Published online Dec 27, 2022. doi: 10.4240/wjgs.v14.i12.1397
Peer-review started: October 2, 2022
First decision: October 30, 2022
Revised: November 6, 2022
Accepted: December 13, 2022
Article in press: December 13, 2022
Published online: December 27, 2022
Processing time: 85 Days and 10.1 Hours
Achieving a clear resection margins for low rectal cancer is technically challenging. Transanal approach to total mesorectal excision (TME) was in
To investigate the outcomes of transanal TME (TaTME) and laparoscopic TME (LaTME) in patients with low rectal cancer.
A comprehensive systematic review of comparative studies was performed in line with Preferred Reporting Items for Systematic Reviews and Meta-Analyses standards. Intraoperative and postoperative complications, anastomotic leak, R0 resection, completeness of mesorectal excision, circumferential resection margin (CRM), distal resection margin (DRM), harvested lymph nodes, and operation time were the investigated outcome measures.
We included twelve comparative studies enrolling 969 patients comparing TaTME (n = 969) and LaTME (n = 476) in patients with low rectal tumours. TaTME was associated with significantly lower risk of postoperative complications (OR: 0.74, P = 0.04), anastomotic leak (OR: 0.59, P = 0.02), and conversion to an open procedure (OR: 0.29, P = 0.002) in comparison with LaTME. Moreover, the rate of R0 resection was significantly higher in the TaTME group (OR: 1.96, P = 0.03). Nevertheless, TaTME and LaTME were comparable in terms of rate of intraoperative complications (OR: 1.87; P = 0.23), completeness of mesoractal excision (OR: 1.57, P = 0.15), harvested lymph nodes (MD: -0.05, P = 0.96), DRM (MD: -0.94; P = 0.17), CRM (MD: 1.08, P = 0.17), positive CRM (OR: 0.64, P = 0.11) and procedure time (MD: -6.99 min, P = 0.45).
Our findings indicated that for low rectal tumours, TaTME is associated with better clinical and short term oncological outcomes compared to LaTME. More randomised controlled trials are required to confirm these findings and to evaluate long term oncological and functional outcomes.
Core Tip: The meta-analysis of best available evidence demonstrated that for low rectal tumours, Transanal total mesorectal excision (TaTME) is associated with better clinical and short term oncological outcomes compared to Laparoscopic TME. More randomised controlled trials with adequate power and high quality are required to not only confirm these findings, but also to evaluate long term oncological and functional outcomes.
- Citation: Bhattacharya P, Patel I, Fazili N, Hajibandeh S, Hajibandeh S. Meta-analysis of transanal vs laparoscopic total mesorectal excision of low rectal cancer: Importance of appropriate patient selection. World J Gastrointest Surg 2022; 14(12): 1397-1410
- URL: https://www.wjgnet.com/1948-9366/full/v14/i12/1397.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i12.1397
The incidence of rectal cancer is increasing making it one of the most common cancers worldwide[1]. Rapidly evolving use of total mesorectal excision (TME) and neoadjuvant chemotherapy have led to considerable improvements in the outcomes of rectal cancer surgery[2]. A clear resection margin associated with a high quality TME is important for an ideal oncological resection, reducing the incidence of local or regional recurrence, and increasing survival from cancer[3,4].
Achieving a negative resection margins during resection of low rectal tumours can be challenging due to existence of diminishing gap between the wall of the rectum and mesorectal fascia towards the anal canal[5]. This has resulted in worse oncological outcomes associated with resection of lower rectal tumours, in comparison with resection of middle or high rectal tumours, because of greater incidence of local recurrence and positive resection margin[6]. Transanal approach to TME was introduced in order to address the challenges associated with the laparoscopic and even open TME in surgical management of low rectal cancers[7].
In 2020, in a comprehensive meta-analysis of comparative studies, we reported that Transanal TME (TaTME) led to higher R0 resection rate and number of harvested lymph nodes while decreasing rates of positive circumferential resection margin (CRM) and conversion to open procedure when compared to laparoscopic TME (LaTME)[8]. Moreover, our findings indicated that TaTME and LaTME may have similar risk of perioperative morbidity[8]. Nevertheless, most of the evaluated studies in the aforementioned meta-analysis compared TaTME and LaTME in patients with middle and low rectal tumours subjecting the findings to bias. Considering the existence of new studies focusing on the clinical outcomes of TaTME and LaTME in management of low rectal cancer, conduction of another meta-analysis is worthwhile in order to help defining more appropriate patient selection.
This study aimed to systematically evaluate the best available comparative evidence surrounding TaTME and LaTME in surgical management of low rectal cancer only and compare the outcome so both approaches using meta-analytical model.
In our review protocol, we highlighted the inclusion and exclusion criteria, our methodology, and evaluated outcome measures. This study was carried out in line with standards of Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement[9].
All comparative studies investigating the outcomes of transanal and laparoscopic TME in patients with low cancer were considered for inclusion. A rectal tumour within 6 cm of anal verge was considered as a low rectal tumour. We considered all adult (aged > 18 years) patients undergoing TaTME or LaTME for low rectal cancer. TaTME was the intervention of interest and LaTME was the comparison of interest.
The primary outcome measures were intraoperative and postoperative complications, and anastomotic leak. The investigated primary oncological outcome measures were R0 resection, CRM, positive CRM, distal resection margin (DRM), completeness of mesorectal excision, and number of harvested lymph nodes. Moreover, conversion to open and operative time were defined as secondary outcome measures.
Following sources: MEDLINE, Web of Science, and CENTRAL were searched by two independent authors. Appendix 1 outlines the used search strategy (Supplementary Table 1). The most recent literature search was carried out on 08 July, 2022. Moreover, we screened the reference lists of the included studies and previous review articles in order to identify more relevant articles.
Two independent review authors screened the title and abstract of the identified studies. This was followed by retrieval of the full-texts of the related studies and their assessment in line with our inclusion and exclusion criteria. Discrepancies in this stage were addressed by discussion among the reviewers.
We created a data extraction tool and extracted details of study-related data, data regarding demographic characteristics of the included patients in each study and outcome data. Two independent reviewers were involved in this process. Disagreements between the authors were resolved following discussion. In case of no resolution, an additional reviewer was consulted.
The methodological quality of the included studies was assessed by 2 review authors who determined their associated risk of bias using the Newcastle-Ottawa scale[10] for observational studies and Cochrane’s tool[11] for randomized controlled trials (RCTs). We resolved disagreements in methodological quality assessment by discussion between the reviewers. However, if disagreement remained unresolved, a third reviewer was consulted as an adjudicator.
For dichotomous outcome measures the odds ratio (OR) was calculated as the summary measures. For continuous outcome parameters, the mean difference (MD) between the two groups was calculated. If mean values were not reported, we extracted data on median and interquartile range and converted those to mean and standard deviation using Hozo et al[12]’s equation.
The unit of analysis for all of the analyzed outcome measures in this study was an individual participant. We did not require contacting the authors of the included studies to ask for any potential missing information.
Data analysis was carried out via Review Manager 5.4 software[11]. One author extracted and entered the data into the software and another author cross-checked the data. Random-effects modelling were used for analysis of all outcomes. We reported outcome of analyses in Forest plots with 95% confidence intervals (CIs).
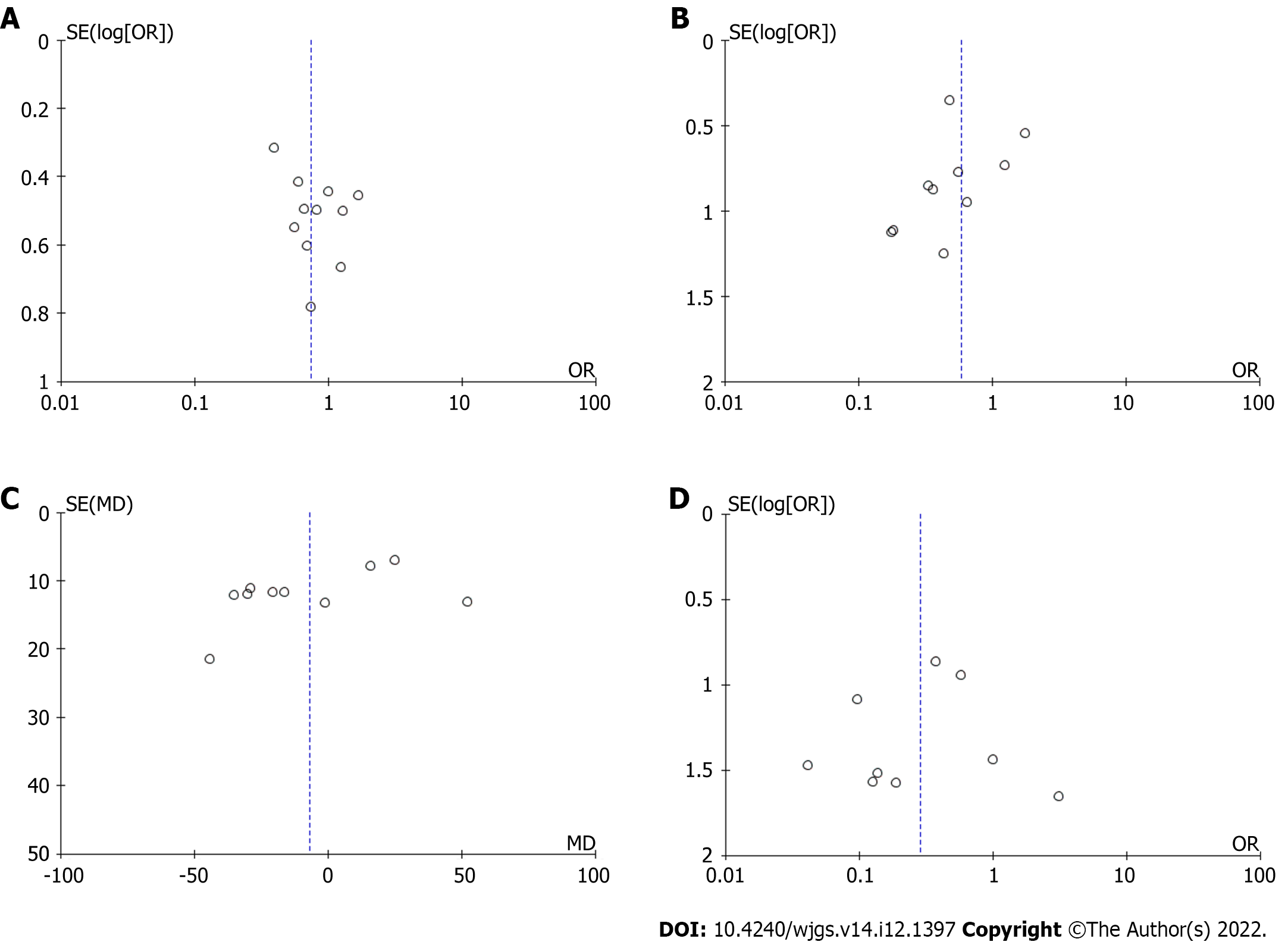
The Cochran Q test (χ2) was used to assess between-study heterogeneity. We calculated I2 and used the following guide for interpreting the degree of heterogeneity: 0% to 50% might not be important; 50% to 75%: May represent moderate heterogeneity; 75% to 100% may represent substantial heterogeneity. Moreover, we constructed funnel plots for any outcome synthesis involving more than 10 studies.
We performed sensitivity analyses to assess for potential sources of heterogeneity and evaluate the robustness of our findings. Finally, we conducted leave-one-out sensitivity analysis to assess the effect of each study on the overall effect size and heterogeneity.
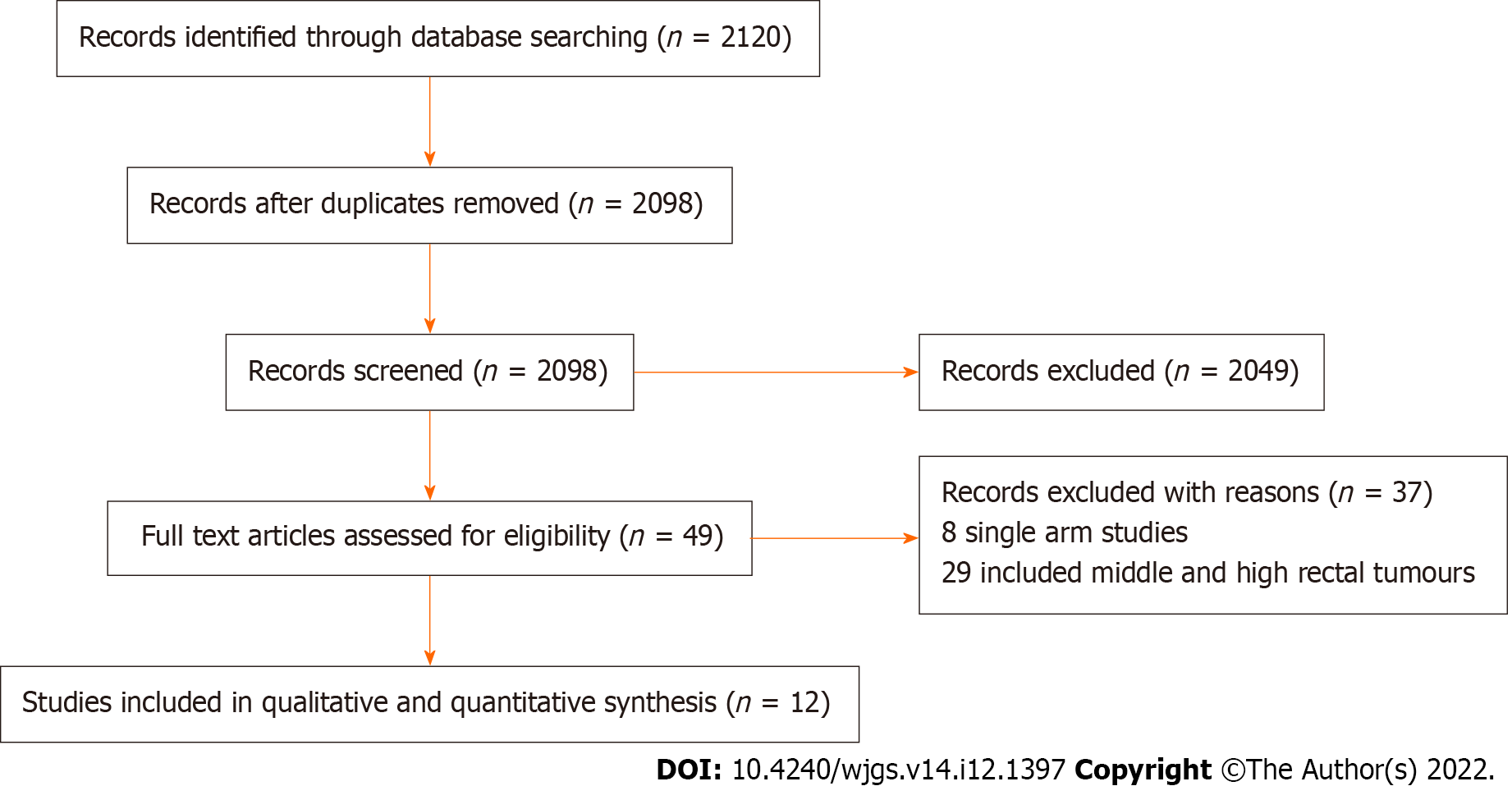
The literature search resulted in 2120 articles. Following further assessment of the aforementioned articles, 12 comparative studies (2 randomised and 10 observational studies)[13-24] met the inclusion criteria (Figure 1). The included studies enrolled 969 patients of whom 493 underwent TaTME and the remaining 476 patients had LaTME for rectal cancer.
Table 1 presents the included studies related data. Table 2 presents baseline demographic and clinical characteristics of the included patients. The patients in the transanal and laparoscopic groups were of similar age (P = 0.53), gender (P = 0.19), and BMI (P = 0.68). No significant difference was found between the TaTME and LaTME groups in rectal cancer stages I (P = 0.29), II (P = 0.30) and III (P = 0.95). Furthermore, the mean distance of the tumour to the anal verge in the TaTME and LaTME groups were 3.4 cm ± 1.4 cm and 3.6 cm ± 1.5 cm, respectively, which was not significantly different (P = 0.07). Neoadjuvant chemotherapy was carried our similarly between two groups (P = 0.22).
| Ref. | Publication year | Journal | Country | Study design | TaTME | LaTME |
| de’Angelis et al[13] | 2015 | Langenbecks Arch Surg | France | Retrospective observational study | 32 | 32 |
| Kanso et al[14] | 2015 | Dis Colon Rectum | France | Retrospective observational study | 51 | 34 |
| Pontallier et al[15] | 2016 | Surg Endosc | France | RCT | 38 | 34 |
| Marks et al[16] | 2016 | Tech. Coloproctol | United States | Retrospective observational study | 17 | 17 |
| Lelong et al[17] | 2017 | J Am Coll Surg | France | Retrospective observational study | 34 | 38 |
| Denost et al[18] | 2018 | Surg Endosc | France | RCT | 50 | 50 |
| Mege et al[19] | 2018 | Colorectal Dis | France | Retrospective observational study | 34 | 34 |
| Rubinkiewicz et al[20] | 2018 | Cancer Manag Res | Poland | Retrospective observational study | 35 | 35 |
| Roodbeen et al[21] | 2019 | Surg Endosc | Netherlands | Retrospective observational study | 41 | 41 |
| Rubinkiewicz et al[22] | 2019 | BMC Surg | Poland | Prospective observational study | 23 | 23 |
| Ren et al[23] | 2021 | Asian J Surg | China | Prospective observational study | 32 | 32 |
| Li et al[24] | 2022 | Gastroenterol Res Pract | China | Prospective observational study | 106 | 106 |
| Ref. | Publication year | Age | Gender | BMI | Neoadjuvant therapy | Tumour stage | Tumour location | Distance of tumour to anal verge |
| de’Angelis[13] | 2015 | 64.91 ± 10.05 vs 67.16 ± 9.61 | 66% vs 66% | 25.19 ± 3.52 vs 24.53 ± 3.19 | 100% vs 100% | I: 65.6% vs 56.3%; II: 31.3% vs 40.6%; III: 3.1% vs 3.1% | Low rectum | 4 (2.5-5.0) vs 3.7 (2.5-5.0) |
| Kanso et al[14] | 2015 | 59 ± 11 (32-79) vs 59 ± 11 (33-82) | 71% vs 77% | 24 ± 4 (17-32) vs 24 ± 4 (15-34) | 80% vs 79% | NR | Lower rectum | 1.6 ± 0.8 (0-3.5) vs 1.8 ± 0.9 (0-3.5) |
| Pontallier et al[15] | 2016 | 62 (39-81) vs 62 (35-82) | 68% vs 62% | 25.5 vs 24.8 | 79% vs 88% | I: 21% vs 21%; II: 19% vs 14%; III: 60% vs 65% | Low rectum | 4 (2-6) vs 4 (2-6) |
| Marks et al[16] | 2016 | 60 vs 59 | NR | 25.9 vs 26.4 | NR | I: 29.4% vs 23.5%; II: 70.6% vs 76.5% | Low rectum | < 4 vs < 4 |
| Lelong et al[17] | 2017 | NR | 68% vs 58% | 24 (18.6-45.0) vs 24.2(17.7-32.7) | 88.2% vs 92.1% | I: 17.6% vs 23.7%; II: 70.6% vs 71.0%; III: 11.8% vs 5.3% | Low rectum | NR |
| Denost et al[18] | 2018 | 64 (39-82) vs 63 (31-90) | 74% vs 64% | 25.1 (17.3-33.2) vs 25.6 (18.3-38.3) | 78% vs 84% | NR | Low rectum | 4 (2-6) vs 4 (2-6) |
| Mege et al[19] | 2018 | 58 ± 14 vs 59 ± 13 | 68% vs 68% | 25 ± 4 vs 25 ± 3 | 85% vs 85% | I: 29.4% vs 11.8%; II: 67.6% vs 82.3%; III: 43.5% vs 47.8%; IV: 2.9% vs 5.9% | Low rectum | NR |
| Rubinkiewicz et al[20] | 2018 | 64.3 ± 10.1 vs 60.3 ± 10.2 | 69% vs 69% | 26.10 ± 4.09 vs 27.10 ± 4.71 | 88.6% vs 88.6% | I: 42.9% vs 45.7%; II: 57.1% vs 54.3% | Low rectum | 2.90 ± 1.17 vs 3.19 ± 1.47 |
| Roodbeen et al[21] | 2019 | 62.5 ± 10.7 vs 66.0 ± 9.2 | 82.9% vs 78% | 26.7 ± 1.9 vs 26.1 ± 4.0 | 43.9% vs 43.9% | I: 22.0% vs 19.5%; II: 36.6% vs 39%; III: 31.7% vs 31.7%; IV: 9.8% vs 9.8% | Low rectum | 2.0 (0.0-4.0) vs 1.5 (0.0-3.0) |
| Rubinkiewicz et al[22] | 2019 | 60 (51-67) vs 64 (58-67) | 69% vs 69% | 26 (22.8-29.7) vs 26.5 (23.8-30.6) | 78.2% vs 82.6% | NR | Low rectum | 3(2-4) vs 4 (3-5) |
| Ren et al[23] | 2021 | 65.78 ± 12.37 vs 67.16 ± 10.03 | 59.3% vs 56.2% | 22.87 ± 2.66 vs 23.05 ± 2.70 | 71.8% vs 65.6% | I: 34.3% vs 37.5%; II: 28.1% vs 31.2%; III: 31.2% vs 21.8% | Low rectum | 5.53 ± 0.98 vs 5.78 ± 0.94 |
| Li et al[24] | 2022 | 55 ± 12 (23-78) vs 56 ± 12 (26-79) | 100% vs 100% | 23:0 ± 2.9 vs 22:9 ± 3.2 | 100% vs 100% | NR | Low rectum | 3.6 ± 0.9 (2.0-5.0) vs 3.8 ± 0.9 (1.4-5.0) |
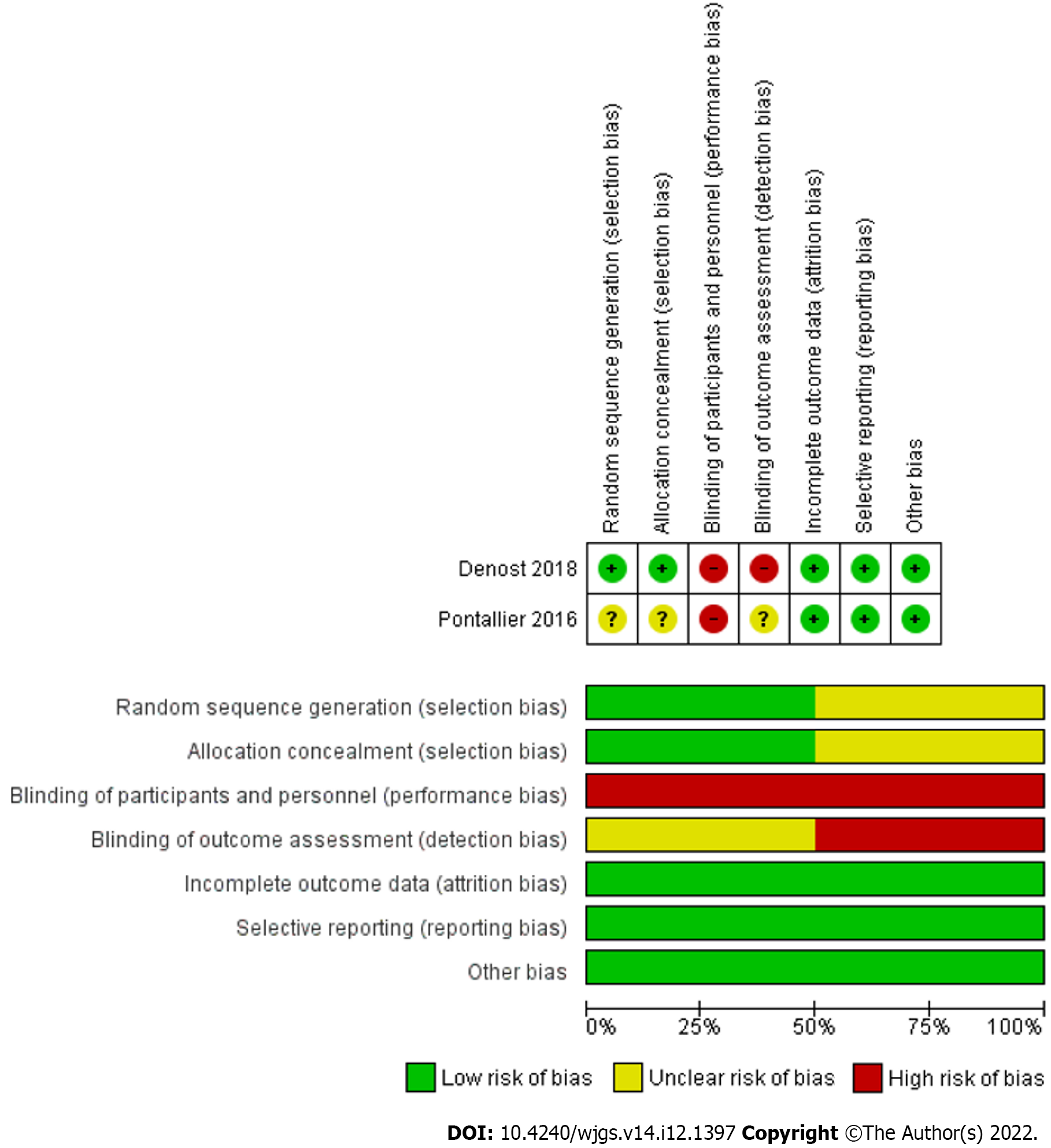
The methodological assessment of 10 observational studies is presented in Table 3. In 7 studies, the risk of bias was low and in 3 studies it was moderate. Moreover, the outcome of methodological assessment of the included randomized controlled trials is demonstrated by Figure 2.
| Author | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts | Total score |
| de’Angelis[13], 2015 | * | * | * | * | ** | * | * | * | 9 |
| Kanso et al[14], 2015 | * | * | * | * | ** | * | * | * | 9 |
| Marks et al[16], 2016 | * | * | * | * | - | * | * | * | 7 |
| Lelong et al[17], 2017 | * | * | * | * | * | * | * | * | 8 |
| Mege et al[19], 2018 | * | * | * | * | ** | * | * | * | 9 |
| Rubinkiewicz et al[20], 2018 | * | * | * | * | ** | * | * | * | 9 |
| Roodbeen et al[21], 2019 | * | * | * | * | ** | * | * | * | 9 |
| Rubinkiewicz et al[22], 2019 | * | * | * | * | * | * | * | * | 8 |
| Ren et al[23], 2021 | * | * | * | * | ** | * | * | * | 9 |
| Li et al[24], 2022 | * | * | * | * | ** | * | * | * | 9 |
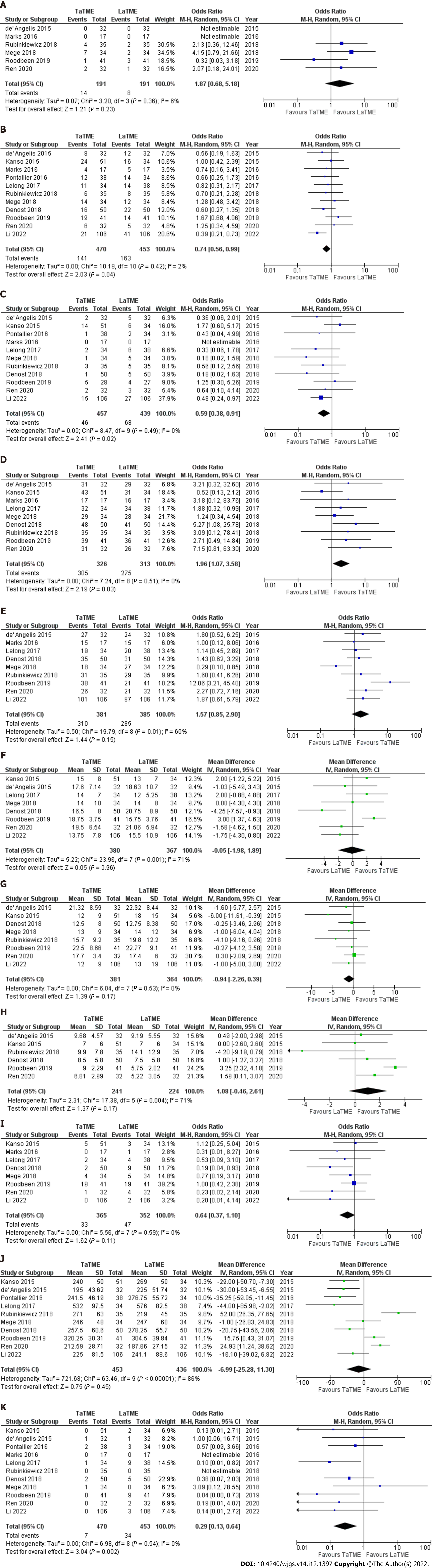
Outcomes are summarised in Figures 3 and 4.
Intraoperative complications: Six studies (382 patients) reported intraoperative complications as an outcome. The rate of intraoperative complications in the TaTME and LaTME were 7.3% and 4.2%, respectively. There was no significant difference in intraoperative complications between TaTME and LaTME (OR: 1.87; 95%CI: 0.68-5.18, P = 0.23). There was low between-study heterogeneity (I2 = 6%, P = 0.36).
Postoperative complications: Eleven studies (923 patients) reported postoperative complications as an outcome. The rate of overall postoperative complications in the TaTME and LaTME were 30.0% and 35.9%, respectively. TaTME significantly reduced postoperative complications when compared to LaTME (OR: 0.74; 95%CI: 0.56-0.99, P = 0.04). There was moderate heterogeneity among the included studies (I2 = 2%, P = 0.42).
Anastomotic leak: This outcome was reported by eleven studies (896 patients). Anastomotic leak occurred in 10.1% and 15.5% of patients in the TaTME and LaTME groups, respectively. TaTME was associated with a significantly lower rate of anastomotic leak compared with LaTME (OR: 0.59; 95%CI: 0.38-0.91, P = 0.02). Heterogeneity among the included studies was low (I2 = 0%, P = 0.49).
R0 resection: Nine studies (609 patients) reported R0 resection as an outcome. An R0 resection was achieved in 93.5% and 87.8% of patients in the TaTME and LaTME groups, respectively. The rate of R0 resection was significantly higher in the TaTME group (OR: 1.96; 95%CI: 1.07-3.58, P = 0.03). Low between-study heterogeneity was detected (I2 = 0%, P = 0.51).
Completeness of mesorectal excision: This outcome was reported by nine studies (766 patients). The rate of completeness of mesorectal excision in the TaTME and LaTME groups were 81.4% and 74.0%, respectively. The pooled analysis did not demonstrated similar rate of completeness of mesorectal excision between two groups (OR: 1.57; 95%CI: 0.85-2.90, P = 0.15). There was moderate between-study heterogeneity (I2 = 60%, P = 0.01).
Number of harvested lymph nodes: Eight studies (747 patients) reported the number of harvested lymph nodes in the TaTME and LaTME groups. The mean number of harvested lymph nodes in the TaTME was 16.1 ± 2.1, while it was 16.3 ± 3.2 in the LaTME group. The pooled analysis demonstrated no significant difference in the number of harvested lymph nodes between two groups (MD: -0.05; 95%CI:
DRM: Eight studies (745 patients) reported DRM in their study groups. The mean DRM in the TaTME group was 15.8 mm ± 3.9 mm whereas it was 17.6 mm ± 3.8 mm in the LaTME group. The pooled analysis found no significant difference in DRM between two groups (MD: -0.94; 95%CI: -2.26-0.39, P = 0.17). There was low heterogeneity among the included studies (I2 = 0%, P = 0.53).
CRM: Six studies (465 patients) reported CRM in their study groups. The mean CRM in the TaTME group was 8.5 mm ± 1.2 mm and it was 8.1 mm ± 2.9 mm in the LaTME group. The pooled analysis did not identify any significant difference in CRM between two groups (MD: 1.08; 95%CI: -0.46-2.61, P = 0.17). There was moderate between-study heterogeneity (I2 = 71%, P = 0.004).
Positive CRM: Eight studies (717 patients) reported the rate of positive CRM in their study groups. The rate of positive CRM in the TaTME group was 9.0% and it was 13.3% in the LaTME group. There was no significant difference in the rate of positive CRM between two groups (OR: 0.64; 95%CI: 0.37-1.10, P = 0.11). Between-study heterogeneity was low (I2 = 0%, P = 0.59).
Procedure time: Ten studies (889 patients) reported the procedure time as an outcome. The mean procedure time in the TaTME and LaTME groups were 274.1 min ± 91.8 min and 282.4 min ± 103.0 min, respectively. There was no significant difference in procedure time between two groups (MD: -6.99 min; 95%CI: -25.28-11.30, P = 0.45). Heterogeneity among the studies was significant (I2 = 86%, P < 0.00001).
Conversion to open: This outcome was reported by eleven studies (923 patients). The rate of conversion to an open procedure in the TaTME group was 1.5% and it was 7.5% in the LaTME group. The conversion rate was significantly lower in the TaTME group compared to the LaTME group (OR: 0.29; 95%CI: 0.13-0.64, P = 0.002). There was low between-study heterogeneity (I2 = 0%, P = 0.54).
Considering that the included study inadequately reported length of hospital stay as an outcome, we were unable to conduct an analysis on this outcome.
There was no change in the direction of pooled effect size when the risk ratio, or risk difference was calculated or during leave-one-out sensitivity analysis.
In view of ongoing debates regarding the best surgical approach for resection of low rectal cancer, we conducted a comprehensive systematic review and meta-analysis to evaluate comparative outcomes of transanal vs laparoscopic TME in management of low rectal cancer. We identified two RCTs and 10 observational studies[13-24] enrolling 969 patients of whom 493 had TaTME and 476 patients had LaTME for low rectal tumour. The subsequent outcome synthesis showed that TaTME significantly reduced rate of postoperative complications, anastomotic leak, and conversion to open in comparison to LaTME. Moreover, TaTME resulted in significantly higher rate of R0 resection. However, no significant difference was found in intraoperative complications, completeness of mesoractal excision, harvested lymph nodes, DRM, CRM, positive CRM and procedure time between TaTME and LaTME
The between-study heterogeneity in the analyses of intraoperative and postoperative complications, anastomotic leak, R0 resection, DRM, positive CRM, and conversion to open were low suggesting that the reported findings with respect to these outcomes can be considered robust. Moderate heterogeneity among the included studies in the analyses of completeness of mesorectal excision, and number of harvested lymph nodes may suggest variation of reporting in the included studies on these outcomes. There was high between-study heterogeneity regarding procedure time suggesting that our findings about procedure time may be less robust.
The findings of our meta-analysis are not consistent with some of the findings of our previous meta-analysis on this topic published in 2020[8]. The simple explanation for such disagreement is the difference in the inclusion criteria of the two studies with regards to the location of the rectal cancer. We only included low rectal cancer patients in this meta-analysis while previously we included both middle and low rectal cancer patients. In fact, as a direction for future research, in our previous meta-analysis we encouraged future studies to consider patients with low rectal cancer only when comparing TaTME and LaTME to evaluate a more realistic comparison between these two management approaches[8]. This is indeed reassuring to observe growing evidence in the context of comparative outcomes of TaTME and LaTME in management of low rectal cancer. The appropriate patient selection in this context is of great importance as inappropriate patient selection for TaTME has been demonstrated to result in unfavourable outcomes of TaTME leading to suspension of TaTME in some countries. Wasmuth et al[25] reported high rate of anastomotic leak and local recurrence associated with TaTME, the findings that led to suspicion of TaTME in Norway. However, only 5% of their included patients had low rectal tumours with the remaining patients having middle or high rectal cancers. Moreover, the study lacked a control group, hence low level of evidence.
In the current meta-analysis, we independently evaluated the baseline characteristics of the study population to assess if the patients in the TaTME and LaTME groups were comparable. We found no significant difference in age, gender, BMI, rate of neoadjuvant chemotherapy, and stage of cancer between two groups. Moreover, we demonstrated similar distance between the distal tumour and anal verge between the TaTME and LaTME patients. This is of a cardinal importance as TaTME has been introduced to address the challenges associated with open and laparoscopic approaches in resecting very low rectal cancers, particularly in male patients with narrow pelvis[8]. Therefore, comparability of our included populations in both groups makes our findings more robust.
We were not able to conduct any analyses on comparative functional outcomes of TaTME and LaTME considering that only two of the included studies reported such outcomes. Lelong et al[17] compared functional outcomes of TaTME and LaTME and demonstrated no significant difference in urinary complications and faecal incontinence between two groups. Rubinkiewicz et al[22] also investigated functional outcomes in patients undergoing TaTME and LaTME for low rectal tumours and reported no significant differences in risk of low anterior resection syndrome between two groups and its severity. The authors found comparable median Wexner score in both groups[22]. Considering the current limited evidence in the context of functional outcomes of TaTME compared with LaTME, no definitive conclusions can be made.
Although we were not able to analyse long term oncological outcomes including disease recurrence, the findings of one of our included RCTs in this context is important. After 5 years follow-up, Denost et al[18] reported no significant differences in long-term outcomes between TaTME and LaTME. Although the authors found a significant association between CRM involvement and local recurrence (P = 0.011), the 5-year local recurrence rate was similar between two groups (3% vs 5%, P = 0.30). Moreover, the authors reported similar 5-year disease-free survival between two groups (72% vs 74%, P = 0.351). The rate of local recurrence in the aforementioned RCT is comparable with the recurrence rate of 4% reported in a review by Deijen et al[26]. Undoubtedly, futures high quality randomized studies with adequate follow-up periods are required to investigate long term oncological outcomes of transanal and laparoscopic approaches to TME.
This study has a number of limitations. Only two of the considered studies were RCTs. Most of the included studies were observational studies with their inherited selection bias. Some of the included studies had small sample sizes which might have introduced Type 2 error to our findings. We were unable to conduct independent analyses on length of hospital stay, functional outcomes or long term oncological outcomes as the data provided by the included studies on such outcomes was inadequate. Finally, there was moderate risk of bias in 3 of our included studies.
Our meta-analysis demonstrated that for low rectal tumours, TaTME is associated with better clinical and short term oncological outcomes compared to LaTME. More randomised controlled trials with adequate power and high quality are required to not only confirm these findings, but also to evaluate long term oncological and functional outcomes.
Achieving a clear resection margins for low rectal cancer is technically challenging. Transanal TME (TaTME) has been introduced in order to address the chalenges associated with the open and laparoscopic TME (LaTME) in resecting low rectal tumours.
Previous meta-analyses have included mixed patients with mid and low rectal tumours when comparing TaTME and LaTME which has made the interpretation of the real differences between two approaches in treating low rectal cancer difficult.
To investigate the outcomes of transanal TaTME and LaTME in patients with low rectal cancer.
A comprehensive systematic review of comparative studies were conducted according to the standards of Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. Intraoperative and postoperative complications, anastomotic leak, completeness of mesorectal excision, R0 resection, distal (DRM) and circumferential resection margin (CRM), number of harvested lymph nodes, and procedure time were the evaluated outcome parameters.
We identified twelve comparative studies enrolling a total of 969 patients comparing the outcomes of TaTME (n = 969) and LaTME (n = 476) in patients with low rectal cancer. The meta-analysis demonstrated that TaTME was associated with significantly lower rate of postoperative complications (OR: 0.74, P = 0.04), anastomotic leak (OR: 0.59, P = 0.02), and conversion to an open procedure (OR: 0.29, P = 0.002) compared with LaTME. Moreover, it was associated with significantly higher rate of R0 resection (OR: 1.96, P = 0.03). However, there was no significant difference in intraoperative complications (OR: 1.87; P = 0.23), completeness of mesoractal excision (OR: 1.57, P = 0.15), harvested lymph nodes (MD: -0.05, P = 0.96), DRM (MD: -0.94; P = 0.17), CRM (MD: 1.08, P = 0.17), positive CRM (OR: 0.64, P = 0.11) and procedure time (MD: -6.99 minutes, P = 0.45) between TaTME and LaTME.
Our findings indicated that for low rectal tumours, TaTME is associated with better clinical and short term oncological outcomes compared to LaTME.
The available evidence does not allow evaluation of long term oncological and functional outcomes. More randomized controlled trials are required to confirm the findings of this meta-analysis regarding clinical and short term oncological outcomes and to evaluate long term oncological and functional outcomes.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lei Y, China; Yao J, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YX
| 1. | Meyer JE, Narang T, Schnoll-Sussman FH, Pochapin MB, Christos PJ, Sherr DL. Increasing incidence of rectal cancer in patients aged younger than 40 years: an analysis of the surveillance, epidemiology, and end results database. Cancer. 2010;116:4354-4359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 2. | Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg. 1998;133:894-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1037] [Cited by in RCA: 1054] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 3. | Quirke P, Steele R, Monson J, Grieve R, Khanna S, Couture J, O’Callaghan C, Myint AS, Bessell E, Thompson LC, Parmar M, Stephens RJ, Sebag-Montefiore D; MRC CR07/NCIC-CTG CO16 Trial Investigators; NCRI Colorectal Cancer Study Group. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet. 2009;373:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 748] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 4. | Martling A, Singnomklao T, Holm T, Rutqvist LE, Cedermark B. Prognostic significance of both surgical and pathological assessment of curative resection for rectal cancer. Br J Surg. 2004;91:1040-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Cecil TD, Taffinder N, Gudgeon AM. A personal view on laparoscopic rectal cancer surgery. Colorectal Dis. 2006;8 Suppl 3:30-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Nagtegaal ID, van de Velde CJ, Marijnen CA, van Krieken JH, Quirke P; Dutch Colorectal Cancer Group; Pathology Review Committee. Low rectal cancer: a call for a change of approach in abdominoperineal resection. J Clin Oncol. 2005;23:9257-9264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 416] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 7. | Bonjer HJ, Deijen CL, Abis GA. A randomized trial of laparoscopic vs open surgery for rectal cancer. N Engl J Med. 2005;372:1324-1332. |
| 8. | Hajibandeh S, Hajibandeh S, Eltair M, George AT, Thumbe V, Torrance AW, Budhoo M, Joy H, Peravali R. Meta-analysis of transanal total mesorectal excision versus laparoscopic total mesorectal excision in management of rectal cancer. Int J Colorectal Dis. 2020;35:575-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13317] [Article Influence: 832.3] [Reference Citation Analysis (0)] |
| 10. | Wells GA, Shea B, O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. November 28, 2019. [cited 3 November 2022]. Available from: http://www.ohri.ca/ programs/clinical_epidemiology/oxford.asp. [DOI] [Full Text] |
| 11. | Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2881] [Article Influence: 480.2] [Reference Citation Analysis (0)] |
| 12. | Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4895] [Cited by in RCA: 6858] [Article Influence: 342.9] [Reference Citation Analysis (0)] |
| 13. | de’Angelis N, Portigliotti L, Azoulay D, Brunetti F. Transanal total mesorectal excision for rectal cancer: a single center experience and systematic review of the literature. Langenbecks Arch Surg. 2015;400:945-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Kanso F, Maggiori L, Debove C, Chau A, Ferron M, Panis Y. Perineal or Abdominal Approach First During Intersphincteric Resection for Low Rectal Cancer: Which Is the Best Strategy? Dis Colon Rectum. 2015;58:637-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Pontallier A, Denost Q, Van Geluwe B, Adam JP, Celerier B, Rullier E. Potential sexual function improvement by using transanal mesorectal approach for laparoscopic low rectal cancer excision. Surg Endosc. 2016;30:4924-4933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Marks JH, Montenegro GA, Salem JF, Shields MV, Marks GJ. Transanal TATA/TME: a case-matched study of taTME versus laparoscopic TME surgery for rectal cancer. Tech Coloproctol. 2016;20:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Lelong B, Meillat H, Zemmour C, Poizat F, Ewald J, Mege D, Lelong JC, Delpero JR, de Chaisemartin C. Short- and Mid-Term Outcomes after Endoscopic Transanal or Laparoscopic Transabdominal Total Mesorectal Excision for Low Rectal Cancer: A Single Institutional Case-Control Study. J Am Coll Surg. 2017;224:917-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Denost Q, Loughlin P, Chevalier R, Celerier B, Didailler R, Rullier E. Transanal versus abdominal low rectal dissection for rectal cancer: long-term results of the Bordeaux’ randomized trial. Surg Endosc. 2018;32:1486-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Mege D, Hain E, Lakkis Z, Maggiori L, Prost À la Denise J, Panis Y. Is trans-anal total mesorectal excision really safe and better than laparoscopic total mesorectal excision with a perineal approach first in patients with low rectal cancer? Colorectal Dis. 2018;20:O143-O151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Rubinkiewicz M, Nowakowski M, Wierdak M, Mizera M, Dembiński M, Pisarska M, Major P, Małczak P, Budzyński A, Pędziwiatr M. Transanal total mesorectal excision for low rectal cancer: a case-matched study comparing TaTME versus standard laparoscopic TME. Cancer Manag Res. 2018;10:5239-5245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Roodbeen SX, Penna M, Mackenzie H, Kusters M, Slater A, Jones OM, Lindsey I, Guy RJ, Cunningham C, Hompes R. Transanal total mesorectal excision (TaTME) versus laparoscopic TME for MRI-defined low rectal cancer: a propensity score-matched analysis of oncological outcomes. Surg Endosc. 2019;33:2459-2467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 22. | Rubinkiewicz M, Zarzycki P, Witowski J, Pisarska M, Gajewska N, Torbicz G, Nowakowski M, Major P, Budzyński A, Pędziwiatr M. Functional outcomes after resections for low rectal tumors: comparison of Transanal with laparoscopic Total Mesorectal excision. BMC Surg. 2019;19:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Ren J, Liu S, Luo H, Wang B, Wu F. Comparison of short-term efficacy of transanal total mesorectal excision and laparoscopic total mesorectal excision in low rectal cancer. Asian J Surg. 2021;44:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Li Z, Xiao J, Hou Y, Zhang X, Jie H, Liu H, Ruan L, Zeng Z, Kang L. Transanal versus Laparoscopic Total Mesorectal Excision in Male Patients with Low Tumor Location after Neoadjuvant Therapy: A Propensity Score-Matched Cohort Study. Gastroenterol Res Pract. 2022;2022:2387464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Wasmuth HH, Faerden AE, Myklebust TÅ, Pfeffer F, Norderval S, Riis R, Olsen OC, Lambrecht JR, Kørner H, Larsen SG; Norwegian TaTME Collaborative Group, on behalf of the Norwegian Colorectal Cancer Group, Forsmo HM, Baekkelund O, Lavik S, Knapp JC, Sjo O, Rashid G. Transanal total mesorectal excision for rectal cancer has been suspended in Norway. Br J Surg. 2020;107:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 181] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 26. | Deijen CL, Tsai A, Koedam TW, Veltcamp Helbach M, Sietses C, Lacy AM, Bonjer HJ, Tuynman JB. Clinical outcomes and case volume effect of transanal total mesorectal excision for rectal cancer: a systematic review. Tech Coloproctol. 2016;20:811-824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |