Published online Sep 27, 2022. doi: 10.4240/wjgs.v14.i9.976
Peer-review started: June 6, 2022
First decision: July 12, 2022
Revised: July 22, 2022
Accepted: August 25, 2022
Article in press: August 25, 2022
Published online: September 27, 2022
Processing time: 108 Days and 9.1 Hours
In orthotopic liver transplantation (OLT) recipients, median arcuate ligament syndrome (MALS) is considered a risk factor for hepatic arterial thrombosis (HAT), which is dreadful for OLT recipients. Different alternative surgical procedures have been proposed to overcome the impact of MALS on trans
To evaluate the feasible surgical management of MALS to reduce complications in OLT patients.
Data for 288 consecutive patients who underwent OLT at The First Hospital of Jilin University between January 2017 and July 2020 were retrospectively revi
Eight patients with MALS were included in this study. The first patient with MALS received no intervention during the primary surgery and developed postoperative HAT. Salvage liver transplantation with MAL division was successfully performed. Gastroduodenal artery (GDA) preservation with splenic artery ligation was performed on three patients, only GDA preservation was performed on two patients, and no intervention was performed on two patients. No patient developed HAT after surgery and postoperative recovery was satisfactory.
The preservation of collateral circulation between the superior mesenteric artery and celiac trunk via the GDA with or without splenic artery ligation is a safe and feasible alternative to MAL division.
Core Tip: This retrospective single-center study analyzed diagnosis, surgical procedure and outcome of 8 patients with median arcuate ligament syndrome (MALS). In eight patients with MALS, orthotopic liver transplantation without median arcuate ligament (MAL) division and celiac trunk-aorta bypass ensured adequate hepatic arterial blood flow. No new onset hepatic arterial thrombosis was observed. The study suggests that without intraoperative MAL release, one cannot ensure adequate hepatic artery flow and prevent hepatic arterial thrombosis.
- Citation: Li SX, Fan YH, Tian GY, Lv GY. Feasible management of median arcuate ligament syndrome in orthotopic liver transplantation recipients. World J Gastrointest Surg 2022; 14(9): 976-985
- URL: https://www.wjgnet.com/1948-9366/full/v14/i9/976.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i9.976
Orthotopic liver transplantation (OLT) is the most effective treatment for end-stage liver disease[1]. Although the operative technique for OLT has been standardized, postoperative hepatic arterial thrombosis (HAT) remains a rare but dreadful complication[2-4]. Previous studies have demonstrated that factors associated with HAT include anastomotic stenosis, anastomosis inversion, arterial tor
The data for 288 consecutive patients who underwent OLT at The First Hospital of Jilin University between January 2017 and July 2020 were retrospectively reviewed. All patients received liver grafts from cardiac death donors. Patients without adequate preoperative images as well as those who received simultaneous liver-kidney transplantation and pediatric liver transplantations were excluded. The collected data included preoperative data on celiac truck stenosis and MALS, surgical procedures for MALS as well as postoperative short- and long-term follow-up details. The investigators obtained approval from the Ethics Committee of The First Hospital of Jilin University. All patients provided written informed consent for the procedures.
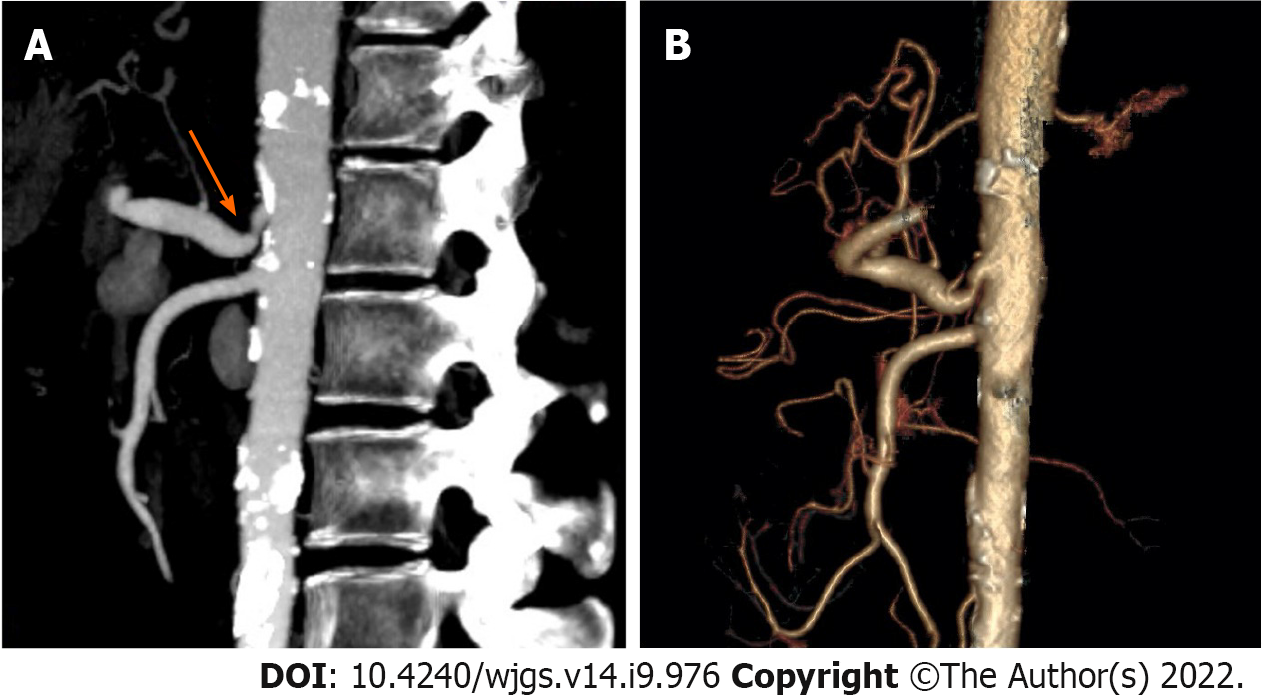
All OLT recipients underwent preoperative computed tomographic angiography (CTA) (Figure 1). End-inspiratory arterial phase, end-expiratory portal venous phase and sagittal arterial reconstruction were examined. Vascular abnormalities were evaluated by a senior staff radiologist and the transplant surgeon to determine the operative approach. According to stenosis rate, length of stenosis and distance from aorta, Sugae et al[14] classified MALS to three types. The rate of type A stenosis should be less than 50%, its length should be less than 3 mm, and its position should be more than 5 mm from the aorta. The rate of type B stenosis should be between 50 and 80 percent, its length should be between 3 and 8 mm, and its position should be greater than 5 mm from the aorta. The rate of type C stenosis should exceed 80%, its length should exceed 8 mm, and its position should be less than 5 mm from the aorta. MALS was defined based on extrinsic compression on the celiac trunk due to MAL, post-stenotic dilatation, and patients diagnosed with MALS should exhibit at least one or more of the following symptoms postprandial pain, weight loss and small meals as described previously[8,15].
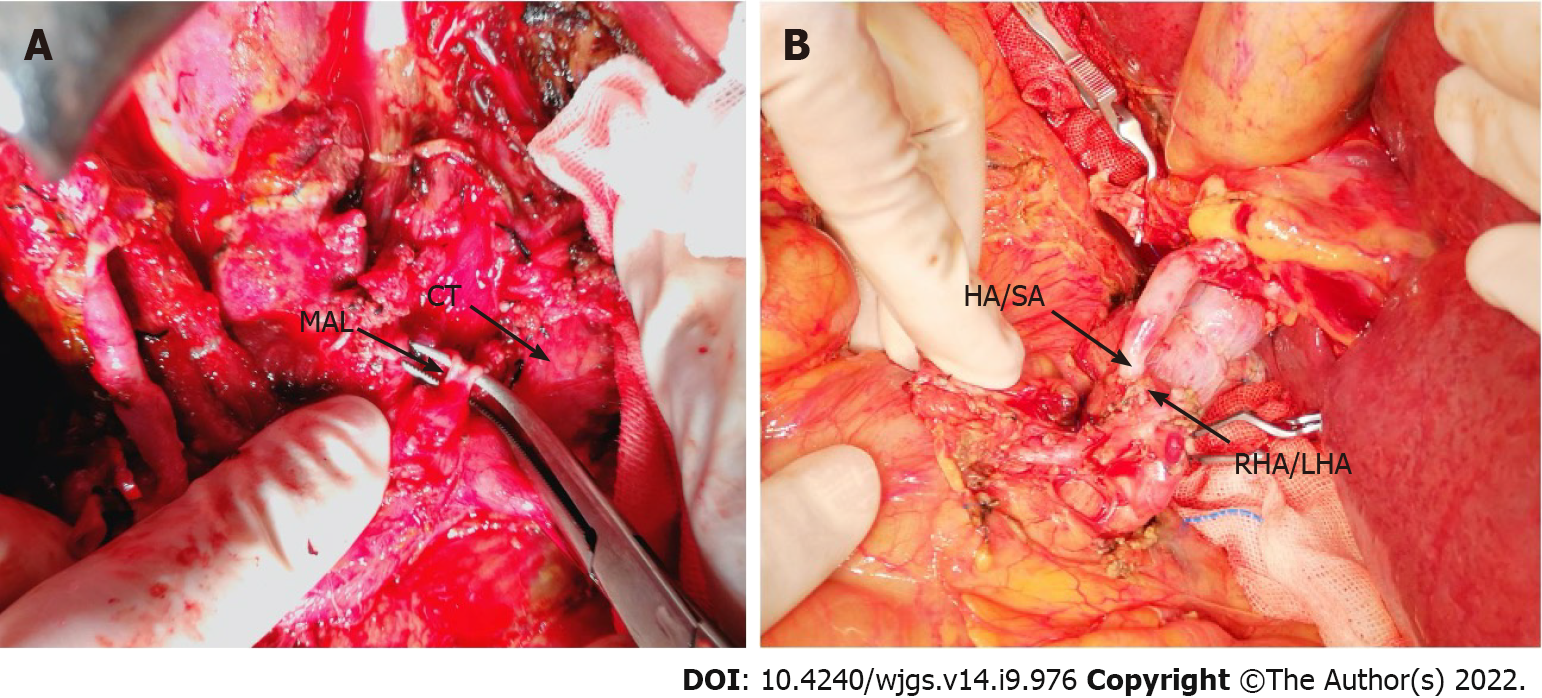
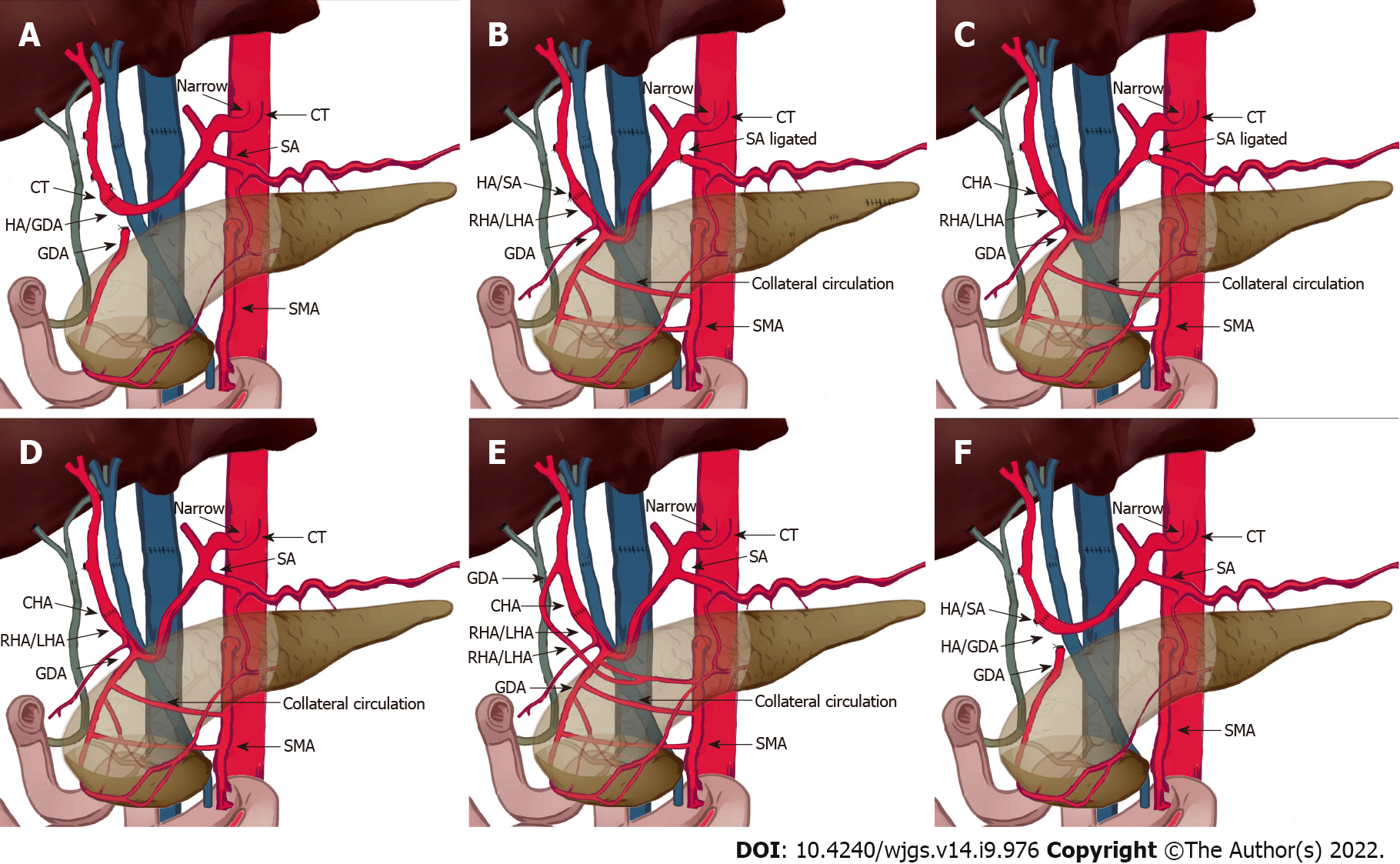
OLT recipients with suspected or confirmed MALS on pre-operative imaging underwent detailed evaluation of the collateral circulation between the superior mesenteric artery and the celiac trunk based on the pre-operative imaging and intraoperative findings. Gastroduodenal arteries (GDAs) with abundant collateral branches were clamped to determine whether the hepatic arterial flow or pulse was reduced. If clamping decreased the hepatic arterial flow, then the GDA and collateral branches were preserved. The hepatic artery/splenic artery patch from the donor and right/left hepatic artery patch from the recipient were used for branch patch anastomosis (Figure 2). If hepatic arterial flow was not affected by GDA clamping, the hepatic artery/GDA patch from the recipient and hepatic artery/splenic artery patch from the donor was used for branch patch anastomosis as a standard arterial revascularization method (Figure 3). After the anastomosis, the intrahepatic arterial blood flow was evaluated using Doppler ultrasound. If the blood flow was not satisfactory (hepatic arterial blood flow rate < 50 cm/s), after assessing the potential for splenic artery steal syndrome, the splenic artery was ligated and the hepatic arterial flow and pulse was tested again. Surgical division of MAL or celiac trunk-aorta bypass was performed when the hepatic arterial flow remained poor despite all the above measures.
Postoperatively, Doppler ultrasound was used periodically: every 12 h during the first week, twice per week until discharged, and once a week for 3 mo to monitor hepatic artery anastomosis. If Doppler ultrasound revealed any abnormal findings, such as HAT as defined by resistive index (RI) < 0.5 and hepatic artery blood flow < 39 cm/s[16] combined with elevated liver enzymes and bilirubin suggestive of hepatocellular injury, CTA was performed immediately to determine the status of hepatic artery anastomosis and initiate the timely salvage of the liver graft if required.
If there were no other signs, the patients received standard prophylaxis of thromboembolism for 6 wk post-OLT and no anticoagulant therapy was used.
Among 288 patients who received OLT, eight were diagnosed with MALS (Figure 1). The mean recipient age was 59 years. There were four men and four women. The warm ischemia time for the liver graft ranged from 12 s to 41 s and the cold ischemia time ranged from 452 min to 632 min. The median follow-up was 20 mo. Other patient characteristics are presented in Table 1. The surgical details for the recipients with MALS are shown in Table 2.
| Characteristics and prognoses | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Age, donor/recipient | 55/52 | 54/53 | 67/66 | 45/48 | 62/63 | 52/62 | 56/38 | 50/63 |
| Sex, donor/recipient | F/F | M/F | M/M | F/M | M/M | M/F | M/M | M/F |
| BMI, donor/recipient | 20/19 | 22/22 | 22/23 | 21/19 | 23/20 | 26/21 | 22/20 | 25/21 |
| Donor cause of death | CVA | CVA | Trauma | CVA | Trauma | CVA | CVA | Trauma |
| The primary disease | PBC | AIH | AIH | Viral | Viral | Viral | Viral | HCC |
| MALS type | B | B | B | C | A | B | A | A |
| Cold ischemic time in min | 608 | 348 | 461 | 582 | 586 | 510 | 550 | 458 |
| Warm ischemic time in s | 19 | 15 | 41 | 29 | 12 | 26 | 15 | 16 |
| Intraoperative blood loss in mL | 1800 | 1500 | 2850 | 3000 | 7000 | 300 | 1000 | 2000 |
| Intra-operative red blood cell transfusions in U | 4 | 20 | 10.5 | 22 | 27 | 9 | 8 | 16.5 |
| Intra-operative fresh frozen plasma transfusions in mL | 1000 | 2350 | 1200 | 950 | 3600 | 960 | 420 | 960 |
| Operation time in min | 485 | 580 | 526 | 538 | 632 | 556 | 560 | 452 |
| Intraoperative hepatic arterial blood flow rate in cm/s | NA | 80 | 90 | 50 | 60 | 65 | 50 | 53 |
| Hepatic arterial blood flow rate on discharge in cm/s | 80 | 85 | 102 | 64 | 65 | 70 | 60 | 68 |
| Hospital stay in d | 17 | 28 | 39 | 18 | 21 | 17 | 17 | 15 |
| No. | Donor arterial patch | Recipient arterial patch | Ligament lysis | GDA preservation | Splenic artery ligation |
| 1 | Celiac truck | Hepatic/gastroduodenal artery patch | Yes | No | No |
| 2 | Hepatic/splenic artery patch | Right/left hepatic artery patch | No | Yes | Yes |
| 3 | Common hepatic artery | Right/left hepatic artery patch | No | Yes | Yes |
| 4 | Hepatic/splenic artery patch | Right/left hepatic artery patch | No | Yes | Yes |
| 5 | Common Hepatic artery | Right/left hepatic artery patch | No | Yes | No |
| 6 | (1) Gastroduodenal artery; (2) common hepatic artery | (1) Right hepatic artery from the superior mesenteric artery; (2) proper hepatic artery | No | Yes | No |
| 7 | Hepatic/splenic artery patch | Hepatic/gastroduodenal artery patch | No | No | No |
| 8 | Hepatic/splenic artery patch | Hepatic/gastroduodenal artery patch | No | No | No |
For the first patient, due to a lack of knowledge about MALS, no intervention for celiac trunk stenosis caused by MAL was performed during the first operation and standard revascularization was performed. On the ninth postoperative day, the total and direct bilirubin reached 210 mmol/L and 130 mmol/L, respectively. Markers of hepatocellular injury increased (alanine aminotransferase 337.5 U/L, aspartate aminotransferase 88.9 U/L). The hepatic flow rate decreased to 10 cm/s and the resistive index dropped to 0.4, suggestive of HAT. On exploratory laparotomy, there was extensive thrombosis in the hepatic artery around the anastomosis. Thrombectomy was performed and hepatic arterial blood flow was restored after re-anastomosis. However, there was no intrahepatic blood flow on Doppler ultrasound, probably due to intrahepatic arterial thrombosis. Thrombolytic therapy with alteplase was given but failed to restore the intrahepatic blood flow. Six hours later, salvage liver transplantation was performed and the MAL was divided (Figure 2A and 3A, Table 1 and 2). Postoperatively, the hepatic blood flow rate increased to 70-87 cm/s.
The remaining six patients had normal preoperative hepatic arterial flow. Four patients had abundant collateral circulation between the superior mesenteric artery and the celiac trunk via GDA (Figure 1B), thus GDA was preserved and the hepatic artery/splenic artery patch from the donor and right/left hepatic artery patch from the recipient were used for branch patch anastomosis (Figure 2B).
In three patients, low hepatic arterial flow rate was detected using Doppler ultrasound during the operation with patent anastomosis. Consequently, splenic artery steal syndrome was evaluated when RI was greater than 0.8 and hepatic artery blood flow was less than 35 cm/s[17]. Hepatic artery blood flow returned to normal after splenic artery ligation, and no HAT occurred after surgery (Figures 3B-D).
Another patient with aberrant right hepatic artery received two anastomoses. The first anastomosis was performed between the recipient right hepatic artery from the superior mesenteric artery and the donor GDA. The second anastomosis was done between the recipient's proper hepatic artery and the donor common hepatic artery (Figure 3E).
Two patients received standard arterial revascularization without preservation of the GDA or splenic artery ligation (Figure 3F).
The seven MALS patients without MAL division had satisfactory hepatic arterial blood flow after the operation. All eight patients had adequate hepatic arterial blood flow at discharge, as presented in Table 1.
The median follow-up was 19 mo (range: 10-29 mo). All the patients are alive. Among these eight patients, seven of them are healthy without complications. One patient developed biliary stricture 2 mo after surgery, which was successfully managed with endoscopic retrograde cholangiography and biliary stenting.
In MALS, the coeliac artery gets compressed by the MAL, leading to reduced blood flow in the hepatic artery[12,13,18-20]. Because the blood flow in the hepatic artery is significantly reduced, it predisposes the patients to HAT after OLT, which leads to graft failure in 50% of cases and re-transplantation[2,21-24]. MALS patients with normal hemodynamics usually have no or little clinical symptoms before OLT. However, in the postoperative phase after OLT, patients may develop severe hemodynamic restrictions in hepatic arterial flow, which increases the risk of HAT[25]. Hence, an appropriate preoperative surgical plan should be developed for OLT patients with MALS. The reported incidence of MALS after liver transplantation varies from 2% to 12%[21,26,27]. The low incidence of MALS in previous reports may be due to insufficient awareness of this disease and limited diagnostic methods. Currently, the extensive application of contrast enhance computed tomographic (CECT) has improved the diagnostic rate of MALS.
Recurrent post-prandial epigastric pain, weight loss, nausea or vomiting and abdominal pain after exercise is common symptoms of MALS. Eight patients in this study had a history of epigastric pain and weight loss, but these symptoms were attributed to chronic hepatitis and decompensated liver cirrhosis. Therefore, the diagnosis of MALS is partly clinical and mainly based on radiology. Celiac axis stenosis caused by MAL appears similar to a hook on CECT during sagittal reconstruction[28]. Abundant collateral branches, post-stenotic dilation and thickening of the MAL can also help in the diagnosis of MALS. Angiography used to be a routine test for detecting aberrant arterial vessels but is now used selectively for suspected cases in arterial dynamic studies[21,28]. Gruber et al[29] found that the combination of a maximum end-expiratory velocity over 350 cm/s in the celiac trunk and a deflection angle higher than 50°, detected using functional ultrasound, was a reliable diagnostic method for MALS. At our center, we routinely perform CTA on OLT patients to detect vascular variations and MALS.
Sugae et al[14] classified MALS into three types according to the stenosis rate, length of stenosis, distance from the aorta and collateral pathways. According to the different types, it has been suggested that type A MALS should not be manipulated, while type B and type C usually require surgery to maintain the blood supply of the hepatic artery.
Cassar et al[24] reported the fourth type in which coeliac artery compression from MAL is at the origin of splenic artery and surgical intervention is required to restore hepatic artery flow during liver transplantation. These suggestions are all based on maintaining the hepatic blood to the liver graft, as it is sensitive to hemodynamic changes. Therefore, whether an intervention should be performed for type A needs to be determined carefully. If MAL-related compression is mild with adequate pre- or intraoperative arterial blood flow, surgical division of MAL is not necessary. However, the perioperative hepatic artery flow is determined by various factors, making it difficult to determine whether the blood flow is adequate[8]. Golse et al[30] used intraoperative contrast-enhanced Doppler ultrasonography to determine the hepatic blood flow in OLT patients. In their reports, MALS patients who required further treatment and six patients with weak arterial flow without intervention underwent MAL division and the incidence of postoperative vascular complications was significantly reduced. In this study, we determined the hepatic blood flow based on the pulse in the hepatic artery and arterial blood flow rate measured using intraoperative Doppler ultrasonography after anastomosis. In MALS patients, postoperative Doppler ultrasound was used routinely to determine hepatic arterial blood flow.
Currently, there is no consensus on the treatment of MALS in patients who undergo liver tran
With the continuous advancements in endovascular interventional therapy, some OLT recipients with MAL have been treated with interventional therapy postoperatively to restore the hepatic blood flow[31,32]. However, the preoperative use of stenting remains controversial, as persistent external compression from the MAL carries a higher risk[21,33].
Recent studies have suggested that regular vascular reconstruction after surgical division of MAL in liver transplant recipients with MALS is safe and effective[13,34]. Czigany et al[21] reported a 7-year retrospective study of 34 MALS patients, in which 26 patients received MAL division and four patients required aorto-hepatic conduit construction. Twenty-six patients who underwent surgical division of MAL or alternative reconstruction had no postoperative complications. Three patients with MALS who did not receive any intervention for MALS developed severe vascular complications and one of them required re-transplantation. In their study, preoperative assessment of vascular aberrations and different surgical approaches were planned before the surgery which led to a relatively low HAT rate.
MAL division is a standard treatment for MALS. However, OLT recipients with MALS usually have gastroesophageal varices and extensive collateral vessels between the celiac trunk and superior mesenteric artery, which increases the risk of bleeding during MAL division. The most common collateral circulation is the superior mesenteric artery-pancreaticoduodenal artery-GDA-hepatic artery network. This collateral circulation helps in maintaining hepatic arterial flow in MALS patients after liver transplantation, even without MAL division. Lubrano et al[27] reported that one out of 10 patients with MALS underwent MAL division while six patients underwent standard hepatic arterial reconstruction without the division of MAL. None of the 10 patients experienced postoperative vascular complications. In this study, one patient with MALS received standard hepatic arterial reconstruction with GDA ligation. The patient developed HAT during the postoperative period and required a salvage liver transplantation with MAL division. The remaining seven MALS patients were diagnosed with MALS before surgery and had adequate hepatic blood flow preoperatively, determined with Doppler ultrasound. Thus MAL was not divided irrespective of the type. Five patients were found to have abundant collateral circulation between the superior mesenteric artery and the celiac trunk before surgery; therefore, the GDA was preserved intraoperatively. The other two patients had no obvious collateral circulation. Consequently, the GDA was clamped and hepatic arterial blood flow was assessed. Since there was adequate hepatic blood flow despite GDA clamping, GDA ligation with standard hepatic arterial anastomosis was performed. All seven patients had good postoperative hepatic blood flow without HAT. Hence, we believe that in OLT recipients with MALS, preservation of the collateral circulation without MAL division is a safe and feasible procedure. The procedure has fewer complications and makes surgery easier. In addition to collateral preservation, the splenic artery can be ligated if necessary. Additionally, we used the left and right hepatic artery bifurcations to enlarge the anastomosis. If the hepatic artery blood flow is still unsatisfactory with the above measures, the division of MAL may be considered. Hepatic artery-abdominal aorta bypass is the most difficult surgical procedure and can be used as a last resort.
This study has certain limitations. First, this study was a single-center retrospective study. Second, the number of patients was limited. Hence, future studies with larger sample sizes are needed to verify the findings of this study.
Preoperative diagnosis of MALS in OLT recipients is important to prevent HAT. Preservation of collateral circulation with or without splenic artery ligation is an easier surgical technique with shorter operation time and a lower risk of intraoperative complications compared to MAL division and celiac trunk-aorta bypass to ensure adequate hepatic arterial blood flow.
In orthotopic liver transplantation (OLT) recipients, median arcuate ligament syndrome (MALS) is regarded as a risk factor for hepatic artery thrombosis (HAT), a devastating complication of OLT. To counteract the influence of MALS on transplantation, a variety of different surgical methods have been proposed, but clinical evidence is still lacking.
To increase the survival rate of MALS patients who receive OLT and decrease postoperative complications.
To evaluate the efficacy of surgical treatment for MALS to reduce complications in OLT patients in order to improve patient survival and decrease the incidence of postoperative complications.
A total of 288 consecutive OLT patients at The First Hospital of Jilin University were retrospectively evaluated. Median arcuate ligament (MAL) surgical treatment and arterial anastomosis modification were recorded. Perioperative and long-term MALS prognoses were noted.
In this investigation, eight patients with MALS were enrolled. The first patient with MALS did not get any intervention during the main operation, and afterward developed HAT. Successful salvage liver transplantation with MAL division was accomplished. Gastroduodenal artery (GDA) preservation with splenic artery ligation was performed on three patients, GDA preservation alone was performed on two patients, and no intervention were performed on two patients. After surgery, no patient got HAT and healing was acceptable.
The preservation of collateral circulation between the superior mesenteric artery and celiac trunk via the GDA, with or without ligation of the splenic artery, provides a safe and practicable alternative to MAL division.
To provide surgeons with effective and feasible surgical options when they need to perform OLT in MALS patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kazmi SSH, Norway; Luyer MDP, Netherlands S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang H
| 1. | Starzl TE, Fung JJ. Themes of liver transplantation. Hepatology. 2010;51:1869-1884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 2. | Oberkofler CE, Reese T, Raptis DA, Kuemmerli C, de Rougemont O, De Oliveira ML, Schlegel A, Dutkowski P, Clavien PA, Petrowsky H. Hepatic artery occlusion in liver transplantation: What counts more, the type of reconstruction or the severity of the recipient's disease? Liver Transpl. 2018;24:790-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Harrison J, Harrison M, Doria C. Hepatic Artery Pseudoaneurysm Following Orthotopic Liver Transplantation: Increasing Clinical Suspicion for a Rare but Lethal Pathology. Ann Transplant. 2017;22:417-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Feltracco P, Barbieri S, Cillo U, Zanus G, Senzolo M, Ori C. Perioperative thrombotic complications in liver transplantation. World J Gastroenterol. 2015;21:8004-8013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 65] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 5. | Giorgakis E, Tedeschi M, Bonaccorsi-Riani E, Khorsandi SE, Menon K, Vilca-Melendez H, Jassem W, Srinivasan P, Prachalias A, Heaton N. The Effect of Recipient Body Mass Index and Its Extremes on Survival and Graft Vascular and Biliary Complications After Liver Transplantation: A Single Center Retrospective Study. Ann Transplant. 2017;22:611-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Puliti Reigada CH, de Ataide EC, de Almeida Prado Mattosinho T, Boin IFSF. Hepatic Artery Thrombosis After Liver Transplantation: Five-Year Experience at the State University of Campinas. Transplant Proc. 2017;49:867-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Gilbo N, Van Praet L, Jochmans I, Sainz-Barriga M, Verslype C, Maleux G, Laleman W, van der Merwe S, Cassiman D, Nevens F, Monbaliu D, Pirenne J. Pre-operative trans-catheter arterial chemo-embolization increases hepatic artery thrombosis after liver transplantation - a retrospective study. Transpl Int. 2018;31:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Lainas P, Fuks D, Gaujoux S, Machroub Z, Fregeville A, Perniceni T, Mal F, Dousset B, Gayet B. Preoperative imaging and prediction of oesophageal conduit necrosis after oesophagectomy for cancer. Br J Surg. 2017;104:1346-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Lipshutz B. A composite study of the coeliac axis artery. Ann Surg. 1917;65:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 173] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 10. | Harjola PT. A rare obstruction of the coeliac artery. Report of a case. Ann Chir Gynaecol Fenn. 1963;52:547-550. [PubMed] |
| 11. | Dunbar JD, Molnar W, Beman FF, Marable SA. Compression of the celiac trunk and abdominal angina. Am J Roentgenol Radium Ther Nucl Med. 1965;95:731-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 263] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Jiang ZJ, Liang TB, Feng XN, Wang WL, Shen Y, Zhang M, Wu J, Xu X, Zheng SS. Arcuate ligament syndrome inducing hepatic artery thrombosis after liver transplantation. Hepatobiliary Pancreat Dis Int. 2008;7:433-436. [PubMed] |
| 13. | Vilatobá M, Zamora-Valdés D, Guerrero-Hernández M, Romero-Talamás H, Leal-Villalpando RP, Mercado MA. Arcuate ligament compression as a cause of early-onset thrombosis of the hepatic artery after liver transplantation. Ann Hepatol. 2011;10:88-92. [PubMed] |
| 14. | Sugae T, Fujii T, Kodera Y, Kanzaki A, Yamamura K, Yamada S, Sugimoto H, Nomoto S, Takeda S, Nakao A. Classification of the celiac axis stenosis owing to median arcuate ligament compression, based on severity of the stenosis with subsequent proposals for management during pancreatoduodenectomy. Surgery. 2012;151:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Horton KM, Talamini MA, Fishman EK. Median arcuate ligament syndrome: evaluation with CT angiography. Radiographics. 2005;25:1177-1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 264] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 16. | Choi EK, Lu DS, Park SH, Hong JC, Raman SS, Ragavendra N. Doppler US for suspicion of hepatic arterial ischemia in orthotopically transplanted livers: role of central versus intrahepatic waveform analysis. Radiology. 2013;267:276-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Pinto S, Reddy SN, Horrow MM, Ortiz J. Splenic Artery Syndrome after orthotopic liver transplantation: a review. Int J Surg. 2014;12:1228-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Agnes S, Avolio AW, Magalini SC, Frongillo F, Castagneto M. Celiac axis compression syndrome in liver transplantation. Transplant Proc. 2001;33:1438-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Sun X, Fan Z, Qiu W, Chen Y, Jiang C, Lv G. Median arcuate ligament syndrome and arterial anastomotic bleeding inducing hepatic artery thrombosis after liver transplantation: A case report. Medicine (Baltimore). 2018;97:e10947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Kazmi SSH, Safi N, Berge ST, Kazmi M, Sundhagen JO, Hisdal J. Laparoscopic Surgery for Median Arcuate Ligament Syndrome (MALS): A Prospective Cohort of 52 Patients. Vasc Health Risk Manag. 2022;18:139-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Czigany Z, Boecker J, Morales Santana DA, Bednarsch J, Meister FA, Amygdalos I, Isfort P, Liebl M, Neumann UP, Lurje G. Median Arcuate Ligament Compression in Orthotopic Liver Transplantation: Results from a Single-Center Analysis and a European Survey Study. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Fujiki M, Hashimoto K, Palaios E, Quintini C, Aucejo FN, Uso TD, Eghtesad B, Miller CM. Probability, management, and long-term outcomes of biliary complications after hepatic artery thrombosis in liver transplant recipients. Surgery. 2017;162:1101-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Silva MA, Jambulingam PS, Gunson BK, Mayer D, Buckels JA, Mirza DF, Bramhall SR. Hepatic artery thrombosis following orthotopic liver transplantation: a 10-year experience from a single centre in the United Kingdom. Liver Transpl. 2006;12:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 192] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 24. | Cassar N, Gregory S, Menon K. New variation of median arcuate ligament compression causing hepatic arterial hypoperfusion during liver transplantation. Hepatobiliary Pancreat Dis Int. 2020;19:394-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Warner P, Fusai G, Glantzounis GK, Sabin CA, Rolando N, Patch D, Sharma D, Davidson BR, Rolles K, Burroughs AK. Risk factors associated with early hepatic artery thrombosis after orthotopic liver transplantation - univariable and multivariable analysis. Transpl Int. 2011;24:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Jurim O, Shaked A, Kiai K, Millis JM, Colquhoun SD, Busuttil RW. Celiac compression syndrome and liver transplantation. Ann Surg. 1993;218:10-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Lubrano J, Scatton O, Randone B, Molinier N, Massault PP, Legmann P, Soubrane O, Dousset B. Median arcuate ligament in orthotopic liver transplantation: relevance to arterial reconstruction. Transplant Proc. 2008;40:3532-3535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Vandermeulen M, Moïse M, Meurisse N, Honoré P, Meurisse M, Meunier P, Detry O. Image in transplantation surgery: median arcuate ligament in liver transplantation. Acta Chir Belg. 2020;120:217-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Gruber H, Loizides A, Peer S, Gruber I. Ultrasound of the median arcuate ligament syndrome: a new approach to diagnosis. Med Ultrason. 2012;14:5-9. [PubMed] |
| 30. | Golse N, Santoni S, Karam V, Ciacio O, Pittau G, Allard MA, Cherqui D, Sa Cunha A, Adam R, Castaing D, Vibert E. Is Routine Intraoperative Contrast-Enhanced Ultrasonography Useful During Whole Liver Transplantation? World J Surg. 2018;42:1523-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Yilmaz S, Ceken K, Gürkan A, Erdoğan O, Demirbaş A, Kabaalioğlu A, Sindel T, Lüleci E. Endovascular treatment of a recipient celiac trunk stenosis after orthotopic liver transplantation. J Endovasc Ther. 2003;10:376-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Sharafuddin MJ, Olson CH, Sun S, Kresowik TF, Corson JD. Endovascular treatment of celiac and mesenteric arteries stenoses: applications and results. J Vasc Surg. 2003;38:692-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Björck M, Koelemay M, Acosta S, Bastos Goncalves F, Kölbel T, Kolkman JJ, Lees T, Lefevre JH, Menyhei G, Oderich G; Esvs Guidelines Committee; Kolh P, de Borst GJ, Chakfe N, Debus S, Hinchliffe R, Kakkos S, Koncar I, Sanddal Lindholt J, Vega de Ceniga M, Vermassen F, Verzini F, Document Reviewers, Geelkerken B, Gloviczki P, Huber T, Naylor R. Editor's Choice - Management of the Diseases of Mesenteric Arteries and Veins: Clinical Practice Guidelines of the European Society of Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2017;53:460-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 431] [Article Influence: 61.6] [Reference Citation Analysis (1)] |
| 34. | Ali M, Patel J. Dunbar syndrome following liver transplantation. BMJ Case Rep. 2016;2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |