Published online Feb 27, 2022. doi: 10.4240/wjgs.v14.i2.174
Peer-review started: October 7, 2021
First decision: December 4, 2021
Revised: December 9, 2021
Accepted: January 25, 2022
Article in press: January 25, 2022
Published online: February 27, 2022
Processing time: 138 Days and 11.8 Hours
Solid pseudopapillary neoplasm (SPN) of the pancreas is a rare neoplasm that mainly affects young women.
To evaluate the impact of parenchyma-preserving surgical methods (PPMs, including enucleation and central pancreatectomy) in the treatment of SPN patients.
From 2013 to 2019, patients who underwent pancreatectomy for SPNs were retrospectively reviewed. The baseline characteristics, intraoperative index, pathological outcomes, short-term complications and long-term follow-up data were compared between the PPM group and the conventional method (CM) group.
In total, 166 patients were included in this study. Of them, 33 patients (19.9%) underwent PPM. Most of the tumors (104/166, 62.7%) were found accidentally. Comparing the parameters between groups, the hospital stay d (12.35 vs 13.5 d, P = 0.49), total expense (44213 vs 54084 yuan, P = 0.21), operation duration (135 vs 120 min, P = 0.71), and intraoperative bleeding volume (200 vs 100 mL, P = 0.49) did not differ between groups. Regarding pathological outcomes, tumor size (45 vs 32 mm, P = 0.07), Ki67 index (P = 0.53), peripheral tissue invasion (11.3% vs 9.1%, P = 0.43) and positive margin status (7.5% vs 6%, P = 0.28) also did not differ between groups. Moreover, PPM did not increase the risk of severe postoperative pancreatic fistula (3.8% vs 3.0%, P = 0.85) or tumor recurrence (3.0% vs 6.0%, P = 0.39). However, the number of patients who had exocrine insufficiency during follow-up was significantly lower in the PPM group (21.8% vs 3%, P = 0.024). CM was identified as an independent risk factor for pancreatic exocrine insufficiency (odds ratio = 8.195, 95% confident interval: 1.067-62.93).
PPM for SPN appears to be feasible and safe for preserving the exocrine function of the pancreas.
Core Tip: Solid pseudopapillary neoplasm (SPN) of the pancreas is a rare neoplasm that mainly affects young women. The prognosis of SPN is excellent following complete surgical resection. However, the conventional surgical method is associated with a high rate of morbidity and a high rate of long-term endocrine/exocrine insufficiency due to the loss of pancreatic parenchyma. Our study identified a parenchyma-preserving surgical method (PPM) for SPN that appears to be feasible and safe for preserving the exocrine function of the pancreas. The risk of PPM did not increase the risk of severe postoperative pancreatic fistula or tumor recurrence. PPM should be taken into consideration in SPN patients with a long life expectancy.
- Citation: Li YQ, Pan SB, Yan SS, Jin ZD, Huang HJ, Sun LQ. Impact of parenchyma-preserving surgical methods on treating patients with solid pseudopapillary neoplasms: A retrospective study with a large sample size. World J Gastrointest Surg 2022; 14(2): 174-184
- URL: https://www.wjgnet.com/1948-9366/full/v14/i2/174.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i2.174
Solid pseudopapillary neoplasms (SPNs) are exceptionally rare. These tumors account for approximately 0.9%-2.7% of all exocrine pancreatic neoplasms[1,2] and approximately 3%-5% of pancreatic cystic neoplasms[3,4]. Although these tumors occur among a wide age range from children to elderly individuals, the mean age at presentation is 28.5 years[5]. SPNs occur predominantly in young women with a female-male ratio of 9.8:1[1]. Moreover, SPN is reported to be the most common pancreatic neoplasm among young females under the age of 40 years[6]. However, the tumor is an epithelial-originated low-grade malignant neoplasm with the possibility of locally advanced, recurrent, and metastatic disease[7]. Complete surgical resection is recommended as the main treatment for SPN[6].
Conventional pancreatic resection (pancreatoduodenectomy (PD), total pancreatectomy (TP) and distal pancreatectomy (DP)) is associated with a high rate of morbidity (40%-50%)[8] and a high rate of long-term endocrine/exocrine insufficiency (8-20% and 20-50%, respectively)[9] due to the loss of pancreatic parenchyma. However, the prognosis of SPN is excellent with a cure rate of approximately 98% following surgical resection[10]. Thus, approximately half of young SPN patients will suffer from lifelong complications.
In addition to drug therapies, improved surgical approaches are one way to address the issue.
The parenchyma preserving surgical methods (i.e., enucleation or central pancreatectomy (CP)) have been explicitly advocated to be used for some benign or low-grade pancreatic neoplasms[11-13]. Parenchyma-sparing surgical approaches decrease the risk of developing endocrine and exocrine dysfunction postoperatively[14], and the subsequent quality of life is significantly higher than that of patients who underwent conventional resection. Recently, a parenchyma-sparing surgical approach was reported to be used for SPN in some retrospective studies with small-sized samples. The increased rate of postoperative pancreatic fistula (POPF) may hinder the utilization of this approach[15,16]. Moreover, although rare, SPN is associated with local recurrence or metastasis after surgery. The long-term outcomes after the parenchyma-sparing surgical approach for SPN remain unclear.
Due to the unsolved issues noted above, we conducted this retrospective study with a large sample size. Our study aimed to compare the intraoperative, short-term, and long-term outcomes in SPN patients who underwent a parenchyma-sparing surgical approach vs conventional pancreatic resection with detailed surgical-related parameters included.
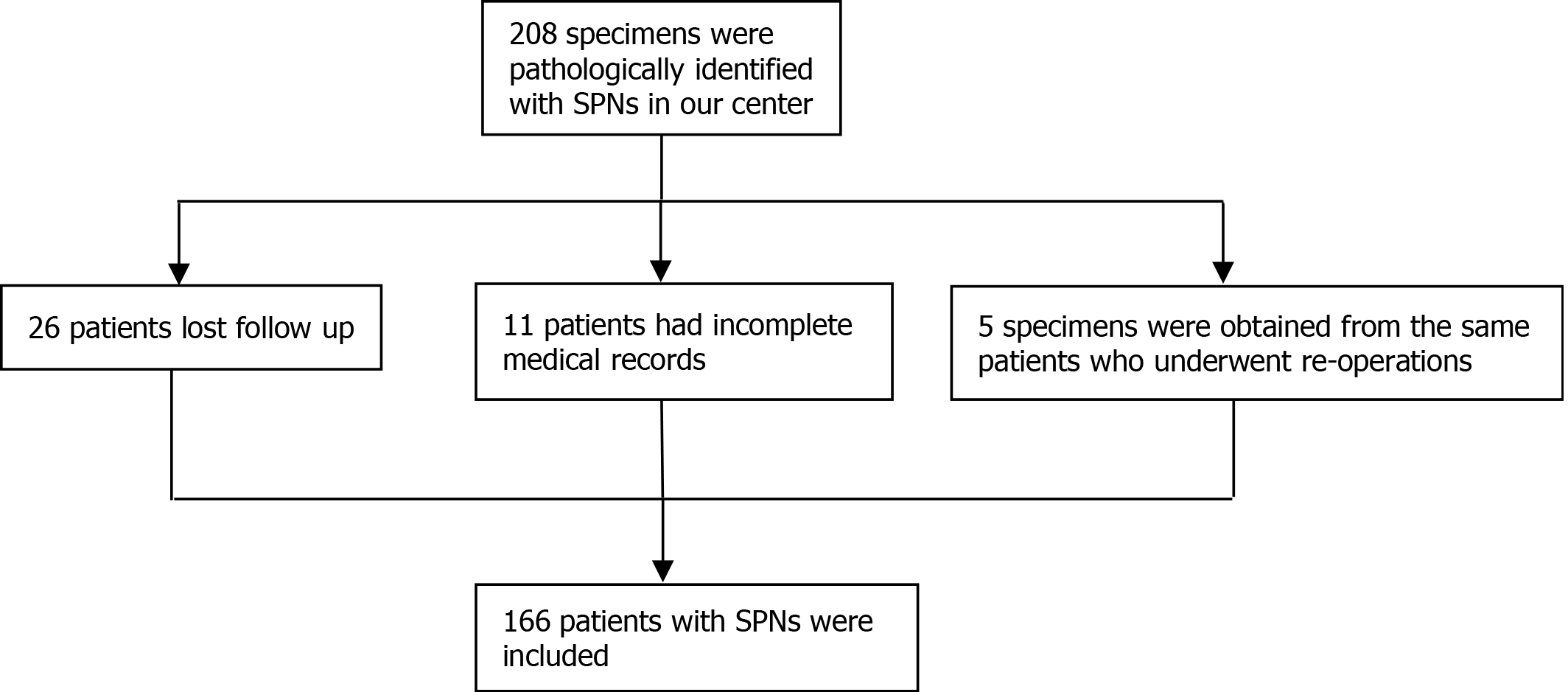
We conducted a retrospective study at Changhai Hospital affiliated with Navy/Second Medical University and Suzhou Science and Technology Town Hospital, Suzhou. The Institutional Review Board of both hospitals approved the study. Patients who underwent surgical resection from January 2013 to December 2018 for pathologically identified SPN were included in our study. The following inclusion criteria were applied: (1) Patients pathologically diagnosed with SPN; (2) Patients whose full electronic medical records could be obtained; and (3) Patients whose follow-up data could be obtained. Exclusion criteria included: (1) Specimens obtained from reresections; (2) Concomitant other neoplasms on final pathology (e.g., neuroendocrine tumor, cholangiocarcinoma); and (3) Patients with unavailable pathological and follow-up data. The selection procedure of the study participants is presented in Figure 1.
The decision on surgical treatment was made by our multidisciplinary hepatopancreatobiliary team. If the lesion was diagnosed as a pancreatic cystic lesion by imaging modality, the surgery indications would follow the International Consensus Guideline[17]. If diagnosed as invasive cancer, the surgery indications would follow the European Society for Medical Oncology guidelines[18]. The choices of surgical procedures depended on the location, degree, extent of diseases and experiences of the surgeons. The surgeons who were qualified to perform pancreatic surgery in our centers had at least 15 years of operation experience with an average of 100 operations per year.
The operations for SPN were performed by experienced surgeons in our center. We performed
Roux-Y loop in patients with a suspicious injury of the main pancreatic duct or a wide wounded area (diameter > 3 cm) of the pancreatic parenchyma.
After surgery, amylase analysis from drainage fluid was performed to determine whether POPF existed. Routine blood examinations were performed to determine whether infection existed and whether antibiotics were used. Plain CT was performed to determine whether pancreatic fluid collection existed and to detect the causes of infection. If any clinically significant complications occurred, further treatments were needed.
The parameters included in our study were composed of five parts: baseline characteristics, intraoperative index, pathological outcomes, short-term complications and long-term follow-up data.
The baseline characteristics included age, gender, symptoms, hospital stay d and total expense.
The intraoperative indices included surgical method, surgical approaches, operation duration, and intraoperative bleeding volume. The surgical methods included conventional methods (PD, TP and DP) and parenchyma-preserving methods (enucleation and CP). Surgical approaches include opening, laparoscopy and robotics. Intraoperative bleeding was noted as dark red liquid aspirated during the operation.
The pathological outcomes were tumor location, tumor size, Ki-67 index, margin status, and peripheral tissue invasion status. The tumor might be located in multiple head/body/tail sites of the pancreas. If multiple tumors occurred, only the size of the largest tumor was measured. The Ki-67 index of the tumor was divided into 3 grades: < 3%, 3%-20% and > 20%[19]. Positive margin status was defined as a tumor component ≤ 5 mm from the incisal margin. Peripheral tissue invasion status consisted of perineural invasion, vascular invasion, cancerization of ducts, lymphatic metastasis, common bile duct invasion, peripancreatic fat invasion, spleen invasion and duodenum invasion.
Short-term complications were adverse events that occurred within 30 d, including POPF, delayed gastric emptying, postoperative hemorrhage, postoperative infection and bile leakage. The grade of complications was based on the Claviene-Dindo score. Complications that scored Claviene Dindo grade III or greater were considered severe complications.
The long-term follow-up data included exocrine insufficiency, endocrine insufficiency, alimentary stricture due to the surgery and whether recurrence occurred. Follow-up data were obtained from telephone interviews and/or outpatient interviews in this study. Endocrine insufficiency was defined as a fasting plasma glucose level > 7.0 mmol/L and/or the need for diet modification, oral medication, or insulin use to control blood. Exocrine insufficiency was defined as symptoms (steatorrhea or weight loss) resolving after pancreatic enzyme supplementation[15]. Recurrence was defined as a local or a metastatic tumor confirmed by radiology or histology during postoperative follow-up.
The patients were divided into 2 groups according to their surgical methods: conventional method (CM) group and parenchyma preserving method (PPM) group. The parameters were compared between the 2 groups. Quantitative parameters were expressed as the medians and range. Continuous data are reported as the mean ± standard deviation (SD) or as the median and range. Categorical parameters were compared between the CM group and the PPM group using χ2 or Fisher's exact test. The nonparametric Mann–Whitney U test was used to compare differences between groups for quantitative parameters. A Kaplan–Meier survival curve was established to estimate the recurrence-free survival (RFS) rate. Statistical analysis was performed using SPSS version 22.0 (SPSS Inc., Chicago, IL, United States). All tests were two-sided, and a P value < 0.05 was considered statistically significant.
From January 2013 to June 2019, 166 patients who underwent pancreatic surgery were included in our study. All tumors were confirmed as SPNs according to the final histology examination. Among them, 33 patients (19.9%) underwent PPM, and 133 patients (80.1%) underwent CM. In the PPM group, 13 patients underwent enucleation and 20 patients underwent CP. The median age of the overall study cohort was 32.5 years (range, 10-68 years), and most of the participants were females (129/166, 77.7%). The majority of the tumors were incidentally found (104/166,62.7%). In the patients who were symptomatic, abdominal pain was the most common symptom (53/62.85.5%) followed by abdominal distension (6/62, 9.7%), nausea and vomiting (2/62, 3.2%) and jaundice (1/62, 1.6%). The mean hospital stay was 12.53 d (SD ± 6.87 d), and no difference was noted between the CM group and the PPM group (t = 0.692, P = 0.49). The mean total expense during hospitalization was 46248 Chinese yuan (SD ± 25414 yuan), and no difference was noted between the 2 groups (t = 1.284, P = 0.21). The baseline characteristics of the study cohort are shown in Table 1.
| Total (n = 166) | CM (n = 133) | PPM (n = 33) | P value | ||
| Female, n (%) | 129 (77.7) | 106 (77.4) | 23 (69.7) | 0.16 | |
| Age (yr), median (range) | 32.5 (10-68) | 32.0 (10-68) | 33 (13-51) | 0.85 | |
| Symptoms, n (%) | |||||
| Accidentally found | 104 (62.7) | 83 (62.4) | 21 (63.6) | 0.84 | |
| Abdominal pain | 53 (31.9) | 43 (32.3) | 10 (30.3) | 0.75 | |
| Abdominal distension | 6 (3.6) | 5 (3.8) | 1 (3.0) | 0.8 | |
| Nausea and vomiting | 2 (1.2) | 1 (0.8) | 1 (3.0) | 0.49 | |
| Jaundice | 1 (0.6) | 1 (0.8) | 0 (0) | 0.32 | |
| Hospital stay (d) ± SD | 12.53 ± 6.87 | 12.35 ± 6.21 | 13.3 ± 9.14 | 0.49 | |
| Total expense (yuan) ± SD | 46248 ± 25414 | 44213 ± 20487 | 54084 ± 38551 | 0.21 | |
In the CM group, 44 patients (33.1%) underwent PD, 81 patients (60.9%) underwent DP, and 8 patients (6.0%) underwent TP. Moreover, 108 (81.2%) patients underwent laparotomy, 11 (8.3%) underwent laparoscopic surgery, and 14 (10.5%) underwent robot surgery. The average operation experiences for surgeons were 19 years. The median operation duration was 135 min (27-381 min), and the median intraoperative bleeding volume was 200 mL (0-2000 mL).
In the PPM group, 11 patients (33.3%) underwent enucleation, and 22 patients (66.6%) underwent CP. Moreover, 31 (93.3%) patients underwent laparotomy, 2 (6.7%) underwent laparoscopic surgery, and no patient underwent robot surgery. The average operation experiences for surgeons were 19.5 years. The median operation duration was 120 min (50-301 min), and the median intraoperative bleeding volume was 100 mL (50-600 mL).
Comparing the intraoperative index between the 2 groups, the surgical approach was not different between the 2 groups (χ2 = 4.15, P = 0.126), and the surgeon experiences, operation duration and intraoperative bleeding volume were also not different between the 2 groups (t = 0.85, 0.385 and 0.695, P = 0.71 and 0.488) (Table 2).
| Total (n = 166) | CM (n = 133) | PPM (n = 33) | P value | |
| Intraoperative index | ||||
| Surgical approach, n (%) | 0.126 | |||
| laparotomy | 139 (80.1) | 108 (81.2) | 31 (93.9) | |
| laparoscopic | 13 (7.8) | 11 (8.3) | 2 (6.1) | |
| Robot | 14 (8.4) | 14 (10.5) | 0 (0) | |
| Surgeon experiences, mean (yr) | 19.3 | 19.0 | 19.5 | 0.85 |
| Operation duration, median (range) | 135 (27-381) | 135 (27-381) | 120 (50-301) | 0.71 |
| Intraoperative bleeding volume, median (± SD) | 200 (0-2000) | 200 (0-2000) | 100 (50-600) | 0.488 |
| Pathological outcomes | ||||
| Median size (mm), median (range) | 40 (3.5-140) | 45 (3.5-140) | 32 (17-140) | 0.069 |
| Tumor location, n (%) | 0.001a | |||
| Head | 69 (41.6) | 46 (34.6) | 23 (69.7) | |
| Body | 19 (11.4) | 15 (11.3) | 4 (12.1) | |
| Tail | 17 (10.2) | 17 (12.8) | 0 (0) | |
| Multiple sites | 64 (38.6) | 55 (41.4) | 6 (18.2) | |
| Ki67 index, n (%) | 0.53a | |||
| I | 141 (84.9) | 115 (86.5) | 26 (78.8) | |
| II | 22 (13.3) | 16 (12.0) | 6 (18.2) | |
| III | 3 (1.8) | 2 (1.5) | 1 (3.0) | |
| Peripheral tissue invasion, n (%) | 18 (10.8) | 15 (11.3) | 3 (9.1) | 0.426 |
| Positive margin status, n (%) | 12 (7.2) | 10 (7.5) | 2 (6.0) | 0.283 |
Regarding the pathological specimens, the median size of tumors in the CM group was 45 mm (3.5-140 mm), with 46 tumors (34.6%) located in the pancreatic head, 15 tumors (11.3%) located in the pancreatic body, 17 tumors (12.8%) located in the pancreatic tail and 55 tumors (41.4%) involving multiple sites. Grade I Ki67 was identified in 115 tumors (86.5%), Grade II Ki67 was identified in 16 tumors (12.0%) and Grade III Ki67 was identified in 2 tumors (1.5%). Positive margin status was observed in 10 patients (7.5%), and peripheral tissue invasion was observed in 15 patients (11.3%).
The median size of tumors in the PPM group was 32 mm (17-140 mm) with 23 tumors (69.7%) located in pancreatic head, 4 tumors (12.1%) located in pancreatic body, no tumors (0%) located in pancreatic tail and 6 tumors (18.2%) involving multiple sites. Grade I Ki67 was identified in 26 tumors (78.8%), Grade II Ki67 was identified in 6 tumors (18.2%), and Grade III Ki67 was identified in 1 tumor (3%). Positive margin status was observed in 2 patients (6%), and peripheral tissue invasion was observed in 3 patients (9.1%).
Comparing the pathological outcomes between the 2 groups, the tumor size was not significantly larger in the CM group compared with the PMM group with a borderline P value (t = 1.832, P = 0.069). Tumors involved in multiple sites were more common in the CM group (χ2 = 15.9, P = 0.001). The Ki67 grade was not different between the 2 groups (χ2 = 1.182, P = 0.53), indicating that the degree of malignancy was not different between the groups. The positive margin status and peripheral tissue invasion were also not different between the 2 groups (χ2 = 1.155 and 0.832, P = 0.283 and 0.425) (Table 2).
In the CM group, perioperative complications occurred in 27 patients (20.3%). POPF grade II or greater developed in 5 patients (5/27, 18.5%), delayed gastric emptying developed in 4 patients (4/27, 14.8%), abdominal infection developed in 12 patients (12/27, 44.4%), bleeding developed in 3 patients (3/27, 11.1%), pancreatitis developed in 2 patients (2/27, 7.4%), and 1 patient (1/27, 3.7%) developed both delayed gastric emptying and abdominal infection. Seven complications (7/27, 25.9%) scored Claviene Dindo grade III or above and were considered severe complications (Table 3).
| Total (n = 166) | CM (n = 133) | PPM (n = 33) | P value | |
| Short-term complications | ||||
| Overall perioperative complication, n (%) | 33 (19.9) | 27 (20.3) | 6 (18.2) | 0.79 |
| Severe POPF | 6 (3.6) | 5 (3.8) | 1 (3.0) | 0.85 |
| delayed gastric emptying | 3 (1.8) | 3 (2.3) | 0 (0) | 0.56 |
| abdominal infection | 13 (7.8) | 12 (9.0) | 1 (3.0) | 0.43 |
| Bleeding | 5 (3.0) | 3 (2.3) | 2 (6.0) | 0.26 |
| Pancreatitis | 2 (1.2) | 2 (1.5) | 0 (0) | 0.49 |
| Multiple complications | 4 (2.4) | 2 (1.5) | 2 (6.0) | 0.18 |
| Severe perioperative complication, n (%) | 9 (5.4) | 7 (5.3) | 2 (6.0) | 1.0 |
| Long-term follow-up data | ||||
| Recurrence, n (%) | 6 (3.6) | 4 (3.0) | 2 (6.0) | 0.39 |
| Local | 4 (2.4) | 3 (2.3) | 1 (3.0) | |
| Distant | 2 (1.2) | 1 (0.8) | 1 (3.0) | |
| Alimentary stricture, n (%) | 6 (3.6) | 5 (3.7) | 1 (3.0) | 1.0 |
| Endocrine insufficiency, n (%) | 7 (4.2) | 6 (4.5) | 1 (3.0) | 1.0 |
| Exocrine insufficiency, n (%) | 30 (18.1) | 29 (21.8) | 1 (3.0) | 0.024 |
In the PPM group, perioperative complications occurred in 6 patients (18.2%). One patient (1/6, 16.7%) developed severe POPF, 1 patient developed abdominal infection (1/6, 16.7%), 2 patients developed bleeding (2/6, 33.3%) and 2 patients (2/6, 33.3%) developed severe POPF, abdominal infection and bleeding. Two complications (2/6, 33.3%) scored Claviene Dindo grade III or greater and were considered severe complications.
The overall perioperative complication rate and severe complication rate were comparable between the groups (χ2 = 0.075 and 0.00, P = 0.79 and 1.0). For each complications, the difference of incidences were not observed, either.
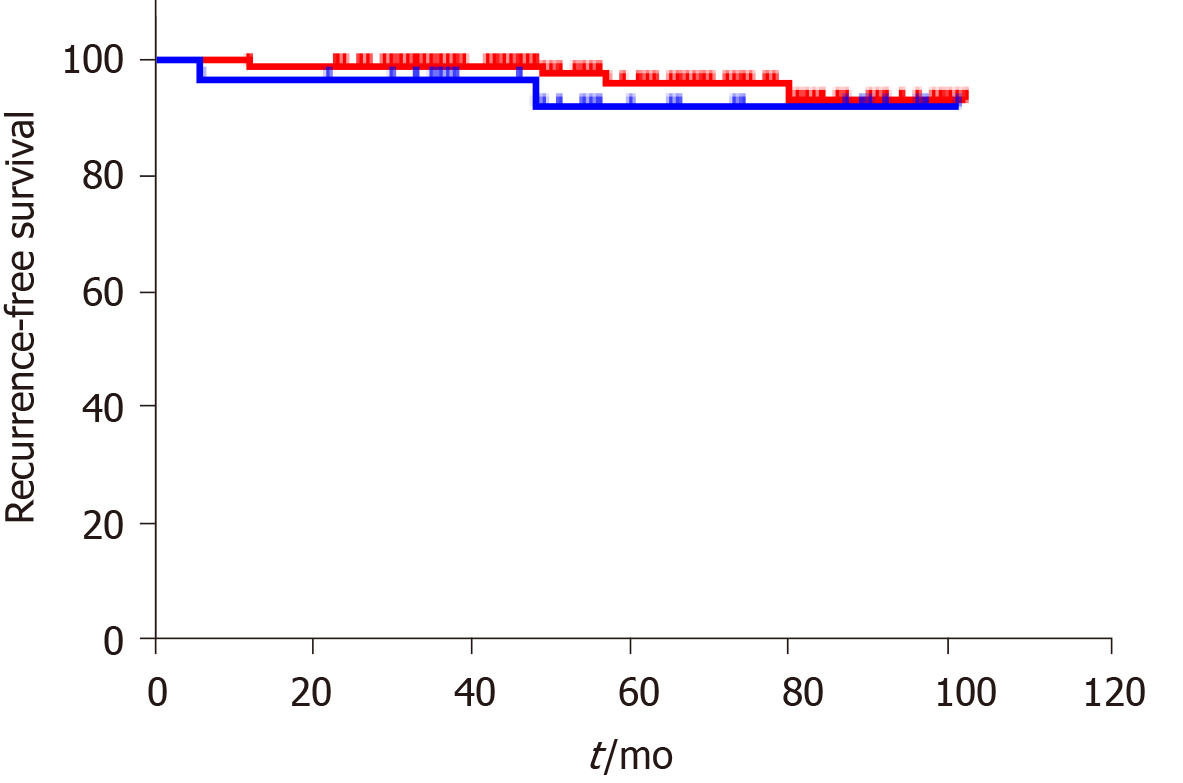
The final follow-up date was June 30, 2021. The median follow-up period was 49 mo (24-102 mo). Only 1 patient died due to perioperative complications, and the 3-, 5-, and 10-year overall survival (OS) rates were estimated to be 99.4%, 99.4%, and 99.4%, respectively. In total, 6 patients (3.6%) developed recurrence in the overall study cohort with 4 patients (3 Local recurrence and 1 Liver metastasis) in the CM group and 2 patients (1 Local recurrence and 1 Liver metastasis) in the PPM group. The median time to recurrence was 48 mo (range 6–84 mo). The 3-, 5-, and 10-year RFS rates were estimated at 98.8%, 97.0%, and 96.4% for the study cohort, respectively. The 3-, 5-, and 10-year RFS rates for the CM group were 99.2%, 97.7%, and 96.9%, respectively. The 3-, 5-, and 10-year RFS rates for the PPM group were 97.0%, 93.9%, and 93.9%, respectively. Kaplan–Meier analysis and the log-rank test showed that the recurrence rate was not significantly different between the groups (P = 0.39) (Figure 2).
The long-term complications were also evaluated. In the CM group, alimentary strictures were observed in 5 patients (3.0%), 3 of whom were treated by digestive tract bypass operations, and the other two were treated by duodenal stents. Six patients (4.5%) experienced pancreatic endocrine insufficiency, and 29 patients (21.8%) experienced exocrine insufficiency. In the PPM group, alimentary strictures were observed in 1 patient (3.0%) and treated by bypass operation. One patient (3.0%) experienced both pancreatic exocrine insufficiency and endocrine insufficiency. No other pancreatic exocrine insufficiency or endocrine insufficiency was observed in the PPM group. The incidence rates of alimentary stricture and pancreatic endocrine insufficiency were comparable between groups (both χ2 = 0.00 and both P = 1.0). However, the incidence of pancreatic exocrine insufficiency was significantly higher in the CM group compared with the PPM group (χ2 = 5.09, P = 0.024). Based on multivariate analysis, CM was identified as an independent risk factor for pancreatic exocrine insufficiency (odds ratio = 8.195, 95% confidence interval (CI): 1.067-62.93) after adjusting for age and sex.
Since it was first described in 1959, SPN has been widely acknowledged as a low-grade malignant neoplasm with a favorable prognosis after complete resection. If completely resected, the OS rate reached greater than 95% in previous studies[1,5]. Even in aggressive SPNs, the 5- and 10-year OS rates reached 71.1% and 65.5%, respectively[20]. In our study, only 1 patient died due to perioperative complications, and the 10-year OS rate reached greater than 99%, indicating that SPNs have very low malignant potential. However, the tumor often occurs in young females whose life expectancy is very long. In our study, the median age of the included patients was 32.5 years. These consistent data highlight the crucial importance of standardizing treatment procedures to guarantee improved quality of life for this small but challenging subset of patients.
According to the current guidelines, complete resection with a negative surgical margin is suggested to be curative for SPN[21,22]. However, the CM (including PD, TP and DP) might bring a negative surgical margin but be accompanied by wide resection of the pancreatic parenchyma. The loss of pancreatic parenchyma may affect the quality of life of young SPN patients. The proper treatment for SPN should balance curative resection and adverse events related to surgery. PPM is increasingly used for low-grade or benign pancreatic neoplasms[23,24]. However, research regarding PPM of SPN is limited and mainly based on case reports and retrospective studies with small sample sizes[25-27] due to the rarity of the disease. However, the results were inconsistent. Christine et al[26] concluded that PPM harbors a significant risk for tumor recurrence. However, only 8 patients who underwent PPM were included in this study. Wang et al[26] found that enucleation for SPNs is feasible and safe for preserving exocrine and endocrine function of the gland, and they concluded that enucleation with a negative surgical margin is adequate with no increased risk of tumor recurrence. In their study, 31 patients who underwent enucleation were included. Yao et al[27] concluded that CP was associated with a lower RFS rate than enucleation. However, only 11 patients were included in this case series, and only 5 of them were diagnosed with SPNs.
Due to the inconsistent data, we conducted a large series retrospective study to evaluate the efficacy of PPM in SPNs with various parameters included. Our results identified that PPM for SPNs had comparable intraoperative indices, pathological outcomes, and short-term complications to CM. The OS and RFS rates were also not different between groups. Long-term exocrine insufficiency was significantly lower (P = 0.024) in the PPM group, and CM was an independent risk factor for exocrine insufficiency. The OR was 8.2 (95%CI: 1.067-62.93). To avoid bias, the baseline characteristics were also compared between groups, and no difference was observed. The baseline characteristics of the patients included in our study were similar to those of resected SPNs previously reported[28], which included young age at diagnosis (mean 32.5 years), female predominance (129/166, 77.7%) and relatively large tumors (median 40 mm). Moreover, SPNs were mostly detected by accident (62.7%). However, the pancreatic head appeared to be the most common site of SPNs in our study. Actually, the body and tail were still the most common location sites because almost all of the SPNs located in multiple sites involved the body and tail of the pancreas (62/64, 96.9%).
The main complication after PPM was POPF, especially after enucleation. The POPF rates after enucleation in previously reported studies were 36-67%[29]. However, the POPF rates in our study were low (3%) because only clinically significant POPF was included in our study. Moreover, we tended to perform pancreaticojejunostomy if the main duct was injured during the operation. Therefore, we deemed PPM to be performed with no significantly increased risk of POPF in specialized centers, which was consistent with the results of Hüttner et al[16]. Moreover, the overall rate of severe complications (Claviene Dindo grade III and above, 6.0%) after PPM of SPN was consistent with other recent studies involving a large series of PPMs (6-18%)[15,16,29].
The intraoperative index was not different in our study, which was inconsistent with previous studies[26,30]. The reason may be due to the high pancreatic surgery volume in our center. The operation duration (median, 135 min) and intraoperative bleeding volume (median, 200 mL) had already reached a very low level. In the meta-analysis by Chua et al[30], the mean operation duration for CM was 325 min, and the mean blood loss was 300 mL. In the study by Wang et al [25], the median operation duration was 245 min, and the median blood loss was 380 mL for CM. Therefore, the benefit of PPM during operations was not identified by our study.
Avoiding tumor recurrence is another important endpoint for the management of SPNs. In addition to the efficacy and safety of PPM for SPNs identified in our study, our results also indicated that PPM did not result in an increased rate of tumor recurrence or metastasis compared with CM (P = 0.39). The risk factors associated with recurrence were not analyzed in our study due to the adequate evidence reported before. The main risk factors were large tumor size, lymphovascular invasion, positive margin status, Ki-67 index and synchronous metastasis[31,32]. However, the influence of these factors on the OS rate was not concluded[28,33]. As the overall prognosis is favorable for SPNs, the factors that worsen the OS rate should be clarified in future studies.
Our study had several limitations worth discussing. First, its retrospective nature prevented us from making stronger conclusions. The second limitation was the small sample size. Due to the rarity of SPNs, SPN cases were not common in our center. Moreover, the patients were often transferred from other referral institutions. Their initial medical records and follow-up data were not fully presented in our medical system.
The 2 factors greatly limited the size of the single institution series. More multicenter prospective studies with large sample sizes are necessary to better understand SPNs.
This retrospective study identified SPN as a rare pancreatic tumor with excellent prognosis after surgical resection. PPM for SPN appears to be feasible and safe for preserving exocrine function of the gland. The risk of recurrence or metastasis did not increase in patients who underwent PPM. PPM can be taken into consideration in SPN patients whose life expectancy is long.
Conventional surgical methods (CM) including pancreatoduodenectomy (PD), total pancreatectomy (TP) and distal pancreatectomy are standard surgical methods in the treatment of patients with Solid pseudopapillary neoplasm (SPN). CM is associated with a high rate of morbidity. However, the tumor mainly affects young women and the prognosis of the tumor is excellent.
The parenchyma-preserving surgical methods (PPM, including enucleation and central pancreatectomy ) are more and more often applied in clinical practice. The role of PPM in treating SPN remains clarified.
To evaluate the impact of PPM in the treatment of SPN patients.
Patients who underwent surgical resection for a pathological identified SPN were included in this study. Patients were divided into 2 groups: PPM group and CM group. The baseline characteristics, intraoperative index, pathological outcomes, short-term complications and long-term follow-up data were compared between the 2 groups.
Patients with SPN had an excellent prognosis. PPM did not increase the surgical risks. After long-term follow-up, we identified PPM did not worsen the prognosis of patients with SPN. However, PPM is suitable for preserving the exocrine function of pancreas in young patients.
PPM can be taken into consideration in SPN patients whose life expectancy is long.
More multicenter prospective studies with large sample sizes are necessary to better understand the best surgical method for patients with SPN.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Ashihara N, Isaji S S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 536] [Article Influence: 26.8] [Reference Citation Analysis (1)] |
| 2. | Machado MC, Machado MA, Bacchella T, Jukemura J, Almeida JL, Cunha JE. Solid pseudopapillary neoplasm of the pancreas: distinct patterns of onset, diagnosis, and prognosis for male vs female patients. Surgery. 2008;143:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 154] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Scholten L, van Huijgevoort NCM, van Hooft JE, Besselink MG, Del Chiaro M. Pancreatic Cystic Neoplasms: Different Types, Different Management, New Guidelines. Visc Med. 2018;34:173-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Stark A, Donahue TR, Reber HA, Hines OJ. Pancreatic Cyst Disease: A Review. JAMA. 2016;315:1882-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 5. | Law JK, Ahmed A, Singh VK, Akshintala VS, Olson MT, Raman SP, Ali SZ, Fishman EK, Kamel I, Canto MI, Dal Molin M, Moran RA, Khashab MA, Ahuja N, Goggins M, Hruban RH, Wolfgang CL, Lennon AM. A systematic review of solid-pseudopapillary neoplasms: are these rare lesions? Pancreas. 2014;43:331-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 6. | Lubezky N, Papoulas M, Lessing Y, Gitstein G, Brazowski E, Nachmany I, Lahat G, Goykhman Y, Ben-Yehuda A, Nakache R, Klausner JM. Solid pseudopapillary neoplasm of the pancreas: Management and long-term outcome. Eur J Surg Oncol. 2017;43:1056-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 7. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2422] [Article Influence: 484.4] [Reference Citation Analysis (3)] |
| 8. | Müller MW, Friess H, Kleeff J, Hinz U, Wente MN, Paramythiotis D, Berberat PO, Ceyhan GO, Büchler MW. Middle segmental pancreatic resection: An option to treat benign pancreatic body lesions. Ann Surg. 2006;244:909-18; discussion 918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Falconi M, Mantovani W, Crippa S, Mascetta G, Salvia R, Pederzoli P. Pancreatic insufficiency after different resections for benign tumours. Br J Surg. 2008;95:85-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Yao J, Song H. A Review of Clinicopathological Characteristics and Treatment of Solid Pseudopapillary Tumor of the Pancreas with 2450 Cases in Chinese Population. Biomed Res Int. 2020;2020:2829647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Divarcı E, Dökümcü Z, Çetingül N, Nart D, Barbet FY, Ergün O, Çelik A. Radical resection of the pancreas should not always be necessary in the surgical management of pancreatic solid pseudopapillary tumor in children. Turk J Gastroenterol. 2017;28:214-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Cauley CE, Pitt HA, Ziegler KM, Nakeeb A, Schmidt CM, Zyromski NJ, House MG, Lillemoe KD. Pancreatic enucleation: improved outcomes compared to resection. J Gastrointest Surg. 2012;16:1347-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Briggs CD, Mann CD, Irving GR, Neal CP, Peterson M, Cameron IC, Berry DP. Systematic review of minimally invasive pancreatic resection. J Gastrointest Surg. 2009;13:1129-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Crippa S, Boninsegna L, Partelli S, Falconi M. Parenchyma-sparing resections for pancreatic neoplasms. J Hepatobiliary Pancreat Sci. 2010;17:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Faitot F, Gaujoux S, Barbier L, Novaes M, Dokmak S, Aussilhou B, Couvelard A, Rebours V, Ruszniewski P, Belghiti J, Sauvanet A. Reappraisal of pancreatic enucleations: A single-center experience of 126 procedures. Surgery. 2015;158:201-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Hüttner FJ, Koessler-Ebs J, Hackert T, Ulrich A, Büchler MW, Diener MK. Meta-analysis of surgical outcome after enucleation vs standard resection for pancreatic neoplasms. Br J Surg. 2015;102:1026-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K; International Association of Pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1614] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 18. | Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, Seufferlein T, Haustermans K, Van Laethem JL, Conroy T, Arnold D; ESMO Guidelines Committee. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v56-v68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 905] [Cited by in RCA: 927] [Article Influence: 92.7] [Reference Citation Analysis (0)] |
| 19. | Inzani F, Petrone G, Rindi G. The New World Health Organization Classification for Pancreatic Neuroendocrine Neoplasia. Endocrinol Metab Clin North Am. 2018;47:463-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 20. | Hao EIU, Hwang HK, Yoon DS, Lee WJ, Kang CM. Aggressiveness of solid pseudopapillary neoplasm of the pancreas: A literature review and meta-analysis. Medicine (Baltimore). 2018;97:e13147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 1154] [Article Influence: 144.3] [Reference Citation Analysis (1)] |
| 22. | Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol. 2018;113:464-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 422] [Article Influence: 60.3] [Reference Citation Analysis (1)] |
| 23. | Strobel O, Cherrez A, Hinz U, Mayer P, Kaiser J, Fritz S, Schneider L, Klauss M, Büchler MW, Hackert T. Risk of pancreatic fistula after enucleation of pancreatic tumours. Br J Surg. 2015;102:1258-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Pärli MS, Müller PC, Müller SA, Ruzza CM, Z'graggen K. Posterior enucleation of the pancreatic head: an alternative route of access for parenchyma-sparing pancreatic resection. Langenbecks Arch Surg. 2019;404:1023-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Wang X, Chen YH, Tan CL, Zhang H, Xiong JJ, Chen HY, Ke NW, Liu XB. Enucleation of pancreatic solid pseudopapillary neoplasm: Short-term and long-term outcomes from a 7-year large single-center experience. Eur J Surg Oncol. 2018;44:644-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Tjaden C, Hassenpflug M, Hinz U, Klaiber U, Klauss M, Büchler MW, Hackert T. Outcome and prognosis after pancreatectomy in patients with solid pseudopapillary neoplasms. Pancreatology. 2019;19:699-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Yao L, Xie ZB, Jin C, Jiang YJ, Li J, Yang F, Lin QJ, Fu DL. Radical resection and enucleation in Chinese adolescents with pancreatic tumors: A 15-year case series. Medicine (Baltimore). 2017;96:e6438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 28. | Jutric Z, Rozenfeld Y, Grendar J, Hammill CW, Cassera MA, Newell PH, Hansen PD, Wolf RF. Analysis of 340 Patients with Solid Pseudopapillary Tumors of the Pancreas: A Closer Look at Patients with Metastatic Disease. Ann Surg Oncol. 2017;24:2015-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Zhang T, Xu J, Wang T, Liao Q, Dai M, Zhao Y. Enucleation of pancreatic lesions: indications, outcomes, and risk factors for clinical pancreatic fistula. J Gastrointest Surg. 2013;17:2099-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Chua TC, Yang TX, Gill AJ, Samra JS. Systematic Review and Meta-Analysis of Enucleation Versus Standardized Resection for Small Pancreatic Lesions. Ann Surg Oncol. 2016;23:592-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Lee G, Sung YN, Kim SJ, Lee JH, Song KB, Hwang DW, Kim J, Lee SS, Kim SC, Hong SM. Large tumor size, lymphovascular invasion, and synchronous metastasis are associated with the recurrence of solid pseudopapillary neoplasms of the pancreas. HPB (Oxford). 2021;23:220-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Yang F, Wu W, Wang X, Zhang Q, Bao Y, Zhou Z, Jin C, Ji Y, Windsor JA, Lou W, Fu D. Grading Solid Pseudopapillary Tumors of the Pancreas: the Fudan Prognostic Index. Ann Surg Oncol. 2021;28:550-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Chen H, Huang Y, Yang N, Yan W, Yang R, Zhang S, Yang P, Li N, Feng Z. Solid-Pseudopapillary Neoplasm of the Pancreas: A 63-Case Analysis of Clinicopathologic and Immunohistochemical Features and Risk Factors of Malignancy. Cancer Manag Res. 2021;13:3335-3343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |