Published online Sep 27, 2022. doi: 10.4240/wjgs.v14.i9.1008
Peer-review started: January 20, 2022
First decision: April 10, 2022
Revised: April 22, 2022
Accepted: August 24, 2022
Article in press: August 24, 2022
Published online: September 27, 2022
Processing time: 245 Days and 1.8 Hours
The role of tumor-infiltrating lymphocytes (TILs) in the growth and progression of hepatocellular carcinoma (HCC) has attracted widespread attention.
To evaluate the feasibility of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) for massive HCC by exploring the role of TIL in the tumor microenvironment.
Fifteen massive HCC patients who underwent ALPPS treatment and 46 who underwent hemi-hepatectomy were selected for this study. Propensity score matching was utilized to match patients in ALPPS and hemi-hepatectomy groups (1:1). Quantitative analysis of TILs in tumor and adjacent tissues between the two groups was performed by immunofluorescence staining and further analyses with oncological characteristics. In the meantime, trends of TILs in peripheral blood were compared between the two groups during the perioperative period.
Continuous measurement of tumor volume and necrosis volume showed that the proportion of tumor necrosis volume on the seventh day after stage-I ALPPS was significantly higher than the pre-operative value (P = 0.024). In the preoperative period of stage-I ALPPS, the proportion of tumor necrosis volume in the high CD8+ T cell infiltration group was significantly higher than that in the low group (P = 0.048).
TIL infiltration level maintained a dynamic balance during the preoperative period of ALPPS. Compared with right hemi-hepatectomy, the ALPPS procedure does not cause severe immu
Core Tip: This study was conducted to evaluate the feasibility of associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) for massive hepatocellular carcinoma by exploring the role of tumor-infiltrating lymphocyte (TIL) subpopulations in the tumor microenvironment. The ALPPS procedure did not cause severe immunosuppression due to reduced TIL infiltration and pathological alterations in peripheral blood immune components. In addition, high perioperative CD8+ T cell infiltration with ALPPS was associated with increased tumor necrosis.
- Citation: Wang W, Deng ZF, Wang JL, Zhang L, Bao L, Xu BH, Zhu H, Guo Y, Wen Z. Change of tumor-infiltrating lymphocyte of associating liver partition and portal vein ligation for staged hepatectomy for hepatocellular carcinoma. World J Gastrointest Surg 2022; 14(9): 1008-1025
- URL: https://www.wjgnet.com/1948-9366/full/v14/i9/1008.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i9.1008
Primary liver cancer is a common digestive system malignancy, with around 906000 new cases and 830000 deaths occurring globally, with the incidence rate and mortality rate increasing yearly. More than 75% of cases of primary liver cancer are hepatocellular carcinoma (HCC)[1]. According to the newly released diagnosis and treatment guidelines, surgery is the primary choice of radical resection of HCC tumors and the principal treatment strategy for prolonging the survival time of patients with HCC[2,3].
In March 2012, Schnitzbauer et al[4] were the first to report associating liver partition and portal vein ligation for staged hepatectomy (ALPPS), an innovative hepatectomy, publicly. ALPPS can block part of the blood flow supplying the tumor and completely block the possible collateral circulation between the two hepatic lobes. Thus, ALPPS can effectively stimulate liver hyperplasia and create more favorable conditions for the second-stage surgical resection of the tumor. With the gradual maturity and improvement in ALPPS technology, the clinical application of ALPPS has gone through an early transition, and the incidence of complications and mortality has been gradually reduced. In HCC patients who have undergone rigorous screening for ALPPS treatment, these risks are comparable to those of traditional hepatectomy and portal vein embolization + hepatectomy, which leads to an increase in the resection rate of massive HCC[5]. As a new method of liver surgery, ALPPS is a promising approach to treating HCC patients.
Tumor-infiltrating lymphocytes (TILs) migrate to the tumor microenvironment (TME) after leaving the peripheral blood circulation system, which involve T and B lymphocytes and natural killer (NK) cells. TILs are an integral part of the TME, and their role in HCC tumor growth and progression has attracted widespread attention. Recent studies have focused on the relationship between TILs and the prognosis of liver cancer patients. Anantha et al[6] reported for the first time that various immunological components of the future liver remnant (FLR) did not change during the perioperative period of ALPPS. This shows that FLR proliferates rapidly and relatively expands the formation of various immune cells and components to maintain immune functions. However, in the perioperative period of ALPPS, patients need to withstand two surgical insults. The impact of subsequent stress or inflammatory response on the changes and effects of immune cells residing or recruited in the TME is still unclear. More specifically, to understand whether ALPPS could be used as a viable alternative to traditional hepatectomy techniques, it is necessary to study the potential mechanism of ALPPS complications and the changes and effects of tumor-infiltrating immune cells or components. Here, we investigated the effect of ALPPS surgery on TIL subsets, analyzed the changes in the immune microenvironment of tumor cells during the two-stage ALPPS surgery, and finally evaluated the safety and effectiveness of ALPPS as an alternative to traditional hemi-hepatectomy for the treatment of massive HCC.
All subjects were HCC cases from the Department of Hepatobiliary Surgery of a single center from August 2018 to August 2019. Surgical resection was performed in all cases, with the types of tumors confirmed by postoperative pathological examination. These data have been uploaded to the International ALPPS Registry (www.alpps.net). This study followed the declaration of Helsinki and was approved by the ethics committee of the center. Patients were not required to give informed consent for the study because the clinical data were obtained retrospectively after each patient agreed to treatment by written consent.
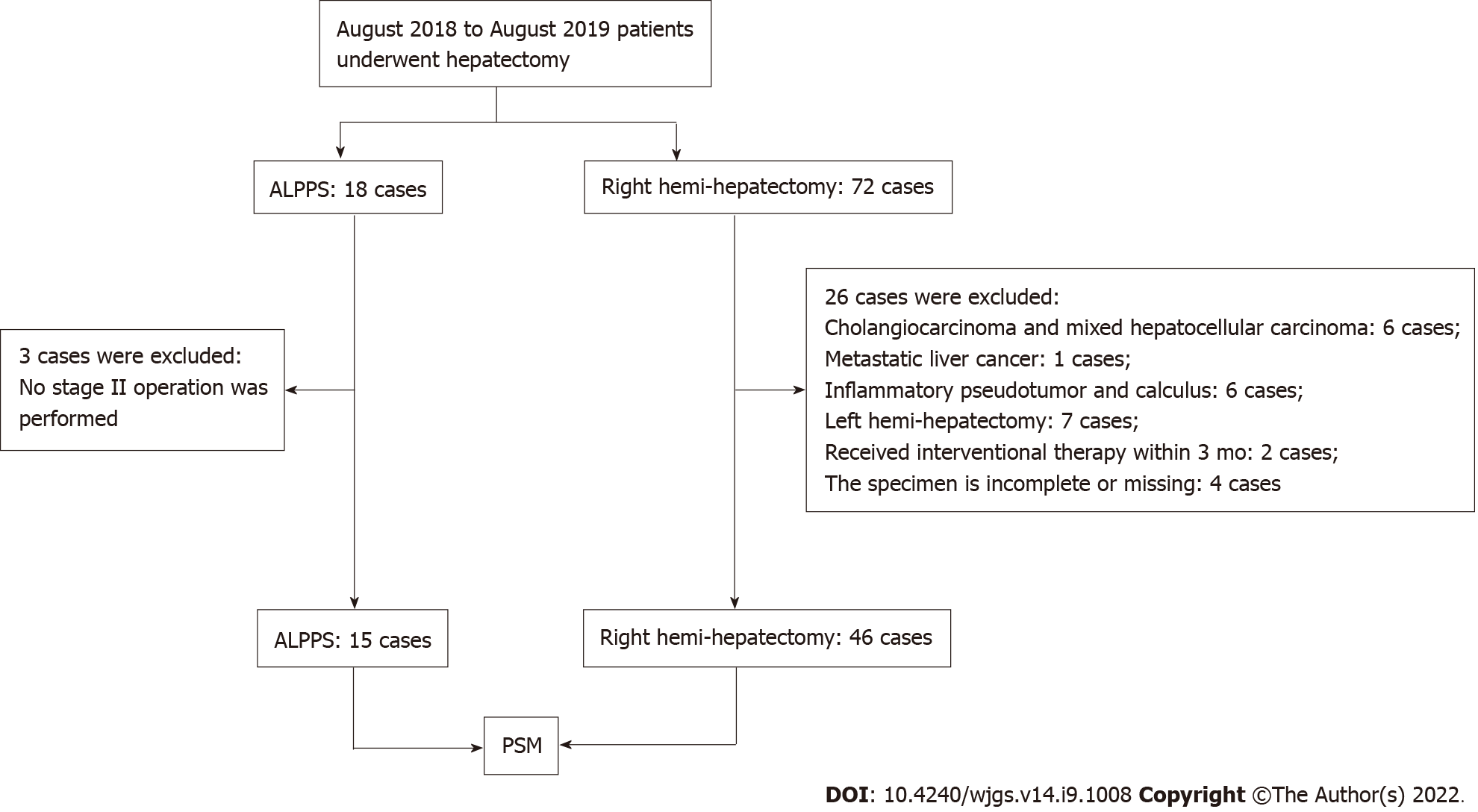
The following inclusion criteria were used for the selection of patients: (1) Patients with an FLR/standard liver volume (SLV) ratio < 30%-50% and who have received stage-I ALPPS treatment; (2) Child-Pugh classification A or B; and (3) All subjects were confirmed to be HCC patients by surgery and pathology. The following exclusion criteria were used for rejecting the patients: (1) Incomplete clinical data or histological specimens; (2) Patients without stage-II ALPPS treatment; and (3) Patients undergoing left hemi-hepatectomy.
Each specimen was numbered according to the chronological order of the included cases and the site of collection, and hematoxylin-eosin-stained sections of HCC tissues kept in the case specimen library were retrieved. After the pathologists read the slides, paraffin specimens with typical HCC characteristics of cancerous and paracancerous tissues were selected. The screened tissues were then arranged on empty white wax blocks in a certain order using a tissue microarray spotter with the assistance of a pathology technician, and the tissue chip was obtained by serially slicing the wax blocks through a slicer, in which each core spot represented a pathological specimen. The prepared tissue chips were placed in slide boxes and refrigerated at 4 °C for storage. Tissue chips were subjected to antigen repair after dewaxing and dehydration. Subsequently, 3% H2O2 was added dropwise to block endogenous peroxidase. Primary antibodies (Abcam, United States) were added and kept at 4 °C overnight. Secondary antibodies were added dropwise at room temperature for 50 min, and then horseradish peroxidase reagent was added dropwise. CD4, CD56, CD3, CD20, CD8, and Foxp3 were stained with different colors of fluorescent dyes. DAPI (Sigma-Aldrich, Germany) was used to stain the nucleus. Dimethyl sulfoxide (1 mL) was added to the tissue chip at room temperature for 5 min, and the slide was covered. Complete images were acquired with the Mantra system (PerkinElmer, Waltham, Massachusetts, United States) to collect multispectral images. The inform image analysis software was used to quantify the amount of fluorescence excitation for each core site and for each fluorophore. The positive expression rate of cells in each sample was calculated as number of positive cells/total number of nucleated cells.
During stage-I ALPPS, the surgeon first opened the abdominal cavity to exclude extrahepatic metastatic tissues. The right portal vein branch would be ligated in the absence of any metastasis. Intra-operative ultrasound-guided anterior hepatic transection was conducted along the middle hepatic vein, and the blood flow of the hepatic artery was preserved. The interval between stage I and stage II of ALPPS depended on the patient’s condition and increased FLR. During stage-II ALPPS, right hepatectomy or enlarged right hepatectomy was performed[7].
To add to the control analysis, patients in the ALPPS group were matched 1:1 with those in the right hemicolectomy group using the propensity score matching (PSM) module built into the SPSS 22.0 software. The independent variables of tumor size and number, alpha-fetoprotein (AFP) level, Child-Pugh score, presence of large vessel cancer thrombi, and presence of distant metastases were used as covariate matching items. Age, gender, body mass index, liver cancer end-stage score, and Barcelona clinic liver cancer (BCLC) staging system were used as balanced matches. The caliper value was set to 0.1.
The liver volume was analyzed using IQQA-3D Liver (EDDA Technology, United States) combined with patient imaging data[8]. SLV was calculated using the Chinese adult standard liver volume estimation formula[9]. FLR/SLV ratio before surgery was used to determine whether FLR was sufficient. The increase in FLR volume confirmed the stage-I ALPPS and stage-II ALPPS. The following conditions were considered acceptable for stage-II ALPPS: (1) FLR/SLV ratio ≥ 50% suggested severe fibrosis or cirrhosis; (2) FLR/SLV ratio ≥ 40% suggested the presence of mild/moderate fibrosis; and (3) FLR/SLV ≥ 30% suggested the absence of liver fibrosis or cirrhosis[10]. A complete tumor image was drawn, and the tumor volume was calculated[11]. The tumor necrosis volume was also calculated. The percentage of tumor necrosis volume was then calculated as tumor necrosis volume/tumor volume × 100%. The tumor size and necrotic volume were analyzed before ALPPS and 3 d and 7 d after stage-I ALPPS.
The patients were followed regularly for 3 mo after discharge and every 3 to 6 mo after that, mainly involving imaging examination (ultrasound, computed tomography, and magnetic resonance imaging), liver function inspection, and AFP level test. After analysis, the overall survival rate of each patient was calculated, with the survival time defined as the time from treatment operation to death. The final events of overall survival included extrahepatic or intrahepatic metastasis, recurrence, and death after primary resection.
The data were analyzed and processed with IBM SPSS22.0. The normally distributed measurement data are expressed as the mean ± SD, and the count data are defined as quantity (%). The student’s t-test was conducted to compare the measurement data between two paired groups. Comparison of counting data was made between two groups using the chi-square test or Fisher’s exact test, and the R × C chi-square test was used for comparison among groups. Repeated measurement data were compared by repeated measurement analysis of variance. Kaplan-Meier method was used for survival analysis and fitting survival curves. The Log-rank test was used to compare the differences in survival curves among different groups. P < 0.05 was considered statistically significant.
The clinical data of 90 patients undergoing hepatectomy in a single center were collected. Fifteen HCC patients treated by ALPPS and 46 patients by right hemi-hepatectomy were included for analysis (Figure 1). A 1:1 match was performed between the ALPPS group and the right hemi-hepatectomy group using the PSM module. After matching, the variables such as age, sex, body mass index, liver cancer end-stage score, BCLC stage, tumor size and number, AFP level, Child-Pugh score, presence of macrovascular tumor thrombus, and distant metastasis were found to be similar between the two groups (P > 0.05, Table 1). In addition, the average FLR/SLV ratio of the ALPPS group measured before the operation was 36.9% (range, 21.6%-45.4%), and the FLR/SLV value of the right hemi-hepatectomy group was 58.9% (range, 35.3%-77.3%).
| Variable | Before matching | After matching | ||||
| ALPPS (15 cases) | Hepatectomy (46 cases) | P valve | ALPPS (15 cases) | Hepatectomy (15 cases) | P valve | |
| Age (yr) | 45.1 ± 11.4 | 49.4 ± 9.6 | 0.157 | 45.1 ± 11.4 | 49.5 ± 9.9 | 0.276 |
| Sex (%) | 0.795 | 0.543 | ||||
| Female | 2 (13.3%) | 5 (10.9%) | 2 (13.3%) | 1 (6.7%) | ||
| Male | 13 (86.7%) | 41 (89.1%) | 13 (86.7%) | 14 (93.3%) | ||
| BMI | 22.4 ± 3.2 | 22.8 ± 3.1 | 0.644 | 22.4 ± 3.2 | 23.0 ± 3.4 | 0.608 |
| HCC end-stage score | 5.8 ± 2.3 | 5.4 ± 2.6 | 0.626 | 5.8 ± 2.3 | 6.1 ± 3.7 | 0.815 |
| BCLC stage | 0.775 | 0.915 | ||||
| A | 3 (20.0%) | 13 (28.3%) | 3 (20.0%) | 3 (20.0%) | ||
| B | 5 (33.3%) | 12 (26.0%) | 5 (33.3%) | 4 (26.7%) | ||
| C | 7 (46.7%) | 21 (45.7%) | 7 (46.7%) | 8 (53.3%) | ||
| AFP (%) | 0.031 | 1.000 | ||||
| ≥ 400 ng/mL | 11 (73.3%) | 19 (41.3%) | 11 (73.3%) | 11 (73.3%) | ||
| < 400ng/mL | 4 (26.7%) | 27 (58.7%) | 4 (26.7%) | 4 (26.7%) | ||
| Child-Pugh class (%) | 0.984 | 1.000 | ||||
| A | 14 (93.3%) | 43 (93.5%) | 14 (93.3%) | 14 (93.3%) | ||
| B | 1 (6.7%) | 3 (6.5%) | 1 (6.7%) | 1 (6.7%) | ||
| Tumor number (%) | 0.125 | 1.000 | ||||
| 1 | 10 (66.7%) | 39 (84.8%) | 10 (66.7%) | 10 (66.67%) | ||
| > 1 | 5 (33.3%) | 7 (15.2%) | 5 (33.3%) | 5 (33.33%) | ||
| Tumor size (cm) | 10.7 ± 4.5 | 7.7 ± 4.8 | 0.033 | 10.7 ± 4.5 | 9.0 ± 4.9 | 0.332 |
| Vascular invasion (%) | 0.952 | 0.705 | ||||
| Yes | 6 (40.0%) | 18 (39.1%) | 6 (40.0%) | 5 (33.3%) | ||
| No | 9 (60.0%) | 28 (60.9%) | 9 (60.0%) | 10 (66.6%) | ||
| Extrahepatic metastasis (%) | 1.000 | 1.000 | ||||
| Yes | 0 (0%) | 1 (2.2%) | 0 (0%) | 0 (0%) | ||
| No | 15 (100%) | 45 (97.8%) | 15 (100%) | 15 (100%) | ||
The average operation time of stage-I ALPPS, stage-II ALPPS, and right hemi-hepatectomy was 342 min (range, 229-459 min), 293 min (range, 167-400 min), and 338 (range, 140-515) min, respectively, while the mean intraoperative bleeding volume was 230 (range, 100-500) mL, 619 mL (range, 200-1800 mL), and 344 (range, 190-638) mL, respectively. There was no allogeneic blood transfusion in stage-I ALPPS, while four cases in stage-II ALPPS required allogeneic blood transfusion and one case received leukocyte-depleted red blood cell suspension 2 U after right hemi-hepatectomy. All surgical margins were resected with R0. The median interval between the first stage of ALPPS and the second one was 15 d (range, 9-27 d).
No ALPPS group patients experienced postoperative bile leakage, while two right hemi-hepatectomy group patients underwent postoperative bile leakage. By the Clavien-Dino criteria[12], for stage-I ALPPS, the number of patients with grade I, grade II, and grade III postoperative complications was 13, 1, and 1, respectively. For stage-II ALPPS, the number of patients with grade I, grade II, grade III, and grade IV postoperative complications was 8, 4, 2, and 1, respectively. Whereas, for right hemi-hepatectomy, the number of patients with grade I, grade II, grade III, and grade IV postoperative complications was 9, 4, 1, and 1, respectively. All other complications were cured, except that a stage-II ALPPS patient rated as grade IV due to postoperative liver failure and a right hepatectomy patient with respiratory failure rated as grade IV died during the perioperative period.
The 15 cases of ALPPS patients underwent postoperative liver failure classification by the International Study Group of Liver Surgery standards[13]. After stage-I ALPPS, four were graded as A, 10 as B, and 1 as C and after stage-II ALPPS, 4 were graded as A, 9 as B, and 2 as C. For the right hepatectomy group, the number of cases graded as A, B, and C was 6, 8, and 1, respectively. One patient of the ALPPS group died on the 32nd d after the second stage, while one of the right hepatectomy group died on the 28th d after the operation (Table 2).
| ALPPS | Hepatectomy | ||
| Stage-I ALPPS | Stage-II ALPPS | ||
| Surgery time (min) | 342 (229-459) | 293 (167-400) | 338 (140-515) |
| Intraoperative blood loss (mL) | 230 (100-500) | 619 (200-1800) | 344 (190-638) |
| Postoperative bile leakage (yes/no) | 0/15 | 0/15 | 2/13 |
| Postoperative complications, Clavien-Dino (I/II/III/IV) | 13/1/1/0 | 8/4/2/1 | 9/4/1/1 |
| Classification of postoperative liver failure, ISGLS (A/B/C) | 4/10/1 | 4/9/2 | 6/8/1 |
| 90 d survival after operation (death/alive) | 0/15 | 1/14 | 1/14 |
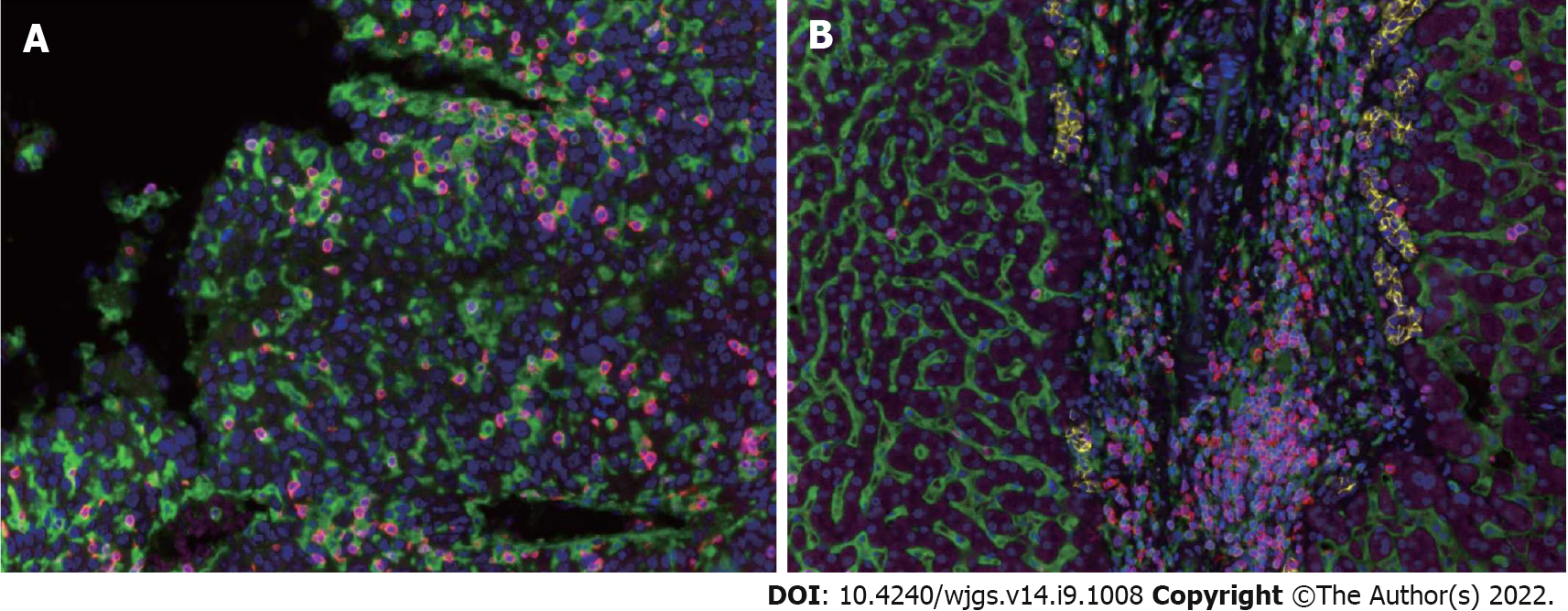
TILs are an important component of the TME involved in the local immune response, and their degree of infiltration greatly affects tumor growth and progression. In order to determine the infiltration degree and trend change of TILs in the HCC microenvironment, we took tissues from 15 cases of ALPPS and 15 matched patients with right hepatectomy. Cancerous tissues and para-cancerous tissues were used to make tissue microarrays. The specific marker molecules of lymphocyte subsets in the TME underwent polychromatic immunohistochemical staining. The results showed that the infiltration pattern of TILs in cancer tissues was significantly different from that in para-cancerous tissues. The infiltration of TILs in cancer tissues was irregular and diffusely distributed. Whereas, in para-cancerous tissues, TILs were mainly concentrated in the connective tissues of the interlobular portal area, often accompanied by three kinds of ducts: Interlobular artery, interlobular vein, and interlobular bile duct (Figure 2).
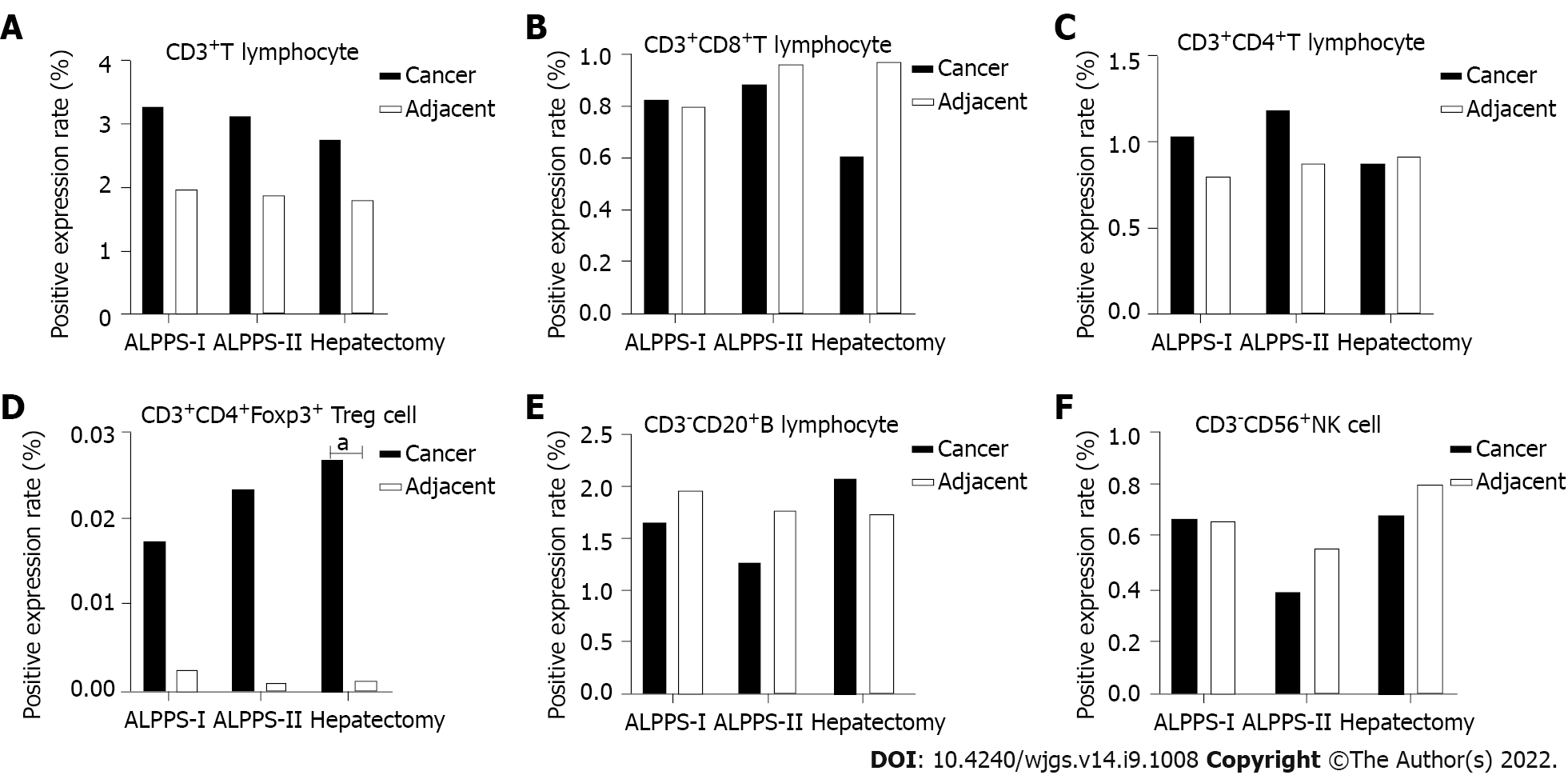
The quantitative analysis showed the number of target cells and the total number of all nucleated cells. The positive expression levels of six TIL subsets of T cells, CD8+ T cells, CD4+ T cells, Treg cells, B cells, and NK cells in the same spatial tissues were calculated. Furthermore, the TILs of the right hemi-hepatectomy group, ALPPS group (including stages I and II), and cancer or para-cancerous tissues were compared and analyzed (Figure 3). The results showed that the positive expression level of Treg cells in the cancer tissues was significantly higher than that of the adjacent tissues (P = 0.043, Tables 3-6).
| ALPPS | Hepatectomy | Variance analysis | |||
| Stage-I ALPPS | Stage-II ALPPS | F valve | P valve | ||
| Total T cells (%) | 3.3 ± 2.9 | 3.1 ± 2.0 | 2.8 ± 1.8 | 0.188 | 0.829 |
| CD4+ T cells (%) | 1.0 ± 0.9 | 1.2 ± 1.1 | 0.9 ± 0.5 | 0.458 | 0.635 |
| CD8+ T cells (%) | 0.8 ± 0.7 | 1.0 ± 0.9 | 0.6 ± 0.4 | 0.546 | 0.583 |
| Treg cells (‰) | 0.2 ± 0.1 | 0.2 ± 0.2 | 0.3 ± 0.1 | 0.166 | 0.848 |
| B cells (%) | 1.7 ± 1.3 | 1.3 ± 0.7 | 2.1 ± 0.8 | 0.726 | 0.490 |
| NK cells (%) | 0.7 ± 0.2 | 0.4 ± 0.2 | 0.7 ± 0.2 | 0.664 | 0.520 |
| ALPPS | Variance analysis | ||||
| Stage-I ALPPS | Stage-II ALPPS | Hepatectomy | F valve | P valve | |
| Total T cells (%) | 2.0 ± 0.6 | 1.9 ± 0.9 | 1.8 ± 1.3 | 0.129 | 0.879 |
| CD4+ T cells (%) | 0.8 ± 0.3 | 0.9 ± 0.3 | 0.9 ± 0.7 | 0.258 | 0.774 |
| CD8+ T cells (%) | 0.8 ± 0.3 | 1.0 ± 0.5 | 1.0 ± 0.7 | 0.510 | 0.604 |
| Treg cells (‰) | 0.0 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.292 | 0.748 |
| B cells (%) | 2.0 ± 1.2 | 1.8 ± 0.8 | 1.7 ± 0.5 | 0.269 | 0.765 |
| NK cells (%) | 0.7 ± 0.7 | 0.6 ± 0.5 | 0.8 ± 0.7 | 0.550 | 0.581 |
| Stage-I ALPPS | Stage-II ALPPS | |||||
| Tumor | Adjacent | P valve | Tumor | Adjacent | P valve | |
| Total T cells (%) | 3.3 ± 2.9 | 2.0 ± 0.6 | 0.116 | 3.1 ± 2.0 | 1.9 ± 0.9 | 0.056 |
| CD4+ T cells (%) | 1.0 ± 0.9 | 0.8 ± 0.3 | 0.403 | 1.2 ± 1.1 | 0.9 ± 0.3 | 0.278 |
| CD8+ T cells (%) | 0.8 ± 0.7 | 0.8 ± 0.3 | 0.902 | 1.0 ± 0.9 | 1.0 ± 0.5 | 0.792 |
| Treg cells (‰) | 0.2 ± 0.1 | 0.0 ± 0.1 | 0.056 | 0.2 ± 0.2 | 0.0 ± 0.0 | 0.156 |
| B cells (%) | 1.7 ± 1.3 | 2.0 ± 1.2 | 0.515 | 1.3 ± 0.7 | 1.8 ± 0.8 | 0.085 |
| NK cells (%) | 0.7 ± 0.2 | 0.7 ± 0.7 | 0.985 | 0.4 ± 0.2 | 0.6 ± 0.5 | 0.403 |
| Right hemi-hepatectomy | |||
| Tumor tissues | Adjacent tissues | P valve | |
| Total T cells (%) | 2.8 ± 1.8 | 1.8 ± 1.3 | 0.105 |
| CD4+ T cells (%) | 0.9 ± 0.5 | 0.9 ± 0.7 | 0.840 |
| CD8+ T cells (%) | 0.6 ± 0.4 | 1.0 ± 0.7 | 0.101 |
| Treg cells (‰) | 0.3 ± 0.1 | 0.0 ± 0.0 | 0.043 |
| B cells (%) | 2.1 ± 0.8 | 1.7 ± 0.5 | 0.645 |
| NK cells (%) | 0.7 ± 0.2 | 0.8 ± 0.7 | 0.678 |
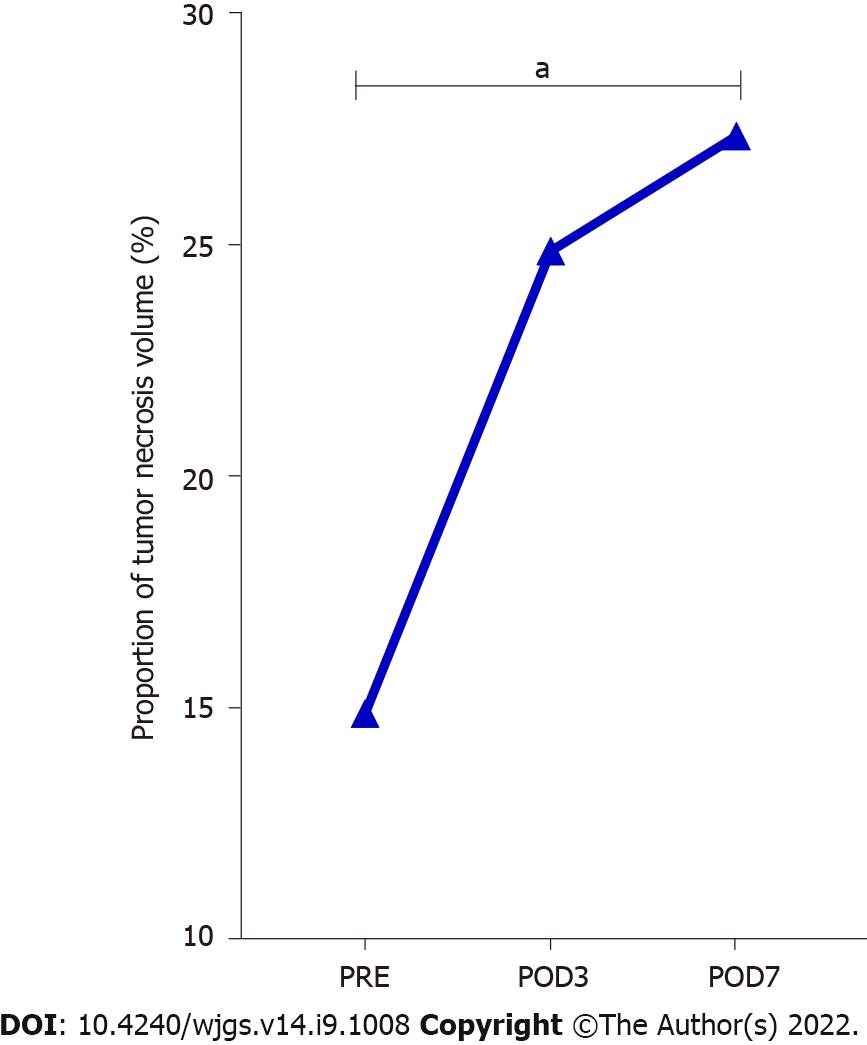
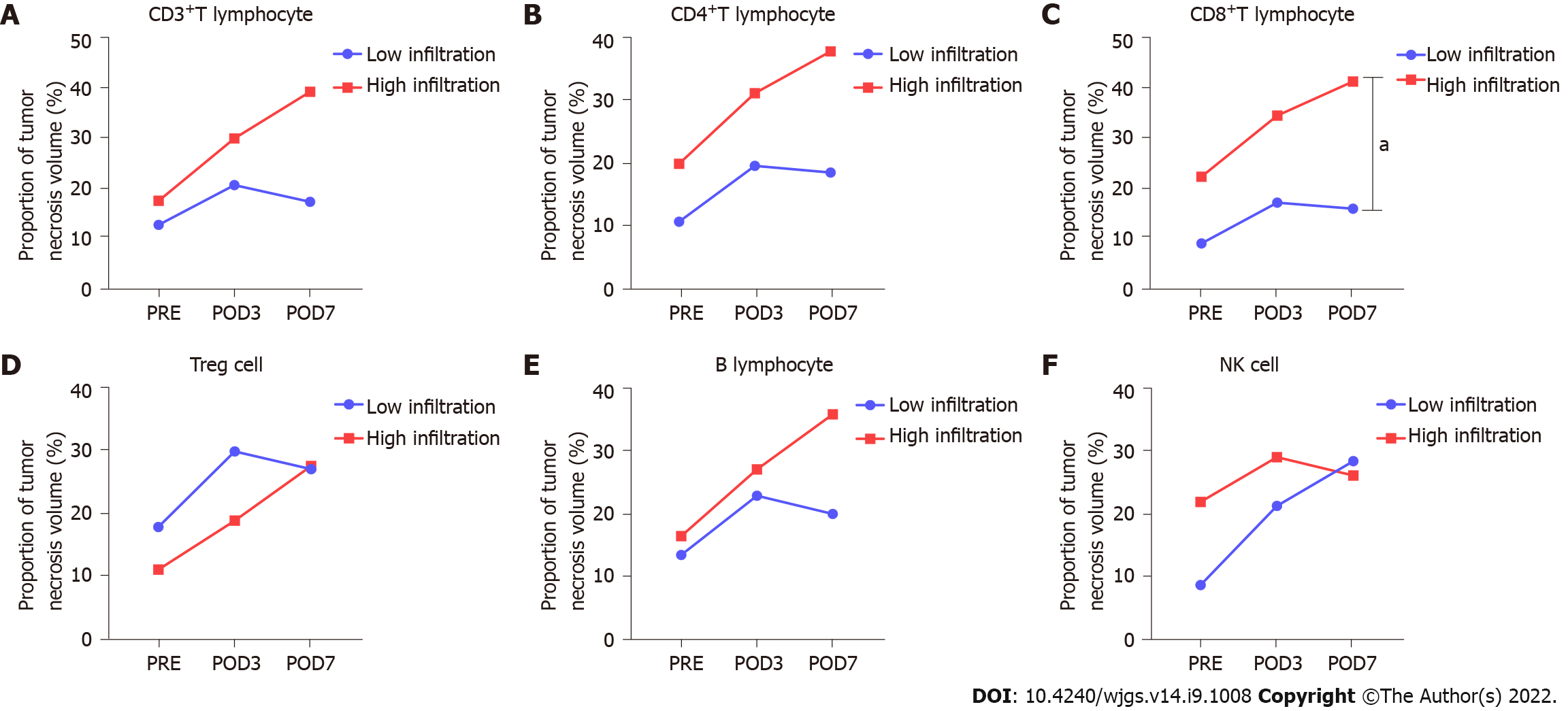
The proportion of tumor necrosis volume was calculated by analyzing the tumor volume and tumor necrosis volume in the perioperative period of stage-I ALPPS (Figure 4). The results showed that the proportion of tumor necrotic volume on the seventh day after stage-I ALPPS was significantly higher than before the operation (P = 0.024, Figure 5). In order to further clarify the relationship between tumor necrosis and TILs in the perioperative period of stage-I ALPPS, the median positive expression level of the six TIL subgroups in stage-I ALPPS cancer tissues was used as the cut-off point. The HCC patients receiving ALPPS treatment were divided into a high-infiltration group and a low-infiltration group. We then compared the difference in the proportion of tumor necrosis volume between the two groups. The results showed that the proportion of tumor necrosis volume in the high CD8+ T cell infiltration group was significantly higher than that in the low CD8+ T cell infiltration group (P = 0.048, Figure 6).
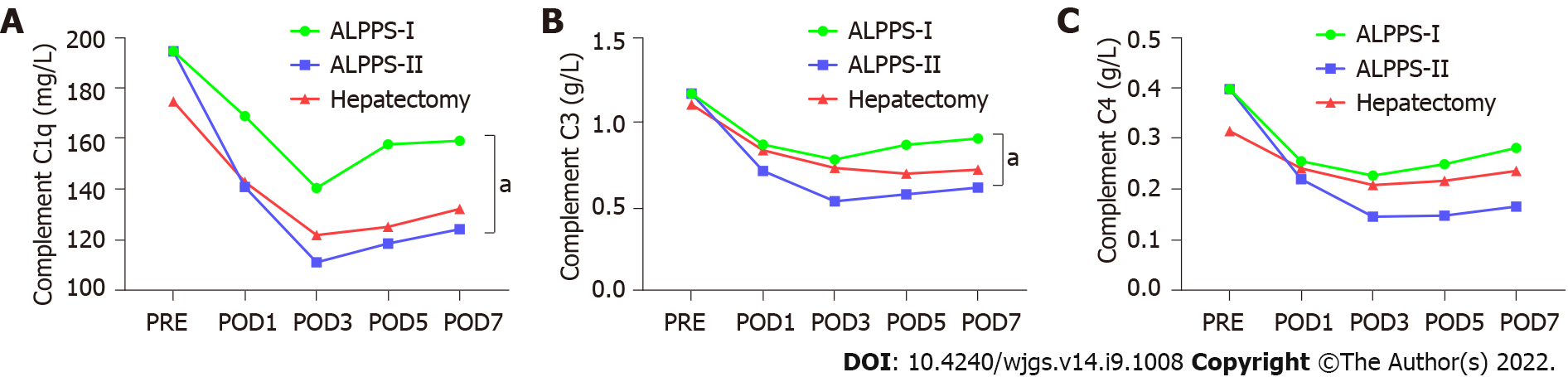
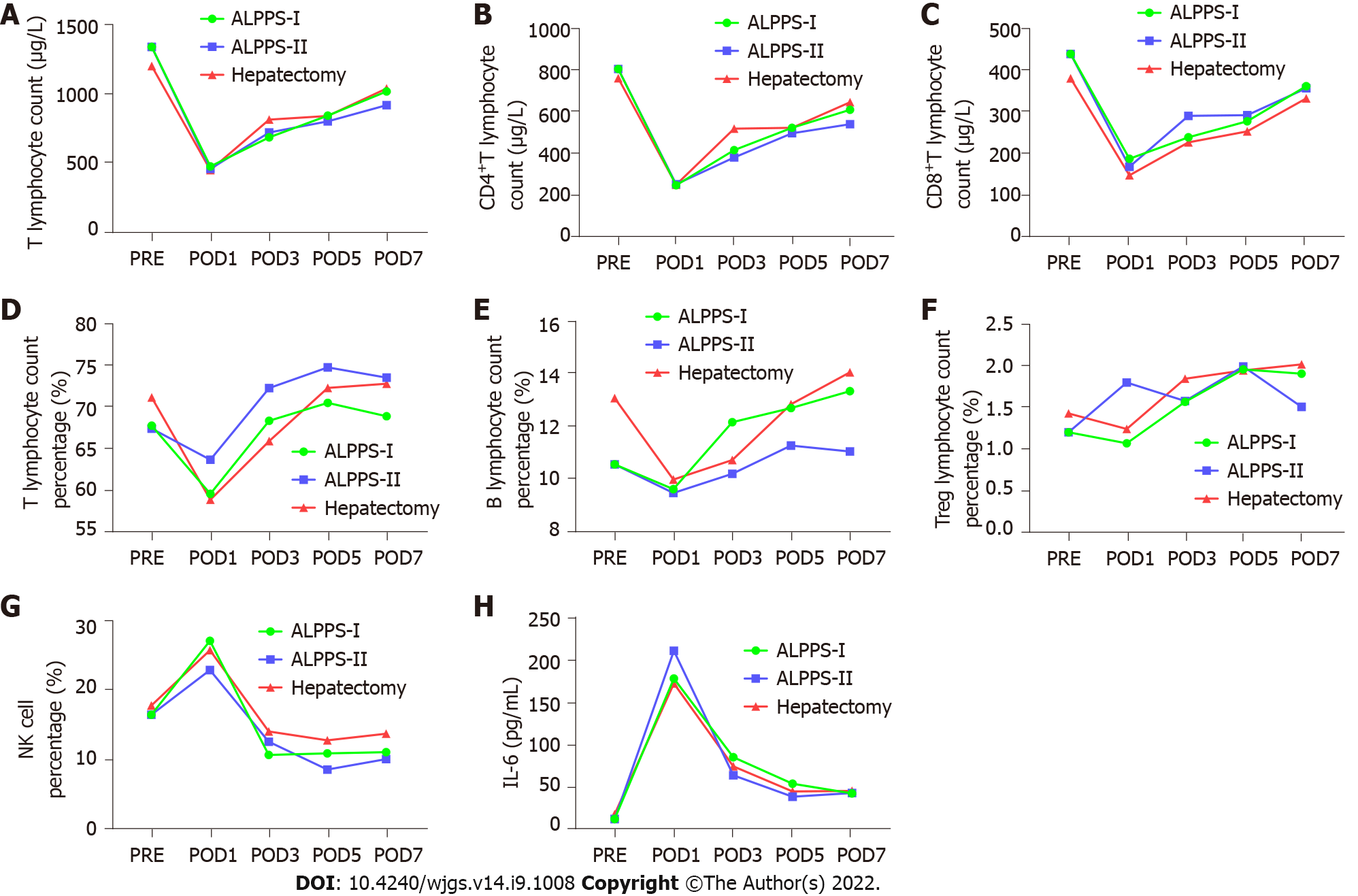
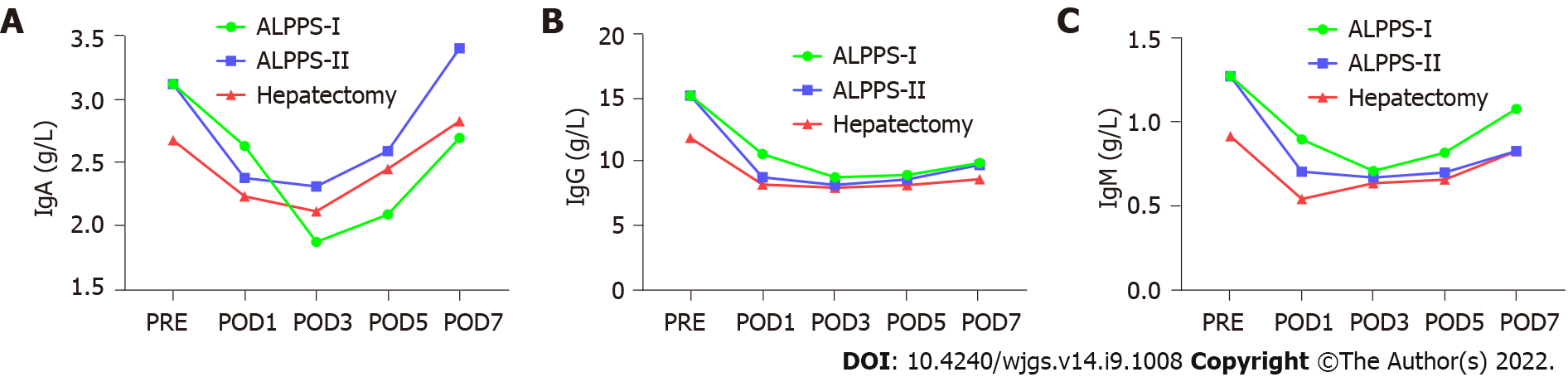
Pairwise comparisons of immune components of peripheral blood were measured between the right hemi-hepatectomy group, stage-I ALPPS group, and stage-II ALPPS group. We found that the components of the complement system, C1q and C3 in peripheral blood in stage-I ALPPS, were significantly higher than those in stage II (C1q: P = 0.007; C3: P = 0.047, Figure 7). In addition, interleukin (IL)-6 levels in the stage-I ALPPS and stage-II ALPPS increased significantly and reached a peak value on the first day after surgery, and then decreased rapidly but were significantly higher than the preoperative level (P1 = 0.000, P2 = 0.002). NK cells in stage-I and stage-II ALPPS temporarily increased on the first day after surgery and gradually decreased on the second day after surgery to figures lower than the preoperative level (Figure 8). There was no significant difference in other remaining peripheral blood indicators among the groups (P > 0.05, Figure 9, Tables 7-9).
| Item | Preoperative | Stage-I ALPPS | F value | P value | |||
| POD1 | POD3 | POD5 | POD7 | ||||
| T lymphocyte count (cells/μL) | 1331.5 ± 600.0 | 472.8 ± 289.9 | 682.0 ± 346.9 | 837.9 ± 383.6 | 1012.5 ± 444.2 | 10.095 | 0.001 |
| Total T lymphocyte percentage (%) | 67.8 ± 8.7 | 59.6 ± 8.5 | 68.4 ± 12.3 | 70.5 ± 11.7 | 68.9 ± 10.3 | 6.717 | 0.000 |
| CD4+ T lymphocytes (cells/μL) | 806.3 ± 428.2 | 241.1 ± 202.2 | 412.3 ± 224.7 | 520.1 ± 255.1 | 608.0 ± 266.1 | 9.049 | 0.002 |
| CD8+ T lymphocyte (cells/μL) | 438.2 ± 194.2 | 187.6 ± 96.6 | 238.9 ± 141.0 | 277.4 ± 143.5 | 361.2 ± 201.6 | 11.294 | 0.001 |
| Natural killer cells (%) | 16.5 ± 7.8 | 27.2 ± 8.6 | 10.8 ± 6.9 | 11.0 ± 3.7 | 11.2 ± 3.7 | 17.341 | 0.000 |
| IgA (g/L) | 3.11 ± 1.28 | 2.63 ± 1.64 | 1.87 ± 0.77 | 2.08 ± 0.79 | 2.69 ± 1.24 | 10.025 | 0.001 |
| IgG (g/L) | 15.11 ± 3.70 | 10.52 ± 2.89 | 8.70 ± 2.72 | 8.89 ± 2.69 | 9.82 ± 2.70 | 62.360 | 0.000 |
| IgM (g/L) | 1.27 ± 0.68 | 0.89 ± 0.40 | 0.71 ± 0.39 | 0.82 ± 0.38 | 1.08 ± 0.53 | 6.114 | 0.008 |
| Complement C1q (mg/L) | 194.5 ± 28.7 | 168.9 ± 44.2 | 140.4 ± 39.6 | 157.7 ± 45.9 | 159.17 ± 55.3 | 6.726 | 0.000 |
| Complement C3 (g/L) | 1.18 ± 0.19 | 0.87 ± 0.15 | 0.79 ± 0.19 | 0.87 ± 0.21 | 0.91 ± 0.26 | 44.808 | 0.000 |
| Complement C4 (g/L) | 0.40 ± 0.18 | 0.26 ± 0.12 | 0.23 ± 0.11 | 0.25 ± 0.12 | 0.28 ± 0.15 | 8.731 | 0.002 |
| Interleukin-6 (g/L) | 10.7 ± 17.0 | 177.4 ± 121.6 | 84.0 ± 62.3 | 52.6 ± 40.9 | 41.2 ± 35.1 | 7.877 | 0.003 |
| CD19 expression rate (%) | 10.5 ± 4.0 | 9.6 ± 5.62 | 12.1 ± 5.6 | 12.7 ± 5.2 | 13.3 ± 6.1 | 1.866 | 0.129 |
| Item | Preoperative | Stage-II ALPPS | F value | P value | |||
| POD1 | POD3 | POD5 | POD7 | ||||
| T lymphocyte count (cells/μL) | 1414.4 ± 634.0 | 455.9 ± 255.4 | 716.3 ± 311.3 | 796.3 ± 282.8 | 913.4 ± 387.1 | 17.626 | 0.000 |
| Total T lymphocyte percentage (%) | 67.8 ± 8.7 | 63.7 ± 9.2 | 72.3 ± 7.5 | 74.8 ± 6.4 | 73.6 ± 7.2 | 8.288 | 0.000 |
| CD4+ T lymphocytes (cells/μL) | 806.3 ± 428.2 | 246.4 ± 168.2 | 375.9 ± 170.1 | 493.9 ± 196.7 | 537.9 ± 231.3 | 7.925 | 0.003 |
| CD8+ T lymphocytes (cells/μL) | 438.2 ± 194.2 | 168.5 ± 89.4 | 290.3 ± 184.7 | 291.9 ± 159.0 | 356.1 ± 210.8 | 8.775 | 0.000 |
| Natural killer cells (%) | 16.5 ± 7.8 | 23.0 ± 7.1 | 12.7 ± 4.6 | 8.7 ± 4.8 | 10.2 ± 3.2 | 15.615 | 0.000 |
| IgA (g/L) | 3.11 ± 1.28 | 2.37 ± 1.88 | 2.31 ± 1.41 | 2.59 ± 1.30 | 3.40 ± 1.66 | 8.900 | 0.002 |
| IgG (g/L) | 15.11 ± 3.70 | 8.71 ± 2.10 | 8.11 ± 1.90 | 8.53 ± 1.66 | 9.68 ± 2.26 | 12.604 | 0.000 |
| IgM (g/L) | 1.27 ± 0.68 | 0.70 ± 0.37 | 0.67 ± 0.28 | 0.70 ± 0.36 | 0.83 ± 0.37 | 1.277 | 0.001 |
| Complement C1q (mg/L) | 194.5 ± 28.7 | 140.8 ± 33.8 | 111.1 ± 39.1 | 118.5 ± 41.3 | 124.2 ± 42.1 | 14.422 | 0.000 |
| Complement C3 (g/L) | 1.18 ± 0.19 | 0.72 ± 0.27 | 0.54 ± 0.20 | 0.58 ± 0.20 | 0.62 ± 0.18 | 24.345 | 0.000 |
| Complement C4 (g/L) | 0.40 ± 0.18 | 0.22 ± 0.16 | 0.15 ± 0.12 | 0.15 ± 0.14 | 0.17 ± 0.16 | 15.305 | 0.000 |
| Interleukin-6 (pg/mL) | 10.7 ± 17.0 | 210.3 ± 160.9 | 62.6 ± 27.6 | 37.1 ± 19.7 | 41.6 ± 61.3 | 12.206 | 0.000 |
| CD19 expression rate (%) | 10.5 ± 4.0 | 9.4 ± 5.1 | 10.2 ± 4.1 | 11.2 ± 5.8 | 11.0 ± 5.6 | 0.522 | 0.720 |
| Item | Preoperative | Conventional hepatectomy | F value | P value | |||
| POD1 | POD3 | POD5 | POD7 | ||||
| T lymphocyte count (cells/μL) | 1194.7 ± 305.4 | 447.1 ± 240.9 | 808.8 ± 313.7 | 835.7 ± 323.7 | 1032.7 ± 323.6 | 123.342 | 0.000 |
| Total T lymphocyte percentage (%) | 71.2 ± 5.2 | 58.9 ± 14.5 | 65.9 ± 11.7 | 72.3 ± 9.1 | 72.8 ± 7.7 | 17.676 | 0.000 |
| CD4+ T lymphocytes (cells/μL) | 761.1 ± 146.6 | 244.1 ± 113.9 | 515.9 ± 155.1 | 520.7 ± 168.7 | 644.8 ± 149.1 | 198.675 | 0.000 |
| CD8+T lymphocytes (cells/μL) | 379.9 ± 119.0 | 147.9 ± 98.5 | 226.8 ± 104.5 | 253.0 ± 110.4 | 331.8 ± 120.9 | 106.219 | 0.000 |
| Natural killer cells (%) | 17.9 ± 4.7 | 25.8 ± 8.4 | 14.1 ± 3.0 | 12.9 ± 4.6 | 13.8 ± 3.8 | 12.893 | 0.000 |
| IgA (g/L) | 2.67 ± 1.49 | 2.23 ± 1.34 | 2.11 ± 1.32 | 2.44 ± 1.50 | 2.82 ± 1.60 | 19.117 | 0.000 |
| IgG (g/L) | 11.78 ± 5.58 | 8.14 ± 3.97 | 7.89 ± 3.98 | 8.09 ± 4.05 | 8.55 ± 3.95 | 35.249 | 0.000 |
| IgM (g/L) | 0.91 ± 0.39 | 0.54 ± 0.31 | 0.63 ± 0.26 | 0.66 ± 0.27 | 0.82 ± 0.30 | 24.051 | 0.000 |
| Complement C1q (mg/L) | 174.6 ± 51.3 | 142.8 ± 49.9 | 121.9 ± 46.9 | 125.1 ± 52.1 | 132.2 ± 47.0 | 39.750 | 0.000 |
| Complement C3 (g/L) | 1.11 ± 0.45 | 0.84 ± 0.41 | 0.74 ± 0.37 | 0.70 ± 0.36 | 0.73 ± 0.37 | 31.517 | 0.000 |
| Complement C4 (g/L) | 0.32 ± 0.13 | 0.24 ± 0.11 | 0.21 ± 0.11 | 0.22 ± 0.11 | 0.24 ± 0.12 | 37.071 | 0.000 |
| Interleukin-6 (pg/mL) | 16.3 ± 17.7 | 171.6 ± 119.2 | 73.3 ± 46.3 | 43.5 ± 28.8 | 44.1 ± 31.1 | 8.981 | 0.002 |
| CD19 expression rate (%) | 13.04 ± 2.21 | 9.93 ± 3.05 | 10.68 ± 3.40 | 12.81 ± 4.37 | 14.03 ± 3.62 | 11.115 | 0.000 |
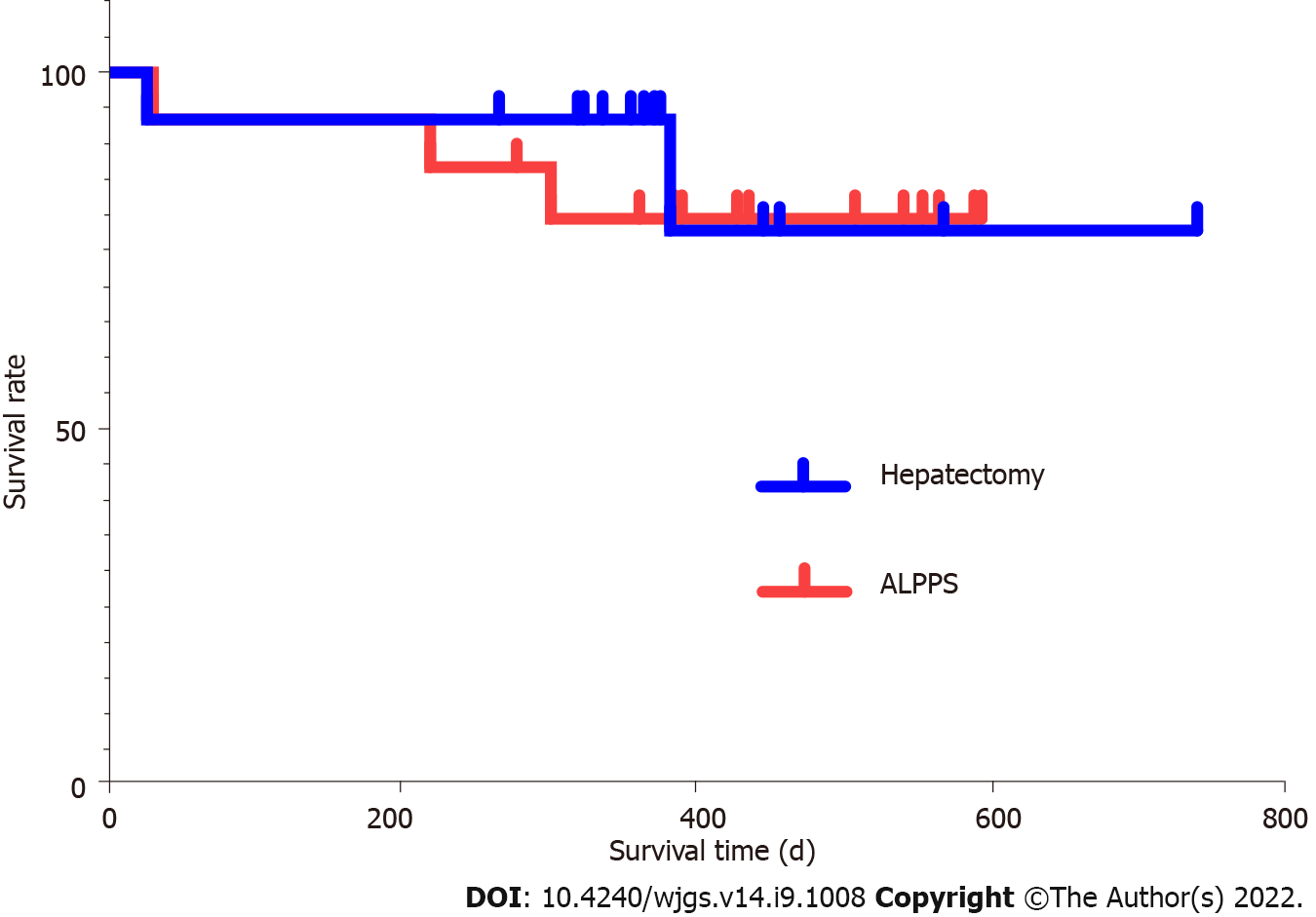
The ALPPS and right hemi-hepatectomy group patients were followed after the surgery. As of May 20, 2020, the median follow-up time of ALPPS group patients and that of right hemi-hepatectomy group patients were 472 d (279-607 d) and 449 d (267-740 d), respectively. There was no significant difference in follow-up time between the two groups (P = 0.528). The survival rate of the ALPPS group and that of the right hemi-hepatectomy group showed no significant difference (Figure 10, log-rank test P = 0.733). During the 90-d follow-up, one person died after stage-II ALPPS, and one died after hemi-hepatectomy; the mortality rate in each group was 6.67% (1/15).
As a planned step-by-step hepatectomy, ALPPS involves strict requirements for liver anatomy, degree of FLR hyperplasia, liver volume evaluation, and patient screening. Stage-I ALPPS separates the left hepatic lobe and the right one and ligates the right hepatic vein, resulting in an inflammatory reaction, hypoxia, tumor necrosis, and other factors, thus leading to a unique and complex immune microenvironment of tumor cells. Therefore, it is necessary to understand such immunological effects of the unique TME formed during HCC treatment by ALPPS from an immunological perspective as anti-tumor effect or tumor-induced immunosuppression. HCC treatment by ALPPS, the subsequent recruitment and change of TILs in the TME, and its effect on the tumor are still not completely understood. To verify the safety of ALPPS in treating massive HCC, more in-depth research on TILs in the TME is needed.
In order to determine the perioperative changes of TILs in patients with massive HCC in the right lobe treated by ALPPS and its effect on the tumor, we used PSM analysis on 15 HCC patients treated by ALPPS and 15 HCC patients treated by right hemi-hepatectomy. The results showed that all clinical baseline and tumor nature trends of the two groups were similar. The PSM method was used to reduce the selection deviation and baseline difference to make the sample data of the two groups more comparable[14]. Meanwhile, cancer and para-cancerous histopathological specimens of the right hemi-hepatectomy group and the ALPPS group were collected. The positive expression levels of TIL subsets were detected by polychromatic immunohistochemical staining. The results showed no significant differences in the six main TIL subsets between the ALPPS and right hepatectomy groups or between the cancerous and adjacent tissues in the same group. Especially during the “isolated” period of tumor-bearing right hepatic lobe between stage-I ALPPS and stage-II ALPPS, the positive expression levels of TIL subsets did not change significantly. It indicated that the degree of TIL infiltration in the TME has not changed due to the traumatic stress of ALPPS surgery and the persistence of stage-I ALPPS to II tumors, which provides a basis for the operation of tumor local immune function and the body’s resistance to tumor invasion. Previous studies have shown that the decrease in the invasion of TILs could promote tumor immune escape and malignant progression and limit the effect of immunotherapy, leading to a poor prognosis. In contrast, the increase in the infiltration degree of TILs produces the opposite result[15-17].
This study showed that the level of TIL infiltration during the perioperative period of ALPPS maintains a dynamic balance, suggesting that there is no adverse effect on TIL infiltration due to the surgical methods of ALPPS. To further verify the correlation between TILs and HCC, we measured the tumor volume and tumor necrotic volume before stage-I ALPPS operation and 3 d and 7 d after the stage-I ALPPS operation. We further calculated the ratio of tumor necrotic volume to tumor volume. We found an increase in tumor necrosis volume proportion, gradually from stage I to stage II of ALPPS, which might be caused by ligation of the right hepatic vein during ALPPS operation[18,19].
TILs play a central role in tumor local immune response, and their infiltration levels largely determine the severity of immune response. This is the main reason for using TILs to evaluate the intensity of immune response induced by ALPPS in this study. T cells not only mediate cellular immune response but also participate in humoral immune response induced by thymus-dependent antigen. CD8+ T cells, also known as cytotoxic T cells, are the primary effector cells of the immune system against tumors. They can kill tumor cells efficiently through the perforin-granzyme pathway, Fas-FasL pathway, and tumor necrosis factor (TNF)-TNF receptor pathway[20,21]. Studies have shown that the local low level of CD8+ T cell infiltration makes the tumor grow and progress more rapidly. Here, we found a correlation between the infiltration level of CD8+ T cells and the degree of tumor necrosis. The proportion of tumor necrotic volume in the perioperative stage-I ALPPS gradually increased with time. Moreover, the proportion of tumor necrotic volume in the high CD8+ T cell infiltration group was significantly higher than that in the low infiltration group. Based on the fact that there was no difference in the expression levels of CD8+ T cells between the cancer tissues of the ALPPS group and the right hepatectomy group, it can be inferred that after stage-I ALPPS, the right lobe of the tumor-bearing liver is segregated and the right hepatic vein is ligated, while CD8+ T cells can still effectively infiltrate the TME, thus exerting cytotoxicity to kill tumor cells. This result also proves that CD8+ T cells do not reduce their infiltration degree due to the ALPPS operation and maintain the stability of the immune system’s killing function.
Components of the peripheral blood circulatory system, including T cells, B cells, Treg cells, NK cells, IL-6, complement components (C1q, C3, and C4), and immunoglobulins (IgA, IgG, and IgM) can comprehensively reflect the immune function of the body. NK cells are the primary killer cells in innate immunity and can produce cytotoxic effects on tumor cells[20]. Among the peripheral blood immune indicators tested, NK cells temporarily increased on the first day after stage-I and stage-II ALPPS. They then gradually decreased to a lower level than the preoperative one. This trend may be related to the inhibitory effect of Treg cells on NK cells. One study has shown that higher serum IL-6 levels are associated with an increased risk of adverse HCC[22]. In this study, IL-6 in stage-I and II ALPPS increased significantly on the first postoperative day, and reached a peak. However, their levels were consistently higher than the preoperative levels. The levels after Stage-I and II ALPPS were significantly higher than that before surgery (P1 = 0.000, P2 = 0.002). This phenomenon might be related to the “waterfall” inflammation and persistent inflammation stimulus caused by surgical strikes. It is reported that the serum complement C1q increases significantly in the occurrence and development of nonalcoholic fatty liver[23]. In addition, complement C3 is involved in the occurrence and development of alcoholic hepatitis, thus inducing liver cancer[24]. In our study, the contents of complement C1q and C3 in peripheral blood after tumor removal in stage-II ALPPS were significantly lower than those in stage-I ALPPS. Finally, there was no significant change in serum IgA, IgG, or IgM levels between stage-I and stage-II ALPPS, indicating that the two-stage surgery performed by ALPPS did not cause excessive physiological stress or inflammation. In summary, comparing the changing trend of peripheral blood immune components in different groups showed that the traumatic stress and inflammatory reaction caused by right hepatectomy and ALPPS are similar. The ALPPS procedure did not cause more severe immunosuppression due to the “radical” surgical strategy, which is consistent with previously reported results[25].
In the past few decades, researchers have gained a deeper understanding of the importance of the TME in the occurrence, development, invasion, and metastasis of HCC[26]. The dynamic changes of the TME significantly affect the tumor biological characteristics of HCC. The TME is thought to have an active interaction with tumors, not just the passive structural support for tumor growth or survival. Therefore, more researchers are actively studying to understand the TME and its interaction with HCC cells. Because each component of the TME plays a complex role and influences one another, targeting a specific component of the TME is usually of little effect. It can be seen that a better understanding of the biological effects and molecular interactions between each component of the TME and tumor cells is crucial for understanding the mechanism and development of tumorigenesis.
In 1988, Rosenberg et al[27] invented the TIL therapy. Lymphocytes were isolated and extracted from the patient’s body, amplified in vitro, and then infused back into the patient’s body, opening up a new avenue in the field of tumor treatment. After years of continuous development and improvement, various new therapies based on TIL therapies have come out, such as chimeric antigen receptor T cell immunotherapy (CAR-T) and T cell receptor chimeric T cell immunotherapy (T cell receptor-modified T cell immunotherapy, TCR-T)[28-30]. CAR-T and TCR-T cells are T cells that have been directionally modified and screened by genetic engineering technology, which strengthens the ability to recognize tumor cells or tumor-associated antigens. They can change the local immune suppression microenvironment induced by tumors and reverse tumor immunity tolerance status, showing good safety and effectiveness in treating various cancers. CAR-T therapy has a significant effect on hematological tumors[31,32], and TCR-T therapy has achieved good results in melanoma[33], multiple myeloma[34], lung cancer[35], and ovarian cancer[36]. The two therapies still face many challenges in treating solid tumors, such as low and uneven treatment response rates, local immunosuppressive effects of the TME, and lack of high-efficiency molecular targets[37,38]. However, the global R&D boom has continued, and several studies on TIL treatment of tumors have entered the clinical trial stage. Given the critical role of TILs in tumor local immunity, various new types of “TIL therapies” have developed rapidly, and significant breakthroughs have been continuously made in the field of tumor treatment. As an essential branch of tumor immunotherapy, TIL therapy is one of the indispensable directions for future medical development. The global multi-center and multi-organization collaboration can promote the standardization of ALPPS surgery and large-scale data statistics. Therefore, it is necessary to deeply understand the trend of TIL changes caused by ALPPS surgery.
From an immunological perspective, this study describes the change in the trend of the TME during the perioperative period of ALPPS. We demonstrate that ALPPS is safe and feasible for massive HCC in the right lobe of the liver. However, this study is a single-center study, with a limited number of patients and clinical data, thus, more in-depth discussion on the conclusions is required.
The level of TIL infiltration can maintain a dynamic balance during the perioperative period of ALPPS, which is the basis for the normal tumor local immune response. Compared with the right hepatectomy, ALPPS does not cause a decrease in TIL infiltration and the pathological changes of immune com
Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) is an innovative approach to hepatectomy. The surgical trauma experienced by ALPPS is relatively high. In addition, stage-I ALPPS separates the right and left liver lobes and ligates the right hepatic vein, which causes inflammatory reactions, hypoxia, and tumor necrosis, resulting in a unique and complex immune microenvironment for tumor cells.
The trends and effects of tumor-infiltrating lymphocytes (TIL) residing or recruited in the tumor microenvironment (TME) are still unexplored in studies on ALPPS for hepatocellular carcinoma (HCC).
From an immunological perspective, the immunological effects exerted by the unique TME formed during the treatment of HCC by ALPPS, such as anti-tumor effects or tumor-induced immunosuppression, were investigated to further evaluate the safety and efficacy of ALPPS in treating massive HCC and conduct an in-depth study of TILs in the TME.
Patients of the ALPPS and hemi-hepatectomy groups were screened using propensity score matching. Immunofluorescence staining was performed to detect and quantify TILs in tumors and adjacent tissues in these two groups of patients. Trends in TILs in peripheral blood during the perioperative period were compared between the two groups.
The proportion of tumor necrosis volume at postoperative day 7 after stage-I ALPPS was significantly higher than the pre-operative value (P = 0.024). The proportion of tumor necrosis volume was significantly higher in the high CD8+ T-cell infiltrated group than in the low group before surgery for stage-I ALPPS (P = 0.048).
From an immunological point of view, ALPPS is safe and feasible for treating right lobe massive HCC. The level of TIL infiltration during the perioperative period is dynamically balanced, and the ALPPS procedure itself does not lead to severe immunosuppression due to reduced TIL infiltration and pathological changes in peripheral blood immune components.
Many studies on TIL therapy for tumors have entered clinical trials. As an important branch of tumor immunotherapy, TIL therapy is one of the potential directions for the future development of medicine.
The authors would like to thank Zong-Rui Jin, Guo-Lin Wu, Jue Wang, Qi-Ling Yi, Zhu-Jing Lan, and Ke-Yu Huang for their help in volumetric measurement of the liver and collection of follow-up data.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Perivoliotis K, Greece; Yang M, United States S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64335] [Article Influence: 16083.8] [Reference Citation Analysis (174)] |
| 2. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1635] [Article Influence: 204.4] [Reference Citation Analysis (0)] |
| 3. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4086] [Article Influence: 583.7] [Reference Citation Analysis (6)] |
| 4. | Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R, Kroemer A, Loss M, Rümmele P, Scherer MN, Padberg W, Königsrainer A, Lang H, Obed A, Schlitt HJ. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 932] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 5. | Linecker M, Björnsson B, Stavrou GA, Oldhafer KJ, Lurje G, Neumann U, Adam R, Pruvot FR, Topp SA, Li J, Capobianco I, Nadalin S, Machado MA, Voskanyan S, Balci D, Hernandez-Alejandro R, Alvarez FA, De Santibañes E, Robles-Campos R, Malagó M, de Oliveira ML, Lesurtel M, Clavien PA, Petrowsky H. Risk Adjustment in ALPPS Is Associated With a Dramatic Decrease in Early Mortality and Morbidity. Ann Surg. 2017;266:779-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 6. | Anantha RV, Shaler CR, Meilleur CE, Parfitt J, Haeryfar SM, Hernandez-Alejandro R. The Future Liver Remnant in Patients Undergoing the Associating Liver Partition with Portal Vein Ligation for Staged Hepatectomy (ALPPS) Maintains the Immunological Components of a Healthy Organ. Front Med (Lausanne). 2016;3:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Deng Z, Jin Z, Qin Y, Wei M, Wang J, Lu T, Zhang L, Zeng J, Bao L, Guo Y, Peng M, Xu B, Wen Z. Efficacy of the association liver partition and portal vein ligation for staged hepatectomy for the treatment of solitary huge hepatocellular carcinoma: a retrospective single-center study. World J Surg Oncol. 2021;19:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | He YB, Bai L, Jiang Y, Ji XW, Tai QW, Zhao JM, Zhang JH, Liu WY, Wen H. Application of a Three-Dimensional Reconstruction Technique in Liver Autotransplantation for End-Stage Hepatic Alveolar Echinococcosis. J Gastrointest Surg. 2015;19:1457-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Fu-Gui L, Lu-Nan Y, Bo L, Yong Z, Tian-Fu W, Ming-Qing X, Wen-Tao W, Zhe-Yu C. Estimation of standard liver volume in Chinese adult living donors. Transplant Proc. 2009;41:4052-4056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Oldhafer KJ, Stavrou GA, van Gulik TM; Core Group. ALPPS--Where Do We Stand, Where Do We Go? Ann Surg. 2016;263:839-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12751] [Cited by in RCA: 13075] [Article Influence: 523.0] [Reference Citation Analysis (0)] |
| 12. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24753] [Article Influence: 1178.7] [Reference Citation Analysis (0)] |
| 13. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, Banting S, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Yokoyama Y, Fan ST, Nimura Y, Figueras J, Capussotti L, Büchler MW, Weitz J. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1224] [Cited by in RCA: 1721] [Article Influence: 122.9] [Reference Citation Analysis (0)] |
| 14. | Yang JY, Webster-Clark M, Lund JL, Sandler RS, Dellon ES, Stürmer T. Propensity score methods to control for confounding in observational cohort studies: a statistical primer and application to endoscopy research. Gastrointest Endosc. 2019;90:360-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Garnelo M, Tan A, Her Z, Yeong J, Lim CJ, Chen J, Lim KH, Weber A, Chow P, Chung A, Ooi LL, Toh HC, Heikenwalder M, Ng IO, Nardin A, Chen Q, Abastado JP, Chew V. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut. 2017;66:342-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 358] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 16. | Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, Porta-Pardo E, Gao GF, Plaisier CL, Eddy JA, Ziv E, Culhane AC, Paull EO, Sivakumar IKA, Gentles AJ, Malhotra R, Farshidfar F, Colaprico A, Parker JS, Mose LE, Vo NS, Liu J, Liu Y, Rader J, Dhankani V, Reynolds SM, Bowlby R, Califano A, Cherniack AD, Anastassiou D, Bedognetti D, Mokrab Y, Newman AM, Rao A, Chen K, Krasnitz A, Hu H, Malta TM, Noushmehr H, Pedamallu CS, Bullman S, Ojesina AI, Lamb A, Zhou W, Shen H, Choueiri TK, Weinstein JN, Guinney J, Saltz J, Holt RA, Rabkin CS; Cancer Genome Atlas Research Network, Lazar AJ, Serody JS, Demicco EG, Disis ML, Vincent BG, Shmulevich I. The Immune Landscape of Cancer. Immunity. 2018;48:812-830.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4007] [Cited by in RCA: 3808] [Article Influence: 544.0] [Reference Citation Analysis (0)] |
| 17. | Kim HD, Song GW, Park S, Jung MK, Kim MH, Kang HJ, Yoo C, Yi K, Kim KH, Eo S, Moon DB, Hong SM, Ju YS, Shin EC, Hwang S, Park SH. Association Between Expression Level of PD1 by Tumor-Infiltrating CD8+ T Cells and Features of Hepatocellular Carcinoma. Gastroenterology. 2018;155:1936-1950.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 202] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 18. | Zalinski S, Scatton O, Randone B, Vignaux O, Dousset B. Complete hepatocellular carcinoma necrosis following sequential porto-arterial embolization. World J Gastroenterol. 2008;14:6869-6872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Chan A, Zhang WY, Chok K, Dai J, Ji R, Kwan C, Man N, Poon R, Lo CM. ALPPS Versus Portal Vein Embolization for Hepatitis-related Hepatocellular Carcinoma: A Changing Paradigm in Modulation of Future Liver Remnant Before Major Hepatectomy. Ann Surg. 2021;273:957-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 20. | Tahmasebi Birgani M, Carloni V. Tumor Microenvironment, a Paradigm in Hepatocellular Carcinoma Progression and Therapy. Int J Mol Sci. 2017;18:405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 21. | Cheng L, Du X, Wang Z, Ju J, Jia M, Huang Q, Xing Q, Xu M, Tan Y, Liu M, Du P, Su L, Wang S. Hyper-IL-15 suppresses metastatic and autochthonous liver cancer by promoting tumour-specific CD8+ T cell responses. J Hepatol. 2014;61:1297-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Ohishi W, Cologne JB, Fujiwara S, Suzuki G, Hayashi T, Niwa Y, Akahoshi M, Ueda K, Tsuge M, Chayama K. Serum interleukin-6 associated with hepatocellular carcinoma risk: a nested case-control study. Int J Cancer. 2014;134:154-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 23. | Rensen SS, Slaats Y, Driessen A, Peutz-Kootstra CJ, Nijhuis J, Steffensen R, Greve JW, Buurman WA. Activation of the complement system in human nonalcoholic fatty liver disease. Hepatology. 2009;50:1809-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 24. | Zhong F, Hu Z, Jiang K, Lei B, Wu Z, Yuan G, Luo H, Dong C, Tang B, Zheng C, Yang S, Zeng Y, Guo Z, Yu S, Su H, Zhang G, Qiu X, Tomlinson S, He S. Complement C3 activation regulates the production of tRNA-derived fragments Gly-tRFs and promotes alcohol-induced liver injury and steatosis. Cell Res. 2019;29:548-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 25. | Joechle K, Moser C, Ruemmele P, Schmidt KM, Werner JM, Geissler EK, Schlitt HJ, Lang SA. ALPPS (associating liver partition and portal vein ligation for staged hepatectomy) does not affect proliferation, apoptosis, or angiogenesis as compared to standard liver resection for colorectal liver metastases. World J Surg Oncol. 2017;15:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Sükei T, Palma E, Urbani L. Interplay between Cellular and Non-Cellular Components of the Tumour Microenvironment in Hepatocellular Carcinoma. Cancers (Basel). 2021;13:5586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319:1676-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1557] [Cited by in RCA: 1598] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 28. | Bonini C, Mondino A. Adoptive T-cell therapy for cancer: The era of engineered T cells. Eur J Immunol. 2015;45:2457-2469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Sermer D, Brentjens R. CAR T-cell therapy: Full speed ahead. Hematol Oncol. 2019;37 Suppl 1:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 30. | Ikeda H. T-cell adoptive immunotherapy using tumor-infiltrating T cells and genetically engineered TCR-T cells. Int Immunol. 2016;28:349-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Zhao Z, Chen Y, Francisco NM, Zhang Y, Wu M. The application of CAR-T cell therapy in hematological malignancies: advantages and challenges. Acta Pharm Sin B. 2018;8:539-551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 32. | Levin A, Shah NN. Chimeric antigen receptor modified T cell therapy in B cell non-Hodgkin lymphomas. Am J Hematol. 2019;94:S18-S23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Hotblack A, Holler A, Piapi A, Ward S, Stauss HJ, Bennett CL. Tumor-Resident Dendritic Cells and Macrophages Modulate the Accumulation of TCR-Engineered T Cells in Melanoma. Mol Ther. 2018;26:1471-1481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Rapoport AP, Stadtmauer EA, Binder-Scholl GK, Goloubeva O, Vogl DT, Lacey SF, Badros AZ, Garfall A, Weiss B, Finklestein J, Kulikovskaya I, Sinha SK, Kronsberg S, Gupta M, Bond S, Melchiori L, Brewer JE, Bennett AD, Gerry AB, Pumphrey NJ, Williams D, Tayton-Martin HK, Ribeiro L, Holdich T, Yanovich S, Hardy N, Yared J, Kerr N, Philip S, Westphal S, Siegel DL, Levine BL, Jakobsen BK, Kalos M, June CH. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med. 2015;21:914-921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 571] [Cited by in RCA: 682] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 35. | Marcinkowski B, Stevanović S, Helman SR, Norberg SM, Serna C, Jin B, Gkitsas N, Kadakia T, Warner A, Davis JL, Rooper L, Hinrichs CS. Cancer targeting by TCR gene-engineered T cells directed against Kita-Kyushu Lung Cancer Antigen-1. J Immunother Cancer. 2019;7:229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Matsuda T, Leisegang M, Park JH, Ren L, Kato T, Ikeda Y, Harada M, Kiyotani K, Lengyel E, Fleming GF, Nakamura Y. Induction of Neoantigen-Specific Cytotoxic T Cells and Construction of T-cell Receptor-Engineered T Cells for Ovarian Cancer. Clin Cancer Res. 2018;24:5357-5367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 37. | Johnson LA, June CH. Driving gene-engineered T cell immunotherapy of cancer. Cell Res. 2017;27:38-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 210] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 38. | Ping Y, Liu C, Zhang Y. T-cell receptor-engineered T cells for cancer treatment: current status and future directions. Protein Cell. 2018;9:254-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |