Published online Sep 27, 2022. doi: 10.4240/wjgs.v14.i9.930
Peer-review started: April 13, 2022
First decision: May 9, 2022
Revised: May 24, 2022
Accepted: August 24, 2022
Article in press: August 24, 2022
Published online: September 27, 2022
Processing time: 161 Days and 23.3 Hours
Splenectomy has previously been found to increase the risk of cancer deve
To compare hepatocellular carcinoma (HCC) recurrence and de novo malignancy between patients undergoing LT with and without simultaneous splenectomy.
We retrospectively analyzed the outcomes of 120 patients with HCC within the University of California San Francisco criteria who received LT with (n = 35) and without (n = 85) simultaneous splenectomy in the Tri-Service General Hospital. Univariate and multivariate Cox regression analyses for cancer-free survival and mortality were established. The comparison of the group survival status and group cancer-free status was done by generating Kaplan–Meier survival curves and log-rank tests.
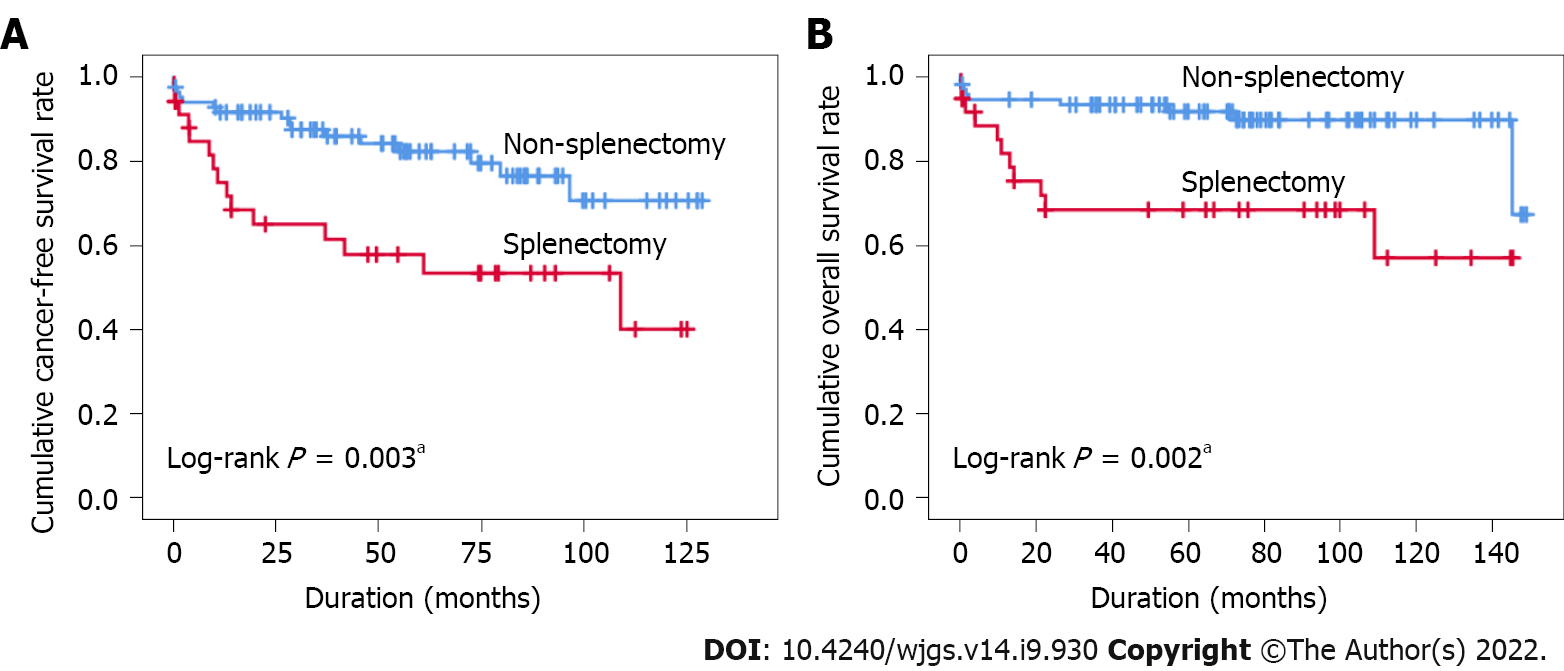
The splenectomy group had more hepatitis C virus infection, lower platelet count, higher -fetoprotein level, and longer operating time. Splenectomy and age were both positive independent factors for prediction of cancer development [hazard ratio (HR): 2.560 and 1.057, respectively, P < 0.05]. Splenectomy and hypertension were positive independent factors for prediction of mortality. (HR: 2.791 and 2.813 respectively, P < 0.05). The splenectomy group had a significantly worse cancer-free survival (CFS) and overall survival (OS) curve compared to the non-splenectomy group (5-year CFS rates: 53.4% vs 76.5%, P = 0.003; 5-year OS rate: 68.1 vs 89.3, P = 0.002).
Our study suggests that simultaneous splenectomy should be avoided as much as possible in HCC patients who have undergone LT.
Core tip: This retrospective study compared the outcomes of hepatocellular carcinoma (HCC) recurrence and de novo malignancy development between HCC patients who underwent liver transplantation (LT) with and without simultaneous splenectomy. Splenectomy leads to a significantly higher risk of cancer development after LT and is a significant risk factor of mortality. Simultaneous splenectomy should be avoided as much as possible.
- Citation: Fan HL, Hsieh CB, Kuo SM, Chen TW. Liver transplantation with simultaneous splenectomy increases risk of cancer development and mortality in hepatocellular carcinoma patients. World J Gastrointest Surg 2022; 14(9): 930-939
- URL: https://www.wjgnet.com/1948-9366/full/v14/i9/930.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i9.930
Hepatocellular carcinoma (HCC) is the fifth most common malignancy in men and the ninth most common in women worldwide[1]. Liver transplantation (LT) is one of the potential curative therapies, according to the Barcelona Clinic Liver Cancer staging classification and treatment schedule[2]. The incidence of recurrent HCC after LT was found to be 7%–25%[3]. Various pre-, intra- and postoperative factors influence the outcomes and disease-free survival (DFS) in patients with HCC after LT[4,5].
The indications for splenectomy are generally divided into traumatic and nontraumatic reasons[6]. Two early studies found an increased risk of cancer after splenectomy, especially in patients with nontraumatic splenectomy[6,7]. The most common post-splenectomy malignancies include lung, nonmelanoma skin cancer, leukemia, lymphoma, Hodgkin’s lymphoma, and ovarian cancer[6,7]. A nationwide population-based cohort study published in 2015 revealed that patients undergoing splenectomy were 1.94 times more likely to develop cancer than patients not undergoing splenectomy[8].
There are a number of indications for simultaneous splenectomy in LT recipients, including the prevention of small-for-size syndrome, ABO-incompatible LT (ABO-iLT), or the prevention of thrombocytopenia during therapy for hepatitis C virus (HCV) after LT[9-12]. The purpose of this study was to compare the outcomes of HCC recurrence and de novo malignancy development between HCC patients who underwent LT with and without simultaneous splenectomy.
Between May 2009 and August 2019, 179 patients with HCC underwent LT and received follow-up management. Among them, 53 patients received simultaneous splenectomy during the LT operation. All patients with HCC met the University of California San Francisco (UCSF) criteria for radiological examinations (a single tumor of ≤ 6.5 cm; a maximum of three tumors with none of them > 4.5 cm; and a cumulative size ≤ 8 cm). The records of these patients were retrospectively reviewed. Fifty-nine patients who had no residual HCCs or who had HCCs without fitting the UCSF criteria on pathological examinations were excluded. Thirty-five of the 120 LT recipients (29.2%) underwent simultaneous splenectomy and were assigned to the splenectomy group. The remaining LT recipients (85/120, 70.8%) did not undergo simultaneous splenectomy and were, thus, assigned to the nonsplenectomy group. The indications for simultaneous splenectomy in our institution include modulation of portal inflow, thrombocytopenia in recipients with HCV, or ABO-iLT recipients. The reasons for simultaneous splenectomy in the 53 recipients were modulation (22/53, 41.5%), thrombocytopenia in recipients with HCV (25/53, 47.2%), and ABO-iLT (6/53, 11.3%). We recorded the recipient characteristics, including age, sex, underlying liver disease, signs of portal hypertension (ascites, hepatic encephalopathy, bleeding varices), preoperative serum biochemistry results (levels of total bilirubin, creatinine, ammonia, albumin, and glucose), international normalized ratio, blood platelet count, Model for End-stage Liver Disease score (MELD score), α-fetoprotein (AFP), operative factors [surgery types in deceased donor LT including split liver, living donor LT, graft weight, graft-to-recipient weight ratio (GRWR), blood loss, and operating time], and pathological results (tumor size, tumor number, tumor necrosis, and lymphovascular invasion). Neutrophil–lymphocyte ratio was calculated by dividing neutrophil count by lymphocyte count. Platelet–lymphocyte ratio was calculated by dividing platelet count by lymphocyte count.
Postsurgical follow-up evaluations included monitoring of AFP levels and performing abdominal sonography, computed tomography (CT), or magnetic resonance imaging every 3 mo and chest radiography yearly. Brain CT was performed in patients with worsening headaches or neurological symptoms, and whole-body bone scans were performed in patients with severe bone pain. Positron emission tomography was performed if the AFP levels were elevated, even if the other above-mentioned examinations showed normal findings. Annual chest radiography and stool examination for occult blood were performed to screen for de novo lung cancer and gastrointestinal tract malignancy, respectively. Chest CT or lung biopsy was performed if lung nodules were found by chest radiography. Esophagogastroduodenoscopy and colonoscopy were performed if occult blood was detected in the stool. In female participants, annual breast sonography was performed to monitor for de novo breast cancer. The time and site of tumor recurrence and patient death were established through follow-up studies. The present study was approved by the institutional review board of Tri-Service General Hospital (IRB No. 2-108-05-127), and informed consent was not required according to the guidance of the Institutional Review Board because this was a retrospective study.
Continuous variables were represented as a median with the corresponding range and comparisons between subgroups were performed using the Mann–Whitney U test. Categorical variables were expressed as the number (percent) and assessed by Fisher’s exact test following Bonferroni correction for comparisons between subgroups. To determine the variables associated with recurrence or death, univariate and multivariate Cox proportional hazard models were established. All factors with P < 0.1 in the univariate analysis were entered into a reverse multivariate hazard model. The duration of cancer-free survival (CFS) was calculated from the date of surgery to the date of HCC recurrence, HCC distant metastases, secondary malignancy, or the date of death for patients who died before the end of follow-up. The overall survival (OS) duration was defined as the period between the date of surgery and the date of death. Kaplan–Meier survival curves were generated, and a log-rank test was performed to compare the group survival status. All two-sided statistical analyses were performed using SPSS version 15.0 (SPSS, Chicago, IL, United States). Significance was defined as P < 0.05.
A total of 120 HCC patients (89 men and 31 women) with a median age of 57 (37–69) years were included in the analyses. Eighty-five patients did not undergo simultaneous splenectomy, whereas 35 (29.2%) patients did. The average follow-up duration was 55 mo (range 0–128 mo). Patients’ characteristics are summarized in Table 1. Age, gender, body mass index, signs of portal hypertension (ascites, hepatic encephalopathy, and varices bleeding), comorbidities (hypertension and diabetes mellitus), preoperative serum tests (white blood count, total bilirubin, creatinine, ammonia, albumin, glucose, INR, and MELD scores), surgical factors (surgical type, graft type, GRWR, and bleeding), and pathology (tumor size, tumor number, tumor necrosis, and lymphovascular invasion) were not significantly different between these two groups (all P > 0.05), indicating that the groups has a similar baseline. Nevertheless, patients who underwent simultaneous splenectomy had a lower hepatitis B virus (HBV) infection rate (40% vs 77.6%, P < 0.001), higher HCV infection rate (65.7% vs 25.9%, P < 0.001), lower platelet count (P < 0.003), higher AFP level (P = 0.012), and longer operating time (P = 0.001) than patients who did not undergo simultaneous splenectomy.
| Nonsplenectomy (n = 85) | Splenectomy (n = 35) | P value | |
| Age (yr), median (range) | 57 (37-69) | 57 (37-69) | 0.667 |
| Gender, n (%) | 0.107 | ||
| Male | 67 (78.8) | 22 (62.9) | |
| Female | 18 (21.2) | 13 (37.1) | |
| BMI, median (range) | 24.2 (17.4-43.8) | 24.6 (18.4-43.3) | 0.707 |
| Underlying liver disease, n (%) | |||
| HBV | 66 (77.6) | 14 (40.0) | < 0.001a |
| HCV | 22 (25.9) | 23 (65.7) | < 0.001a |
| Alcoholism | 13 (15.3) | 4 (11.4) | 0.775 |
| Signs of portal hypertension, n (%) | |||
| Ascites | 43 (50.6) | 19 (54.3) | 0.841 |
| Hepatic encephalopathy | 35 (41.2) | 13 (37.1) | 0.838 |
| Varices bleeding | 19 (22.4) | 12 (34.3) | 0.251 |
| Comorbidity, n (%) | |||
| Hypertension | 20 (23.5) | 9 (25.7) | 0.817 |
| Diabetes mellitus | 40 (47.1) | 11 (31.4) | 0.155 |
| Preoperative serum tests, median (range) | |||
| White blood count (/uL) | 4600 (1480-11200) | 3500 (1350-12200) | 0.120 |
| Platelet count (/uL) | 80000 (26000-279000) | 64000 (27000-155000) | 0.003a |
| Neutrophil–lymphocyte ratio | 2.44 (0.51-24.18) | 3.2 (0.91-21.33) | 0.273 |
| Platelet–lymphocyte ratio | 78.49 (36.80-284.01) | 71.19 (28.53-188.08) | 0.386 |
| Total bilirubin (mg/dL) | 1.4 (0-38.9) | 1.6 (0.4-57.1) | 0.984 |
| Creatinine (mg/dL) | 0.9 (0.4-10.1) | 0.8 (0.5-1.3) | 0.578 |
| Ammonia (ug/dL) | 99 (0-337) | 99 (30-560) | 0.737 |
| Albumin (g/dL) | 3.2 (1.2-5.3) | 3.3 (2.2-5.1) | 0.922 |
| Glucose (mg/dL) | 115 (0-457) | 118 (82-312) | 0.956 |
| INR | 1.1 (0.9-2.7) | 1.2 (0.9-2.1) | 0.819 |
| MELD scores | 11 (6-32) | 11 (6-30) | 0.494 |
| AFP (ng/mL) | 7.0 (0.5-1190.0) | 14.0 (2.0-2170.0) | 0.012a |
| Surgical factors | |||
| Surgical type, n (%) | 0.276 | ||
| DDLT | 26 (30.6) | 6 (17.1) | |
| LDLT | 56 (65.9) | 28 (80) | |
| SLT | 3 (3.5) | 1 (2.9) | |
| Graft type, n (%) | 0.120 | ||
| Whole graft | 27 (31.8) | 6 (17.1) | |
| Partial graft | 58 (68.2) | 29 (82.9) | |
| GRWR < 0.8 | 12 (14.1) | 6 (17.1) | 0.673 |
| Blood loss (mL), median (range) | 1600 (200-14400) | 1350 (260-11000) | 0.519 |
| Operative time (minutes), median (range) | 552 (360-1035) | 630 (420-870) | 0.001a |
| Pathology | |||
| Tumor size (cm) | 2.2 (0-6.5) | 2.5 (0-6.2) | 0.140 |
| Tumor number, n (%) | 0.404 | ||
| 0 or 1 | 58 (68.2) | 21 (60.0) | |
| 2 or 3 | 27(31.8) | 14 (40.0) | |
| Tumor necrosis, n (%) | 49 (58.3) | 20 (57.1) | 1.000 |
| Lymphovascular invasion, n (%) | 6 (7.1) | 5 (14.3) | 0.297 |
| Outcomes | |||
| Hospital stays, median (range) (d) | 21 (0-85) | 18 (5-116) | 0.810 |
| HCC Recurrence, n (%) | 16 (18.8) | 15 (42.9) | 0.011a |
| Secondary cancer, n (%) | 5 (6.4) | 0 | 0.322 |
| Mortality, n (%) | 9 (10.6) | 11 (31.4) | 0.013a |
Upon completion of the analysis, the splenectomy group was found to have a higher proportion of HCC recurrence (42.9% vs 18.8%, P = 0.011) and mortality (31.4% vs 10.6%, P = 0.013) compared with that in the nonsplenectomy group (Table 1). Five of the 85 patients (6.4%) in the nonsplenectomy group had de novo cancer development. Of five patients with de novo cancer development, one each had lung cancer, urothelial carcinoma, squamous cell carcinoma of the tongue, breast cancer, and adenocarcinoma of the esophagus. In the splenectomy group, no de novo cancer development was found. However, the length of hospital stay was not significantly different between these two groups (P > 0.05, Table 1).
Subsequently, the Cox regression model was used to investigate cancer development and mortality (Tables 2 and 3). In the univariate Cox regression analysis, splenectomy, age and HBV were significantly associated with cancer development (all P < 0.05, Table 2), while splenectomy, HBV, HCV and hypertension were associated with mortality (all P < 0.05, Table 3). In the multivariate Cox regression analysis, splenectomy [hazard ratio (HR) = 2.560; 95% confidence interval (CI): 1.198–5.471, P = 0.015] and age (HR = 1.057, 95%CI: 1.001–1.117, P = 0.048) were positive independent factors for prediction of cancer development (Table 2). Splenectomy (HR = 2.791, 95%CI: 1.081–7.206, P = 0.034), hypertension (HR = 2.813, 95%CI: 1.111–7.123, P = 0.029) and HBV (HR = 4.077, 95%CI: 1.001–16.615, P = 0.050) were positive independent factors for prediction of mortality (Table 3). In addition, Kaplan–Meier curve analyses revealed that splenectomy could identify subjects at higher risk for cancer development or mortality (all P < 0.05, Figure 1). The cumulative CFS (5-year CFS rates: 76.5% in nonsplenectomy group; 53.4% in splenectomy group) and cumulative OS rates (5-year OS rate: 89.3% in the nonsplenectomy group; 68.1% in the splenectomy group) differed significantly between the two groups.
| Univariate | Multivariate | |||
| Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | |
| Age | 1.055 (1.001, 1.112) | 0.047a | 1.057 (1.001, 1.117) | 0.048a |
| Gender/male | 1.346 (0.614, 2.950) | 0.459 | - | |
| BMI | 0.937 (0.850, 1.033) | 0.191 | - | |
| HBV | 2.070 (1.005, 4.263) | 0.048a | 1.371 (0.632, 2.978) | 0.425 |
| HCV | 0.687 (0.332-1.423) | 0.313 | - | |
| Alcoholism | 1.751 (0.532-5.769) | 0.357 | - | |
| Diabetes mellitus | 1.062 (0.523, 2.157) | 0.868 | - | |
| Hypertension | 1.704 (0.777, 3.736) | 0.183 | - | |
| Tumor size | 1.057 (0.817, 1.368) | 0.672 | - | |
| Tumor number (2/3 vs 0/1) | 1.577 (0.777, 3.199) | 0.207 | - | |
| Lymphovascular invasion | 1.722 (0.600, 4.945) | 0.312 | - | |
| Splenectomy | 2.754 (1.359, 5.581) | 0.005a | 2.560 (1.198, 5.471) | 0.015a |
| PLT | 1.000 (1.000, 1.000) | 0.579 | - | |
| AFP | 1.001 (1.000, 1.002) | 0.070 | - | |
| Univariate | Multivariate | |||
| Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | |
| Age | 1.063 (0.994, 1.136) | 0.075 | - | |
| Gender/male | 1.424 (0.540, 3.757) | 0.475 | - | |
| BMI | 0.942 (0.834, 1.063) | 0.333 | - | |
| HBV | 4.386 (1.719, 11.193) | 0.002a | 4.077 (1.001, 16.615) | 0.050 |
| HCV | 2.853 (1.145, 7.114) | 0.024a | 0.661 (0.166, 2.640) | 0.558 |
| Alcoholism | 0.696 (0.161, 3.018) | 0.629 | - | |
| Diabetes mellitus | 1.640 (0.679, 3.958) | 0.271 | - | |
| Hypertension | 2.872 (1.142, 7.221) | 0.025a | 2.813 (1.111, 7.123) | 0.029a |
| Tumor size | 0.944 (0.679, 1.312) | 0.732 | - | |
| Tumor number (2-3 vs 0-1) | 1.911 (0.795, 4.596) | 0.148 | - | |
| Lymphovascular invasion | 2.054 (0.597, 7.062) | 0.254 | - | |
| Splenectomy | 3.656 (1.510, 8.848) | 0.004a | 2.791 (1.081, 7.206) | 0.034a |
| PLT | 1.000 (1.000, 1.000) | 0.409 | - | |
| AFP | 1.001 (1.000, 1.002) | 0.081 | - | |
The present study analyzed the outcomes of patients with HCC within the UCSF criteria who underwent LT with and without simultaneous splenectomy. In the past, simultaneous splenectomy was performed in cases of ABO-incompatible living donor LT (ABO-iLDLT) because of immunological concerns, or in patients with HCV for prevention of thrombocytopenia. In recent years, simultaneous splenectomy is performed less due to the advancement of the desensitization protocol in ABO-iLT and the development of direct-acting antiviral agents as anti-HCV therapy. However, inflow modulation was still necessary in many LDLT patients. The topic of simultaneous splenectomy still deserves attention. In our cohort, simultaneous splenectomy was independently correlated with cancer development and OS, suggesting that simultaneous splenectomy should be a factor for concern in patients with HCC who undergo LT.
The increased cancer risk associated with splenectomy was reported in previous clinical studies and in a nationwide Taiwanese population-based cohort study[6-8]. In the Taiwanese study, the HR was 2.06 in the splenectomy cohort[8]. Cancer risk was higher in cases of nontraumatic splenectomy than in traumatic splenectomy, especially in splenectomy cases caused by hematological conditions[6,8]. Splenectomy significantly increases the risk of all malignant neoplasms, especially those of the lung, nonmelanoma skin cancer, leukemia, lymphoma, and Hodgkin’s lymphoma[6]. A study published by Linet et al[7] revealed a higher incidence of lung and ovarian cancers in patients who underwent splenectomy[7]. Buccal, esophagus, liver, colon, pancreas, lung, prostate, and multiple hematological malignancies were observed in a cohort of cancer-free American veterans after splenectomy[13]. The previously mentioned Taiwanese study found that the most common cancers after a splenectomy were those of the gastrointestinal tract, head and neck and liver, as well as hematological malignancies[8]. The relationship between splenectomy and cancer has also been proven in animal experiments[14-17]. An early experiment inferred that the ability of the spleen to protect a rat from cancer is due to the preservation of immunological surveillance and not due to the DNA repair mechanism[14]. Splenectomy enhances metastatic ability through the immunological tolerance of regulatory T cells[15]. Splenectomy was also found to enhance tumor growth and peritoneal seeding in an orthotopic syngeneic murine pancreatic cancer mouse model, which is explained by its immunological effects[16,17].
To the best of our knowledge, there are no studies discussing the oncological effects of simultaneous splenectomy in LT. Therefore, we reviewed the oncological effects of simultaneous splenectomy and hepatectomy in patients with HCC to gain a greater understanding of this relationship. Some studies have found that the results of hepatectomy with simultaneous splenectomy in HCC patients with hypersplenism were positive. Chen et al[18] showed that the 5-year DFS rate was significantly higher in patients with HCC who underwent hepatectomy and splenectomy than in those who underwent hepatectomy alone (37% vs 27.3%; P = 0.003)[18]. Zhang et al[19-21] also found that HCC patients with hypersplenism who underwent hepatectomy and simultaneous splenectomy exhibited significantly better DFS and OS rates than those who underwent hepatectomy alone[19-21]. It seems, therefore, that splenectomy benefits surgical management in selected cases of HCC. The role of splenectomy in improving oncological outcomes has also been reported in animal studies[22,23]. Spleen cells release tumor-enhancing factors that promote tumor growth activity in vivo[22], and the spleen may also evoke a complex vascular response[23], which suggests that splenectomy could inhibit tumor growth. Besides inhibiting tumor growth, simultaneous splenectomy has been reported to decrease tumor metastasis[24]. However, some papers have put forth opposing views, suggesting that simultaneous splenectomy and hepatectomy did not benefit OS and DFS rates, in comparison to hepatectomy alone[25,26]. The oncological benefits of simultaneous splenectomy in patients with liver cirrhosis are, therefore, still controversial.
The relationship between cancer risk after splenectomy and LT gained little attention in previous clinical studies. Ito et al[27] pointed out that simultaneous splenectomy was associated with reoperation due to postoperative hemorrhage, prolonged operating time, increased intraoperative blood loss, and increased incidence of lethal infectious disease[27]. A meta-analysis found that simultaneous splenectomy during LT was associated with prolonged operating time, increased intraoperative blood loss, increased need for intraoperative blood transfusions, and increased incidence of postoperative hemorrhage, thrombosis, infection and mortality[28]. Another study revealed that splenectomy significantly increases the rates of postoperative splenic vein thrombosis and cytomegalovirus infection in LDLT[29]. These three studies suggest that splenectomy has a number of short-term risks and should be performed only in carefully selected patients. Our study shed light on the increased long-term cancer risk after LT, which was associated with simultaneous splenectomy. In brief, LT with simultaneous splenectomy should be avoided as much as possible, whether the risks lie in the short or long term.
The role of age in the oncological outcomes of HCC after LT is still uncertain. There are reports demonstrating that younger patients tend to have more aggressive tumors and a higher risk of recurrence than older patients[30,31]. In the present study, old age was associated with poor outcomes in patients with HCC after LT. A possible explanation is that older patients have been exposed to HBV and HCV infections for a longer period.
Hypertension is the most common cardiovascular complication to occur after LT, with a prevalence reported to be between 40%[32] and 85%[33]. The mechanisms are multifactorial, and hypertension is one of the main risk factors leading to post-transplant mortality[34]. An early diagnosis of hypertension, as well as implementation of lifestyle changes and antihypertensive medications is essential for increasing the long-term survival of LT patients[35].
The limitations of this study were the patient selection methods and the small sample size. Because of surgical indications for simultaneous splenectomy, more HCV patients underwent simultaneous splenectomy. There may have been biases in terms of patient selection. However, SupplementaryTable 1 shows that the HCV subgroup analysis was like that of the whole group. Nevertheless, this study only analyzed patients with HCC within the UCSF criteria and that were confirmed by both radiological and postoperative pathological examinations. The study did not analyze patients who primarily had HCCs outside the UCSF criteria and had successfully treated HCCs to fit the USCF criteria upon radiological examination on the day of LT. The reason for this was that the percentage of tumor necrosis would make it difficult for pathological examination to accurately determine whether patients complied with the UCSF criteria or not. Besides, splenic artery ligation is often considered, instead of splenectomy, for achieving the goal of modulation of portal inflow[36]. The effects of splenic artery ligation, compared to splenectomy, were not discussed in this study.
Our study revealed that the patients with HCC who met the UCSF criteria and who underwent LT and simultaneous splenectomy had poorer DFS and OS than patients who did not undergo simultaneous splenectomy. Therefore, simultaneous splenectomy should be avoided in patients with HCC undergoing LT.
Patients undergoing splenectomy were more likely to develop cancer than patients not undergoing splenectomy. There are a number of indications for simultaneous splenectomy in liver transplantation (LT) recipients.
The hypothesis is that simultaneous splenectomy has bad outcomes on cancer and mortality in LT recipients.
The purpose of this study was to compare the outcomes of hepatocellular carcinoma (HCC) recurrence and de novo malignancy development between HCC patients who underwent LT with and without simultaneous splenectomy.
Of 120 patients with HCC who received LT with (n = 35) and without (n = 85) simultaneous splenectomy were analyzed by Cox regression analysis, Kaplan–Meier survival curves and log-rank tests.
Splenectomy and age were both positive independent factors for prediction of cancer development. Splenectomy and hypertension were positive independent factors for prediction of mortality. The splenectomy group had a significantly worse cancer-free survival and overall survival curve compared to the nonsplenectomy group.
Simultaneous splenectomy should be avoided in patients with HCC undergoing LT.
Splenic artery ligation is often considered, instead of splenectomy, for achieving the goal of modulation of portal inflow. The direction of the future research is the comparison on cancer outcome between splenectomy and splenic artery ligation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Djuric O, Italy; Du GS, China; Xu X, China S-Editor: Fan JR L-Editor: Kerr C P-Editor: Fan JR
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21374] [Article Influence: 2137.4] [Reference Citation Analysis (3)] |
| 2. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4111] [Article Influence: 587.3] [Reference Citation Analysis (6)] |
| 3. | Zhang JA, Kwee SA, Wong LL. Late recurrence of hepatocellular carcinoma after liver transplantation. Hepatoma Res. 2017;3:58-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Mazzola A, Costantino A, Petta S, Bartolotta TV, Raineri M, Sacco R, Brancatelli G, Cammà C, Cabibbo G. Recurrence of hepatocellular carcinoma after liver transplantation: an update. Future Oncol. 2015;11:2923-2936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Yoon YI, Lee SG. Living Donor Liver Transplantation for Hepatocellular Carcinoma: An Asian Perspective. Dig Dis Sci. 2019;64:993-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Mellemkjoer L, Olsen JH, Linet MS, Gridley G, McLaughlin JK. Cancer risk after splenectomy. Cancer. 1995;75:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Linet MS, Nyrén O, Gridley G, Mellemkjaer L, McLaughlin JK, Olsen JH, Adami HO, Fraumeni JF Jr. Risk of cancer following splenectomy. Int J Cancer. 1996;66:611-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Sun LM, Chen HJ, Jeng LB, Li TC, Wu SC, Kao CH. Splenectomy and increased subsequent cancer risk: a nationwide population-based cohort study. Am J Surg. 2015;210:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 9. | Yoshizumi T, Itoh S, Shimokawa M, Inokuchi S, Harada N, Takeishi K, Mano Y, Yoshiya S, Kurihara T, Nagao Y, Ikegami T, Soejima Y, Mori M. Simultaneous splenectomy improves outcomes after adult living donor liver transplantation. J Hepatol. 2021;74:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 10. | Usui M, Isaji S, Mizuno S, Sakurai H, Uemoto S. Experiences and problems pre-operative anti-CD20 monoclonal antibody infusion therapy with splenectomy and plasma exchange for ABO-incompatible living-donor liver transplantation. Clin Transplant. 2007;21:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Yoshizumi T, Mori M. Portal flow modulation in living donor liver transplantation: review with a focus on splenectomy. Surg Today. 2020;50:21-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Jiang WT, Yang J, Xie Y, Guo QJ, Tian DZ, Li JJ, Shen ZY. Simultaneous partial splenectomy during liver transplantation for advanced cirrhosis patients combined with severe splenomegaly and hypersplenism. World J Gastroenterol. 2021;27:654-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Kristinsson SY, Gridley G, Hoover RN, Check D, Landgren O. Long-term risks after splenectomy among 8,149 cancer-free American veterans: a cohort study with up to 27 years follow-up. Haematologica. 2014;99:392-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 236] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 14. | Hull CC, Galloway P, Gordon N, Gerson SL, Hawkins N, Stellato TA. Splenectomy and the induction of murine colon cancer. Arch Surg. 1988;123:462-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Higashijima J, Shimada M, Chikakiyo M, Miyatani T, Yoshikawa K, Nishioka M, Iwata T, Kurita N. Effect of splenectomy on antitumor immune system in mice. Anticancer Res. 2009;29:385-393. [PubMed] |
| 16. | Hwang HK, Murakami T, Kiyuna T, Kim SH, Lee SH, Kang CM, Hoffman RM, Bouvet M. Splenectomy is associated with an aggressive tumor growth pattern and altered host immunity in an orthotopic syngeneic murine pancreatic cancer model. Oncotarget. 2017;8:88827-88834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Hwang HK, Kang CM, Lee SH, Murakami T, Kiyuna T, Kim SH, Hoffman RM, Bouvet M. Fluorescence-guided Surgery with Splenic Preservation Prevents Tumor Recurrence in an Orthotopic Nude-mouse Model of Human Pancreatic Cancer. Anticancer Res. 2018;38:665-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Chen XP, Wu ZD, Huang ZY, Qiu FZ. Use of hepatectomy and splenectomy to treat hepatocellular carcinoma with cirrhotic hypersplenism. Br J Surg. 2005;92:334-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Zhang X, Li C, Wen T, Peng W, Yan L, Li B, Yang J, Wang W, Xu M, Zeng Y. Synchronous splenectomy and hepatectomy for patients with small hepatocellular carcinoma and pathological spleen: neutrophil to lymphocyte ratio changes can predict the prognosis. Oncotarget. 2017;8:46298-46311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Zhang XY, Li C, Wen TF, Yan LN, Li B, Yang JY, Wang WT, Jiang L. Synchronous splenectomy and hepatectomy for patients with hepatocellular carcinoma and hypersplenism: A case-control study. World J Gastroenterol. 2015;21:2358-2366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Zhang XF, Liu Y, Li JH, Lei P, Zhang XY, Wan Z, Lei T, Zhang N, Wu XN, Long ZD, Li ZF, Wang B, Liu XM, Wu Z, Chen X, Wang JX, Yuan P, Li Y, Zhou J, Pawlik M, Lyu Y. Effect of splenectomy on the right of hepatocellular carcinoma development amoung patients with liver cirrhosis and portal hypertension: a multi-institutional cohort study. Zhonghua Waike Zazhi. 2021;59:821-828. [DOI] [Full Text] |
| 22. | Eiján AM, Jasnis MA, Kohan SS, Oisgold-Dagá S. Nature of the spleen cell populations capable of releasing tumor enhancing factor. J Surg Oncol. 1987;36:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Davel L, Miguez M, Jasnis MA, Eiján AM, Oisgold-Dagá S, Sacerdote de Lustig E. Angiogenic activity by spleen cell supernatants from tumor-bearing and tumor-resected mice. J Surg Oncol. 1988;37:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 24. | Klein S, de Bonaparte YP, de D'Elia I. Enhancement of the incidence of metastasis in tumor-resected mice. Influence of soluble tumor extract and splenectomy. Invasion Metastasis. 1985;5:309-316. [PubMed] |
| 25. | Shi R, Zhang YM, Zhu ZJ, Deng YL, Pan C, Zheng H, Shen ZY. Synchronous splenectomy and hepatectomy in patients with hepatocellular carcinoma, hypersplenism and liver cirrhosis. Hepatogastroenterology. 2014;61:1363-1367. [PubMed] |
| 26. | Wang C, Li C, Wen TF, Yan LN, Li B, Liang GL, Li KW. Safety of synchronous hepatectomy and splenectomy for patients with hepatocellular carcinoma and hypersplenism. Hepatogastroenterology. 2012;59:526-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 27. | Ito K, Akamatsu N, Ichida A, Ito D, Kaneko J, Arita J, Sakamoto Y, Hasegawa K, Kokudo N. Splenectomy is not indicated in living donor liver transplantation. Liver Transpl. 2016;22:1526-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 28. | He C, Liu X, Peng W, Li C, Wen TF. Evaluation the efficacy and safety of simultaneous splenectomy in liver transplantation patients: A meta-analysis. Medicine (Baltimore). 2018;97:e0087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Badawy A, Hamaguchi Y, Satoru S, Kaido T, Okajima H, Uemoto S. Evaluation of safety of concomitant splenectomy in living donor liver transplantation: a retrospective study. Transpl Int. 2017;30:914-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Wang P, Wang C, Li H, Shi B, Wang J, Zhong L. Impact of age on the prognosis after liver transplantation for patients with hepatocellular carcinoma: a single-center experience. Onco Targets Ther. 2015;8:3775-3781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Wai CT, Woon WA, Tan YM, Lee KH, Tan KC. Younger age and presence of macrovascular invasion were independent significant factors associated with poor disease-free survival in hepatocellular carcinoma patients undergoing living donor liver transplantation. Transplant Proc. 2012;44:516-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Martínez-Saldivar B, Prieto J, Berenguer M, de la Mata M, Pons JA, Serrano T, Rafael-Valdivia L, Aguilera V, Barrera P, Parrilla P, Lorente S, Rubin A, Fraga E, Rimola A. Control of blood pressure in liver transplant recipients. Transplantation. 2012;93:1031-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Albeldawi M, Aggarwal A, Madhwal S, Cywinski J, Lopez R, Eghtesad B, Zein NN. Cumulative risk of cardiovascular events after orthotopic liver transplantation. Liver Transpl. 2012;18:370-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 34. | Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Charlton MR. Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant. 2010;10:1420-1427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 617] [Cited by in RCA: 587] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 35. | Jiménez-Pérez M, González-Grande R, Omonte Guzmán E, Amo Trillo V, Rodrigo López JM. Metabolic complications in liver transplant recipients. World J Gastroenterol. 2016;22:6416-6423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (2)] |
| 36. | Kelly DM, Miller C. Understanding the splenic contribution to portal flow: the role of splenic artery ligation as inflow modification in living donor liver transplantation. Liver Transpl. 2006;12:1186-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |