Published online Feb 27, 2022. doi: 10.4240/wjgs.v14.i2.78
Peer-review started: March 20, 2021
First decision: October 3, 2021
Revised: October 18, 2021
Accepted: January 25, 2022
Article in press: January 25, 2022
Published online: February 27, 2022
Processing time: 338 Days and 15.5 Hours
Although gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) have always been considered rare tumors, their incidence has risen over the past few decades. They represent a highly heterogeneous group of neoplasms with several prognostic factors, including disease stage, proliferative index (Ki67), and tumor differentiation. Most of these neoplasms express somatostatin receptors on the cell surface, a feature that has important implications in terms of prognosis, diagnosis, and therapy. Although International Guidelines propose algorithms aimed at guiding therapeutic strategies, GEP-NEN patients are still very different from one another, and the need for personalized treatment continues to increase. Radical surgery is always the best option when feasible; however, up to 80% of cases are metastatic upon diagnosis. Regarding medical treatments, as GEP-NENs are characterized by relatively long overall survival, multiple therapy lines are adopted during the lifetime of these patients, but the optimum sequence to be followed has never been clearly defined. Furthermore, although new molecular markers aimed at predicting the response to therapy, as well as prognostic scores, are currently being studied, their application is still far from being part of daily clinical practice. As they represent a complex disease, with therapeutic protocols that are not completely standardized, GEP-NENs require a multidisciplinary approach. This review will provide an overview of the available therapeutic options for GEP-NENs and attempts to clarify the possible approaches for the management of these patients and to discuss future perspectives in this field.
Core Tip: Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) have shown an increasing incidence over the past few decades. Although International Guidelines propose algorithms aimed guiding therapeutic strategies, the need for personalized treatment continues to increase. Radical resection is always the best option when feasible; however, up to 80% of cases are metastatic upon diagnosis. Several medical therapies are available for unresectable cases: Somatostatin analogs, peptide receptor radionuclide therapy, targeted drugs (primarily everolimus and sunitinib), chemotherapy and immunotherapy. This review provides an updated overview of the available therapeutic options for GEP-NENs and attempts to discuss future perspectives in this field.
- Citation: Merola E, Michielan A, Rozzanigo U, Erini M, Sferrazza S, Marcucci S, Sartori C, Trentin C, de Pretis G, Chierichetti F. Therapeutic strategies for gastroenteropancreatic neuroendocrine neoplasms: State-of-the-art and future perspectives. World J Gastrointest Surg 2022; 14(2): 78-106
- URL: https://www.wjgnet.com/1948-9366/full/v14/i2/78.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i2.78
Elettra Merola, MD, PhD: She is a gastroenterologist and researcher currently working at the Department of Gastroenterology of Santa Chiara Hospital (APSS), Trento (Italy). She received her MD degree with honors in 2005 from Campus Bio Medico University, Rome (Italy), and during medical school she was visiting student and researcher at the University of Illinois in Chicago (United States) and at Temple University in Philadelphia (United States). In 2009, she completed her residency in Gastroenterology and in 2013 her PhD in Digestive Oncology at Sant’Andrea Hospital, Sapienza University, Rome (Italy), where she developed a particular interest in neuroendocrine neoplasms (NENs). Her experience in this field grew, joining as a visiting researcher the European Neuroendocrine Tumor Society (ENETS) center of excellence of Charité University, Berlin (Germany) (2015-2017), and then working as an NEN specialist at the NEN center of FAU Erlangen University, Erlangen (Germany) (2017-2018). Dr. Merola is internationally recognized for her expertise in NENs and she has led several cooperative studies aimed at improving the clinical management of these patients. In March 2017 she was awarded the ENETS Center of Excellence Academy Fellowship Grant for an international, cooperative research project regarding curative surgery in neuroendocrine tumors. She moved to Santa Chiara Hospital (APSS) in Trento (Italy) in 2018, where she promoted the management of NEN patients in a multidisciplinary setting, coordinating a dedicated NEN tumor board. She is also responsible for the outpatient clinic of neuroendocrine neoplasms in the Gastroenterology Department (Figure 1).
Although gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) have always been considered rare tumors, their incidence has risen in recent decades, up to 3-5 cases per 100000 persons per year[1,2]. They represent a highly heterogeneous group of neoplasms with varying biological behavior. Several prognostic factors have an impact on GEP-NEN survival, including the proliferative index (Ki67)[3], disease stage according to the European Neuroendocrine Tumor Society (ENETS) tumor-node-metastasis (TNM) staging system[4,5], and the World Health Organization (WHO) classification[6].
In particular, if the definition of NENs is adopted for all neoplasms with a neuroendocrine differen
The expression of somatostatin receptors (SSTRs) also has an important role in therapy selection and characterizes nearly 90% of NENs. This feature is mainly identified by functional imaging tests, which are pivotal in diagnosis, disease staging, and the therapeutic management of NENs. They include octreotide scintigraphy with radiolabeled somatostatin analogs (SSAs) (Octreoscan®), limited by the low accuracy in detecting small lesions (< 1 cm in diameter) and by a difficult semiquantitative analysis[7]. The subsequent development of different radiolabeled DOTA-conjugated peptides (DOTANOC, DOTATOC, DOTATATE) for positron emission tomography/computed tomography (PET/CT) has changed the landscape of nuclear medicine. Following the first published paper introducing
Although International Guidelines propose algorithms aimed at guiding therapeutic strategies[9-13], NEN patients are still very different from one another and the need for personalized treatments continues to increase. Although radical surgery is always the best option when feasible, up to 80% of cases are metastatic upon diagnosis and data on adjuvant treatments are still insufficient for this disease. Regarding medical treatments, as NENs are characterized by a relatively long overall survival (OS), multiple therapy lines are adopted for these patients during their lifetime, but the best sequence to be followed has never been clearly defined. Furthermore, new molecular markers aimed at predicting therapy response and prognostic scores[14,15] are currently being studied, but their application is still far from being part of daily clinical practice. A recent network meta-analysis including only phase-III randomized controlled trials (RCTs) has attempted to identify the best therapeutic strategy for controlling tumor growth, proposing the combination of peptide receptor radionuclide therapy (PRRT) and SSAs as the option with the best progression-free survival (PFS). However, this analysis seems very speculative and hard to apply to real-life settings.
This review will explore the available antiproliferative therapeutic options for GEP-NENs, based on evidence reported in the literature and on many years of experience in the field. It also includes the contribution of the specialists working in the multidisciplinary setting dedicated to NEN patients at Santa Chiara Hospital (APSS) in Trento (Italy). A separate session will be dedicated to new frontiers in the therapy landscape. Genetic syndromes and management of clinical syndrome (i.e. carcinoid syndrome) will not be discussed in this manuscript.
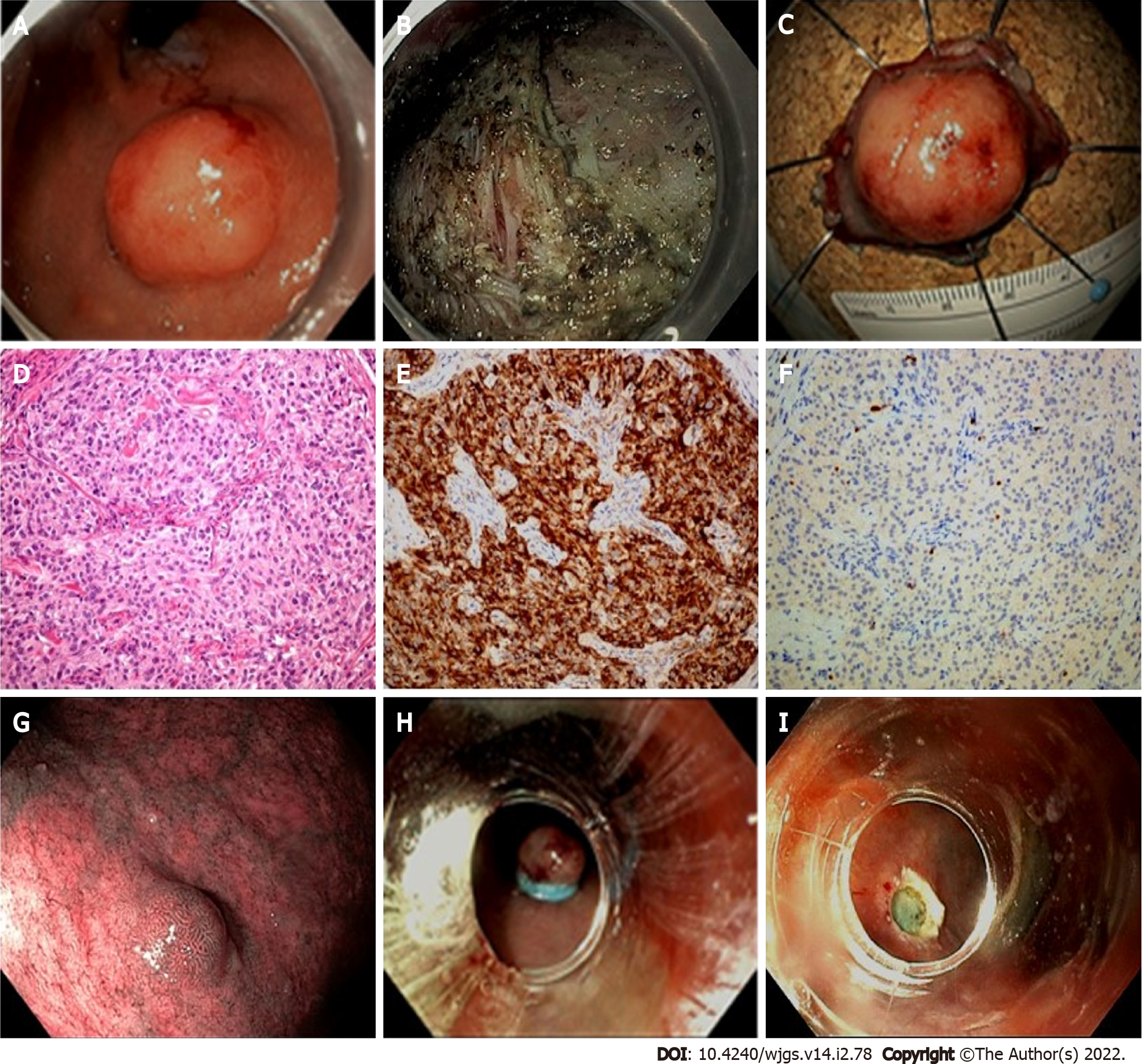
The incidence of GEP-NENs has increased in the last two decades also due to the extensive use of endoscopy, particularly following the worldwide implementation of bowel cancer screening programs. Endoscopic resection is reserved to small, localized NETs, mainly located in the rectum, stomach and duodenum. The endoscopist must have extensive knowledge of the macroscopic appearance of these lesions and perform endoscopic ultrasound (EUS) for staging when an invasive NET is suspected, and perform a biopsy when lesions arise from the deep mucosal layer and then extend into the submucosa[16]. A thorough evaluation of tumor location, size, and depth of invasion are mandatory and a multidisciplinary consultation is recommended prior to resection even in case of small and low-grade lesions[17,18].
In this session, the endoscopic approach for gastrointestinal NETs will be discussed according to site, and our proposal for endoscopic management is reported in Table 1.
| r-NETs | g-NETs | d-NETs | e-NETs | |
| Prevalence (% of GI-NETs) | 8-30 | 4.6-7 | 1-3 | 0.2 |
| Indications to EUS | ≥ 10 mm | (1) Type I ≥ 10 mm; and (2) Type II-III | Always | Always |
| Indications to endoscopic resection | < 20 mm, no signs of deep invasion or lymphadenopathy | G1/G2, 10-20 mm, no signs of deep invasion or lymphadenopathy | (1) < 10 mm, no signs of deep invasion or lymphadenopathy; (2) 10-20 mm, G1/G2, no signs of deep invasion or lymphadenopathy (debated); and (3) Periampullary region: G1, no signs of deep invasion or lymphadenopathy(debated) | ≤ 10 mm, confined to submucosa, no ulceration |
| Resection techniques | (1) EMR-C, EMR-L (< 10 mm); and (2) ESD (10-20 mm) | (1) EMR-C, EMR-L (Type I < 10 mm); and (2) ESD (Type I 10-20 mm, Type II-III) | (1) EMR, EMR-C, EMR-L, ESD; and (2) Endoscopic papillectomy in referral centers | EMR-C, EMR-L, ESD |
Colorectal NETs: Colonic NETs are located in the right colon in 70% of cases, can reach a very large size without obstructive symptoms, and are usually aggressive[19]. Given their advanced stage at the time of diagnosis, endoscopic treatment has only been reported in case series, with a significant burden of complications and incomplete resections[17].
Rectal NETs (r-NETs) appear as small, sessile lesions, located within 5-10 cm of the anal verge, with overlying normal or yellowish mucosa. Larger lesions may also be semi-pedunculated or have central depression or ulceration[19].
Staging with EUS is not required for lesions < 10 mm in size due to the negligible risk of invasion[16,20]. The endoscopist may be tempted to perform a standard snare resection but must bear in mind that the complete removal rate for polypectomy is approximately 30%, and for conventional endoscopic mucosal resection (EMR) it is highly variable (17%-90%) due to the submucosal nature of these nodules[19,21,22].
Modified EMR techniques have been employed to obtain a deeper resection. Cap-assisted EMR (EMR-C) uses a dedicated cap with a circumferential rim that can lodge a crescent snare. After saline injection of the submucosa, the lesion is suctioned within the cap and cut. Band-ligation EMR (EMR-L) also requires saline injection. Once the lesion has been adequately captured by the deployment of an elastic band (usually employed for variceal ligation), a snare resection is performed below the band.
The rate of histologically complete resection by modified EMR is high, particularly for EMR-L (93%-100% vs 71%-100% for EMR-C) and comparative studies and a meta-analysis confirmed a higher complete resection rate than conventional EMR[20,23,24]. Resection by EMR-C and EMR-L are both used for r-NETs, and the only comparative retrospective study available to date demonstrated similar effectiveness[23]. The higher en bloc resection rate for EMR-L was explained by the authors by the larger quantity of submucosa captured by the thickness of the elastic band.
Another technique for advanced endoscopic resection is endoscopic submucosal dissection (ESD). This technique is superior in terms of radical histologic resection in r-NETs ≥ 10 mm[17], but has similar outcomes to EMR-C and EMR-L for small r-NETs (< 10 mm) despite a longer procedure time[20,25].
Gastric NETs: Gastric NENs (g-NENs) usually arise from enterochromaffin-like (ECL) cells and are divided into three types. More specifically, Type I arises in the setting of a chronic atrophic gastritis, Type II is associated with gastrinomas, and Type III is sporadic and independent from gastrin levels. Two additional categories of g-NENs have been recently described and are currently being investigated: Type IV lesions arise from non-ECL endocrine cells, whereas another subtype of g-NETs might be determined by the chronic use of proton pump inhibitors[19,26,27].
Type I and II g-NETs have a highly variable endoscopic aspect (red or yellow, depending on the vascular supply) and are sometimes characterized by a central depression. They usually appear as smooth and rounded multiple polypoid lesions, with size < 20 mm and located in the gastric body and fundus[19,28,29]. As Type I g-NETs are mainly characterized by indolent behavior, conservative management with endoscopic surveillance +/- resection is safe and effective also in the case of recurrent lesions[17,30,31].
Disease staging by EUS prior to resection is not required for small Type I g-NETs (< 10 mm) but it is mandatory when lesions are ≥ 10 mm, when Ki67 is > 3% or in the case of Type II g-NETs[17]. The data regarding ESD show complete resection achieved in 75%-100% of cases, with a lower rate of positive vertical margins at histology compared to standard EMR[32-34]. Modified EMR techniques (EMR-L or EMR-C) are currently being used for Type I g-NETs, and should be considered for small lesions (≤ 10 mm) that can be completely suctioned within the cap in order to obtain the en bloc resection (Figure 2).
Type II lesions are extremely rare, and in the absence of high-quality level data, their management is generally similar to Type I[27]. However, considering their size upon presentation (≥ 10 mm) they usually require ESD for complete en bloc resection that is better than EMR.
Type III g-NENs are larger, solitary lesions located anywhere in the stomach, sometimes with a broad fixed base and ulceration indicating deeper invasion[17,28,29]. They require a complete disease staging, including EUS. As lymph node involvement is present in more than 50% of cases upon diagnosis and liver metastases is in 22%-75%, an endoscopic approach is not frequent in these cases[18,27]. A recent systematic review included 121 patients from eight studies with small localized Type III g-NETs who underwent endoscopic resection. The complete resection rate varied from 72% to 87%, but details about the endoscopic technique were often not reported, preventing comparisons of the EMR and ESD outcomes[18].
Type IV g-NENs are described as aggressive lesions, with a size of > 40 mm upon diagnosis, and in the case of localized disease, surgical resection is preferable[19].
Small bowel NETs: Jejunal and ileal NETs are usually > 20 mm, multifocal in 40% of cases, and with lymphatic involvement upon diagnosis in 70% of cases[19]. Due to these features, and as they are often beyond the reach of a device-assisted enteroscopy, a surgical approach is recommended for localized disease. Endoscopy may instead be helpful for diagnosis, in the case of bleeding, or for tattooing of the lesion[19].
Duodenal NETs (d-NETs) are usually small, sessile and solitary lesions, mainly located in the duodenal bulb or second part[28]. As even sub centimetric tumors present lymphatic spread in 40%-60% upon diagnosis, EUS is mandatory, and resection by EMR or ESD is reserved to submucosal lesions < 10 mm with no lymphatic involvement[17,19]. The management of intermediate (10-20 mm) lesions is controversial and based on local expertise[28]. Considering the thin duodenal wall, some authors prefer to use standard EMR rather than modified EMR[29]. Standard EMR has indeed shown outcomes that are comparable to EMR-C and EMR-L, although higher rates of complete histological removal (70%-92%) has been reported for EMR-L in a small case series[35-38]. Some authors even suggest the autoamputation of small d-NETs using band ligation without snare resection[39].
The rate of radical resection by ESD in the duodenum is variable (67%-100%), due to the technical challenge of scope maneuvering in this anatomical district and the scarce submucosal lifting[36,40]. Moreover, the complication rate may be higher than in other gastrointestinal districts, especially perforation (13%-67% in small case series)[36,41,42]. Based on these considerations, ESD may be offered depending on local expertise and preferentially reserved to poor surgically-suited candidates.
Endoscopic full-thickness resection (EFTR) is usually reserved for subepithelial tumors originating from the muscularis propria. It has only been described for NETs in small case series and ideally should not provide a clear advantage compared to ESD as most NETs remain submucosal[17,43]. The ability of EFTR to secure the intestinal wall with an over-the-scope clip under the cutting plane may overcome the risks of endoscopic resection in the duodenum[40]. However, this advantage may be hampered by the technical drawbacks of operating this unwieldy device in the already difficult duodenal anatomy.
Duodenal NETs originate from the periampullary region in 20% of patients. In these cases, current guidelines recommend surgical resection because they have a more aggressive biology and their metastatic potential is independent of tumor size[18,28,30,35]. Nevertheless, a growing body of evidence favors a prior attempt with endoscopic papillectomy[21,44]. Prospective data are needed to evaluate the efficacy of this approach.
Esophageal NETs: Esophageal NETs (e-NETs) account for only 0.2% of total gastrointestinal NETs. Their appearance is similar to other gastrointestinal NETs, but they tend to have a central ulceration and may sometimes be multiple[29]. Endoscopic resection can be considered in low-risk cases: Lesions ≤ 10 mm, without ulceration and confined to the submucosa according to EUS evaluation. Both en bloc EMR and ESD have been effectively used for complete removal. However, the exceptionally rare incidence of e-NETs does not allow high level comparative studies for these techniques[17]. Regarding EMR, EMR-C and EMR-L are advocated to obtain a deeper submucosal resection than standard EMR.
Future perspectives and open questions: The available data regarding the use of SSAs in the management of Type I g-NETs derive from small, retrospective cohorts, resulting in controversial conclusions[45]. Prospective trials exploring this approach would be useful in understanding the indications and the potential benefit of this alternative option which is currently considered only experimental. A prospective study describing the endoscopic appearance of gastrointestinal NETs and proposing an endoscopic classification would help recognize these lesions and select the suitable technique for endoscopic resection.
Surgery with radical intent is the preferred option in the management of all GEP-NENs, when feasible. Preoperative work should include complete disease staging with both morphological and functional imaging tests. We will discuss the surgical approach of these patients according to the tumor primary site and focusing on the main critical issues regarding this therapeutic option.
NETs of the appendix: Appendiceal NETs are usually incidental found during surgery for acute appendicitis. For this reason, radicality of the intervention and the indications to right hemicolectomy with lymphadenectomy still represent critical issues in the management of these patients. The European Guidelines for NETs have established, based on the literature, certain criteria aimed at guiding this decision according to the features of the tumor[46]. More specifically, appendicectomy is considered sufficient when the tumor is < 1 cm and resection is R0. Right hemicolectomy is instead recommended when the tumor is > 2 cm. Regarding the “grey zone” of intermediate tumor size (1-2 cm), additional risk factors indicating a surgical re-intervention are represented by a G2 histology, signs of histological vascular or lymphatic invasion (V1 and/or L1) or a mesoappendiceal infiltration > 3 mm.
Small bowel NETs: Pre-operative tests to be performed in the case of small bowel NETs (Sb-NETs) should also include echocardiography (to evaluate carcinoid heart disease) and colonoscopy. The surgical procedures for resection should include the intraoperative exploration of all abdominal cavities and extensive lymphadenectomy, as one-third of the cases (regardless of primary tumor size) have lymph node metastases upon diagnosis. As these lesions are in almost 80% of cases small, multiple nodules, undetectable by conventional imaging tests, palpation of the entire jejunum and ileum is mandatory to achieve radical resection. These tumors are often characterized by mesenteric fibrosis, and in 5% of cases by small peritoneal implants. For this reason, Sb-NETs are sometimes diagnosed for acute intestinal obstruction. Resection of mesenteric metastases is usually feasible, unless in cases of complete vascular encasement or retroperitoneal involvement[12,47].
Pan-NETs: Regarding pre-operative evaluation for Pan-NETs, vascular involvement (superior mesenteric vein, superior mesenteric artery, coeliac axis and common hepatic artery) must be accurately assessed in order to discuss the feasibility of a curative resection. When patients are candidate to enucleation, EUS or magnetic resonance cholangiopancreatography help evaluate the relationship of the tumor with the pancreatic duct[12]. There is an open debate about the management of non-functioning Pan-NETs < 2 cm and with no involvement of the main pancreatic duct. The two possible proposals are surgical resection vs follow-up. As long-term data concerning safety of the conservative management are insufficient, surgery can be considered in young, healthy patients. Parenchyma-sparing pancreatic resections (enucleation or central pancreatectomy) can be performed in these cases; however, complete surgery with these techniques is uncertain because lymphadenectomy is crucial to reach the radicality. In fact, recent data report that 12% of resected small Pan-NETs have lymph nodal metastases at surgery, with poorer recurrence-free survival (RFS) rates in the case of tumors of 15-20 mm[12,48]. The decision to operate or just observe these patients also needs to be based on the general conditions of patients, as the benefit of surgery can be counterbalanced by significant morbidity and mortality rates compared to conservative management[49].
Locally advanced or metastatic disease: Regarding advanced Sb-NETs, surgery can be considered when patients suffer from symptoms due to mesenteric involvement but must be performed in specialized centers. In fact, radical resection or debulking surgery can significantly improve the quality of life of these patients[12]. Encouraging results of curative resection are also available for GEP-NEN patients with TNM stage IV disease, but after ruling out the presence of extra-abdominal disease. When radical resection is feasible, survival rates are indeed better than debulking or medical treatments. For Pan-NETs, median OS for these three options accounts for 97, 89, and 36 mo, respectively[50]. However, careful patient selection is mandatory in order to reduce the risk of complications. The data regarding the use of neoadjuvant treatment associated with radical surgery are scarce. The RMPanNET trial will compare the survival outcomes of metastatic Pan-NETs treated with resection on the primary tumor and metastases after neoadjuvant systemic treatment (SSAs, targeted therapy or chemotherapy) vs continuing only systemic treatment (Supplementary Table 1). The NEONEC trial will instead investigate the role of neoadjuvant treatment in terms of RFS in patients with localized NECs, adopting a cisplatin (or carboplatin)/etoposide regimen (Supplementary Table 1).
Role of adjuvant treatments: Unlike other cancers, the data regarding adjuvant treatments in GEP-NENs after curative surgery are scarce, and this approach is not routinely applied in clinical practice. This limitation is probably due to the relatively long survival rates after radical resection without any other treatments (especially for GEP-NETs G1-G2) and to the lack of validated risk scores aimed at identifying patients at high risk of disease recurrence.
A recent retrospective, multicenter study from the United States has reported survival outcomes of 91 GEP-NETs treated with adjuvant treatments (chemotherapy or SSAs) after curative-intended surgery, compared to patients receiving surgery only[51]. The results showed that adjuvant therapy had negative impact on RFS rates, with no benefit in terms of OS. Another piece of analysis from the Surveillance, Epidemiology, and End Results-Medicare (SEER) database included 318 colorectal NENs treated with radical surgery. Focusing on stage I-III TNM disease, no benefit in terms of OS or RFS was observed when adopting adjuvant chemotherapy compared to surgery only[52]. These data discourage the use of adjuvant treatments, but might be read with caution due to the inevitable selection bias of retrospective studies. In fact, patients receiving post-surgical treatment, in a retrospective analysis, are characterized by more aggressive tumor features, and should not be compared to patients with theoretically less aggressive tumors.
Focusing on NENs G3, the available data concerning the use of adjuvant treatments after curative surgery are derived from retrospective cohorts and provide controversial results. In a series of 73 digestive NECs, with the majority having a colorectal primary tumor site, 43 received chemotherapy, either neoadjuvant and/or adjuvant. The median OS and RFS for patients receiving chemotherapy were 62 and 13 mo, respectively, showing the potential prognostic impact of chemotherapy on survival outcomes[53]. Another study compared the survival rates of 394 radically resected non-metastatic colorectal NECs receiving adjuvant chemotherapy vs 412 undergoing radical surgery only. The median OS was significantly longer for patients treated with adjuvant therapy [57.4 vs 38.2 mo for patients treated with surgery only; hazard ratio (HR): 0.73, P < 0.01], especially in the subgroup of patients with left-sided NECs[54]. Discouraging results were reported by Lin et al[55], who analyzed the data of 804 gastric NECs or MiNENs treated with radical surgery +/- adjuvant therapy. The study showed no statistically significant different OS between the two groups. In another retrospective series of 60 GEP-NENs G3 with TNM stage I-III disease receiving radical surgery, the 2-year OS of the total population was 64.5% and the median RFS was 14 mo. Adjuvant therapy, adopted in 20 patients, did not improve either the OS or RFS rates[56].
Future perspectives and open questions: The ASPEN study is prospectively assessing clinical outcomes of patients with Pan-NENs < 2 cm managed by radical surgery vs follow-up[57] (Supple-mentary Table 1). The validation of risk scores in prospective cohorts might help stratify resected GEP-NENs according to the risk of disease recurrence. Patients at high risk might be enrolled in RCT evaluating the potential benefit of adjuvant therapies compared to curative surgery only. Studies evaluating response to adjuvant treatments should also include NETs, as data showing a potential benefit of this therapeutic option so far available were mainly obtained in the setting of NEC patients.
Beyond the need for debulking in uncontrolled functioning syndrome, resection of the primary tumor is another possible surgical indication in metastatic disease. Some series have recently proved that, in addition to symptomatic relief (for example, for obstruction due to the mesenteric involvement in Sb-NETs), this approach has also a prognostic impact. In fact, in a retrospective series of 14510 GEP-NETs, a benefit in terms of survival has been observed for G1 and G2 patients[58]. A very recent publication from the SEER Registry, including 2219 GEP-NETs, confirms these results for all sites excluding the rectum, with an overall HR of 0.65. In addition, the study highlights the importance of a careful patient selection in a multidisciplinary setting[59]. These conclusions may however be limited by a selection bias, as in retrospective analysis the surgical approach might be reserved to patients with a better performance status or more localized disease[60].
Future perspectives and open questions: Prospective studies comparing the survival outcomes of patients with metastatic GEP-NENs treated with primary tumor resection vs patients not undergoing this option would assess the potential prognostic impact of this surgical approach.
Indications, efficacy, and safety: Up to 80% of GEP-NETs present liver metastases at the time of initial diagnosis. Current guidelines recommend vascular and ablative locoregional treatments only for NETs G1-G2 in the case of metastases involving only or predominantly the liver with stable extrahepatic disease. The goals are the relief of symptoms caused by hormone secretion or mass effect in order to improve quality of life, and survival prolongation by slowing the growth of liver lesions. In very select cases, locoregional treatments can be bridging therapies to liver transplantation[61,62]. These treatments should be offered after discussion in a multidisciplinary team consultation, in the case of hepatic disease progression (DP), and might be also considered in conjunction with other systemic therapies or combined with surgery. The choice is based on liver tumor burden, patient symptoms, general clinical condition, but also on the local expertise and availability of the various procedures.
Liver-directed therapies for metastatic GEP-NETs include thermal ablation, transarterial embolization (TAE) or transarterial chemoembolization (TACE) and transarterial radioembolization (TARE), also known as selective internal radiation therapy (SIRT). The data regarding their anti-tumor efficacy primarily comes from retrospective studies using heterogeneous protocols, and consequently it is currently unclear which technique is preferable.
Ablation techniques (radiofrequency ablation, microwave ablation, and cryotherapy) require imaging guidance, and are only applied in the case of limited liver disease: Less than three lesions ≤ 3 cm, or a single lesion < 5 cm, or even in association with liver surgery[61]. When feasible, thermal ablation shows lower complication rates than surgery (3.9% vs 20%, respectively) and a good clinical response (with relief of symptoms in up to 92%)[63]. Unfortunately, the benefits of ablation alone in terms of survival rates are difficult to demonstrate, due to the influence of subsequent lines of therapy in the calculation.
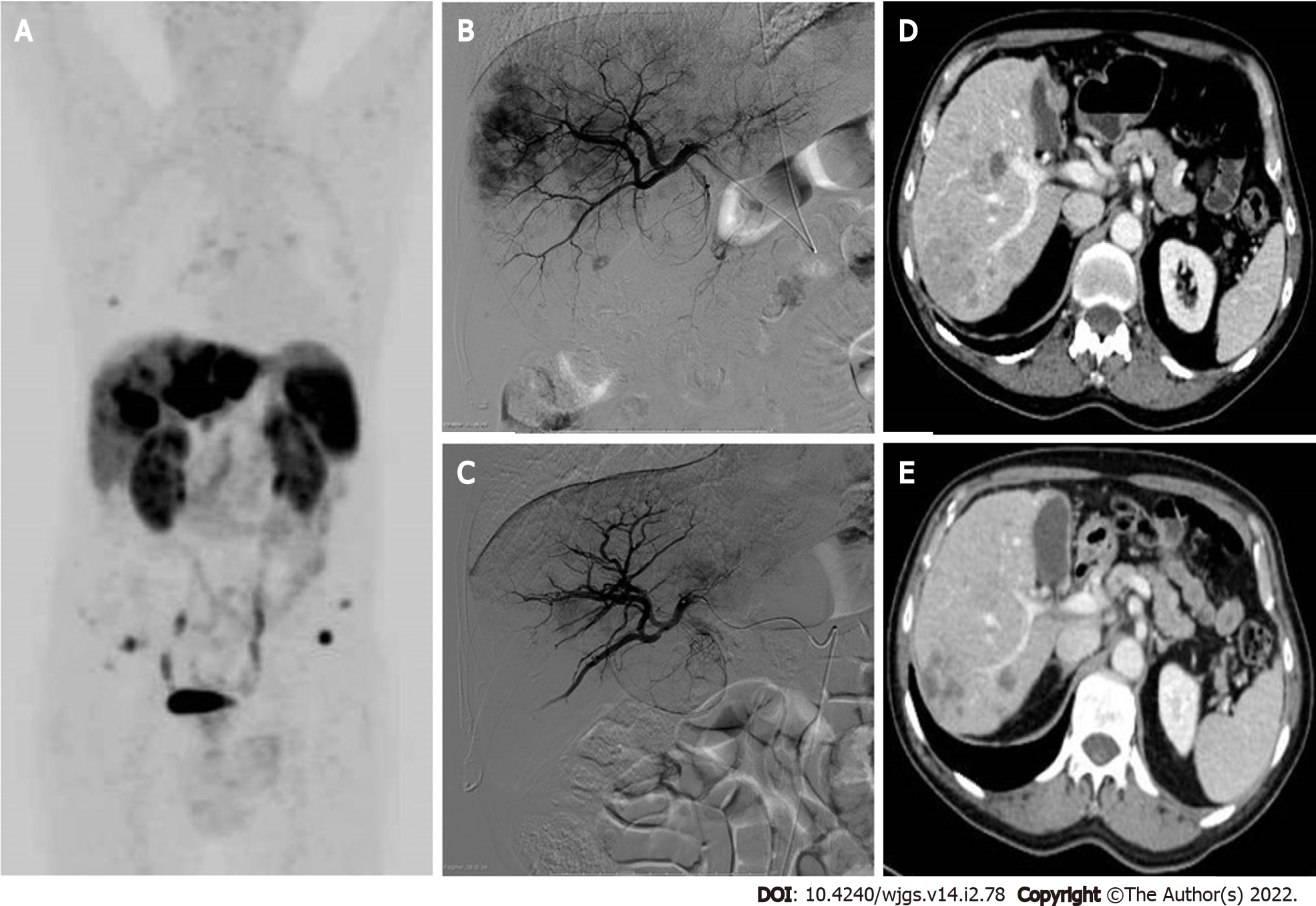
Vascular treatments are based on the rationale that neuroendocrine liver metastases are hypervascular, deriving all their blood supply from the hepatic artery, whereas the normal hepatic parenchyma is mainly supplied by the portal vein (75%). Arterial embolization makes it possible to deliver a tumoricidal dose of chemotherapy (TACE) or β-radiation (SIRT) in association with the ischemic effect on the lesions, thereby reducing systemic adverse effects (AEs) and limiting toxicity for the normal liver parenchyma through the use of a selective technique. The feared carcinoid crisis due to massive release of serotonin or vasoactive peptides in the case of secreting GEP-NETs is prevented by octreotide premedication and by scheduling the presence of the anesthesiologist during the procedure[11]. A multicenter retrospective study showed better results for catheter-based therapies in terms of OS and hepatic PFS for lower grade NETs and for liver tumor burden ≤ 50%, regardless of the primary tumor site (Pan-NETs or Sb-NETs)[64]. Previous studies have instead reported a higher morphological response rate (RR) and/or better OS for non-pancreatic cases[65].
The TACE uses a mixture of chemotherapy drugs and a temporary embolic agent (degradable starch microspheres of 50 μm, with a half-life of approximately 35–50 min), with the aim of preserving arterial patency for further cycles of treatment (Figure 3). Negative predictive factors for response to TACE treatment in GEP-NETs are represented by impaired liver function (ascites, bilirubin ≥ 2 mg/dL, albumin ≤ 3.5 mg/dL), tumor burden ≥ 70% and previous treatment with three or more systemic lines of therapy[61]. In the case of bilobar liver involvement, a sequential approach with multiple selective or lobar TACE treatment sessions is recommended, usually at a 6-8 wk interval, with assessments for patient tolerance and response after each course. Possible complications include portal vein narrowing or thrombosis, bile duct dilatation leading to biloma formation, and liver necrosis with the possible development of abscesses. Caution is therefore recommended especially in the case of bilio-enteric anastomoses, when initial bile duct dilatation or segmental portal vein thrombosis is detected by pretreatment imaging, representing relative contraindications to the performing of TACE.
Another technique, TARE with 90Y-loaded microspheres, has a more favorable safety profile than TACE or TAE, with fewer AEs (pain, post-embolization syndrome, liver/biliary toxicity) in the early post-treatment period; however, hepatic cirrhosis with portal hypertension may appear as a long-term complication, especially in the case of bilobar treatment[66]. Patients should undergo preprocedural evaluation for hepatopulmonary shunts to ensure that no more than 20% of the blood flow is diverted to the lungs to avoid radiation pneumonitis.
A recent meta-analysis revealed that patients treated with TACE had significantly better OS than those treated with TARE[67]. TARE proved to be more effective than TAE/TACE when Ki67 ≥ 3%, whereas Ki67 < 3% predicts a greater benefit with TACE[68]. TARE is indicated in the case of TACE failure or in patients at risk for TACE including major portal vein thrombosis, bilio-enteric anastomoses, and heart problems contraindicating doxorubicin administration. The cost per procedure for TARE is nearly double that of TACE; however, there is usually no need for multiple treatment sessions[61]. The ArTisaN study will provide data on the efficacy of TARE in metastatic NETs in a phase II-designed study (Supplementary Table 1).
Future perspectives and open questions: Studies also including GEP-NETs G3 might explore the efficacy of locoregional treatments for liver metastases in these patients, especially cases with a lower proliferative index (e.g., Ki67 < 55%). The LUTIA trial will investigate the efficacy of the intraarterial administration of
Indications, efficacy, and safety: The expression of SSTRs is the prerequisite for benefiting from SSAs. These drugs bind with high affinity to the G protein-coupled transmembrane SSTR2 and with moderate affinity to SSTR5. They are usually adopted at the first-line stage in advanced GEP-NETs, with good tolerability. They have a double effect: Clinical syndrome control in functionally active NENs (i.e. carcinoid syndrome or duodenopancreatic functioning tumors), and antiproliferative effect[13].
Different formulations are available. The short-acting Octreotide is administered subcutaneously, usually to test the tolerability of the therapy. Long-acting formulations for antiproliferative treatment include Octreotide LAR (10, 20, or 30 mg) with intramuscular injection, and Lanreotide autogel (60, 90, or 120 mg) with deep subcutaneous injection. Pasireotide will not be discussed in this review, due to the limited and controversial results regarding its role as an antineoplastic treatment.
The antiproliferative effect of SSAs compared to placebo has been proved by two double-blind RCTs: The PROMID study[70] for Octreotide LAR and the CLARINET trial[71] for Lanreotide. Thanks to these publications, Octreotide LAR was registered for intestinal NETs and NETs of unknown primary tumor site, whereas Lanreotide autogel for intestinal NETs, Pan-NETs, or for cases with unknown primary tumor site. The recommended dosage for the antiproliferative use is the maximum available (Octreotide LAR 30 mg or Lanreotide autogel 120 mg, administered every 4 wk)[13].
The cumulative antineoplastic effect of Octreotide and Lanreotide compared to placebo has been assessed by a meta-analysis with an overall population of 289 patients, showing a reduction of DP risk of 41% by adopting SSAs compared to placebo [HR: 0.41; 95% confidence interval (CI): 0.29-0.58, P < 0.01][72]. The meta-analysis also showed no statistically significant difference in terms of serious AEs (SAEs) between the two arms. However, a higher frequency of biliary stones occurred in the treatment arm (10.5% vs 2.7%, respectively)[72]. The elective prophylactic cholecystectomy in advanced GEP-NETs undergoing primary tumor resection represents a possible, but still debated, option in case SSAs are required.
Other possible side effects observed during treatment with SSAs are hypo/hyperglycemia, gastrointestinal symptoms (abdominal pain and diarrhea), and pancreatic insufficiency, which can be confirmed by fecal elastase test, and treated by pancreatic enzyme supplementation[73].
SSAs for highly proliferating Pan-NETs: Focusing on Pan-NETs, the available data regarding the efficacy of SSAs as antineoplastic treatment are limited to the CLARINET study, which however included only G2 cases with Ki67 < 10%[71]. Thus the question regarding their use in the case of higher proliferative index remains open. A recent cooperative real-world study analyzed the antiproliferative effect of SSAs when adopted at the first-line stage for non-functioning, metastatic Pan-NETS with Ki67 ≥ 10%[74]. The total population of 73 patients also included five Pan-NETs G3. The median PFS was 11.9 mo (95%CI: 8.6, 14.1), but a higher efficacy was shown in G2 patients and with limited hepatic tumor involvement. In detail, the median PFS was 12.4 mo in G2 patients vs 4 mo in G3 cases (P < 0.01). Patients with liver load ≤ 25% had a median PFS of 15 mo vs 9.7 mo in the case of higher hepatic tumor load (P = 0.04).
Dose escalation: After the occurrence of DP during the treatment with SSAs, GEP-NET patients receive more aggressive and less tolerable drugs. A possible alternative option to this approach is a dose escalation of SSAs. A recent systematic review regarding this therapeutic strategy has reported a disease control rate (DCR) of 30%-100%, and a median PFS of 6.8-32 mo. These wide ranges are probably due to the heterogeneity of the included studies, as they are both retrospective and prospective and they adopt different SSA formulations and at different disease statuses[75].
The NETTER-1 study evaluated the administration of Octreotide 60 mg every 4 wk, but in clinical practice, the dose increase is usually performed by shortening the time interval between injections[76]. The CLARINET FORTE study recently investigated, for the first time in a prospective setting, the potential benefit of this strategy in a series of Sb-NETs G1-G2 or Pan-NETs (NCT02651987)[77]. After experiencing DP during monthly injections of Lanreotide 120 mg, patients were treated with the same dosage but every 2 wk, respectively for 48 and 24 cycles. The results were presented at the last ESMO Conference 2020, showing a duration of stable disease of 13.8 mo for Sb-NETs and 8.3 mo for Pan-NETs. The DCR after 48 wk was 33.3% and 22.9%, respectively. Toxicity was similar to the data observed in the CLARINET trial[71], additionally highlighting the good safety profile of SSAs also after dose escalation, with rare Grade 3 side effects. Considering the efficacy, the good safety profile and the absence of deterioration of quality of life with SSA dose escalation, this approach might represent a valid option for progressive NENs, as it can delay the switch to other potentially more toxic drugs.
Novel biomarkers: Measuring the transcript profile of blood in NET patients is more sensitive and specific than chromogranin A or other blood tests available, and might overcome the limits of imaging tests in assessing the tumor response. The “NETest” represents a transcriptomic signature of NETs, being a multianalyte algorithm analysis PCR-based test. It evaluates, using peripheral blood real-time PCR, the tumor biological activity by measuring the expression of 51 genes, which are associated with neoplastic behavior. In a prospective study, its role in predicting tumor progression during SSAs for GEP-NETs was assessed, showing an earlier prediction of DP than chromogranin A, with an accuracy of 80%-100%[15]. Besides the potential applications of the NETest both for NET diagnosis and follow-up, this test is currently only experimental and it is unavailable in daily clinical practice[78].
Future perspectives and open questions: Besides the use of SSAs at the first-line stage in advanced GEP-NETs G1-G2, the role of these drugs in maintaining therapy is being explored. The REMINET trial is assessing whether Lanreotide 120 mg can maintain a stable disease in duodenopancreatic NETs G1-G2, after response to first-line chemotherapy. The preliminary results were presented at the last ENETS Conference 2021, but a phase III trial is needed for their validation (Supplementary Table 1). The TNE-IDC-COLE trial is evaluating, in a prospective randomized setting, the potential benefit of prophylactic cholecystectomy in advanced GEP-NETs receiving SSAs (Supplementary Table 1). The indication of SSAs in G3 cases needs to be further investigated, as well as the potential benefit of SSAs in cases with low or heterogeneous expression of SSTRs. Prospective studies assessing the role of NETest in predicting response to SSAs, as well as other therapeutic options, are needed for validation of this test in clinical practice.
Interferon alpha (IFN-α) is licensed in Europe for functioning GEP-NETs, but it can also control tumor growth. This latter function is based both on a direct antiproliferative effect (influencing the cell cycle, the production of growth factors, and angiogenesis), and an indirect immunomodulatory effect. Several prospective studies have investigated its efficacy as antineoplastic therapy, with conflicting results.
Bajetta et al[79] prospectively enrolled 53 patients affected by progressive, metastatic NETs. Patients received IFN-α-2a with the following scheme: 3 × l06 IU for the first 3 d, progressively increased to 6 × l06 IU for 8 wk, and then three times per week. After a median treatment duration of 6 mo, 64% of patients showed partial or complete tumor regression, lasting 1-11 mo. Less enthusiastic results were reported by Faiss et al[80], showing no benefit in terms of PFS adopting in naïve GEP-NETs the association of IFN-α/Lanreotide alone. Regarding comparison with chemotherapy, a study showed, in naïve patients with functioning tumor, a better DCR with IFN-α than with streptozotocin (STZ)/5-fluorouracil (5-FU) (P < 0.01)[81].
The most common clinical AEs that occur during IFN-therapy (nearly 50% of the patients) are: Flu-like syndrome (fatigue, fever), which can be prevented by paracetamol, neurological disorders (depression), weight loss, abdominal pain, alopecia, pain at the injection site, and headache. Biochemical toxicity includes: Impaired liver functional test (one third of patients), leukopenia, autoimmune diseases (thyroiditis) in 20% of cases, anemia (31%), thrombocytopenia, hyper/hypoglycemia, and the production of neutralizing interferon antibodies. Considering the balance of pros and cons, and the fact that we currently have several alternative options for unresectable GEP-NENs, IFN therapy is currently reserved for only very select cases, mostly syndromic[13]. Regarding the increase in dosage of IFN at DP, as well as its use in G3 patients, no consistent data are available in the literature.
Indications, efficacy, and safety: PRRT is based on radiolabeled somatostatin receptor agonists binding SSTRs on tumor cells. After binding, they are internalized and stored in lysosomes, thereby delivering the radioactivity to the tumor cells. The target of PRRT is DNA damage induced by radiation and suboptimal repair, and this effect is more active during mitosis. Before PRRT begins, a basal Octreoscan®, 68Ga-DOTA-PET/CT or 64Cu-DOTA-PET/CT is mandatory in order to obtain in vivo mapping of all lesions expressing SSTRs. Suitable patients for PRRT have strong SSTR expression, whereas extensive hepatic and/or bone disease, as well as decreased renal function, may limit its indication. According to ENETS Consensus Guidelines “PRRT is a therapeutic option in progressive SSTR-positive NET with homogenous SSTR expression (all lesions are positive)”[82].
Radiolabeled DOTA pharmaceuticals include 90Y- or 177Lu-DOTATOC, and currently, 177Lu-DOTATATE (LutaThera®), which was approved for GEP-NETs by the United States Food and Drug Administration in 2018. Due to the high renal toxicity, 90Y is now used for the locoregional treatments of liver metastases. The usual schedule for PRRT comprises four cycles of 177Lu-DOTATATE over 6-8 mo, achieving total radioactivity of 25-30 GBq. Toxicity includes myelotoxicity, which can be mitigated with extracorporeal affinity adsorption treatment. This side effect is usually mild and reversible; however, up to 10% of patients may develop WHO Grade 3/4 hematotoxicity, and rarely myelodysplastic syndrome or leukemia[10,83]. Nephrotoxicity may also be caused by PRRT, as the radiopeptides accumulate in the renal interstitium; however, this AE can be reduced by administering a positively charged amino acid infusion. Nausea, vomiting, or (rarely) carcinoid crisis may also occur with PRRT[10].
After a long series of retrospective studies investigating PRRT and proving its ability to inhibit tumor growth in 50%-70% of GEP-NETs[84], the first phase III RCT (the NETTER-1 study)[76] was published. It included 229 patients affected by progressive, unresectable, Sb-NETs G1-G2, and showed an improved outcome with Lutathera® + best supportive care (including Octreotide 30 mg) than with Octreotide 60 mg administered every 4 wk. More specifically, PFS rates at month 20 were 65.2% in the
In a recent consensus, the indication for PRRT was confirmed as a second-line treatment for GEP-NETs with
PRRT for G3 patients: The data regarding the use of PRRT in GEP-NENs G3 are derived from retrospective series, suggesting the potential active role of this treatment for highly proliferating cases. A recent review of the literature with the same topic has shown a median PFS of 19 mo when adopting PRRT in NETs G3 patients vs 11 mo for NECs with Ki67 < 55%, and only 4 mo for NECs with higher Ki67[91]. Based on these results, PRRT can be considered for patients with increased uptake on somatostatin-based imaging tests, both in GEP-NETs G3 and NECs, but with a Ki67 < 55%, inoperable disease, life expectancy of at least 3–6 mo, and reasonable performance status (Karnofski Score > 50%)[82]. A potential role for highly proliferating NEC patients might be reserved to very selected cases, and probably a dual tracer using somatostatin-based imaging tests and 18Fluorine-fluorodeoxyglucose (18F-FDG) PET/CT might be necessary for these patients.
Novel biomarkers and potential role of 18F-FDG-PET/CT: DP during PRRT is reported in 15%–30% of patients, and the lack of predictive biomarkers helping identify responders vs non-responders represents an open issue for NEN management. Proposed tests are the PRRT prediction quotient (PPQ), which is a blood-based assay for eight genes useful to predict PRRT efficacy with an accuracy of 97%, and the NETest, showing an accuracy of 98% in assessing response to PRRT. Trends of NETest correlate with PPQ prediction, but no tests can predict toxicity[92,93]. The 18F-FDG-PET/CT might also help select patients who are candidate for PRRT. It is commonly used in many tumors, but its value for NENs had been initially reserved only for poorly differentiated cases. The recent International Consensus regarding the role of theragnostic in NENs considered it suitable to employ 18F-FDG PET/CT in NECs, in NETs G3 and also in NETs G1-G2, in order to identify the mismatched (18F-FDG-PET/CT-positive/
Re-treatment with PRRT: The opportunity to perform a second PRRT regimen, in patients already undergoing this therapy, is currently being discussed. Rudisile et al[95] re-treated 35 patients, who had previously received four cycles with 177Lu-DOTATATE, obtaining a stable disease in 26 patients (81.3%). They concluded that salvage therapy with 177Lu-DOTATATE is safe and effective, even in patients with extensive previous multimodal therapies during DP. The experience from Denmark reports a better response for G1-G2 cases than G3, but shorter survival outcomes upon retreatment (median PFS 19 mo, median OS 54 mo)[96]. In 2021, a meta-analysis of seven studies regarding PRRT re-treatment in 414 patients with advanced NETs showed a median PFS of 12.52 mo, with a safety profile similar to the initial PRRT treatment[97]. These encouraging data have been recently supported by a consensus on theragnostic in NENs, proposing PRRT rechallenge in patients with a stable disease for at least 1 year following therapy completion[89].
Neoadjuvant PRRT: The use of pre-surgical PRRT, aimed at obtaining disease downstaging, primarily derives from small retrospective series. The largest series includes 57 GEP-NETs with unresectable primary tumor due to vascular involvement, with or without liver metastases. After receiving pre-operative 177Lu-DOTATATE, resectable primary tumor was observed in 15 (26.3%) cases. The estimated PFS rate at 2 years was 90%-95%, and OS accounted for 92.1%. A better response was observed in the case of: Duodenal NETs, GEP-NETs with no regional lymph node involvement, primary tumor < 5 cm, liver lesions ≤ 1.5 cm, number of liver lesions ≤ 3, and 18FDG-uptake as a maximum standard uptake value < 5 in the primary tumor[98]. Regarding Pan-NETs, neoadjuvant PRRT seems to reduce the size of the primary tumor, the size of metastatic lymph nodes, and the risk of pancreatic fistula, maintaining the same post-operative survival outcomes[99].
Future perspectives and open questions: Besides the available data supporting PRRT as a second-line treatment after SSA-failure, the efficacy of PRRT at first line will be evaluated by the NETTER-2 study, which adopts Lutathera® in combination with long-acting Octreotide in advanced GEP-NETs G2-G3 compared to high-dose (60 mg) long-acting Octreotide (Supplementary Table 1). The RCT is including both naïve patients and cases previously treated with SSAs in the absence of DP. The study will also provide more data regarding the use of PRRT in the treatment of GEP-NETs G3, probably also at first line. The identification of novel biomarkers helping select the right candidates for PRRT from the NENs would pave the way for the application of precision medicine in this field. The NeoLuPaNET trial will assess the role of neoadjuvant PRRT in resectable Pan-NETs at high risk of disease recurrence. The study endpoints will include post-operative 90-d morbidity and mortality rates, and objective RRs (Supplementary Table 1). Somatostatin receptor antagonists rather than agonists, labeled with radionuclides, are being investigated and seem to provide a longer tumor residence time of the administered dose. New alpha, beta, gamma, and Auger electron-emitting radionuclides are being investigated. In particular, 212Pb-DOTAMTATE seems to be a possible alternative to 177Lutethium (NCT03466216). The first results from a dose-escalation study on 6 patients were presented at the NANETS 2020 Conference[100], and the results are promising.
Indications, efficacy, and safety: Everolimus is an inhibitor of the mammalian target of rapamycin, which is an intracellular protein kinase downstream of the phosphoinositide 3-kinase (PI3K)/Akt pathway involved in tumorigenesis. It has been approved as an antineoplastic drug for progressive GEP-NETs as a result of several trials and also “real-life” experiences. It is usually prescribed at a standard dosage of 10 mg/d as continuous oral intake, but in the case of toxicity it can be reduced to 5 mg/d or interrupted (in the case of Grade 3 or 4 side effects).
Focusing on metastatic Pan-NETs, the phase II trial RADIANT-1 proved the efficacy in tumor control after chemotherapy failure of both everolimus alone (10 mg/d) and combined with Octreotide LAR, led to a median PFS of 9.7 mo and 16.7, respectively[101]. The subsequent phase III RADIANT-3 study assessed tumor control by everolimus in 140 progressive Pan-NETs, and showed a significantly different median PFS compared to placebo: 11.0 mo vs 4.6 mo, respectively (P < 0.01)[102].
Regarding non-pancreatic NETs, the RADIANT-4 RCT evaluated the efficacy of everolimus 10 mg/d compared to placebo in progressive, well-differentiated, non-functioning lung and non-pancreatic digestive NETs[103]. A significantly higher PFS was observed in the treatment arm compared to placebo (11 mo vs 3.9 mo; P < 0.001), with a rate of disease stabilization respectively of 81% vs 64%. The efficacy of everolimus was also proved in terms of OS, with a 36% reduction in the risk of death (HR: 0.64; P = 0.037). However, a recent meta-analysis of all available trials adopting everolimus for NENs confirmed the benefit in terms of PFS, but not in terms of OS[104].
The efficacy of everolimus and the good safety profile in advanced progressive GEP-NETs were also confirmed in the real-world setting. In 169 patients receiving this drug for compassionate use, the median PFS was 12 mo and the median OS was 32 mo. The results of the study also suggested the use of everolimus before chemotherapy and PRRT, as the subgroup of patients previously treated with these therapies had suffered due to higher toxicity[105].
Reported toxicity during treatment with everolimus includes: Stomatitis (up to 67% of cases), skin rash (29%–49%), fatigue (33%), infections (20%), diarrhea (30%), cytopenias (< 20%), pulmonary toxicity (10.4%), metabolic impairment (hyperglycemia 5%-13%, increased triglyceride and cholesterol levels 39%-66%, hypophosphatemia 40%), peripheral oedema (13%-20%), and renal impairment (rare and transient)[13,106,107]. Regarding stomatitis, a systematic review observed a longer PFS when it occurs within 8 wk from the start of therapy[106].
Everolimus for G3 patients: A potential antiproliferative effect of everolimus in NENs G3 far been reported in well-differentiated cases. A median PFS of 6 mo and a median OS of 28 mo were observed in a small, retrospective cohort of 15 cases with Ki67 20%-55%[108]. In this series, disease stabilization was maintained in 40% of cases for at least 1 year. Focusing on prospective studies, the NECTOR study (a phase II multicenter trial) has evaluated the safety and efficacy of everolimus after failure of platinum-containing chemotherapy in Pan-NECs, providing discouraging results[109]. In the enrolled 25 patients, the median PFS was only 1.2 mo and median OS was 7.5 mo. Disease control was obtained in 39.1% of cases, with no objective response.
Resistance to everolimus: The antiproliferative effect of everolimus may be limited by primary and secondary drug resistance. In detail, patients showing DP at their first evaluation after starting treatment are primary refractory, whereas cases facing DP after an initial tumor response are patients with acquired resistance[110]. Several strategies are being investigated to overcome the resistance to everolimus. Retreatment after a pause might be an option, but this strategy is only supported by clinical experience and not by published data. A possibility reported in the literature is represented by BEZ-235, which is a dual inhibitor for PI3K and mammalian target of rapamycin (mTOR) (PI3K/mTOR kinase inhibitors), and has a potential synergistic effect when adopted in combination with everolimus. Passing from preclinical to clinical studies, about 250 patients affected by several tumor types were treated with this drug. Since the patients experienced high toxicity of the gastrointestinal tract and bone marrow, as well as early progression, the trials including Pan-NETs were prematurely stopped[111,112].
Future perspectives and open questions: The EVINEC study is currently enrolling patients with G3 neuroendocrine disease, after platinum-based chemotherapy failure, to be treated with everolimus (Supplementary Table 1). This trial will provide further data regarding the use of this therapy in NEC patients. The possibility to retreat patients with everolimus, alone or in combination with other drugs, has never been investigated but may represent another option to be evaluated in future studies. This strategy might also help overcome the resistance to everolimus.
Indications, efficacy, and safety: Sunitinib is an oral multikinase inhibitor competing with ATP for binding within the intracellular domain of various wild-type and/or mutated receptor tyrosine kinases. This antiangiogenetic drug acts against vascular endothelial growth factor receptors, platelet-derived growth factor receptors, KIT, fms-like tyrosine kinase 3, and RET. It has been registered for advanced progressive Pan-NETs at a standard oral daily dose of 37.5 mg, based on a double-blind phase III RCT including 171 well-differentiated, advanced, progressive Pan-NETs receiving sunitinib or placebo[113]. The trial was interrupted early due to the significantly different outcomes and toxicity observed in the two arms: Median PFS 11.4 mo with sunitinib vs only 5.5 mo in the placebo arm (P < 0.01), OS at 6 mo 92.6% vs 85.2%, respectively (P = 0.02). A re-analysis of this study[114] showed no significant difference in terms of quality of life between the two arms, with the exception of a worsening of diarrhea observed in the treated patients (P < 0.05). Reported toxicity observed during treatment with sunitinib generally includes gastrointestinal symptoms (diarrhea, nausea, vomiting) in 33%-59% of cases, and fatigue (41% of patients). Other possible side effects can be hypertension, headache, the hand-foot syndrome, and neutropenia (Grade 3-4 in 12%). Treatment discontinuation due to side effects occurs in 15% of patients, and 31% require a dose reduction[13]. Experiences from the real-world setting reported, in 62 Pan-NETs receiving Sunitinib for a median time of 165 d, objective response in 13.7% of patients, but the need for dose reduction in 41.9%[115]. In an Italian retrospective study[116] of 80 pre-treated Pan-NETs receiving sunitinib, the median PFS was very close to the results of the trial by Raymond et al[113] (10 mo), with 7.5% of patients stopping the treatment due to toxicity. The data concerning the efficacy of sunitinib in non-pancreatic NENs are scare and disappointing. One study from Korea[117] adopted sunitinib in 10 non-pancreatic patients, observing a disease stabilization in 50% of the series, but a poorer median PFS than in cases treated with everolimus: 1.7 mo vs 14.7 mo, respectively (P < 0.01).
Sunitinib for G3 patients: Regarding G3 disease, data regarding the use of sunitinib are scarce. Mizuno et al[118] observed, in 15 unresectable Pan-NENs G3 receiving sunitinib, a significantly better outcome for Pan-NETs G3 than Pan-NECs (P < 0.05), and no significant difference between Pan-NETs G3 and G1-G2 cases. A tumor response in G3 cases treated with sunitinib was also observed by Pellat et al[119] in an open-label study, who described in 31 GEP-NENs G3 a median PFS of 42 d, and median OS of 181 d. However, this study was primarily focused on biomarkers, and did not report further details regarding survival.
Future perspectives and open questions: Prospective studies should assess the efficacy of sunitinib in non-pancreatic, digestive NENs, as well as in GEP-NENs G3.
Surufatinib is an oral tyrosine kinase inhibitor targeting immune cells and angiogenesis. To date, few data are available on its efficacy in GEP-NENs, but they are encouraging. Results of the SANET-ep RCT[120] enrolling 198 patients with progressive, unresectable or metastatic, well differentiated, extra-pancreatic NETs showed a better median PFS for the surufatinib arm compared to placebo (9.2 mo vs 3.8 mo, respectively, P < 0.01). The SANET-p trial included 172 progressive, advanced, Pan-NETs, receiving surufatinib or placebo. The median PFS rates were 10.9 mo vs 3.7 mo, respectively (P < 0.01)[121]. Based on these results, surufatinib might represent a possible further therapeutic option for advanced GEP-NENs, but it also needs to be evaluated in a real-life setting to draw definitive conclusions, especially if we consider the reported toxicity. The two available trials[120,121], in fact, showed more frequent AEs, the occurrence of Grade 3 or worse hypertension, proteinuria, and hypertriglyceridemia. SAEs were reported in 22%-25% of cases in the surufatinib group, and death was observed in 3 patients in both trials.
According to the ENETS Guidelines[9], chemotherapy in general represents a valid option for progressive or advanced Pan-NETs and GEP-NENs G3. Besides these indications, it may also be considered in other particular situations, such as GEP-NENs G2 with high Ki67, in the case of rapidly progressive disease, after the failure of other treatments, or even in cases not expressing SSTRs.
Indications, efficacy, and safety: STZ is generally adopted in advanced/metastatic Pan-NETs G1-G2 with high tumor burden, with the aim of obtaining an objective response. STZ is an alkylating agent, usually administered intravenously as a daily regimen for a 6-wk schedule, by rapid injection or short (15–30 min) infusion with a maximum single dose of 1500 mg/m2. The data concerning its efficacy are controversial, and this drug is not available in some European countries (including Italy). A retrospective study from Germany adopted STZ/5-FU in 96 Pan-NETs, including 56.3% naïve patients, and 6.3% G3. Objective response was reached in 42.7% of patients and stable disease in 40.6%. The median time to progression and OS were 19.4 and 54.8 mo, respectively. A better outcome was observed for Pan-NETs with Ki67 < 15%[122]. Besides the association with 5-FU, an alternative combination of STZ with doxorubicin (or even the STZ/5-FU/doxorubicin regimen) has been investigated, and a better response was observed compared to STZ/5-FU; however, the application of these regimens was limited by a significant cardiotoxicity[123,124].
The most frequent AEs caused by STZ are renal toxicity (dose-related and cumulative), gastrointestinal symptoms (nausea, vomiting, diarrhea), glucose intolerance, liver dysfunction, and hematotoxicity. STZ is mutagenic and carcinogenic and its extravasation causes necrotic tissue lesions[9]. With regard to toxicity, a Japanese retrospective, multicenter study[125] reported in 110 patients the same efficacy adopting a daily vs weekly administration of STZ-based chemotherapy, and with monotherapy vs combination therapy, but with a significantly better tolerability when STZ was adopted as a monotherapy. The objective response observed in the overall population was 21.8%, with median PFS of 9.8 mo. Schrader et al[126] proposed maintaining therapy with STZ/5-FU, using an extended cycle protocol. After the 6-wk protocol, resulting in a median PFS of 21 mo and a median OS of 69 mo, 13 of the 28 included patients were switched to an extended 3-mo cycle protocol for maintaining therapy. This treatment provided an additional median PFS of 23 mo.
Future perspectives and open questions: The use of STZ for non-pancreatic GEP-NETs needs to be further investigated, and might represent a potential option as a neoadjuvant treatment. There are a few studies that evaluate a potential role of STZ in the management of G3 cases and these provided conflicting results[122,127,128]. This option should be further investigated in a prospective setting. Therapy combination with PRRT might be explored as a possible additional therapeutic option for GEP-NETs.
Indications, efficacy, and safety: Temozolomide is an oral alkylator, whereas capecitabine is an oral prodrug for 5-FU. Their association (CAPTEM) usually follows a scheme consisting of capecitabine 750 mg/m2 twice daily (days 1–14) and temozolomide 200 mg/m2 once daily at bedtime (days 10–14) every 28 d[129]. Chemotherapy with CAPTEM has been initially adopted in advanced Pan-NETs G1-G2, based on retrospective studies showing a synergistic effect of these two drugs against tumor proliferation. A randomized phase II study (NCT01824875) including Pan-NETs has definitely proved its superiority in disease control compared to only temozolomide, observing a median PFS of 22.7 mo vs 14.4 mo, respectively (P = 0.023), whereas median OS was not reached vs 38 mo[130].
The cumulative antineoplastic effect of CAPTEM regimen has been calculated by a recent meta-analysis including 15 studies and a total population of 384 NENs: Median OS was at least 12 mo and DCR was 72.89%[131]. The efficacy of CAPTEM has also been assessed at first line for Pan-NETs, resulting in an objective response in 70% of patients and median PFS of 18 mo[129]. Regarding toxicity, most frequent AEs due to temozolomide are gastrointestinal symptoms (vomiting, mild nausea, constipation, anorexia), rash, headache, and fatigue, but convulsions may also occur. Grade 3-4 events have been observed in more than 40% of cases after 4 mo of therapy, and may remain in more than 30% for 12 mo following the stopping of treatment. They include thrombocytopenia (3.36%), neutropenia (0.69%), lymphopenia (0.65%), anemia (0.59%), mucositis (0.57%), and transaminase elevation (0.13%)[9,131]. Capecitabine is associated with hand-foot syndrome and liver toxicity (usually hyperbilirubinemia). Less frequently, hematological toxicity may also occur. Side effects are usually reversible and do not require permanent drug discontinuation, but only a dose reduction[9].
CAPTEM for non-pancreatic GEP-NETs and G3 patients: Some series report the use of CAPTEM regimen also for non-pancreatic GEP-NETs. Ostwal et al[132] included in their series of 29 NENs G2-G3 also 12 Sb-NENs, obtaining a median PFS for the overall cohort of 33.7 mo. Spada et al[133] analyzed data regarding 170 NETs treated with temozolomide-based chemotherapy, including 21 gastrointestinal primary cases and G1 cases. Objective response of the overall population was 28%, median OS 35.6 mo, and median PFS 14.7 mo. The efficacy and safety of CAPTEM regimen have also been proven after prolonged administration in a retrospective study from Israel[134] including 79 NENs with median treatment duration of 12.1 mo (range 0.6-55.6). The median PFS was 10.1 mo and median OS 102.9 mo, with DCR achieved in 59.5% patients. SAEs were rare, with a low discontinuation rate. Regarding the use of temozolomide-based therapy for NENs G3, data from the literature describes CAPTEM as the most commonly used treatment for NETs G3, with a DCR of 65% (35% objective response) and a median PFS of 9.4-12 mo[135]. Instead, the few data available regarding the use of this temozolomide-based regimen in GEP-NECs report poorer disease control in this subset of patients compared to all NETs (HR: 2.70)[136], with a median PFS of 1.8 mo and a median OS of 7.8 mo observed in unresectable extra-pulmonary NECs after platinum-based chemotherapy failure[137].
Neoadjuvant use of TEMCAP: Only two series sought to exploit the downstaging effect of TEMCAP as a neoadjuvant treatment. In a series of 30 Pan-NETs with advanced disease or hepatic metastases, partial response after CAPTEM was achieved in 43% of cases[138]. Another report from the United States adopted in six Pan-NETs with borderline criteria for resectability the CAPTEM regimen +/- radiotherapy before surgery, and obtained in all the patients a radiologic response, and a R0 resection in four[139].
Future perspectives and open questions: Alkylating agents (temozolomide, dacarbazine, DTZ) transfer methyl adducts on DNA bases. Of these, O6-methylguanine accounts for many of their cytotoxic effects and can be repaired by the O6-methylguanine-methyltransferase (MGMT). Approximately half of Pan-NETs are MGMT-deficient, as determined by impaired tumor MGMT expression or by MGMT promoter methylation[133]. An open issue is whether the MGMT deficiency may be a relevant biomarker for increased response and improved survival in these patients. Prospective studies evaluating this possibility and attempting to standardize the assessment of MGMT status are needed. Prospective studies investigating the potential benefit from neoadjuvant CAPTEM for advanced GEP-NETs would provide data regarding a possible further cytotoxic role of this chemotherapy regimen.
Indications, efficacy, and safety: Platinum-based chemotherapies are considered the standard of care for unresectable GEP-NECs[9]. Sorbye et al[140] showed a better outcome for advanced GEP-NENs G3 adopting palliative chemotherapy compared to best supportive care. Median OS was indeed 11 mo vs 1 mo, respectively. Patients with Ki67 < 55% had a lower RR to the treatment (15% vs 42%, P < 0.001) but a better OS than cases with higher Ki67 (14 mo vs 10 mo, P < 0.001). Furthermore, the analysis identified negative prognostic factors for survival a poor performance status, a primary tumor colorectal site, and elevated platelets or lactate dehydrogenase levels.
A recent study performed a reclassification of G3 patients previously treated with platinum-based chemotherapy based on the new WHO classification[6]. In this analysis, a higher RR was observed for NECs with Ki67 ≥ 55% (44%) than NECs with Ki67 < 55% (25%) or NETs G3 (24%). Median PFS was instead 5 mo for all the subgroups[141]. The cisplatin-etoposide regimen (or alternatively carboplatin-etoposide, or irinotecan-cisplatin) is usually adopted at first line in these neoplasms, with an expected RR of 30%-70% and high toxicity. An adequate organ function and performance status are thereby required to receive this systemic treatment[9,142,143].
Cisplatin is usually administered by intravenous infusion, after intensive pre- and post-treatment intravenous hydration +/- osmotic diuretic (to prevent renal toxicity)[9]. Etoposide is usually administered by intravenous infusion, but oral formulation is also available[144]. Regarding toxicity, cisplatin is contraindicated in the case of renal impairment or allergic reactions against platinum compounds, whereas dose reduction is not needed in the case of liver function impairment. Side effects (involving at least 10% of patients) include gastrointestinal symptoms (anorexia, nausea, vomiting, and diarrhea), hematotoxicity (leukopenia, thrombocytopenia, and anemia), renal disorders, hearing impairment, fever, and peripheral sensory neurotoxicity (transient or permanent)[9]. The data concerning the possibility to adopt carboplatin in the case of renal failure as an alternative to cisplatin are still scarce; however, AEs, including liver failure, may occur also with carboplatin[9]. Etoposide is carcinogenic and mutagenic. The dose-limiting effect of etoposide is myelosuppression. Impaired hepatic or renal function may increase etoposide concentration in tissue. Gastrointestinal symptoms may also occur, as well as stomatitis and temporary hair loss[9]. The association of oxaliplatin-based chemotherapy with 5-FU, leucovorin, and oxaliplatin (FOLFOX) or with capecitabine (XELOX) are usually adopted for NECs as a second or further line of therapy, with an expected DCR of 62%-84%[145,146]. Some series have shown activity also in GEP-NENs G1-G2[145,147], and a retrospective series has reported promising results with FOLFOX also adopted at first line for GEP-NETs G2 and GEP-NENs G3[148]. Toxicity from FOLFOX includes hematotoxicity (84.1%), chemotherapy-induced peripheral sensory neuropathy, renal toxicity, and infections[148].
Platinum-based chemotherapy for GEP-NETs G3: The efficacy of platinum-based chemotherapy in unresectable GEP-NETs G3 is uncertain. A recent retrospective series analyzed the efficacy of platinum-based treatment, regardless of tumor differentiation and grading[149]. The data regarding 50 Pan-NETs and 29 Pan-NECs were collected, observing partial response in 20% and 41%, respectively. Median OS was 10.9 mo vs 29.2 mo, respectively, and no statistically significant difference in terms of PFS was observed. A potential role of cisplatin-etoposide and FOLFOX-regimens in NETs G3 have been suggested, but with a short-lived response[135]. These data also suggest a potential role of platinum-based regimen in Pan-NETs, but patient selection still represents a critical issue. Some molecular markers have been proposed to help select patients (e.g., retinoblastoma protein, KRAS, and TP53 mutations), but the data are still scarce and only based on retrospective series[150-152].
Future perspectives and open questions: Prospective studies investigating new biomarkers predicting tumor response to platinum-based chemotherapy would help select the right candidates for this treatment, including a subgroup of GEP-NETs G3.
Prospective studies adopting FOLFOX at first line in GEP-NENs G3 would definitely assess the potential efficacy of this regimen in these aggressive neoplasms.
Regarding GEP-NECs, chemotherapy with fluorouracil, leucovorin, and irinotecan (FOLFIRI) is a possible second-line option for GEP-NENs after cisplatin-etoposide failure. The series published to date are small and retrospective[153]. A randomized, non-comparative, multicenter phase II trial (the SENECA study) will assess the efficacy of CAPTEM vs FOLFIRI in GEP-NECs as a second-line treatment after failure of platinum-based therapy (Supplementary Table 1).
Indications, efficacy, and safety: In the last decade, immunotherapy has revolutionized the prognosis of many solid tumors, such as melanoma and non-small cell lung cancer. However, the efficacy of immune checkpoint inhibitors (ICIs) in NENs is disappointing. The reasons for this failure might be related to their tumor biology, since NETs are usually characterized by a slow growth rate, a relatively low tumor mutational burden and a rare microsatellite instability[154,155]. Instead, although NECs are highly aggressive neoplasms with high tumor mutational burden, ICIs have not achieved the expected results with these patients[154,156,157].
One of the first ICIs tested in NENs is pembrolizumab, a highly selective, humanized monoclonal antibody blocking the interaction of programmed cell death protein 1 (PD-1) with its ligands [programmed death-ligand 1 (PD-L1) and PD-L2]. The multicohort, single-arm, phase 1 KEYNOTE-028 basket trial evaluated the safety and efficacy of pembrolizumab monotherapy across 20 tumor cohorts, including a cohort of 25 non-pancreatic NETs and a cohort of 16 Pan-NETs. Patients had a PD-L1-positive tumor and were mostly heavily pre-treated. The median follow-up was 20 mo and the overall RR 12.0% in non-pancreatic NETs and 6.3% in Pan-NETs, respectively. The range of response duration was 6.9-17.6 mo. No complete response was observed[158]. In the subsequent phase II KEYNOTE-158 basket trial, pembrolizumab was administered in a cohort of 107 progressive NETs. Patients were enrolled regardless of PD-L1 expression. Objective (only partial) response was achieved in 3.7% of patients, and they all had PD-L1-negative neoplasms. The treatment provided disease stabilization se in 57% of cases, a median PFS of 4.1 mo and a median OS of 24.2 mo. Although these results seem encouraging, they need to be read with caution, as NETs are characterized by a slow growth[159].
ICI for G3 patients: The role of ICIs was also analyzed in NENs G3. Vijayvergia et al[160] published a joint analysis of two prospective, non-randomized trials with pembrolizumab in 29 advanced NENs G3 after failure of platinum-based treatment. In 1 patient (3.4%), an objective response was observed, while 6 (20.7%) achieved stable disease. The median PFS was 8.9 wk, with no significant differences between the PD-L1-positive and PD-L1-negative groups. Similar and no clinically relevant results were obtained with avelumab[161]. Another humanized anti-PD-1 antibody, spartalizumab, was evaluated in a phase II, multicenter, single-arm study of 95 patients including 55 GEP-NETs and 21 GEP-NECs[162]. All patients were progressive at study entry and had received prior treatment for advanced disease. The DCR was 64.2% in the NET group and 19% in the GEP-NEC group, with a better outcome observed for thoracic NETs. However, this study was formally negative because the primary endpoint (objective response > 10%) was not reached.
Combination immunotherapy: Studies of combination immunotherapy with dual blockade of PD-1 and cytotoxic T-lymphocyte antigen 4 (CTLA-4) have shown more promising results. In the DART SWOG 1609 basket trial, ipilimumab was adopted in combination with nivolumab[163]. The cohort of rare tumors also included 32 extra-pancreatic NENs (18 with high-grade disease). One patient obtained complete response (3%), whereas 7 (22%) achieved a partial response, with a better outcome achieved by NECs than NETs (P = 0.004). The combination of durvalumab with tremelimumab in patients with progressive NETs was investigated in the phase II DUNE trial. This study recruited 123 patients, including GEP-NENs after the failure of standard therapies. The immune-related RECIST objective response was 0% for gastrointestinal NETs, 6.3% for Pan-NETs, and 9.1% for GEP-NENs G3[164].
Future perspectives and open questions: Considering the poor results obtained by adopting immunotherapy in NENs, compared to other solid cancers, new biomarkers able to identify the right candidates for immunotherapy are needed. New prospective trials investigating further immunotherapy combination are also needed to provide further therapeutic options to progressive, heavily pretreated patients. More data concerning immunotherapy for GEP-NECs are also needed, as therapeutic options for these aggressive cases are still scarce.
The concept of new frontiers in the field of therapy for GEP-NENs can have several interpretations. Besides the introduction of novel medications, new perspectives also include: Endoscopic ablation for Pan-NETs, medical therapy combination, and the optimization of therapy sequences.
The development of specifically designed accessories and suitable technologies for locoregional treatments with EUS guidance has made it possible to perform tumor ablations in Pan-NETs not eligible for surgery, resulting in a lower morbidity rate.
These treatments include EUS-guided radiofrequency ablation (EUS-RFA), which is utilized for the treatment of small functional Pan-NETs to improve symptoms without serious complications[165]. The same technique has also been used to treat non-functional asymptomatic Pan-NETs, with complete response obtained in 71.4%-85.7%[166].
Another technique that has demonstrated good safety and reproducible results is ablation by ethanol injection. Multiple case reports and case series have been published with a procedure success rate ranging from 50% to 60% for non-functioning Pan-NETs and 93% for functioning Pan-NETs[167]. Similar results were achieved in a larger cohort by Choi et al[168], treating 33 Pan-NETs with a mixture of 1:1 ethanol and lipidiol. Complete ablation was observed in 60% of the lesions, with complete necrosis at the 3-year follow-up in 41%.
The RAPNEN trial is currently recruiting patients with Pan-NENs to be treated with EUS-RFA, and will investigate the efficacy of this treatment in both functioning and non-functioning cases, including G3 patients (Supplementary Table 1).
Several studies have investigated the antiproliferative effect of therapy combinations, thereby exploiting the potential synergistic effect of the different treatments. This approach must however be investigated with caution, also considering the possible superior toxicity compared to single treatments.
Therapy combination with PRRT: In one phase II clinical trial[169], 177Lu-DOTATATE PRRT was implemented with capecitabine and temozolomide in advanced low-grade NETs, achieving DCR in 71% of patients, a median PFS of 31 mo and median OS not reached. AEs were mild to moderate, and most frequently represented by nausea, thrombocytopenia and neutropenia. In another phase II study[170], the efficacy and toxicity of PRRT with 177Lu-DOTATATE were assessed in combination with metronomic capecitabine, as a radio-sensitizer agent, in advanced, progressive 18FDG-positive GEP NETs with Ki67 < 55%. The DCR was obtained in 85% of cases, with a median PFS of 31.4 mo, and a median OS not reached. No renal toxicity was observed in this series.
The combination with CAPTEM was also investigated as a sandwich chemo-PRRT treatment. More specifically, within 2 wk after PRRT, CAPTEM was administered followed by a 2-wk rest period; the next cycle of CAPTEM was repeated similarly and followed by 1 mo break, and the next cycle of PRRT was administered at about 3 mo. Two cycles of CAPTEM were therefore sandwiched between two cycles of PRRT. With this treatment schedule, DCR was observed in 84% of cases, while the median PFS and OS were not reached at a median follow-up of 36 mo[171]. Regarding first-line PRRT, a series from India investigated its efficacy in association with Capecitabine in 45 consecutive unresectable NETs, with favorable outcomes. In detail, partial response was observed in 30% of cases, and the median PFS was 48 mo[172].
Therapy combination with everolimus: Bajetta et al[173] adopted the everolimus + LAR Octreotide combination regimen in naïve advanced NETs, and obtained positive conclusions. More specifically, 18% and 74% of the cases showed objective response and disease stabilization for at least 6 mo, respectively. The EVERLAR study[174] reported prospective data on everolimus in combination with SSAs in non-functioning gastrointestinal NETs, with encouraging results in terms of both safety and efficacy. Indeed, the 24-mo PFS rate was 43.6%, with objective response achieved in 2.3% and stable disease in 58.1%. The median OS was not reached after 24 mo. Focusing on chemotherapy, the combination of everolimus and temozolomide offered interesting results in advanced Pan-NETs. In 40 patients treated with these two therapies for 6 mo, no synergistic toxicities were observed and 40% of patients experienced a partial response. The median PFS rate was 15.4 mo, whereas the median OS was not reached[175]. A single-arm trial (NCT02248012) will show the potential synergy of everolimus and temozolomide in NET G3 patients with Ki67 ranging from 20% to 55% (Supplementary Table 1).
Therapy combination with sunitinib: The combination of sunitinib and SSAs adopted in 50 NET patients lead to a "not reached" median PFS, with DCR of 86%. These results come from real-world studies and may be limited by retrospective design and heterogeneous population[176]. Sunitinib was also investigated to potentiate tumor control after TAE in 23 NETs, administered for 1 year after the procedure, achieving a median PFS of 15.2 mo and RR of 72%[177].
Chemotherapy combination: A recent retrospective study has evaluated response to treatment with 5-FU, doxorubicin and STZ (FAS) in Pan-NETs. Median PFS was 20 mo and median OS 63 mo. A better outcome was observed when adopting the FAS regimen at first line, without significant safety concerns[178]. The BEVANEC trial is currently recruiting GEP-NEC patients, after failure of platinum-based chemotherapy, to receive a combination of bevacizumab with FOLFIRI vs FOLFIRI alone (Supple-mentary Table 1)[179].
Although the therapeutic landscape for NENs offers several options, the correct therapy sequence to be adopted is so far unknown. Several trials are attempting to compare different sequences in order to understand, on the basis of benefit and toxicity, which alternative option should be preferred. The safety and efficacy of everolimus after prior treatment with PRRT was investigated in a multicenter study including 24 GEP-NETs[180]. Major clinical AEs during treatment with everolimus were hyperglycemia (20.8%), thrombocytopenia (8.3%), fatigue (8.3%) and elevated alanine transaminase levels (8.3%). The median PFS was 13.1 mo, longer than observed in previous trials, suggesting that pretreatment with PRRT might not affect response to everolimus. A retrospective series of Pan-NETs pretreated with chemotherapy followed by PRRT showed that previous treatment with more than one chemotherapy line was a negative prognostic factor for survival outcome, whereas resection of the primary tumor had a positive impact on survival[181]. The COMPETE trial is currently recruiting unresectable, progressive GEP-NETs G1-G2 to receive treatment by PRRT with 177Lu-Edotreotide vs everolimus (SupplementaryTable 1). The SEQTOR study, which has been developed in Europe, aims instead to investigate the optimal sequence for everolimus and chemotherapy (Supplementary Table 1).
In summary, as GEP-NENs represent a highly heterogeneous disease treated with therapeutic protocols that are not fully standardized, a multidisciplinary approach is mandatory for their management. Great advances have been made in the last decade in terms of treatments. Current trials will help answer the open questions regarding therapies for these patients, offering new perspectives in terms of novel drugs, therapy sequence and therapy combination. These data, together with molecular profiling and the application of radiomics in the understanding of tumor features and behavior, will also pave the way for precision medicine in this oncological field.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ramirez RA, Wang WQ S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2464] [Article Influence: 308.0] [Reference Citation Analysis (4)] |
| 2. | Leoncini E, Boffetta P, Shafir M, Aleksovska K, Boccia S, Rindi G. Increased incidence trend of low-grade and high-grade neuroendocrine neoplasms. Endocrine. 2017;58:368-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 3. | Klöppel G, La Rosa S. Ki67 Labeling index: assessment and prognostic role in gastroenteropancreatic neuroendocrine neoplasms. Virchows Arch. 2018;472:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 4. | Rindi G, Klöppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, Erikssson B, Falchetti A, Falconi M, Komminoth P, Körner M, Lopes JM, McNicol AM, Nilsson O, Perren A, Scarpa A, Scoazec JY, Wiedenmann B; all other Frascati Consensus Conference participants; European Neuroendocrine Tumor Society (ENETS). TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449:395-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1196] [Cited by in RCA: 1084] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 5. | Rindi G, Klöppel G, Couvelard A, Komminoth P, Körner M, Lopes JM, McNicol AM, Nilsson O, Perren A, Scarpa A, Scoazec JY, Wiedenmann B. TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2007;451:757-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 680] [Cited by in RCA: 653] [Article Influence: 36.3] [Reference Citation Analysis (1)] |
| 6. | WHO. Classification of Tumors Editorial Board: digestive system tumors, 5th ed. WHO 2019. |
| 7. | Bakker WH, Albert R, Bruns C, Breeman WA, Hofland LJ, Marbach P, Pless J, Pralet D, Stolz B, Koper JW. [111In-DTPA-D-Phe1]-octreotide, a potential radiopharmaceutical for imaging of somatostatin receptor-positive tumors: synthesis, radiolabeling and in vitro validation. Life Sci. 1991;49:1583-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 214] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, Kovacs P, Von Guggenberg E, Bale R, Virgolini IJ. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 688] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 9. | Garcia-Carbonero R, Rinke A, Valle JW, Fazio N, Caplin M, Gorbounova V, O Connor J, Eriksson B, Sorbye H, Kulke M, Chen J, Falkerby J, Costa F, de Herder W, Lombard-Bohas C, Pavel M; Antibes Consensus Conference participants. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasms. Systemic Therapy 2: Chemotherapy. Neuroendocrinology. 2017;105:281-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 10. | Hicks RJ, Kwekkeboom DJ, Krenning E, Bodei L, Grozinsky-Glasberg S, Arnold R, Borbath I, Cwikla J, Toumpanakis C, Kaltsas G, Davies P, Hörsch D, Tiensuu Janson E, Ramage J; Antibes Consensus Conference participants. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasia: Peptide Receptor Radionuclide Therapy with Radiolabeled Somatostatin Analogues. Neuroendocrinology. 2017;105:295-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 242] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 11. | Kaltsas G, Caplin M, Davies P, Ferone D, Garcia-Carbonero R, Grozinsky-Glasberg S, Hörsch D, Tiensuu Janson E, Kianmanesh R, Kos-Kudla B, Pavel M, Rinke A, Falconi M, de Herder WW; Antibes Consensus Conference participants. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pre- and Perioperative Therapy in Patients with Neuroendocrine Tumors. Neuroendocrinology. 2017;105:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 12. | Partelli S, Bartsch DK, Capdevila J, Chen J, Knigge U, Niederle B, Nieveen van Dijkum EJM, Pape UF, Pascher A, Ramage J, Reed N, Ruszniewski P, Scoazec JY, Toumpanakis C, Kianmanesh R, Falconi M; Antibes Consensus Conference participants. ENETS Consensus Guidelines for Standard of Care in Neuroendocrine Tumours: Surgery for Small Intestinal and Pancreatic Neuroendocrine Tumours. Neuroendocrinology. 2017;105:255-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 13. | Pavel M, Valle JW, Eriksson B, Rinke A, Caplin M, Chen J, Costa F, Falkerby J, Fazio N, Gorbounova V, de Herder W, Kulke M, Lombard-Bohas C, O'Connor J, Sorbye H, Garcia-Carbonero R; Antibes Consensus Conference Participants; Antibes Consensus Conference participants. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Neoplasms: Systemic Therapy - Biotherapy and Novel Targeted Agents. Neuroendocrinology. 2017;105:266-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 14. | Malczewska A, Oberg K, Kos-Kudla B. NETest is superior to chromogranin A in neuroendocrine neoplasia: a prospective ENETS CoE analysis. Endocr Connect. 2021;10:110-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Ćwikła JB, Bodei L, Kolasinska-Ćwikła A, Sankowski A, Modlin IM, Kidd M. Circulating Transcript Analysis (NETest) in GEP-NETs Treated With Somatostatin Analogs Defines Therapy. J Clin Endocrinol Metab. 2015;100:E1437-E1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Standards of Practice Committee, Faulx AL, Kothari S, Acosta RD, Agrawal D, Bruining DH, Chandrasekhara V, Eloubeidi MA, Fanelli RD, Gurudu SR, Khashab MA, Lightdale JR, Muthusamy VR, Shaukat A, Qumseya BJ, Wang A, Wani SB, Yang J, DeWitt JM; Standards of Practice Committee. The role of endoscopy in subepithelial lesions of the GI tract. Gastrointest Endosc. 2017;85:1117-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 180] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 17. | Yazici C, Boulay BR. Evolving role of the endoscopist in management of gastrointestinal neuroendocrine tumors. World J Gastroenterol. 2017;23:4847-4855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Exarchou K, Howes N, Pritchard DM. Systematic review: management of localised low-grade upper gastrointestinal neuroendocrine tumours. Aliment Pharmacol Ther. 2020;51:1247-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Ahmed M. Gastrointestinal neuroendocrine tumors in 2020. World J Gastrointest Oncol. 2020;12:791-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 153] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (11)] |
| 20. | Yang DH, Park Y, Park SH, Kim KJ, Ye BD, Byeon JS, Myung SJ, Yang SK. Cap-assisted EMR for rectal neuroendocrine tumors: comparisons with conventional EMR and endoscopic submucosal dissection (with videos). Gastrointest Endosc. 2016;83:1015-22; quiz 1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Lee SH, Lee TH, Jang SH, Choi CY, Lee WM, Min JH, Cho HD, Park SH. Ampullary neuroendocrine tumor diagnosed by endoscopic papillectomy in previously confirmed ampullary adenoma. World J Gastroenterol. 2016;22:3687-3692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Kim J, Kim JH, Lee JY, Chun J, Im JP, Kim JS. Clinical outcomes of endoscopic mucosal resection for rectal neuroendocrine tumor. BMC Gastroenterol. 2018;18:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Lee J, Park YE, Choi JH, Heo NY, Park J, Park SH, Moon YS, Nam KH, Kim TO. Comparison between cap-assisted and ligation-assisted endoscopic mucosal resection for rectal neuroendocrine tumors. Ann Gastroenterol. 2020;33:385-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Zhou X, Xie H, Xie L, Li J, Cao W, Fu W. Endoscopic resection therapies for rectal neuroendocrine tumors: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2014;29:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Harada H, Suehiro S, Murakami D, Nakahara R, Shimizu T, Katsuyama Y, Miyama Y, Hayasaka K, Tounou S. Endoscopic submucosal dissection for small submucosal tumors of the rectum compared with endoscopic submucosal resection with a ligation device. World J Gastrointest Endosc. 2017;9:70-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Trinh VQ, Shi C, Ma C. Gastric neuroendocrine tumours from long-term proton pump inhibitor users are indolent tumours with good prognosis. Histopathology. 2020;77:865-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Roberto GA, Rodrigues CMB, Peixoto RD, Younes RN. Gastric neuroendocrine tumor: A practical literature review. World J Gastrointest Oncol. 2020;12:850-856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (8)] |
| 28. | Sato Y, Hashimoto S, Mizuno K, Takeuchi M, Terai S. Management of gastric and duodenal neuroendocrine tumors. World J Gastroenterol. 2016;22:6817-6828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 86] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (5)] |
| 29. | Chin JL, O'Toole D. Diagnosis and Management of Upper Gastrointestinal Neuroendocrine Tumors. Clin Endosc. 2017;50:520-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Delle Fave G, O'Toole D, Sundin A, Taal B, Ferolla P, Ramage JK, Ferone D, Ito T, Weber W, Zheng-Pei Z, De Herder WW, Pascher A, Ruszniewski P; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology. 2016;103:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 353] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 31. | Merola E, Sbrozzi-Vanni A, Panzuto F, D'Ambra G, Di Giulio E, Pilozzi E, Capurso G, Lahner E, Bordi C, Annibale B, Delle Fave G. Type I gastric carcinoids: a prospective study on endoscopic management and recurrence rate. Neuroendocrinology. 2012;95:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 32. | Ngamruengphong S, Ferri L, Aihara H, Draganov PV, Yang DJ, Perbtani YB, Jue TL, Munroe CA, Boparai ES, Mehta NA, Bhatt A, Kumta NA, Othman MO, Mercado M, Javaid H, Aadam AA, Siegel A, James TW, Grimm IS, DeWitt JM, Novikov A, Schlachterman A, Kowalski T, Samarasena J, Hashimoto R, Chehade NEH, Lee J, Chang K, Su B, Ujiki MB, Mehta A, Sharaiha RZ, Carr-Locke DL, Chen A, Chen M, Chen YI, Pourmousavi Khoshknab M, Wang R, Kerdsirichairat T, Tomizawa Y, von Renteln D, Kumbhari V, Khashab MA, Bechara R, Karasik M, Patel NJ, Fukami N, Nishimura M, Hanada Y, Wong Kee Song LM, Laszkowska M, Wang AY, Hwang JH, Friedland S, Sethi A, Kalloo AN. Efficacy of Endoscopic Submucosal Dissection for Superficial Gastric Neoplasia in a Large Cohort in North America. Clin Gastroenterol Hepatol. 2021;19:1611-1619.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 33. | Sato Y, Takeuchi M, Hashimoto S, Mizuno K, Kobayashi M, Iwafuchi M, Narisawa R, Aoyagi Y. Usefulness of endoscopic submucosal dissection for type I gastric carcinoid tumors compared with endoscopic mucosal resection. Hepatogastroenterology. 2013;60:1524-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (1)] |
| 34. | Kim HH, Kim GH, Kim JH, Choi MG, Song GA, Kim SE. The efficacy of endoscopic submucosal dissection of type I gastric carcinoid tumors compared with conventional endoscopic mucosal resection. Gastroenterol Res Pract. 2014;2014:253860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 35. | Gincul R, Ponchon T, Napoleon B, Scoazec JY, Guillaud O, Saurin JC, Ciocirlan M, Lepilliez V, Pioche M, Lefort C, Adham M, Pialat J, Chayvialle JA, Walter T. Endoscopic treatment of sporadic small duodenal and ampullary neuroendocrine tumors. Endoscopy. 2016;48:979-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 36. | Kim GH, Kim JI, Jeon SW, Moon JS, Chung IK, Jee SR, Kim HU, Seo GS, Baik GH, Lee YC; Korean College of Helicobacter and Upper Gastrointestinal Research. Endoscopic resection for duodenal carcinoid tumors: a multicenter, retrospective study. J Gastroenterol Hepatol. 2014;29:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 37. | Fujimoto A, Sasaki M, Goto O, Maehata T, Ochiai Y, Kato M, Nakayama A, Akimoto T, Kuramoto J, Hayashi Y, Kameyama K, Yahagi N. Treatment Results of Endoscopic Mucosal Resection with a Ligation Device for Duodenal Neuroendocrine Tumors. Intern Med. 2019;58:773-777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Oono Y, Shinmura K, Hori K, Yoda Y, Ishii G, Ikematsu H, Yano T. Endoscopic submucosal resection using a ligation device without injection for duodenal neuroendocrine tumors. Surg Endosc. 2019;33:2008-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Scherer JR, Holinga J, Sanders M, Chennat J, Khalid A, Fasanella K, Singhi AD, McGrath K. Small duodenal carcinoids: a case series comparing endoscopic resection and autoamputation with band ligation. J Clin Gastroenterol. 2015;49:289-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Kobara H, Miyaoka Y, Ikeda Y, Yamada T, Takata M, Fujihara S, Nishiyama N, Fujita K, Tani J, Kobayashi N, Chiyo T, Yachida T, Okano K, Suzuki Y, Mori H, Masaki T. Outcomes of Endoscopic Submucosal Dissection for Subepithelial Lesions Localized Within the Submucosa, Including Neuroendocrine Tumors: A Multicenter Prospective Study. J Gastrointestin Liver Dis. 2020;29:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Suzuki S, Ishii N, Uemura M, Deshpande GA, Matsuda M, Iizuka Y, Fukuda K, Suzuki K, Fujita Y. Endoscopic submucosal dissection (ESD) for gastrointestinal carcinoid tumors. Surg Endosc. 2012;26:759-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 42. | Nishio M, Hirasawa K, Ozeki Y, Sawada A, Ikeda R, Fukuchi T, Kobayashi R, Makazu M, Sato C, Maeda S. Short- and long-term outcomes of endoscopic submucosal dissection for non-ampullary duodenal neuroendocrine tumors. Ann Gastroenterol. 2020;33:265-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Cai MY, Martin Carreras-Presas F, Zhou PH. Endoscopic full-thickness resection for gastrointestinal submucosal tumors. Dig Endosc. 2018;30 Suppl 1:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 44. | Fukasawa H, Tounou S, Nabetani M, Michida T. Endoscopic Resection of Ampullary Neuroendocrine Tumor. Intern Med. 2017;56:499-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Rossi RE, Invernizzi P, Mazzaferro V, Massironi S. Response and relapse rates after treatment with long-acting somatostatin analogs in multifocal or recurrent type-1 gastric carcinoids: A systematic review and meta-analysis. United European Gastroenterol J. 2020;8:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 46. | Pape UF, Niederle B, Costa F, Gross D, Kelestimur F, Kianmanesh R, Knigge U, Öberg K, Pavel M, Perren A, Toumpanakis C, O'Connor J, Krenning E, Reed N, O'Toole D; Vienna Consensus Conference participants. ENETS Consensus Guidelines for Neuroendocrine Neoplasms of the Appendix (Excluding Goblet Cell Carcinomas). Neuroendocrinology. 2016;103:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 178] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 47. | de Mestier L, Lardière-Deguelte S, Brixi H, O'Toole D, Ruszniewski P, Cadiot G, Kianmanesh R. Updating the surgical management of peritoneal carcinomatosis in patients with neuroendocrine tumors. Neuroendocrinology. 2015;101:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Dong DH, Zhang XF, Poultsides G, Rocha F, Weber S, Fields R, Idrees K, Cho C, Maithel SK, Pawlik TM; other members of the US Neuroendocrine Tumor Study Group. Impact of tumor size and nodal status on recurrence of nonfunctional pancreatic neuroendocrine tumors ≤2 cm after curative resection: A multi-institutional study of 392 cases. J Surg Oncol. 2019;120:1071-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 49. | Barenboim A, Lahat G, Nachmany I, Nakache R, Goykhman Y, Geva R, Osher E, Scapa E, Wolf I, Orbach L, Brazowski E, Isakov O, Klausner JM, Lubezky N. Resection Versus Observation of Small Asymptomatic Nonfunctioning Pancreatic Neuroendocrine Tumors. J Gastrointest Surg. 2020;24:1366-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 50. | Partelli S, Inama M, Rinke A, Begum N, Valente R, Fendrich V, Tamburrino D, Keck T, Caplin ME, Bartsch D, Thirlwell C, Fusai G, Falconi M. Long-Term Outcomes of Surgical Management of Pancreatic Neuroendocrine Tumors with Synchronous Liver Metastases. Neuroendocrinology. 2015;102:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 51. | Barrett JR, Rendell V, Pokrzywa C, Lopez-Aguiar AG, Cannon J, Poultsides GA, Rocha F, Crown A, Beal E, Michael Pawlik T, Fields R, Panni RZ, Smith P, Idrees K, Cho C, Beems M, Maithel S, Weber S, Erik Abbott D. Adjuvant therapy following resection of gastroenteropancreatic neuroendocrine tumors provides no recurrence or survival benefit. J Surg Oncol. 2020;121:1067-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 52. | Wu Z, Yu D, Zhao S, Gao P, Song Y, Sun Y, Chen X, Wang Z. The efficacy of chemotherapy and operation in patients with colorectal neuroendocrine carcinoma. J Surg Res. 2018;225:54-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Pellat A, Walter T, Augustin J, Hautefeuille V, Hentic O, Do Cao C, Lievre A, Coriat R, Hammel P, Dubreuil O, Cohen R, Couvelard A, André T, Svrcek M, Baudin E, Afchain P. Chemotherapy in Resected Neuroendocrine Carcinomas of the Digestive Tract: A National Study from the French Group of Endocrine Tumours. Neuroendocrinology. 2020;110:404-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Mao R, Li K, Cai JQ, Luo S, Turner M, Blazer D 3rd, Zhao H. Adjuvant Chemotherapy Versus Observation Following Resection for Patients With Nonmetastatic Poorly Differentiated Colorectal Neuroendocrine Carcinomas. Ann Surg. 2021;274:e126-e133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Lin JP, Zhao YJ, He QL, Hao HK, Tian YT, Zou BB, Jiang LX, Lin W, Zhou YB, Li Z, Xu YC, Zhao G, Xue FQ, Li SL, Fu WH, Li YX, Zhou XJ, Li Y, Zhu ZG, Chen JP, Xu ZK, Cai LH, Li E, Li HL, Xie JW, Huang CM, Li P, Lin JX, Zheng CH. Adjuvant chemotherapy for patients with gastric neuroendocrine carcinomas or mixed adenoneuroendocrine carcinomas. Br J Surg. 2020;107:1163-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 56. | Merola E, Rinke A, Partelli S, Gress TM, Andreasi V, Kollár A, Perren A, Christ E, Panzuto F, Pascher A, Jann H, Arsenic R, Cremer B, Kaemmerer D, Kump P, Lipp RW, Agaimy A, Wiedenmann B, Falconi M, Pavel ME. Surgery with Radical Intent: Is There an Indication for G3 Neuroendocrine Neoplasms? Ann Surg Oncol. 2020;27:1348-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 57. | Partelli S, Ramage JK, Massironi S, Zerbi A, Kim HB, Niccoli P, Panzuto F, Landoni L, Tomazic A, Ibrahim T, Kaltsas G, Bertani E, Sauvanet A, Segelov E, Caplin M, Coppa J, Armstrong T, Weickert MO, Butturini G, Staettner S, Boesch F, Cives M, Moulton CA, He J, Selberherr A, Twito O, Castaldi A, De Angelis CG, Gaujoux S, Almeamar H, Frilling A, Vigia E, Wilson C, Muffatti F, Srirajaskanthan R, Invernizzi P, Lania A, Kwon W, Ewald J, Rinzivillo M, Nessi C, Smid LM, Gardini A, Tsoli M, Picardi EE, Hentic O, Croagh D, Toumpanakis C, Citterio D, Ramsey E, Mosterman B, Regi P, Gasteiger S, Rossi RE, Smiroldo V, Jang JY, Falconi M. Management of Asymptomatic Sporadic Nonfunctioning Pancreatic Neuroendocrine Neoplasms (ASPEN) ≤2 cm: Study Protocol for a Prospective Observational Study. Front Med (Lausanne). 2020;7:598438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 58. | Tierney JF, Chivukula SV, Wang X, Pappas SG, Schadde E, Hertl M, Poirier J, Keutgen XM. Resection of primary tumor may prolong survival in metastatic gastroenteropancreatic neuroendocrine tumors. Surgery. 2019;165:644-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 59. | Tsilimigras DI, Hyer JM, Paredes AZ, Ejaz A, Cloyd JM, Beane JD, Dillhoff M, Tsung A, Pawlik TM. Resection of Primary Gastrointestinal Neuroendocrine Tumor Among Patients with Non-Resected Metastases Is Associated with Improved Survival: A SEER-Medicare Analysis. J Gastrointest Surg. 2021;25:2368-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 60. | Zhou B, Zhan C, Ding Y, Yan S, Zheng S. Role of palliative resection of the primary pancreatic neuroendocrine tumor in patients with unresectable metastatic liver disease: a systematic review and meta-analysis. Onco Targets Ther. 2018;11:975-982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 61. | de Baere T, Deschamps F, Tselikas L, Ducreux M, Planchard D, Pearson E, Berdelou A, Leboulleux S, Elias D, Baudin E. GEP-NETS update: Interventional radiology: role in the treatment of liver metastases from GEP-NETs. Eur J Endocrinol. 2015;172:R151-R166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 62. | Kennedy A, Bester L, Salem R, Sharma RA, Parks RW, Ruszniewski P; NET-Liver-Metastases Consensus Conference. Role of hepatic intra-arterial therapies in metastatic neuroendocrine tumours (NET): guidelines from the NET-Liver-Metastases Consensus Conference. HPB (Oxford). 2015;17:29-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 63. | Eriksson J, Stålberg P, Nilsson A, Krause J, Lundberg C, Skogseid B, Granberg D, Eriksson B, Akerström G, Hellman P. Surgery and radiofrequency ablation for treatment of liver metastases from midgut and foregut carcinoids and endocrine pancreatic tumors. World J Surg. 2008;32:930-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 64. | Chen JX, Rose S, White SB, El-Haddad G, Fidelman N, Yarmohammadi H, Hwang W, Sze DY, Kothary N, Stashek K, Wileyto EP, Salem R, Metz DC, Soulen MC. Embolotherapy for Neuroendocrine Tumor Liver Metastases: Prognostic Factors for Hepatic Progression-Free Survival and Overall Survival. Cardiovasc Intervent Radiol. 2017;40:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 65. | Hur S, Chung JW, Kim HC, Oh DY, Lee SH, Bang YJ, Kim WH. Survival outcomes and prognostic factors of transcatheter arterial chemoembolization for hepatic neuroendocrine metastases. J Vasc Interv Radiol. 2013;24:947-56; quiz 957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 66. | Tomozawa Y, Jahangiri Y, Pathak P, Kolbeck KJ, Schenning RC, Kaufman JA, Farsad K. Long-Term Toxicity after Transarterial Radioembolization with Yttrium-90 Using Resin Microspheres for Neuroendocrine Tumor Liver Metastases. J Vasc Interv Radiol. 2018;29:858-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 67. | Ngo L, Elnahla A, Attia AS, Hussein M, Toraih EA, Kandil E, Killackey M. Chemoembolization Versus Radioembolization for Neuroendocrine Liver Metastases: A Meta-analysis Comparing Clinical Outcomes. Ann Surg Oncol. 2021;28:1950-1958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 68. | Singla S, LeVea CM, Pokuri VK, Attwood KM, Wach MM, Tomaszewski GM, Kuvshinoff B, Iyer R. Ki67 score as a potential predictor in the selection of liver-directed therapies for metastatic neuroendocrine tumors: a single institutional experience. J Gastrointest Oncol. 2016;7:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 69. | Hickey RM, Kulik LM, Nimeiri H, Kalyan A, Kircher S, Desai K, Riaz A, Lewandowski RJ, Salem R. Immuno-oncology and Its Opportunities for Interventional Radiologists: Immune Checkpoint Inhibition and Potential Synergies with Interventional Oncology Procedures. J Vasc Interv Radiol. 2017;28:1487-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 70. | Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Bläker M, Harder J, Arnold C, Gress T, Arnold R; PROMID Study Group. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656-4663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1609] [Cited by in RCA: 1739] [Article Influence: 108.7] [Reference Citation Analysis (0)] |
| 71. | Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P; CLARINET Investigators. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1288] [Article Influence: 117.1] [Reference Citation Analysis (0)] |
| 72. | Merola E, Panzuto F, Delle Fave G. Antiproliferative effect of somatostatin analogs in advanced gastro-entero-pancreatic neuroendocrine tumors: a systematic review and meta-analysis. Oncotarget. 2017;8:46624-46634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 73. | Lamarca A, McCallum L, Nuttall C, Barriuso J, Backen A, Frizziero M, Leon R, Mansoor W, McNamara MG, Hubner RA, Valle JW. Somatostatin analogue-induced pancreatic exocrine insufficiency in patients with neuroendocrine tumors: results of a prospective observational study. Expert Rev Gastroenterol Hepatol. 2018;12:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 74. | Merola E, Alonso Gordoa T, Zhang P, Al-Toubah T, Pellè E, Kolasińska-Ćwikła A, Zandee W, Laskaratos F, de Mestier L, Lamarca A, Hernando J, Cwikla J, Strosberg J, de Herder W, Caplin M, Cives M, van Leeuwaarde R. Somatostatin Analogs for Pancreatic Neuroendocrine Tumors: Any Benefit When Ki-67 Is ≥10%? Oncologist. 2021;26:294-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 75. | Chan DL, Ferone D, Albertelli M, Pavlakis N, Segelov E, Singh S. Escalated-dose somatostatin analogues for antiproliferative effect in GEPNETS: a systematic review. Endocrine. 2017;57:366-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 76. |
Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, Mittra E, Kunz PL, Kulke MH, Jacene H, Bushnell D, O'Dorisio TM, Baum RP, Kulkarni HR, Caplin M, Lebtahi R, Hobday T, Delpassand E, Van Cutsem E, Benson A, Srirajaskanthan R, Pavel M, Mora J, Berlin J, Grande E, Reed N, Seregni E, Öberg K, Lopera Sierra M, Santoro P, Thevenet T, Erion JL, Ruszniewski P, Kwekkeboom D, Krenning E; NETTER-1 Trial Investigators. Phase 3 Trial of |
| 77. | Pavel M, Dromain C, Massien C, Houchard A. Safety and efficacy of lanreotide autogel/depot (LAN) every 14 days for patients with pancreatic or midgut neuroendocrine tumours (NETs) progressing on LAN every 28 days: The prospective, international CLARINET FORTE study. Proceedings of the ESMO; 2020. Ann Oncol. 2020;31 (suppl_4). |
| 78. | Malczewska A, Kos-Kudła B, Kidd M, Drozdov I, Bodei L, Matar S, Oberg K, Modlin IM. The clinical applications of a multigene liquid biopsy (NETest) in neuroendocrine tumors. Adv Med Sci. 2020;65:18-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 79. | Bajetta E, Zilembo N, Di Bartolomeo M, Di Leo A, Pilotti S, Bochicchio AM, Castellani R, Buzzoni R, Celio L, Dogliotti L. Treatment of metastatic carcinoids and other neuroendocrine tumors with recombinant interferon-alpha-2a. A study by the Italian Trials in Medical Oncology Group. Cancer. 1993;72:3099-3105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 80. | Faiss S, Pape UF, Böhmig M, Dörffel Y, Mansmann U, Golder W, Riecken EO, Wiedenmann B; International Lanreotide and Interferon Alfa Study Group. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors--the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol. 2003;21:2689-2696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 336] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 81. | Oberg K, Norheim I, Alm G. Treatment of malignant carcinoid tumors: a randomized controlled study of streptozocin plus 5-FU and human leukocyte interferon. Eur J Cancer Clin Oncol. 1989;25:1475-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 82. | Kwekkeboom DJ, Krenning EP, Lebtahi R, Komminoth P, Kos-Kudła B, de Herder WW, Plöckinger U; Mallorca Consensus Conference participants; European Neuroendocrine Tumor Society. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: peptide receptor radionuclide therapy with radiolabeled somatostatin analogs. Neuroendocrinology. 2009;90:220-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 83. | Merola E, Capurso G, Campana D, Panzuto F, Monarca B, Tomassetti P, Delle Fave G. Acute leukaemia following low dose peptide receptor radionuclide therapy for an intestinal carcinoid. Dig Liver Dis. 2010;42:457-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 84. | Valkema R, Pauwels S, Kvols LK, Barone R, Jamar F, Bakker WH, Kwekkeboom DJ, Bouterfa H, Krenning EP. Survival and response after peptide receptor radionuclide therapy with [90Y-DOTA0,Tyr3]octreotide in patients with advanced gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med. 2006;36:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 213] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 85. | Strosberg J, Wolin E, Chasen B, Kulke M, Bushnell D, Caplin M, Baum RP, Kunz P, Hobday T, Hendifar A, Oberg K, Sierra ML, Thevenet T, Margalet I, Ruszniewski P, Krenning E; NETTER-1 Study Group. Health-Related Quality of Life in Patients With Progressive Midgut Neuroendocrine Tumors Treated With 177Lu-Dotatate in the Phase III NETTER-1 Trial. J Clin Oncol. 2018;36:2578-2584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 277] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 86. | Pavel ME, Broberg P, Caplin M, Ruszniewski P, Strosberg J, Santoro P, Ravasi L, Krenning E. Relation between objective tumor shrinkage and progression-free survival (PFS) in the NETTER-1 population. ESMO. Ann Oncol. 2019;30 (suppl_5):v564-v573. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 87. | Zhang J, Kulkarni HR, Singh A, Baum RP. Delayed Response (Partial Remission) 3 Years After Peptide Receptor Radionuclide Therapy in a Patient Participating in the NETTER-1 Trial. Clin Nucl Med. 2019;44:223-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 88. | Wang LF, Lin L, Wang MJ, Li Y. The therapeutic efficacy of 177Lu-DOTATATE/DOTATOC in advanced neuroendocrine tumors: A meta-analysis. Medicine (Baltimore). 2020;99:e19304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 89. | Ambrosini V, Kunikowska J, Baudin E, Bodei L, Bouvier C, Capdevila J, Cremonesi M, de Herder WW, Dromain C, Falconi M, Fani M, Fanti S, Hicks RJ, Kabasakal L, Kaltsas G, Lewington V, Minozzi S, Cinquini M, Öberg K, Oyen WJG, O'Toole D, Pavel M, Ruszniewski P, Scarpa A, Strosberg J, Sundin A, Taïeb D, Virgolini I, Wild D, Herrmann K, Yao J. Consensus on molecular imaging and theranostics in neuroendocrine neoplasms. Eur J Cancer. 2021;146:56-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 156] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 90. | Strosberg JR, Kunz PL, Ruszniewski PB, Bodei L, Hendifar AE, Mittra E, Wolin EM, Yao JC, Pavel ME, Grande E, Van Cutsem E, Seregni E, Duarte H, Gericke G, Bartalotta A, Demange A, Mutevelic S, Krenning E, On behalf of the NETTER-1 study group. Final overall survival in the phase 3 NETTER-1 study of lutetium-177-DOTATATE in patients with midgut neuroendocrine tumors. ASCO: J Clin Oncol. 2021;4112-4112. |
| 91. | Sorbye H, Kong G, Grozinsky-Glasberg S. PRRT in high-grade gastroenteropancreatic neuroendocrine neoplasms (WHO G3). Endocr Relat Cancer. 2020;27:R67-R77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 92. | Bodei L, Schöder H, Baum RP, Herrmann K, Strosberg J, Caplin M, Öberg K, Modlin IM. Molecular profiling of neuroendocrine tumours to predict response and toxicity to peptide receptor radionuclide therapy. Lancet Oncol. 2020;21:e431-e443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 93. | Bodei L, Kidd MS, Singh A, van der Zwan WA, Severi S, Drozdov IA, Malczewska A, Baum RP, Kwekkeboom DJ, Paganelli G, Krenning EP, Modlin IM. PRRT neuroendocrine tumor response monitored using circulating transcript analysis: the NETest. Eur J Nucl Med Mol Imaging. 2020;47:895-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 94. | Binderup T, Knigge U, Johnbeck CB, Loft A, Berthelsen AK, Oturai P, Mortensen J, Federspiel B, Langer SW, Kjaer A. 18F-FDG PET is Superior to WHO Grading as a Prognostic Tool in Neuroendocrine Neoplasms and Useful in Guiding PRRT: A Prospective 10-Year Follow-up Study. J Nucl Med. 2021;62:808-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 95. | Rudisile S, Gosewisch A, Wenter V, Unterrainer M, Böning G, Gildehaus FJ, Fendler WP, Auernhammer CJ, Spitzweg C, Bartenstein P, Todica A, Ilhan H. Salvage PRRT with 177Lu-DOTA-octreotate in extensively pretreated patients with metastatic neuroendocrine tumor (NET): dosimetry, toxicity, efficacy, and survival. BMC Cancer. 2019;19:788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 96. | Zacho MD, Iversen P, Villadsen GE, Baunwall SMD, Arveschoug AK, Grønbaek H, Dam G. Clinical efficacy of first and second series of peptide receptor radionuclide therapy in patients with neuroendocrine neoplasm: a cohort study. Scand J Gastroenterol. 2021;56:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 97. | Strosberg J, Leeuwenkamp O, Siddiqui MK. Peptide receptor radiotherapy re-treatment in patients with progressive neuroendocrine tumors: A systematic review and meta-analysis. Cancer Treat Rev. 2021;93:102141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 98. | Parghane RV, Bhandare M, Chaudhari V, Ostwal V, Ramaswamy A, Talole S, Shrikhande SV, Basu S. Surgical Feasibility, Determinants, and Overall Efficacy of Neoadjuvant 177Lu-DOTATATE PRRT for Locally Advanced Unresectable Gastroenteropancreatic Neuroendocrine Tumors. J Nucl Med. 2021;62:1558-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 99. | Partelli S, Bertani E, Bartolomei M, Perali C, Muffatti F, Grana CM, Schiavo Lena M, Doglioni C, Crippa S, Fazio N, Zamboni G, Falconi M. Peptide receptor radionuclide therapy as neoadjuvant therapy for resectable or potentially resectable pancreatic neuroendocrine neoplasms. Surgery. 2018;163:761-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 100. | Delpassand E. 212Pb-AlphaMedixTM Targeted Alpha Therapy (TAT): a potential breakthrough in treatment of metastatic SSTR expressing NET. NANET 2020: Virtual. Abstract 193, 2020. |
| 101. | Yao JC, Lombard-Bohas C, Baudin E, Kvols LK, Rougier P, Ruszniewski P, Hoosen S, St Peter J, Haas T, Lebwohl D, Van Cutsem E, Kulke MH, Hobday TJ, O'Dorisio TM, Shah MH, Cadiot G, Luppi G, Posey JA, Wiedenmann B. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J Clin Oncol. 2010;28:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 474] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 102. | Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, Tomassetti P, Pavel ME, Hoosen S, Haas T, Lincy J, Lebwohl D, Öberg K; RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2039] [Cited by in RCA: 2113] [Article Influence: 150.9] [Reference Citation Analysis (0)] |
| 103. | Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, Tomasek J, Raderer M, Lahner H, Voi M, Pacaud LB, Rouyrre N, Sachs C, Valle JW, Fave GD, Van Cutsem E, Tesselaar M, Shimada Y, Oh DY, Strosberg J, Kulke MH, Pavel ME; RAD001 in Advanced Neuroendocrine Tumours, Fourth Trial (RADIANT-4) Study Group. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387:968-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 898] [Article Influence: 99.8] [Reference Citation Analysis (0)] |
| 104. | Zhuo ZG, Zhu YK, Deng HY, Li G, Luo J, Alai GH, Lin YD. Role of everolimus in the treatment of advanced neuroendocrine tumor: a meta-analysis of randomized trials. J BUON. 2019;24:368-373. [PubMed] |
| 105. | Panzuto F, Rinzivillo M, Fazio N, de Braud F, Luppi G, Zatelli MC, Lugli F, Tomassetti P, Riccardi F, Nuzzo C, Brizzi MP, Faggiano A, Zaniboni A, Nobili E, Pastorelli D, Cascinu S, Merlano M, Chiara S, Antonuzzo L, Funaioli C, Spada F, Pusceddu S, Fontana A, Ambrosio MR, Cassano A, Campana D, Cartenì G, Appetecchia M, Berruti A, Colao A, Falconi M, Delle Fave G. Real-world study of everolimus in advanced progressive neuroendocrine tumors. Oncologist. 2014;19:966-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 106. | Rugo HS, Hortobagyi GN, Yao J, Pavel M, Ravaud A, Franz D, Ringeisen F, Gallo J, Rouyrre N, Anak O, Motzer R. Meta-analysis of stomatitis in clinical studies of everolimus: incidence and relationship with efficacy. Ann Oncol. 2016;27:519-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 107. | Iacovelli R, Palazzo A, Mezi S, Morano F, Naso G, Cortesi E. Incidence and risk of pulmonary toxicity in patients treated with mTOR inhibitors for malignancy. A meta-analysis of published trials. Acta Oncol. 2012;51:873-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 108. | Panzuto F, Rinzivillo M, Spada F, Antonuzzo L, Ibrahim T, Campana D, Fazio N, Delle Fave G. Everolimus in Pancreatic Neuroendocrine Carcinomas G3. Pancreas. 2017;46:302-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 109. | Okuyama H, Ikeda M, Okusaka T, Furukawa M, Ohkawa S, Hosokawa A, Kojima Y, Hara H, Murohisa G, Shioji K, Asagi A, Mizuno N, Kojima M, Yamanaka T, Furuse J. A Phase II Trial of Everolimus in Patients with Advanced Pancreatic Neuroendocrine Carcinoma Refractory or Intolerant to Platinum-Containing Chemotherapy (NECTOR Trial). Neuroendocrinology. 2020;110:988-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 110. | Tijeras-Raballand A, Neuzillet C, Couvelard A, Serova M, de Gramont A, Hammel P, Raymond E, Faivre S. Resistance to targeted therapies in pancreatic neuroendocrine tumors (PNETs): molecular basis, preclinical data, and counteracting strategies. Target Oncol. 2012;7:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 111. | Vandamme T, Beyens M, de Beeck KO, Dogan F, van Koetsveld PM, Pauwels P, Mortier G, Vangestel C, de Herder W, Van Camp G, Peeters M, Hofland LJ. Long-term acquired everolimus resistance in pancreatic neuroendocrine tumours can be overcome with novel PI3K-AKT-mTOR inhibitors. Br J Cancer. 2016;114:650-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 112. | Marone R, Erhart D, Mertz AC, Bohnacker T, Schnell C, Cmiljanovic V, Stauffer F, Garcia-Echeverria C, Giese B, Maira SM, Wymann MP. Targeting melanoma with dual phosphoinositide 3-kinase/mammalian target of rapamycin inhibitors. Mol Cancer Res. 2009;7:601-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 113. | Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Hörsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2032] [Cited by in RCA: 1826] [Article Influence: 130.4] [Reference Citation Analysis (0)] |
| 114. | Vinik A, Bottomley A, Korytowsky B, Bang YJ, Raoul JL, Valle JW, Metrakos P, Hörsch D, Mundayat R, Reisman A, Wang Z, Chao RC, Raymond E. Patient-Reported Outcomes and Quality of Life with Sunitinib Versus Placebo for Pancreatic Neuroendocrine Tumors: Results From an International Phase III Trial. Target Oncol. 2016;11:815-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 115. | Sato K, Toyoshima Y, Moriyama S, Endo Y, Ito T, Ohki E. Real-world use of sunitinib in Japanese patients with pancreatic neuroendocrine tumors: results from a post-marketing surveillance study. Cancer Chemother Pharmacol. 2019;83:201-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 116. | Rinzivillo M, Fazio N, Pusceddu S, Spallanzani A, Ibrahim T, Campana D, Marconcini R, Partelli S, Badalamenti G, Brizzi MP, Catena L, Schinzari G, Carnaghi C, Berardi R, Faggiano A, Antonuzzo L, Spada F, Gritti S, Femia D, Gelsomino F, Bongiovanni A, Ricci S, Brighi N, Falconi M, Delle Fave G, Panzuto F. Sunitinib in patients with pre-treated pancreatic neuroendocrine tumors: A real-world study. Pancreatology. 2018;18:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 117. | Yoo C, Cho H, Song MJ, Hong SM, Kim KP, Chang HM, Chae H, Kim TW, Hong YS, Ryu MH, Kang YK, Kim SC, Ryoo BY. Efficacy and safety of everolimus and sunitinib in patients with gastroenteropancreatic neuroendocrine tumor. Cancer Chemother Pharmacol. 2017;79:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 118. | Mizuno Y, Kudo A, Akashi T, Akahoshi K, Ogura T, Ogawa K, Ono H, Mitsunori Y, Ban D, Tanaka S, Tateishi U, Tanabe M. Sunitinib shrinks NET-G3 pancreatic neuroendocrine neoplasms. J Cancer Res Clin Oncol. 2018;144:1155-1163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 119. | Pellat A, Dreyer C, Couffignal C, Walter T, Lombard-Bohas C, Niccoli P, Seitz JF, Hentic O, André T, Coriat R, Faivre S, Zappa M, Ruszniewski P, Pote N, Couvelard A, Raymond E. Clinical and Biomarker Evaluations of Sunitinib in Patients with Grade 3 Digestive Neuroendocrine Neoplasms. Neuroendocrinology. 2018;107:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 120. | Xu J, Shen L, Zhou Z, Li J, Bai C, Chi Y, Li Z, Xu N, Li E, Liu T, Bai Y, Yuan Y, Li X, Wang X, Chen J, Ying J, Yu X, Qin S, Yuan X, Zhang T, Deng Y, Xiu D, Cheng Y, Tao M, Jia R, Wang W, Fan S, Peng M, Su W. Surufatinib in advanced extrapancreatic neuroendocrine tumours (SANET-ep): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:1500-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 126] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 121. | Xu J, Shen L, Bai C, Wang W, Li J, Yu X, Li Z, Li E, Yuan X, Chi Y, Yin Y, Lou W, Xu N, Bai Y, Zhang T, Xiu D, Wang X, Yuan Y, Chen J, Qin S, Jia R, Lu M, Cheng Y, Zhou Z, He J, Su W. Surufatinib in advanced pancreatic neuroendocrine tumours (SANET-p): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:1489-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 122. | Dilz LM, Denecke T, Steffen IG, Prasad V, von Weikersthal LF, Pape UF, Wiedenmann B, Pavel M. Streptozocin/5-fluorouracil chemotherapy is associated with durable response in patients with advanced pancreatic neuroendocrine tumours. Eur J Cancer. 2015;51:1253-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 123. | Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326:519-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 583] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 124. | Krug S, Boch M, Daniel H, Nimphius W, Müller D, Michl P, Rinke A, Gress TM. Streptozocin-Based Chemotherapy in Patients with Advanced Neuroendocrine Neoplasms--Predictive and Prognostic Markers for Treatment Stratification. PLoS One. 2015;10:e0143822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 125. | Shibuya H, Hijioka S, Sakamoto Y, Ito T, Ueda K, Komoto I, Kobayashi N, Kudo A, Yasuda H, Miyake H, Arita J, Kiritani S, Ikeda M, Imaoka H, Ueno M, Kobayashi S, Furuta M, Nagashio Y, Murohisa G, Aoki T, Matsumoto S, Motoya M, Azemoto N, Itakura J, Horiguchi S, Yogi T, Kawagoe T, Miyaoka Y, Imamura F, Senju M, Arioka H, Hara K, Imamura M, Okusaka T. Multi-center clinical evaluation of streptozocin-based chemotherapy for advanced pancreatic neuroendocrine tumors in Japan: focus on weekly regimens and monotherapy. Cancer Chemother Pharmacol. 2018;82:661-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 126. | Schrader J, Henes FO, Blaeker M, Zimmermann-Fraedrich K, Pace A, Perez D, Izbicki JR, Lohse AW, Benten D. Extended cycle streptozotocin/5-FU chemotherapy for maintenance therapy in pancreatic neuroendocrine tumors. Endocrine. 2019;65:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 127. | Ono H, Kudo A, Akahoshi K, Ogura T, Ogawa K, Ban D, Tanaka S, Tanabe M. Combination of weekly streptozocin and oral S-1 treatment for patients of unresectable or metastatic pancreatic neuroendocrine neoplasms. J Cancer Res Clin Oncol. 2020;146:793-799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 128. | Clewemar Antonodimitrakis P, Sundin A, Wassberg C, Granberg D, Skogseid B, Eriksson B. Streptozocin and 5-Fluorouracil for the Treatment of Pancreatic Neuroendocrine Tumors: Efficacy, Prognostic Factors and Toxicity. Neuroendocrinology. 2016;103:345-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 129. | Strosberg JR, Fine RL, Choi J, Nasir A, Coppola D, Chen DT, Helm J, Kvols L. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011;117:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 548] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 130. | Kunz PL, Catalano PJ, Nimeiri H, Fisher GA, Longacre TA, Suarez CJ, Yao JC, Kulke MH, Hendifar AE, Shanks JC, Shah MH, Zalupski M, Schmulbach EL, Reidy DL, Strosberg JR, O'Dwyer PJ, Benson AB. A randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors: a trial of the ECOG-ACRIN Cancer Research Group (E2211). J Clin Oncol. 2018;. |
| 131. | Lu Y, Zhao Z, Wang J, Lv W, Lu L, Fu W, Li W. Safety and efficacy of combining capecitabine and temozolomide (CAPTEM) to treat advanced neuroendocrine neoplasms: A meta-analysis. Medicine (Baltimore). 2018;97:e12784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 132. | Ostwal V, Basu S, Bhargava P, Shah M, Parghane RV, Srinivas S, Chaudhari V, Bhandare MS, Shrikhande SV, Ramaswamy A. Capecitabine-Temozolomide in Advanced Grade 2 and Grade 3 Neuroendocrine Neoplasms: Benefits of Chemotherapy in Neuroendocrine Neoplasms with Significant 18FDG Uptake. Neuroendocrinology. 2021;111:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 133. | Spada F, Maisonneuve P, Fumagalli C, Marconcini R, Gelsomino F, Antonuzzo L, Campana D, Puliafito I, Rossi G, Faviana P, Messerini L, Barberis M, Fazio N. Temozolomide alone or in combination with capecitabine in patients with advanced neuroendocrine neoplasms: an Italian multicenter real-world analysis. Endocrine. 2021;72:268-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 134. | Chatzellis E, Angelousi A, Daskalakis K, Tsoli M, Alexandraki KI, Wachuła E, Meirovitz A, Maimon O, Grozinsky-Glasberg S, Gross D, Kos-Kudła B, Koumarianou A, Kaltsas G. Activity and Safety of Standard and Prolonged Capecitabine/Temozolomide Administration in Patients with Advanced Neuroendocrine Neoplasms. Neuroendocrinology. 2019;109:333-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 135. | Apostolidis L, Dal Buono A, Merola E, Jann H, Jäger D, Wiedenmann B, Winkler EC, Pavel M. Multicenter Analysis of Treatment Outcomes for Systemic Therapy in Well Differentiated Grade 3 Neuroendocrine Tumors (NET G3). Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 136. | Bongiovanni A, Liverani C, Foca F, Fausti V, Di Menna G, Mercatali L, De Vita A, Riva N, Calpona S, Miserocchi G, Spadazzi C, Cocchi C, Ibrahim T. Temozolomide Alone or Combined with Capecitabine for the Treatment of Metastatic Neuroendocrine Neoplasia: A "Real-World" Data Analysis. Neuroendocrinology. 2021;111:895-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 137. | Kobayashi N, Takeda Y, Okubo N, Suzuki A, Tokuhisa M, Hiroshima Y, Ichikawa Y. Phase II study of temozolomide monotherapy in patients with extrapulmonary neuroendocrine carcinoma. Cancer Sci. 2021;112:1936-1942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 138. | Squires MH, Worth PJ, Konda B, Shah MH, Dillhoff ME, Abdel-Misih S, Norton JA, Visser BC, Dua M, Pawlik TM, Schmidt CR, Poultsides G, Cloyd JM. Neoadjuvant Capecitabine/Temozolomide for Locally Advanced or Metastatic Pancreatic Neuroendocrine Tumors. Pancreas. 2020;49:355-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 139. | Ambe CM, Nguyen P, Centeno BA, Choi J, Strosberg J, Kvols L, Hodul P, Hoffe S, Malafa MP. Multimodality Management of "Borderline Resectable" Pancreatic Neuroendocrine Tumors: Report of a Single-Institution Experience. Cancer Control. 2017;24:1073274817729076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 140. | Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, Dueland S, Hofsli E, Guren MG, Ohrling K, Birkemeyer E, Thiis-Evensen E, Biagini M, Gronbaek H, Soveri LM, Olsen IH, Federspiel B, Assmus J, Janson ET, Knigge U. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 708] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 141. | Elvebakken H, Perren A, Scoazec JY, Tang LH, Federspiel B, Klimstra DS, Vestermark LW, Ali AS, Zlobec I, Myklebust TÅ, Hjortland GO, Langer SW, Gronbaek H, Knigge U, Tiensuu Janson E, Sorbye H. A Consensus-Developed Morphological Re-Evaluation of 196 High-Grade Gastroenteropancreatic Neuroendocrine Neoplasms and Its Clinical Correlations. Neuroendocrinology. 2021;111:883-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 142. | Kulke MH, Wu B, Ryan DP, Enzinger PC, Zhu AX, Clark JW, Earle CC, Michelini A, Fuchs CS. A phase II trial of irinotecan and cisplatin in patients with metastatic neuroendocrine tumors. Dig Dis Sci. 2006;51:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 143. | Lu ZH, Li J, Lu M, Zhang XT, Zhou J, Wang XC, Gong JF, Gao J, Li Y, Shen L. Feasibility and efficacy of combined cisplatin plus irinotecan chemotherapy for gastroenteropancreatic neuroendocrine carcinomas. Med Oncol. 2013;30:664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 144. | Ali AS, Grönberg M, Langer SW, Ladekarl M, Hjortland GO, Vestermark LW, Österlund P, Welin S, Grønbæk H, Knigge U, Sorbye H, Janson ET. Intravenous vs oral etoposide: efficacy and correlation to clinical outcome in patients with high-grade metastatic gastroenteropancreatic neuroendocrine neoplasms (WHO G3). Med Oncol. 2018;35:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 145. | Spada F, Antonuzzo L, Marconcini R, Radice D, Antonuzzo A, Ricci S, Di Costanzo F, Fontana A, Gelsomino F, Luppi G, Nobili E, Galdy S, Cella CA, Sonzogni A, Pisa E, Barberis M, Fazio N. Oxaliplatin-Based Chemotherapy in Advanced Neuroendocrine Tumors: Clinical Outcomes and Preliminary Correlation with Biological Factors. Neuroendocrinology. 2016;103:806-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 146. | Bajetta E, Catena L, Procopio G, De Dosso S, Bichisao E, Ferrari L, Martinetti A, Platania M, Verzoni E, Formisano B, Bajetta R. Are capecitabine and oxaliplatin (XELOX) suitable treatments for progressing low-grade and high-grade neuroendocrine tumours? Cancer Chemother Pharmacol. 2007;59:637-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 147. | Faure M, Niccoli P, Autret A, Cavaglione G, Mineur L, Raoul JL. Systemic chemotherapy with FOLFOX in metastatic grade 1/2 neuroendocrine cancer. Mol Clin Oncol. 2017;6:44-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 148. | Merola E, Dal Buono A, Denecke T, Arsenic R, Pape UF, Jann H, Wiedenmann B, Pavel ME. Efficacy and Toxicity of 5-Fluorouracil-Oxaliplatin in Gastroenteropancreatic Neuroendocrine Neoplasms. Pancreas. 2020;49:912-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 149. | Pulvirenti A, Raj N, Cingarlini S, Pea A, Tang LH, Luchini C, Chou JF, Grego E, Marinova I, Capanu M, Landoni L, Scarpa A, Allen PJ, Klimstra DS, Reidy-Lagunes DL. Platinum-Based Treatment for Well- and Poorly Differentiated Pancreatic Neuroendocrine Neoplasms. Pancreas. 2021;50:138-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 150. | Lacombe C, De Rycke O, Couvelard A, Turpin A, Cazes A, Hentic O, Gounant V, Zalcman G, Ruszniewski P, Cros J, de Mestier L. Biomarkers of Response to Etoposide-Platinum Chemotherapy in Patients with Grade 3 Neuroendocrine Neoplasms. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 151. | Hijioka S, Hosoda W, Matsuo K, Ueno M, Furukawa M, Yoshitomi H, Kobayashi N, Ikeda M, Ito T, Nakamori S, Ishii H, Kodama Y, Morizane C, Okusaka T, Yanagimoto H, Notohara K, Taguchi H, Kitano M, Yane K, Maguchi H, Tsuchiya Y, Komoto I, Tanaka H, Tsuji A, Hashigo S, Kawaguchi Y, Mine T, Kanno A, Murohisa G, Miyabe K, Takagi T, Matayoshi N, Yoshida T, Hara K, Imamura M, Furuse J, Yatabe Y, Mizuno N. Rb Loss and KRAS Mutation Are Predictors of the Response to Platinum-Based Chemotherapy in Pancreatic Neuroendocrine Neoplasm with Grade 3: A Japanese Multicenter Pancreatic NEN-G3 Study. Clin Cancer Res. 2017;23:4625-4632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 152. | Tanaka H, Hijioka S, Hosoda W, Ueno M, Kobayashi N, Ikeda M, Ito T, Kodama Y, Morizane C, Notohara K, Taguchi H, Kitano M, Komoto I, Tsuji A, Hashigo S, Kanno A, Miyabe K, Takagi T, Ishii H, Kojima Y, Yoshitomi H, Yanagimoto H, Furuse J, Mizuno N. Pancreatic neuroendocrine carcinoma G3 may be heterogeneous and could be classified into two distinct groups. Pancreatology. 2020;20:1421-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 153. | Sugiyama K, Shiraishi K, Sato M, Nishibori R, Nozawa K, Kitagawa C. Salvage Chemotherapy by FOLFIRI Regimen for Poorly Differentiated Gastrointestinal Neuroendocrine Carcinoma. J Gastrointest Cancer. 2021;52:947-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 154. | Cives M, Pelle' E, Quaresmini D, Rizzo FM, Tucci M, Silvestris F. The Tumor Microenvironment in Neuroendocrine Tumors: Biology and Therapeutic Implications. Neuroendocrinology. 2019;109:83-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 155. | Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K, Quinn MC, Robertson AJ, Fadlullah MZ, Bruxner TJ, Christ AN, Harliwong I, Idrisoglu S, Manning S, Nourse C, Nourbakhsh E, Wani S, Wilson PJ, Markham E, Cloonan N, Anderson MJ, Fink JL, Holmes O, Kazakoff SH, Leonard C, Newell F, Poudel B, Song S, Taylor D, Waddell N, Wood S, Xu Q, Wu J, Pinese M, Cowley MJ, Lee HC, Jones MD, Nagrial AM, Humphris J, Chantrill LA, Chin V, Steinmann AM, Mawson A, Humphrey ES, Colvin EK, Chou A, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Pettitt JA, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N, Jamieson NB, Graham JS, Niclou SP, Bjerkvig R, Grützmann R, Aust D, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Falconi M, Zamboni G, Tortora G, Tempero MA; Australian Pancreatic Cancer Genome Initiative, Gill AJ, Eshleman JR, Pilarsky C, Scarpa A, Musgrove EA, Pearson JV, Biankin AV, Grimmond SM. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2130] [Cited by in RCA: 1982] [Article Influence: 198.2] [Reference Citation Analysis (1)] |
| 156. | Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, Kiezun A, Hammerman PS, McKenna A, Drier Y, Zou L, Ramos AH, Pugh TJ, Stransky N, Helman E, Kim J, Sougnez C, Ambrogio L, Nickerson E, Shefler E, Cortés ML, Auclair D, Saksena G, Voet D, Noble M, DiCara D, Lin P, Lichtenstein L, Heiman DI, Fennell T, Imielinski M, Hernandez B, Hodis E, Baca S, Dulak AM, Lohr J, Landau DA, Wu CJ, Melendez-Zajgla J, Hidalgo-Miranda A, Koren A, McCarroll SA, Mora J, Crompton B, Onofrio R, Parkin M, Winckler W, Ardlie K, Gabriel SB, Roberts CWM, Biegel JA, Stegmaier K, Bass AJ, Garraway LA, Meyerson M, Golub TR, Gordenin DA, Sunyaev S, Lander ES, Getz G. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3848] [Cited by in RCA: 4112] [Article Influence: 342.7] [Reference Citation Analysis (0)] |
| 157. | Vijayvergia N, Boland PM, Handorf E, Gustafson KS, Gong Y, Cooper HS, Sheriff F, Astsaturov I, Cohen SJ, Engstrom PF. Molecular profiling of neuroendocrine malignancies to identify prognostic and therapeutic markers: a Fox Chase Cancer Center Pilot Study. Br J Cancer. 2016;115:564-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 158. | Mehnert JM, Bergsland E, O'Neil BH, Santoro A, Schellens JHM, Cohen RB, Doi T, Ott PA, Pishvaian MJ, Puzanov I, Aung KL, Hsu C, Le Tourneau C, Hollebecque A, Élez E, Tamura K, Gould M, Yang P, Stein K, Piha-Paul SA. Pembrolizumab for the treatment of programmed death-ligand 1-positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. Cancer. 2020;126:3021-3030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 159. | Strosberg J, Mizuno N, Doi T, Grande E, Delord JP, Shapira-Frommer R, Bergsland E, Shah M, Fakih M, Takahashi S, Piha-Paul SA, O'Neil B, Thomas S, Lolkema MP, Chen M, Ibrahim N, Norwood K, Hadoux J. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Neuroendocrine Tumors: Results From the Phase II KEYNOTE-158 Study. Clin Cancer Res. 2020;26:2124-2130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 160. | Vijayvergia N, Dasari A, Deng M, Litwin S, Al-Toubah T, Alpaugh RK, Dotan E, Hall MJ, Ross NM, Runyen MM, Denlinger CS, Halperin DM, Cohen SJ, Engstrom PF, Strosberg JR. Pembrolizumab monotherapy in patients with previously treated metastatic high-grade neuroendocrine neoplasms: joint analysis of two prospective, non-randomised trials. Br J Cancer. 2020;122:1309-1314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 161. | Fottner CA, Ferrata M, Krug S, Michl P, Schad A, Roth W, Jaeger D, Galle PR, Weber MM. A phase II, open label, multicenter trial of avelumab in patients with advanced, metastatic high-grade neuroendocrine carcinomas NEC G3 (WHO 2010) progressive after first-line chemotherapy (AVENEC). J Clin Oncol. 2019;. |
| 162. | Yao JC, Strosberg J, Fazio N, Pavel ME, Bergsland E, Ruszniewski P, Halperin DM, Li D, Tafuto S, Raj N, Campana D, Hijioka S, Raderer M, Guimbaud R, Gajate P, Pusceddu S, Reising A, Degtyarev E, Shilkrut M, Eddy S, Singh S. Spartalizumab in metastatic, well/poorly-differentiated neuroendocrine neoplasms. Endocr Relat Cancer. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 163. | Patel SP, Othus M, Chae YK, Giles FJ, Hansel DE, Singh PP, Fontaine A, Shah MH, Kasi A, Baghdadi TA, Matrana M, Gatalica Z, Korn WM, Hayward J, McLeod C, Chen HX, Sharon E, Mayerson E, Ryan CW, Plets M, Blanke CD, Kurzrock R. A Phase II Basket Trial of Dual Anti-CTLA-4 and Anti-PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin Cancer Res. 2020;26:2290-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 234] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 164. | Capdevila JT, López C, García-Carbonero R, Benavent M, Custodio A, Cubillo A, Alonso V, Alonso Gordoa T, Carmona-Bayonas A, Crespo G, Blanco-Codesido M, Jimenez-Fonseca P, Viúdez A, La Casta Muñoa A, Sevilla I, Llanos M, Segura A, Hernando-Cubero J, Manzano JL. A multi-cohort phase II study of durvalumab plus tremelimumab for the treatment of patients (pts) with advanced neuroendocrine neoplasms (NENs) of gastroenteropancreatic or lung origin: The DUNE trial (GETNE 1601). J Clin Oncol. 2020;. |
| 165. | Limmer S, Huppert PE, Juette V, Lenhart A, Welte M, Wietholtz H. Radiofrequency ablation of solitary pancreatic insulinoma in a patient with episodes of severe hypoglycemia. Eur J Gastroenterol Hepatol. 2009;21:1097-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 166. | Barthet M, Giovannini M, Lesavre N, Boustiere C, Napoleon B, Koch S, Gasmi M, Vanbiervliet G, Gonzalez JM. Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors and pancreatic cystic neoplasms: a prospective multicenter study. Endoscopy. 2019;51:836-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 167. | Larghi A, Rimbaș M, Rizzatti G, Carbone C, Gasbarrini A, Costamagna G, Alfieri S, Tortora G. Endoscopic ultrasound-guided therapies for pancreatic solid tumors: An overview. Semin Oncol. 2021;48:95-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 168. | Choi JH, Park DH, Kim MH, Hwang HS, Hong SM, Song TJ, Lee SS, Seo DW, Lee SK. Outcomes after endoscopic ultrasound-guided ethanol-lipiodol ablation of small pancreatic neuroendocrine tumors. Dig Endosc. 2018;30:652-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 169. | Claringbold PG, Price RA, Turner JH. Phase I-II study of radiopeptide 177Lu-octreotate in combination with capecitabine and temozolomide in advanced low-grade neuroendocrine tumors. Cancer Biother Radiopharm. 2012;27:561-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 170. | Nicolini S, Bodei L, Bongiovanni A, Sansovini M, Grassi I, Ibrahim T, Monti M, Caroli P, Sarnelli A, Diano D, Di Iorio V, Grana CM, Cittanti C, Pieri F, Severi S, Paganelli G. Combined use of 177Lu-DOTATATE and metronomic capecitabine (Lu-X) in FDG-positive gastro-entero-pancreatic neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2021;48:3260-3267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 171. | Parghane RV, Ostwal V, Ramaswamy A, Bhandare M, Chaudhari V, Talole S, Shrikhande SV, Basu S. Long-term outcome of "Sandwich" chemo-PRRT: a novel treatment strategy for metastatic neuroendocrine tumors with both FDG- and SSTR-avid aggressive disease. Eur J Nucl Med Mol Imaging. 2021;48:913-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 172. | Satapathy S, Mittal BR, Sood A, Kapoor R, Gupta R. Peptide Receptor Radionuclide Therapy as First-Line Systemic Treatment in Advanced Inoperable/Metastatic Neuroendocrine Tumors. Clin Nucl Med. 2020;45:e393-e399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 173. | Bajetta E, Catena L, Fazio N, Pusceddu S, Biondani P, Blanco G, Ricci S, Aieta M, Pucci F, Valente M, Bianco N, Mauri CM, Spada F. Everolimus in combination with octreotide long-acting repeatable in a first-line setting for patients with neuroendocrine tumors: an ITMO group study. Cancer. 2014;120:2457-2463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 174. | Capdevila J, Teulé A, Barriuso J, Castellano D, Lopez C, Manzano JL, Alonso V, García-Carbonero R, Dotor E, Matos I, Custodio A, Casanovas O, Salazar R; EVERLAR study investigators. Phase II Study of Everolimus and Octreotide LAR in Patients with Nonfunctioning Gastrointestinal Neuroendocrine Tumors: The GETNE1003_EVERLAR Study. Oncologist. 2019;24:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 175. | Chan JA, Blaszkowsky L, Stuart K, Zhu AX, Allen J, Wadlow R, Ryan DP, Meyerhardt J, Gonzalez M, Regan E, Zheng H, Kulke MH. A prospective, phase 1/2 study of everolimus and temozolomide in patients with advanced pancreatic neuroendocrine tumor. Cancer. 2013;119:3212-3218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 176. | Capdevila J, Sevilla I, Alonso V, Antón Aparicio L, Jiménez Fonseca P, Grande E, Reina JJ, Manzano JL, Alonso Lájara JD, Barriuso J, Castellano D, Medina J, López C, Segura Á, Carrera S, Crespo G, Fuster J, Munarriz J, García Alfonso P. Evaluation of the efficacy and safety of lanreotide in combination with targeted therapies in patients with neuroendocrine tumours in clinical practice: a retrospective cross-sectional analysis. BMC Cancer. 2015;15:495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 177. | Strosberg JR, Weber JM, Choi J, Campos TL, Valone TL, Han G, Schell MJ, Kvols LK. A phase II clinical trial of sunitinib following hepatic transarterial embolization for metastatic neuroendocrine tumors. Ann Oncol. 2012;23:2335-2341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 178. | Rogers JE, Lam M, Halperin DM, Dagohoy CG, Yao JC, Dasari A. Fluorouracil, Doxorubicin with Streptozocin and Subsequent Therapies in Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2022;112:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 179. | Walter T, Malka D, Hentic O, Lombard-Bohas C, Le Malicot K, Smith D, Ferru A, Assenat E, Cadiot G, Lievre A, Kurtz JE, Dahan L, Dubreuil O, Hautefeuille V, Lepere C, Gangloff A, Elhajbi F, Coriat R, Roquin G, Bouarioua N, Granger V, Scoazec JY, Lepage C. Evaluating bevacizumab in combination with FOLFIRI after the failure of platinum-etoposide regimen in patients with advanced poorly differentiated neuroendocrine carcinoma: The PRODIGE 41-BEVANEC randomized phase II study. Dig Liver Dis. 2018;50:195-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 180. | Kamp K, Gumz B, Feelders RA, Kwekkeboom DJ, Kaltsas G, Costa FP, de Herder WW. Safety and efficacy of everolimus in gastrointestinal and pancreatic neuroendocrine tumors after (177)Lu-octreotate. Endocr Relat Cancer. 2013;20:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 181. | Fröss-Baron K, Garske-Roman U, Welin S, Granberg D, Eriksson B, Khan T, Sandström M, Sundin A. 177Lu-DOTATATE Therapy of Advanced Pancreatic Neuroendocrine Tumors Heavily Pretreated with Chemotherapy: Analysis of Outcome, Safety, and Their Determinants. Neuroendocrinology. 2021;111:330-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |