Published online Nov 27, 2022. doi: 10.4240/wjgs.v14.i11.1310
Peer-review started: July 15, 2022
First decision: September 12, 2022
Revised: September 24, 2022
Accepted: October 19, 2022
Article in press: October 19, 2022
Published online: November 27, 2022
Processing time: 133 Days and 7.8 Hours
Celiac trunk stenosis or occlusion is a common condition observed in patients undergoing pancreaticoduodenectomy (PD). The risk of upper abdominal organ ischemia or failure increases if the blood circulation in the celiac arterial system is not maintained after the surgery.
We present two cases of elderly patients with distal cholangiocarcinoma and celiac trunk occlusion who underwent PD. We performed blood circulation modification preoperatively with transcatheter coil embolization of the arterial arcades of the pancreatic head via the superior mesenteric artery to develop collateral communication between the superior mesenteric artery and the common hepatic or splenic arteries to ensure arterial blood flow to the upper abdominal organs. The postoperative course was marked by delayed gastric emptying, but no major surgical complications, such as biliary or pancreatic fistula, or clinical, biochemical, or radiological evidence of ischemic disease, was observed.
Preoperative blood circulation modification may be a valid alternative procedure for elderly patients with celiac trunk occlusion who are ineligible for interventional or surgical revascularization.
Core Tip: Celiac trunk stenosis or occlusion is a common condition observed in patients undergoing pancreaticoduodenectomy (PD). Celiac trunk occlusion may increase the risk of upper abdominal organ ischemia or failure. In this case report, we present two elderly patients who underwent PD for distal cholangiocarcinoma with celiac trunk occlusion. We performed blood circulation modification preoperatively with transcatheter coil embolization of the arterial arcades of the pancreatic head to develop collateral communication between the superior mesenteric and the common hepatic or splenic artery.
- Citation: Colella M, Mishima K, Wakabayashi T, Fujiyama Y, Al-Omari MA, Wakabayashi G. Preoperative blood circulation modification prior to pancreaticoduodenectomy in patients with celiac trunk occlusion: Two case reports. World J Gastrointest Surg 2022; 14(11): 1310-1319
- URL: https://www.wjgnet.com/1948-9366/full/v14/i11/1310.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i11.1310
Celiac trunk stenosis is a common condition observed in up to 10% of patients undergoing pancreaticoduodenectomy (PD)[1,2]. If undiagnosed, it can lead to fatal ischemia of the upper abdominal organs after the surgery because the blood supply via the pancreatic head arcades is sacrificed intraoperatively due to the ligation of the gastroduodenal artery (GDA)[1-4]. Herein we describe two elderly patients who underwent PD following a novel blood circulation modification with transcatheter coil embolization of the arterial arcades of the pancreatic head.
Case 1: In June 2019, an 83-year-old man with a history of hypertension, chronic kidney disease, and chronic obstructive pulmonary disease was referred to our hospital for liver dysfunction during a blood test, fever, and anorexia.
Case 2: An 84-year-old man with a history of cervical spondylosis, chronic obstructive pulmonary disease, and atrial fibrillation was admitted to our institution in August 2019 with a 1-mo history of epigastric pain, weight loss, and dyspepsia.
Case 1: A computed tomography (CT) scan revealed celiac artery (CeA) occlusion due to atherosclerosis, with thickening of the extrahepatic bile duct wall and luminal stenosis, resulting in mild upstream bile duct dilatation, and the possibility of cholangitis or distal cholangiocarcinoma was enlightened.
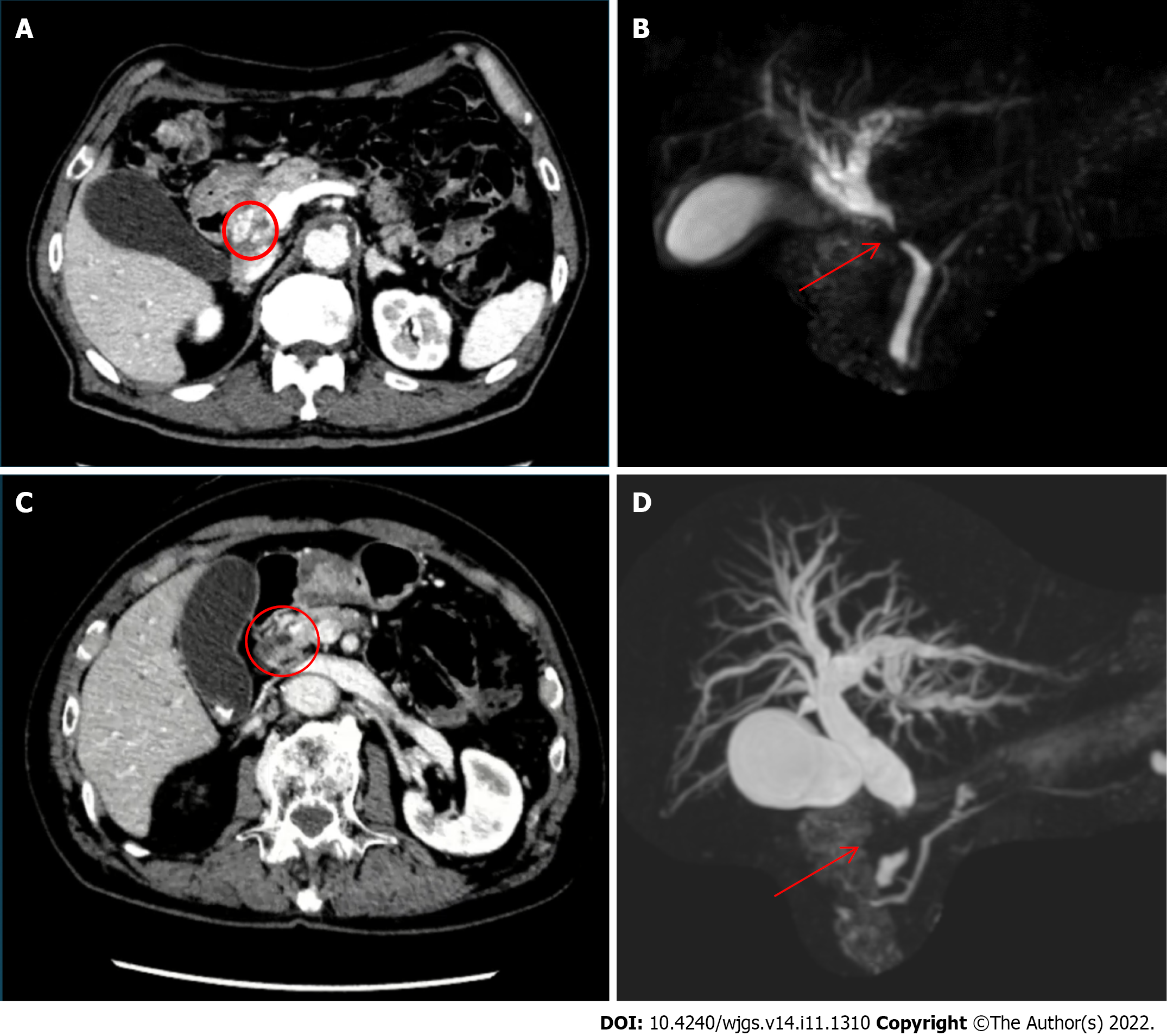
Distal cholangiocarcinoma was suspected following magnetic resonance cholangiopancreatography (MRCP), which revealed a stricture of the extrahepatic bile duct and mild dilatation of the intrahepatic bile duct (Figure 1A and B). An ERCP with brushing cytology was performed to confirm the diagnosis, and a plastic stent was placed.
Case 2: CT revealed dilatation of the entire bile duct system, with 15-mm tissue obstructing the wall and lumen of the pancreatic tract of the common bile duct without pancreatic duct dilatation. Additionally, a small lymph node was observed around the hepatic hilum and abdominal aorta (Figure 1C and D). Distal bile duct cancer was suspected, and the patient underwent MRCP and ERCP with brushing cytology, which confirmed the diagnosis.
Case 1: Distal cholangiocarcinoma cT2N0M0 stage IIA was diagnosed according to the American Joint Committee on Cancer (AJCC) 8th classification, and PD was scheduled.
Case 2: According to the AJCC 8th edition classification, a diagnosis of distal cholangiocarcinoma cT2N1M0 stage IIB was made, and PD was scheduled. A plastic stent was placed in the common hepatic duct preoperatively.
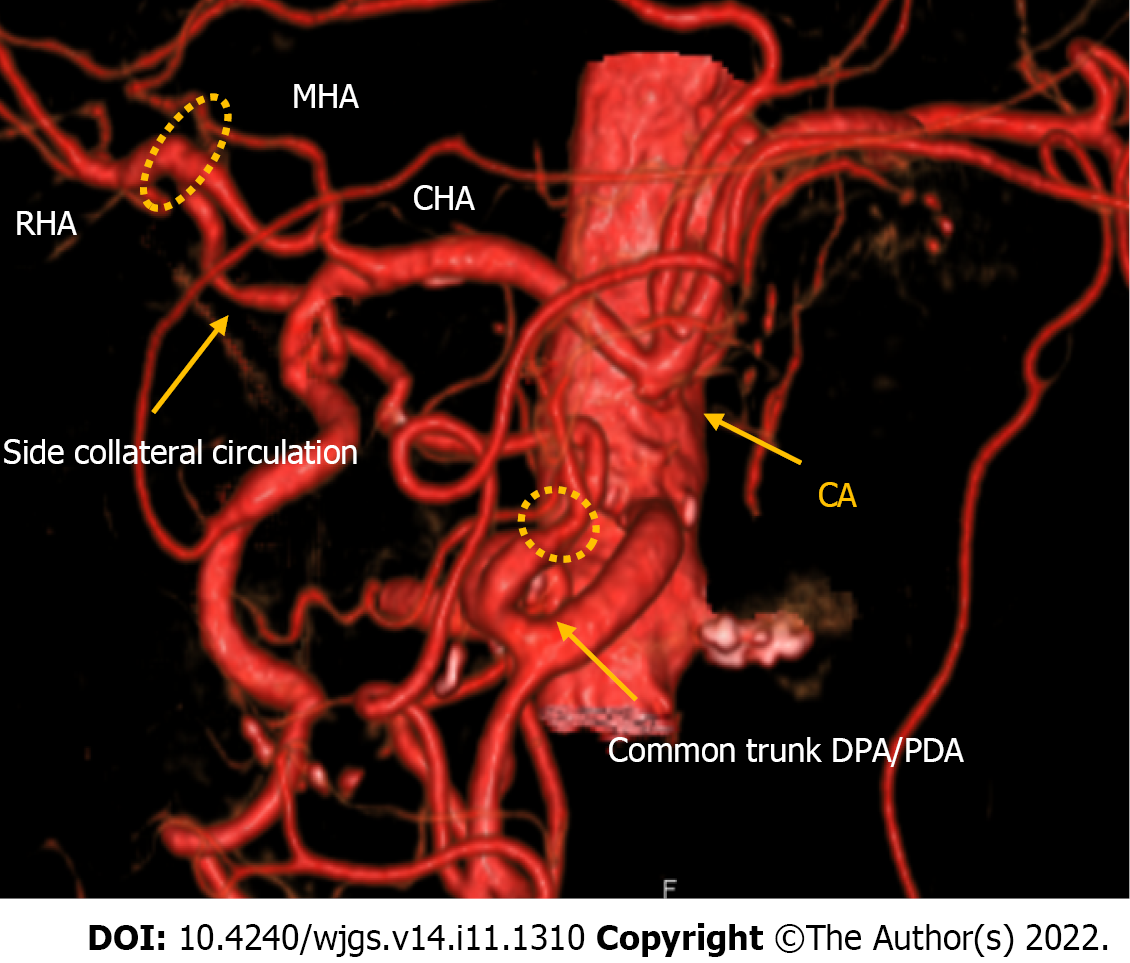
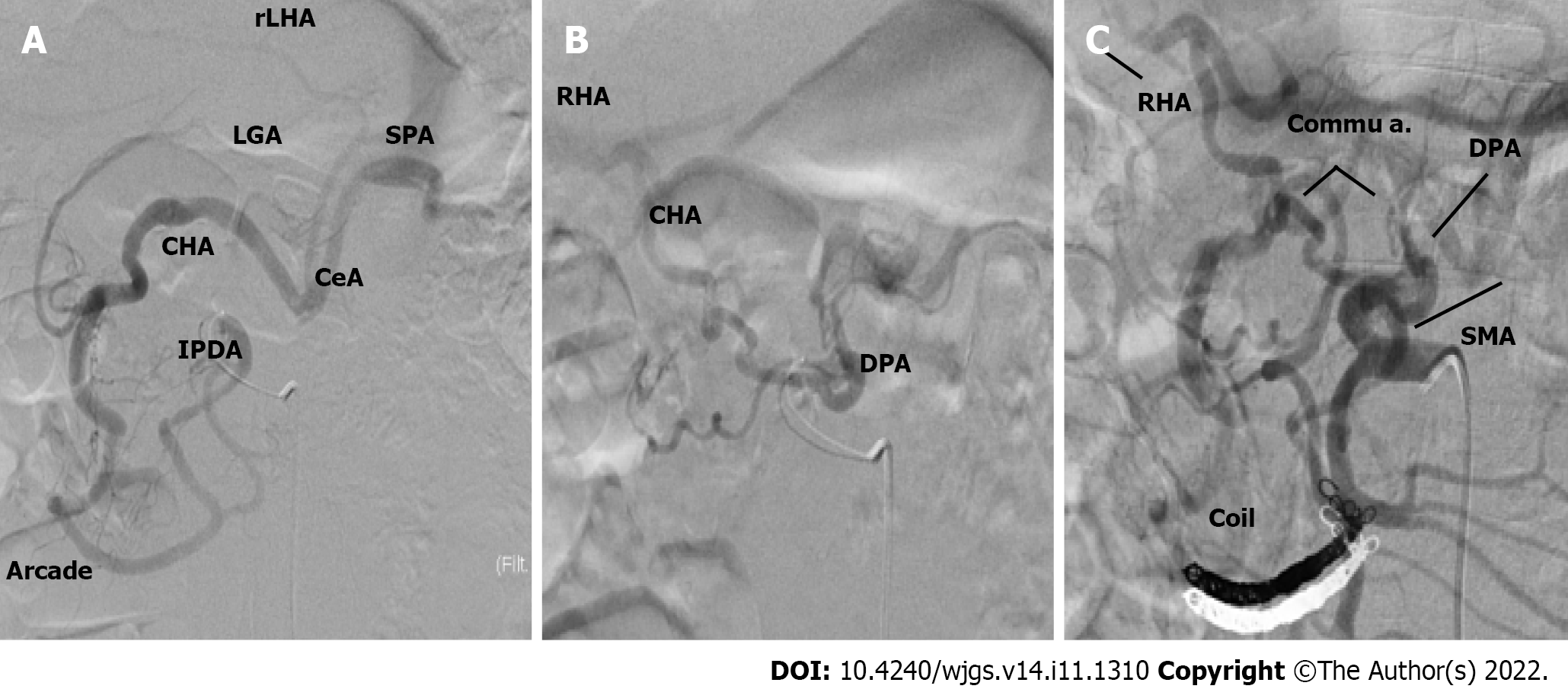
Case 1: An angiographic study and CT with 3D reconstruction were performed; complete occlusion of the CeA and complex anomalous arterial anatomy were observed (Figure 2). The inferior pancreaticoduodenal artery (IPDA), which branches from the superior mesenteric artery (SMA), supplied the celiac arterial system's primary blood supply, while the dorsal pancreatic artery (DPA) supplied the right hepatic arteries (RHA) and splenic arteries (SPA). Preoperatively, we embolized the arterial arcades of the pancreatic head using a coil to increase blood flow via the SMA to the RHA and SPA (Figure 3). Both post-procedural CT and angiography confirmed the development of blood flow sustained by the DPA, and no radiological signs of ischemic complications were observed.
Case 2: On preoperative CT, complete CeA obstruction due to atherosclerosis and a well-developed collateral pathway between the SMA and CeA were observed (Figure 4). Angiography revealed complete celiac trunk occlusion, maintenance of the major backflow to the celiac arterial system by two main pathways (the pancreatic head arcades), and linkage of the DPA to the common hepatic artery (CHA). We speculated that celiac arterial blood flow could be supplied via the DPA; however, ligating the GDA would cause blood flow reduction. Hence, there was a high risk of severe ischemia of the upper abdominal organs.
Pathological diagnosis identified pT3apN1pM0, stage IIB, poorly differentiated biliary tract cancer. Of the 24 lymph nodes retrieved, only one was involved, and the lesion was completely included in the resection margin (R0 resection). The operative time was 7 h and 55 min, and the estimated blood loss was 700 mL.
Pathological diagnosis identified pT2pN1pM0, stage IIB, intermediate-grade biliary tract cancer, which involved 3 of 20 lymph nodes retrieved.
The nodes and the lesion were completely included in the resection margin (R0 resection). The operation time was 6 h and 32 min, and the estimated blood loss was 200 mL.
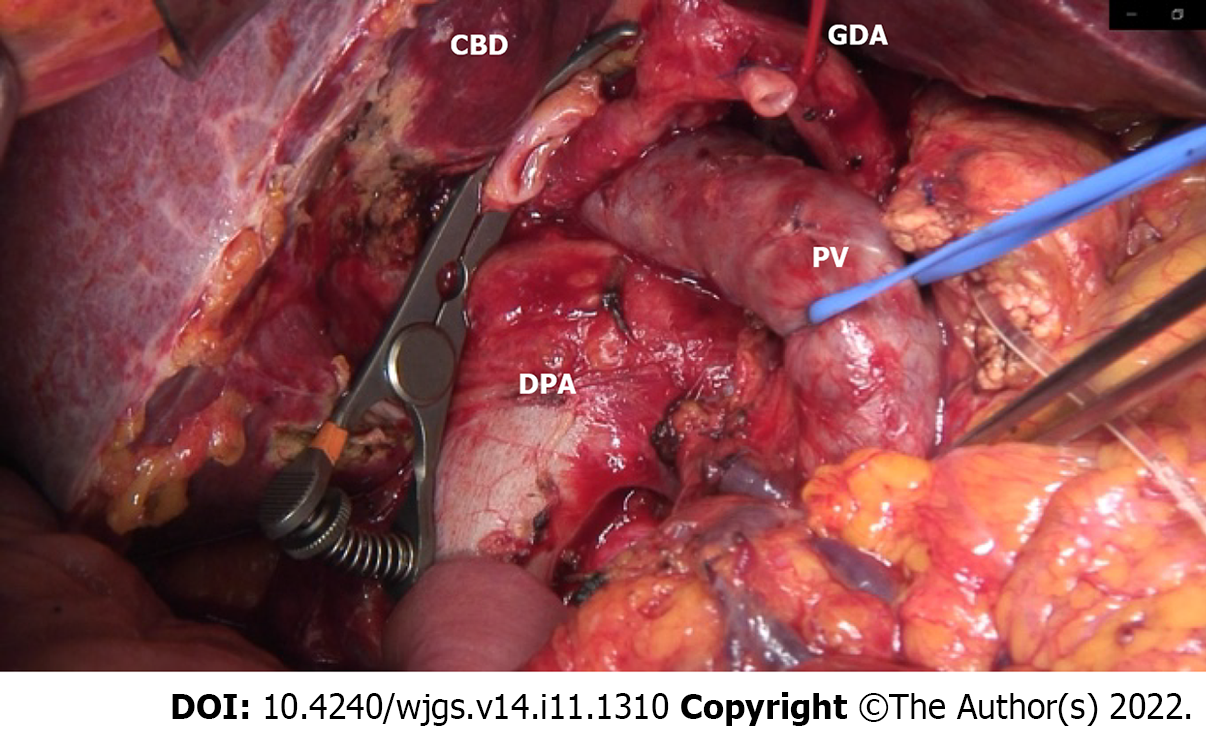
An open-approach Whipple procedure with D2 lymphadenectomy was performed in August 2019 (10 d after the TAE). During lymph node dissection around the hepatoduodenal ligament, the collateral artery from the DPA to RHA was successfully preserved.
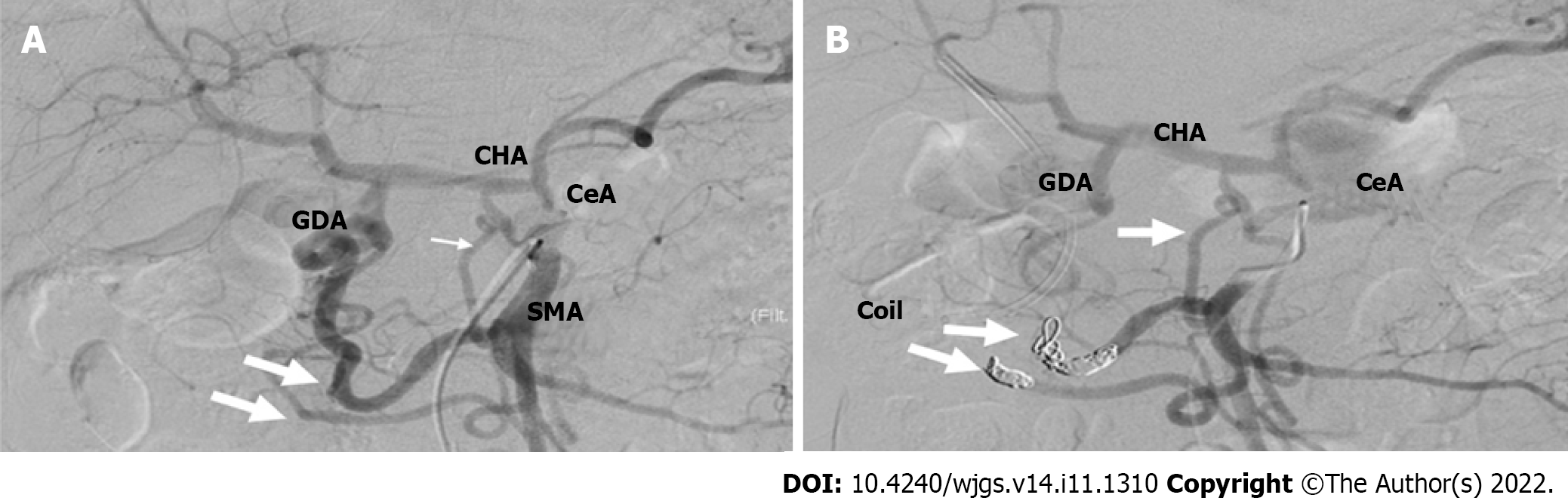
To reduce this risk, we decided to embolize the arterial arcades of the pancreatic head using a coil to increase the blood flow from the DPA to the CHA preoperatively. After embolization, angiography and CT confirmed blood re-flow from the DPA through the entire celiac arterial system, and no radiological signs of parenchyma or bowel ischemia were found. A standard open-approach Whipple procedure with D2 Lymphadenectomy was performed in September 2019 (10 d after the TAE). During the operation, the DPA was identified and preserved (Figure 5).
Postoperatively, the patient developed blue toe syndrome and delayed gastric emptying but did not show any major surgical complications, such as biliary or pancreatic fistula; additionally, there was no clinical, biochemical, or radiological evidence of ischemic disease. The patient was discharged on postoperative day (POD) 56. The patient was not followed up; hence, no evidence of recurrence or delayed complications was obtained on the scheduled 6-mo CT scan.
Postoperatively, the patient developed pneumonia, pulmonary embolism, and delayed gastric emptying but did not show any major surgical complications and was discharged on POD 41. No evidence of recurrence was observed at the 3-year follow-up.
Stenosis of the celiac trunk is a frequent occlusive vascular disease that can be observed in 2%-11% of patients who undergo PD[2-5]. Occlusion of the celiac axis is a rare situation encountered in 1%-3% of patients and is associated with a higher risk of ischemic consequences on the liver and both hepaticojejunal and pancreatico-jejunal anastomoses[6].
The main causes of stenosis or total occlusion of the celiac trunk are compression by the medial arcuate ligament (MAL) followed by atherosclerosis, which accounts for nearly 90% of the causes, including aortic dissection, congenital causes, inflammatory disease, invasion of malignancy, and iatrogenicity[7].
The typical symptoms of celiac trunk stenosis include postprandial abdominal pain, nausea, vomiting, and weight loss; nevertheless, clinically significant ischemic disease is rarely encountered owing to the development of rich collateral vessels from the SMA[8]. The diagnosis of CeA stenosis can be easily accessed through CT and arteriography, with a detection rate of 91.5%[4]; evaluation of all the collateral pathways is essential for any preoperative planning. When considering PD, any anatomical variant that may affect surgical planning should be evaluated using CT along with 3D reconstruction[9] and, in selective cases, angiographic studies. However, most surgeons do not routinely perform angiographic studies, and while preoperative imaging is often diagnostic, if an angiographic study is not performed in case of doubt, vascular sufficiency can be assessed intraoperatively.
Bull et al[3] endorsed a well-known maneuver that tested the pulsation of the hepatic artery before GDA ligation to ensure adequate patency of the collateral pathways. GDA ligation at its origin is an essential step during PD, and in the event of celiac trunk stenosis, the procedure can lead to ischemia of the liver, stomach, spleen, and residual pancreas, resulting in complications such as organ failure, abscess, and anastomotic leakage[4,5]. Berney et al[9] reported two cases of postoperative transient ischemic liver dysfunction and two cases of disruption of the pancreatic jejunal anastomosis in patients with CeA stenosis without preoperative or intraoperative revascularization procedures. Gaujoux et al[4] reported 545 patients who underwent PD and identified 23 CeA compressions by MAL, 2 CeA, and 2 SMA atherosclerotic stenoses. After PD, ischemic complications accounted for one-third of the 2.6% postoperative mortality, suggesting that ischemic complications account for significant morbidity and mortality after PD. Zhou et al[10] retrospectively analyzed the risk for biliary fistula in 508 patients who underwent PD, of which 84 had CeA stenosis due to atherosclerotic disease. The incidence of biliary fistulas was 2.1% in patients with mild CeA stenosis (1/47) and 27% in those with severe stenosis.
Different options have been proposed to treat CeA stenosis encountered during PD, majorly depending on the underlying disease. In cases of extrinsic compression caused by MAL, division of the median arcuate ligament can be performed intraoperatively[11]. In cases of atherosclerotic stenosis, percutaneous transcatheter angioplasty or stenting can be performed[12]. In cases of severe stenosis or complete occlusion, percutaneous revascularization with angioplasty or stenting of the CeA was not possible. Therefore, endovascular treatment via arcades of the pancreatic head[13] or surgical treatment with vascular reconstruction should be considered. Several surgical revascularization methods have been reported in the literature: Bypass grafting using autologous or prosthetics graft[14,15], end-to-end or end-to-side arterial anastomosis method[14-16] (Table 1), etc. Vascular reconstruction with PD increases the risk of thromboembolism and postoperative bleeding (caused by pancreatic fistula) and carries an intrinsic risk of thrombosis and leakage of the vascular anastomosis.
In our study, we treated two elderly patients with severe comorbidities and atherosclerotic diseases. In such cases, arterial bypass could be technically difficult because of diffuse atherosclerotic disease, and the risk of thrombosis and leakage of vascular anastomoses could be very high.
Considering the patients’ advanced age and risk of comorbidities as well as to avoid the risk of fatal ischemic complications after PD, we decided to embolize the arterial arcades of the pancreatic head before the operation to increase the blood flow from the SMA to the celiac arterial system.
This expedient minimizes the risk of fatal ischemic complications after PD, ensuring that the entire collateral pathway and hypertrophy of the collateral vessels are preserved during the operation. We observed delayed gastric emptying, which was correlated with slight transient gastric ischemia. Repetitive postoperative CT showed strong hypertrophy of the collateral vessels, with good blood flow of the celiac arterial system and no vascular complications.
In elderly patients with severe stenosis or complete occlusion who are not eligible for interventional revascularization or surgical reconstruction, this technique is simple, feasible, repeatable in case of failure, and less prone to complications than any previous vascular reconstruction method. However, the use of CT and angiographic studies to evaluate vascular anatomy and eventually plan blood flow modification prior to the surgical procedure is crucial.
| Ref. | Number of cases | Age, yr | Disease | Degree of CA stenosis | Treatment | Time between vascular reconstruction and surgery | Outcome | Discharged POD |
| Berney et al[9], 1999 | 13 | 69 median ages | Pancreatic adenocarcinoma | 9 occlusion, 5 subtotal stenosis, 1 partial stenosis | 2 GDA preservation | IO | Pancreatic fistula | N/A |
| Pancreatic adenocarcinoma | 1 aortohepatic bypass | IO | No complications | |||||
| 2 Pancreatic adenocarcinoma, 5 chronic pancreatitis, 1 ampullary cancer, 1 duodenal adenoma | 9 no reconstruction | IO | 2 liver ischemia3 pancreatic fistulas | |||||
| Pancreatic adenocarcinoma | 1 CeA reimplantation | IO | No complications | |||||
| Nara et al[2], 2005 | 2 | 57 | Duodenal cancer | Occlusion | Middle colic right gastroepiploic anastomosis | IO | Transient liver ischemia | 35 d |
| 61 | Duodenal cancer | Occlusion | Preservation of replaced RHA | IO | Pancreatic fistula | 128 d | ||
| Hayashibe et al[17], 2005 | 1 | 75 | Duodenal cancer | Occlusion | Aorta-CHA venous bypass | IO | No complications | 35 d |
| Halazun et al[16], 2006 | 1 | 65 | CCA | 50% | Preoperative stent | 1 d | No complications | N/A |
| Soonawalla et al[5], 2007 | 1 | 60 | Pancreatic adenocarcinoma | Occlusion | CeA reimplantation into aorta | IO | No complications | N/A |
| Smith et al[18], 2007 | 10 | 76 median ages (73-86) | Adenocarcinoma, ampullary tumor, islet cell tumor, papillary tumor | 3 pt 30%; 1 pt 20%; 2 pt 50%; 1 pt 25%; 2 pt 60%; 1 pt occlusion | 10 no reconstruction | IO | 1 death for MOF, 1 GI bleeding | N/A |
| Gaujoux et al[4], 2009 | 3 | 72 | Malignant ampulloma | N/A | Aortohepatic bypass | IO | Pancreatic fistula | N/A |
| 59 | Pancreatic adenocarcinoma | N/A | Preoperative CeA stent | 3 wk | Pancreatic fistula | N/A | ||
| 77 | Malignant ampulloma | N/A | Postoperative CeA stent | 0 POD | Pancreatic fistula | N/A | ||
| El-Ghazaly et al[19], 2009 | 1 | 70 | Pancreatic adenocarcinoma | Occlusion | Anterior pancreaticoduodenal arcade resected and anastomosed end-to-end | IO | No complications | 10 d |
| Berselli et al[20], 2010 | 1 | 72 | Branch type IPMN | Occlusion | Side to side anastomosis SPPDA to the IPPDA | IO | Pancreatic fistula, GI bleeding, Splenic artery pseudoaneurysm | 97 d |
| Yi et al[21], 2014 | 1 | 51 | NET | N/A | Preoperative angioplasty and stent of CA | 1 mo | No complications | 7 d |
| Beane et al[22], 2017 | 1 | 69 | Pancreatic cancer | Occlusion | SMA to HA venous bypass | IO | Pancreatic fistula, GI bleeding, pseudoaneurysm of graft; hepatic abscess (4 mo after discharge) | 30 d |
| Zhou et al[10], 2018 | 84 | 73 median ages (61-88) | N/A | 47 pt (mild 1%-49%); 37 pt (substantial 50%-99%) | No treatment | IO | 2 deaths, 2 GI bleeding, 11 biliary fistulas, 8 pancreatic fistulas | 21 d median (8-21 POD) |
| Tagkalos et al[23], 2018 | 1 | 64 | Pancreatic adenocarcinoma | Occlusion (postoperative diagnosis) | Heparinization | 3 POD | Transient liver ischemia | 14 d |
| Oikawa et al[7], 2022 | 1 | 80 | CCA | Occlusion | No reconstruction | IO | Pancreatic fistula | 41 d |
In elderly patients with celiac trunk occlusion, PD can lead to a severe risk of postoperative complications, and preoperative blood circulation modification can reduce the risk of ischemic accidents. Precise preoperative anatomical studies of the vascular pathway and an optimal surgical or radiological technique must be chosen on a case-by-case basis to avoid unfavorable postoperative outcomes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chiu CC, Taiwan; Zhao K, China S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YL
| 1. | Sugae T, Fujii T, Kodera Y, Kanzaki A, Yamamura K, Yamada S, Sugimoto H, Nomoto S, Takeda S, Nakao A. Classification of the celiac axis stenosis owing to median arcuate ligament compression, based on severity of the stenosis with subsequent proposals for management during pancreatoduodenectomy. Surgery. 2012;151:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Nara S, Sakamoto Y, Shimada K, Sano T, Kosuge T, Takahashi Y, Onaya H, Yamamoto J. Arterial reconstruction during pancreatoduodenectomy in patients with celiac axis stenosis--utility of Doppler ultrasonography. World J Surg. 2005;29:885-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Bull DA, Hunter GC, Crabtree TG, Bernhard VM, Putnam CW. Hepatic ischemia, caused by celiac axis compression, complicating pancreaticoduodenectomy. Ann Surg. 1993;217:244-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Gaujoux S, Sauvanet A, Vullierme MP, Cortes A, Dokmak S, Sibert A, Vilgrain V, Belghiti J. Ischemic complications after pancreaticoduodenectomy: incidence, prevention, and management. Ann Surg. 2009;249:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 5. | Soonawalla Z, Ganeshan A, Friend P. Celiac artery occlusion encountered during pancreatic resection: a case report. Ann R Coll Surg Engl. 2007;89:W15-W17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Sakorafas GH, Sarr MG, Peros G. Celiac artery stenosis: an underappreciated and unpleasant surprise in patients undergoing pancreaticoduodenectomy. J Am Coll Surg. 2008;206:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Oikawa R, Ito K, Takemura N, Mihara F, Shida Y, Tajima T, Kokudo N. Risk Factors of Atherosclerotic Celiac Artery Stenosis Among Patients Undergoing Pancreaticoduodenectomy. Pancreas. 2022;51:e15-e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Wakabayashi T, Felli E, Pessaux P. Celiac axis stenosis caused by median arcuate ligament in pancreaticoduodenectomy. J Pancreas 2019; 20: 101-102. |
| 9. | Berney T, Prêtre R, Chassot G, Morel P. [Ischemic risk in the case of celiac trunk occlusion in patients undergoing pancreatodeodenectomy. Multicenter study and review of literature]. Ann Chir. 1999;53:273-279. [PubMed] |
| 10. | Zhou Y, Wang W, Shi Y, Lu X, Zhan Q, Chen H, Deng X, Peng C, Shen B. Substantial atherosclerotic celiac axis stenosis is a new risk factor for biliary fistula after pancreaticoduodenectomy. Int J Surg. 2018;49:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Kurosaki I, Hatakeyama K, Nihei K, Oyamatsu M. Celiac axis stenosis in pancreaticoduodenectomy. J HepatoBil Pancreat Surg 2004; 11: 119-124. [RCA] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Ikeda O, Tamura Y, Nakasone Y, Yamashita Y. Celiac artery stenosis/occlusion treated by interventional radiology. Eur J Radiol. 2009;71:369-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Farma JM, Hoffman JP. Nonneoplastic celiac axis occlusion in patients undergoing pancreaticoduodenectomy. Am J Surg. 2007;193:341-4; discussion 344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Okamoto H, Suminaga Y, Toyama N, Konishi F, Kawahito H. Autogenous vein graft from iliac artery to splenic artery for celiac occlusion in pancreaticoduodenectomy. J Hepatobiliary Pancreat Surg. 2003;10:109-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Portolani N, Tiberio GA, Coniglio A, Baiocchi G, Vettoretto N, Giulini SM. Emergency celiac revascularization for supramesocolic ischemia during pancreaticoduodenectomy: report of a case. Surg Today. 2004;34:616-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Halazun KJ, Kotru A, Menon KV, Patel J, Prasad KR. Stenting of coeliac axis stenosis facilitates pancreatectomy. Eur J Surg Oncol. 2006;32:811-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | 17 Hayashibe A, Sakamoto K, Shinbo M, Makimoto S, Nakamoto T, Higashiue S, Toyonaga T. A resected case of advanced duodenal carcinoma with occlusion of the celiac artery. J Surg Oncol. 2005;91:270-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Smith SL, Rae D, Sinclair M, Satyadas T. Does moderate celiac axis stenosis identified on preoperative multidetector computed tomographic angiography predict an increased risk of complications after pancreaticoduodenectomy for malignant pancreatic tumors? Pancreas. 2007;34:80-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | El-Ghazaly Harb ST, O'Sullivan AW, Marangoni G, Heaton ND, Rela M. Managing arterial collaterals due to coeliac axis stenosis during pancreaticoduodenectomy. JOP. 2009;10:547-549. [PubMed] |
| 20. | Berselli M, Sperti C, Ballotta E, Beltrame V, Pedrazzoli S. Pancreaticoduodenectomy with unusual artery reconstruction in a patient with celiac axis occlusion: report of a case. Updates Surg. 2010;62:117-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Yi HT, Lee WL, Liu TJ, Ting CT, Su CS. Endovascular Angioplasty of Celiac Axis Obstruction Prior to Pancreaticoduodenectomy in a Patient with Pancreatic Neuroendocrine Carcinoma. Acta Cardiol Sin. 2014;30:165-168. [PubMed] |
| 22. | Beane JD, Schwarz RE. Vascular challenges from pancreatoduodenectomy in the setting of coeliac artery stenosis. BMJ Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Tagkalos E, Jungmann F, Lang H, Heinrich S. One visceral artery may be enough; successful pancreatectomy in a patient with total occlusion of the celiac and superior mesenteric arteries. BMC Surg. 2018;18:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |