaka S. Clinical biomarkers in adjuvant chemotherapy for gastric cancer after D2 dissection by a pooled analysis of individual patient data from large randomized controlled trials. Gastric Cancer. 2021;24:1184-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Shitara K, Chin K, Yoshikawa T, Katai H, Terashima M, Ito S, Hirao M, Yoshida K, Oki E, Sasako M, Emi Y, Tsujinaka T. Phase II study of adjuvant chemotherapy of S-1 plus oxaliplatin for patients with stage III gastric cancer after D2 gastrectomy. Gastric Cancer. 2017;20:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 5. | Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, Zhang J, Sun LY, Lin RB, Qiu H, Wang C, Qiu MZ, Cai MY, Wu Q, Liu H, Guan WL, Zhou AP, Zhang YJ, Liu TS, Bi F, Yuan XL, Rao SX, Xin Y, Sheng WQ, Xu HM, Li GX, Ji JF, Zhou ZW, Liang H, Zhang YQ, Jin J, Shen L, Li J, Xu RH. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). 2021;41:747-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 461] [Article Influence: 115.3] [Reference Citation Analysis (1)] |
| 6. | Li Y, Zhang X. Prognostic nomograms for gastric carcinoma after surgery to assist decision-making for postoperative treatment with chemotherapy cycles <9 or chemotherapy cycles ≥9. Front Surg. 2022;9:916483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Westerhoff M, Osecky M, Langer R. Varying practices in tumor regression grading of gastrointestinal carcinomas after neoadjuvant therapy: results of an international survey. Mod Pathol. 2020;33:676-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the Common Terminology Criteria for Adverse Events (CTCAE - Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr (Engl Ed). 2021;112:90-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 403] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 9. | Yao L, Pang Z, Wang M, Sun X, Cui M, Zheng Y, Li X, Dong H, Zhang Q, Xu Y. The choice of a neoadjuvant chemotherapy cycle for breast cancer has significance in clinical practice: results from a population-based, real world study. Cancer Biol Med. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Nong S, Pan X, Chen K, Li Y, Zhu X. Therapeutic Effect of Chemotherapy Cycle in Nasopharyngeal Carcinoma (NPC) Patients Who Developed Bone-Only Metastasis. Med Sci Monit. 2020;26:e922244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | D'Andrea D, Black PC, Zargar H, Dinney CP, Soria F, Cookson MS, Montgomery JS, Kassouf W, Dall'Era MA, Sridhar SS, McGrath JS, Wright JL, Thorpe AC, Holzbeierlein JM, Carrión DM, Di Trapani E, Bivalacqua TJ, North S, Barocas DA, Lotan Y, Grivas P, Stephenson AJ, van Rhijn BW, Daneshmand S, Spiess PE, Shariat SF; Contributors. Identifying the Optimal Number of Neoadjuvant Chemotherapy Cycles in Patients with Muscle Invasive Bladder Cancer. J Urol. 2022;207:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Chen Q, Li X, Zhao J, Bi X, Li Z, Huang Z, Zhang Y, Zhou J, Zhao H, Cai J. What is the optimal number of neoadjuvant chemotherapy cycles for resectable colorectal liver oligometastases? Ann Transl Med. 2021;9:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Liu YL, Zhou QC, Iasonos A, Chi DS, Zivanovic O, Sonoda Y, Gardner G, Broach V, O'Cearbhaill R, Konner JA, Grisham R, Aghajanian CA, Abu-Rustum NR, Tew W, Long Roche K. Pre-operative neoadjuvant chemotherapy cycles and survival in newly diagnosed ovarian cancer: what is the optimal number? Int J Gynecol Cancer. 2020;30:1915-1921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Chen L, Hou Y, Xia Y, Chang L, Diao X, Wang L, Li L, Long Q, Liu Y, Li W. Radiotherapy Dose and Induction Chemotherapy Cycles Are Associated With Prognosis and Toxicity Risk: A Retrospective Study of 227 Patients With Unresectable Stage III Non-Small-Cell Lung Cancer. Technol Cancer Res Treat. 2020;19:1533033820951802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Kuo YH, Lai CH, Huang CY, Chen CJ, Huang YC, Huang WS, Chin CC. Monthly tegafur-uracil maintenance for increasing relapse-free survival in ypStage III rectal cancer patients after preoperative radiotherapy, radical resection, and 12 postoperative chemotherapy cycles: a retrospective study. BMC Cancer. 2019;19:815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Sgouros J, Aravantinos G, Kouvatseas G, Rapti A, Stamoulis G, Bisvikis A, Res H, Samantas E. Impact of Dose Reductions, Delays Between Chemotherapy Cycles, and/or Shorter Courses of Adjuvant Chemotherapy in Stage II and III Colorectal Cancer Patients: a Single-Center Retrospective Study. J Gastrointest Cancer. 2015;46:343-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Jeong SH, Yoo MW, Son YG, Oh SJ, Kim JH, Kim HI, Park JM, Hur H, Jee YS, Hwang SH, Jin SH, Lee SE, Lee YJ, Seo KW, Park S, Lee CM, Kim CH, Jeong IH, Lee HH, Choi SI, Lee SI, Kim CY, Chae H, Son MW, Pak KH, Kim S, Lee MS, Min JS. Appropriate Number of Adjuvant Chemotherapy Cycles for Patients with Stage 2 or 3 Gastric Cancer After Curative Gastrectomy: A Multicenter Cohort Study. Ann Surg Oncol. 2021;28:4458-4470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Li Y, Zhao H. Postoperative recurrence of gastric cancer depends on whether the chemotherapy cycle was more than 9 cycles: Based on a retrospective and observational study of follow-up within 3 years of 843 patients. Medicine (Baltimore). 2022;101:e28620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |