Published online Nov 27, 2022. doi: 10.4240/wjgs.v14.i11.1285
Peer-review started: July 25, 2022
First decision: September 4, 2022
Revised: September 16, 2022
Accepted: October 31, 2022
Article in press: October 31, 2022
Published online: November 27, 2022
Processing time: 122 Days and 19.9 Hours
Overlapped esophagojejunostomy (OEJ) is a secure purely laparoscopic reconstruction after laparoscopic total gastrectomy (LTG). However, long-term surgical results have not been documented well.
In this paper, we report unusual patients who manifested jejunal limb stricture near the esophageal hiatus without anastomotic stenosis during long-term observation after surgery.
From April 2009 until May 2020, we retrospectively reviewed 211 patients underwent LTG following by OEJ for gastric carcinoma and took a standard surveillance program. We aimed to characterize a novel complicated disorder observed in these patients to assist treatment and prevention.
Five patients (2.4%) had unusual jejunal limb stricture after LTG and OEJ, occurring at a mean of 10 mo after initial radical LTG. All five patients had dis
Disturbed passage through the jejunal limb near the hiatus can occur after some types of OEJ following LTG. We speculate that it may result from a short remnant esophagus, excessive mobilization of the jejunal limb that permits bending or tortuosity and adhesions on the left crus at the hiatus. Prevention for this complication is possible during the original LTG procedure.
Core Tip: Overlapped esophagojejunostomy (OEJ) is a secure purely laparoscopic reconstruction after laparoscopic total gastrectomy (LTG). However, disturbed passage through the jejunal limb near the esophageal hiatus can occur. In this paper, mechanisms and prevention for this complication are described. Five patients (2.4%) had disturbed oral intake and marked weight loss, all had unusual jejunal limb stricture after LTG and OEJ. Reoperation for adhesiolysis and division of the left crus and rearrangement of the jejunal limb was required. Prevention for this complication is possible during the original LTG procedure.
- Citation: Noshiro H, Okuyama K, Yoda Y. Disturbed passage of jejunal limb near esophageal hiatus after overlapped esophagojejunostomy following laparoscopic total gastrectomy. World J Gastrointest Surg 2022; 14(11): 1285-1296
- URL: https://www.wjgnet.com/1948-9366/full/v14/i11/1285.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i11.1285
Since the development of safe and feasible intracorporeal anastomosis under laparoscopy[1,2], purely laparoscopic total gastrectomy (LTG) has been widely used to treat gastric carcinoma occupying the upper third of the stomach[3-7]. Overlapped esophagojejunostomy (OEJ) is a secure purely laparoscopic side-to-side reconstruction method that uses an endoscopic linear stapler after LTG[2]. Very few cases of postoperative anastomotic leakage or stenosis have been reported in patients treated with OEJ after LTG[8,9]. In addition, this technique is applicable even to patients treated by LTG with long esophageal excision[10]. However, we have experienced five unusual cases of jejunal limb stricture near the esophageal hiatus without anastomotic stenosis during long-term observation after LTG with OEJ. All five patients required reoperation for this complication. In this report, we sequentially analyzed these five patients and describe the characteristics of this complication to assist treatment and prevention.
This study was approved by the Institutional Review Board of Saga University Hospital (2020-07-06). All data and clinical findings were obtained retrospectively from the medical charts and videos, which were stored in our department library.
Since April 2009, all patients with curable gastric carcinoma at Saga University Hospital have been treated basically with laparoscopic surgery. Open surgery was performed in one patient for systemic para-aortic lymphadenectomy and in four patients who underwent other open surgery concomitantly. From April 2009 until May 2020, 925 patients underwent laparoscopic gastrectomy with at least 5 years of follow-up. During the study period, no patient had conversion to open surgery and only four patients missed postoperative surveillance appointments. Among the 925 patients, six had intrathoracic OEJ through a thoracoscopic approach because they required excision of over 4 cm of the esophagus. In one patient who underwent proximal gastrectomy, OEJ was performed as a part of double-tract reconstruction. After exclusion of these seven patients, 211 patients who underwent LTG following by OEJ for reconstruction of the alimentary tract were enrolled in this study.
To provide information that allows speculation about the mechanisms of the complication, we summarize the detailed surgical procedures below. Five abdominal ports were placed during robotic surgery and laparoscopic surgery. Lymph node dissection extended to the stations along the hepatic, splenic and celiac arteries[11], and splenectomy was occasionally performed for complete dissection around the splenic hilum[12]. The esophago-cardiac branch of the subphrenic artery was divided, but the main artery was generally preserved. The esophageal hiatus was enlarged to a variable extent in the ventral direction in the tendinous portion of the diaphragm for the subsequent OEJ procedure. When further enlargement of the hiatus was requested for operative views and procedures, division of the left crus of the diaphragm was added. We intentionally transected the isolated esophagus vertically, using an endoscopic linear stapler, to create the OEJ on the posterior side on the esophageal stump. However, the transection often seemed to be horizontal after division. The jejunum was transected with a stapler 15 cm to 20 cm from the ligament of Treitz, and the mesentery was divided up to the bifurcation of the jejunal arteries and veins. If the approximation between the jejunal limb and the esophageal stump needed improvement, a combined jejunal artery and vein were divided after a clamp test to confirm blood supply. The jejunal limb was raised through the antecolic route as the first choice; the retrocolic route was used when the antecolic route was not possible. A small hole for insertion of the stapler fork was created on the posterior portion of the esophageal stump when the esophagus was transected vertically. Otherwise, the right portion was selected in most patients because of facilitation of the procedures and proper arrangement of the jejunal limb. A small enterotomy was also created on the antimesenteric side of the jejunal limb 45 mm from the stump. A 45-mm endoscopic linear stapler was used to create an overlapped side-to-side anastomosis. The stapling device for creation of the anastomosis was commonly introduced through the left abdominal port by the assistant’s left hand. After adjusting, approximating and firing of the linear stapler, the entry hole was closed with continuous hand-sewing with absorbable 4-0 monofilament suture so that the V-shaped anastomosis was maximally widened. Barbed 3-0 suture was often available in more recent procedures[13]. The esophagus was generally not fixed at the hiatus. The jejunal limb was rarely fixed to the hiatus or to the other structures unless arrangement of the limb looked tortuous. When the jejunal limb passed via the retrocolic route, it was always fixed to the transverse mesocolon with a couple of nonabsorbable sutures. Prevention of Petersen’s internal hernia was carefully performed with nonabsorbable sutures. A drainage tube was placed when there were concerns about anastomotic leakage, massive accumulation of lymphorrhea or pancreatic fistula.
Postoperative management after LTG was carried out according to the regular critical care protocol. Patient without postoperative complications usually left the hospital on POD 10 to 14. After discharge, patients who were diagnosed with pathological stage II or higher received adjuvant chemotherapy[14]. Postoperative surveillance was performed every 2 to 3 mo for patients with advanced-stage gastric cancer and every 6 to 12 mo for patients with early-stage cancer, for at least 5 years after surgery. During the observation period, body weight measurement, blood sampling and computed tomography (CT) examination were routinely performed. Endoscopic examination or upper gastrointestinal X-ray series was added for patients with any unusual complaints.
Among the 211 patients who underwent LTG following by OEJ, the mean age was 69 years (range 25–88 years), and the female-to-male ratio was 42:169. The clinical stages according to the 8th edition of the TNM classification system[15] were as follows: 94 patients were stage I, 46 were stage II, 55 were stage III and 16 were stage IV. Five patients (2.4%) had unusual jejunal limb stricture after LTG and OEJ. The characteristics of these five patients at the first radical LTG are listed in Table 1. The group included one woman and four men. The age range at first LTG was 65 to 80 years. Three patients had gastric carcinoma located in the upper stomach, and one had Siewert type III esophagogastric junctional carcinoma invading 1 cm of the esophagus. The fifth patient had remnant gastric carcinoma after open distal gastrectomy and Billroth I reconstruction 13 years prior to LTG. The clinical depth of invasion was T1 in three patients and T2 in two patients; all patients were diagnosed as free from lymph node metastasis preoperatively, corresponding to clinical stage I in all patients. Therefore, none of the patients was treated with neoadjuvant chemotherapy. After pathological examination of the excised stomach specimens, one patient was diagnosed with pathological T3 and two had lymph node metastasis. One patient diagnosed with pathological stage IIB with T3 and N1 disease was treated with oral adjuvant chemotherapy for 1 year.
| Case | 1 | 2 | 3 | 4 | 5 |
| Sex | M | M | M | M | F |
| Age | 69 | 67 | 80 | 74 | 65 |
| Original disease | |||||
| Location | Upper | Remnant stomach | Upper | EGJ | Upper |
| Histological type | Well | Well | Moderately | Well | Poorly |
| Clinical Stage1 | I | I | I | I | I |
| Pathological Stage1 | IA | IA | IIB | IA | IB |
| Neoadjuvant chemotherapy | - | - | - | - | - |
| Adjuvant chemotherapy | - | - | + | - | - |
| Type of gastrectomy | Total | Total (Complete) | Total | Total | Total |
| Approach | Laparoscopic | Laparoscopic | Laparoscopic | Laparoscopic | Robotic |
| Operation time (min) | 336 | 475 | 339 | 438 | 368 |
| Blood loss (mL) | 130 | 76 | 93 | 35 | 34 |
| Splenectomy | - | - | - | - | - |
| Lymph node dissection | D2 | D2 | D2 | D2 | D2 |
| Length of excised esophagus (cm) | 1.5 | 1.5 | 1.5 | 3.5 | 2.0 |
| Direction of esophageal transection | Horizontal | Vertical | Horizontal | Horizontal | Horizontal |
| Site of esophagostomy | Right | Posterior | Right | Right | Right |
| Enlarged hiatus | Large | Large | Small | Large | Small |
| Direction | Ventral | Ventral | Ventral | Ventral | Ventral |
| Closure of enlarged hiatus | - | - | - | - | - |
| Route of jejunum | Antecolic | Retrocolic | Antecolic | Retrocolic | Antecolic |
| Insertion of stapler | Left | Left | Left | Left | Left |
| Fixation of esophagus | - | - | - | - | - |
| Fixation of jejunum | - | - | - | + | - |
| Anastomosis site (common channel level) | at hiatus | above hiatus | below hiatus | above hiatus | below hiatus |
| Drainage | + | + | + | + | + |
| Resume of oral intake | 3 | 1 | 3 | 4 | 3 |
| Length of hospital stay | 11 | 49 | 12 | 17 | 19 |
| Postoperative complications | - | - | - | - | - |
A summary of the initial radical LTG in the five patients is shown in Table 1. All five patients had LTG without splenectomy; four patients were treated with laparoscopic surgery and one with robotic surgery. The mean length of excised esophagus was 2.0 cm (range 1.5–3.5 cm). The direction of the esophageal transection was horizontal in four patients and vertical in one. In the four patients with horizontal transection, the entry hole was created on the right side of the esophageal stump. The esophageal hiatus was slightly enlarged toward the ventral side in the tendinous portion of the diaphragm in two patients and was greatly enlarged in the same portion in three patients. After the reconstructive procedures, the enlarged hiatus was not closed in any patient. The jejunal limb was raised to the esophageal stump via the antecolic route in three patients and via the retrocolic route in two patients. In all patients, a stapling device was introduced through the left abdominal port as usual for the anastomosis. At surgery, the level of the closed entry hole was above the hiatus in two patients, at the hiatus in one patient and below the hiatus in two patients. No patient had fixation of the esophagus to the hiatus. One patient whose anastomotic level was high had fixation of the jejunal limb using absorbable sutures around the hiatus to achieve proper positioning. A drainage tube was placed in all patients.
None of the five patients had abnormal findings on postoperative upper gastrointestinal X-ray series with contrast medium at the first admission for LTG. Four of the five patients had a typical postoperative clinical course and were discharged from the hospital. The patient who was treated for remnant gastric cancer had persistent anorexia resulting from a feeling of abdominal fullness and had a prolonged hospital stay.
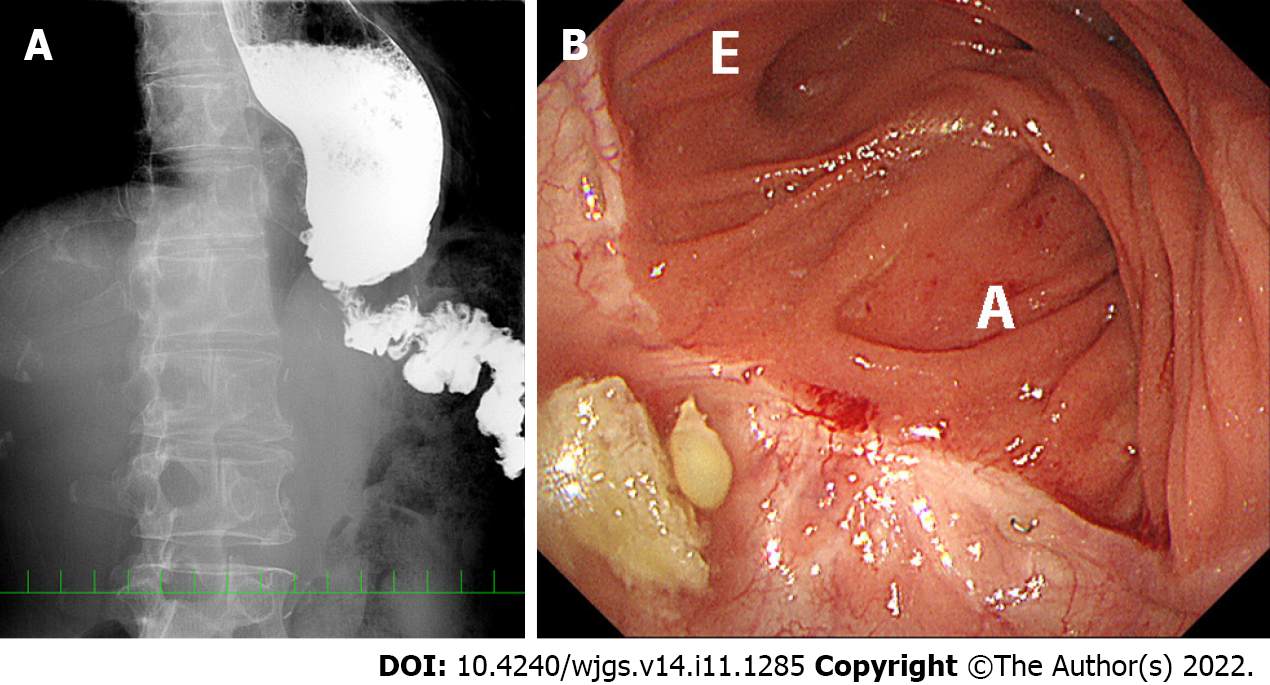
Severe symptoms developed within nine months after LTG in all patients. After a mean interval of 10 mo (range 5–21 mo), the five patients underwent reoperation to treat ongoing complications. Clinical findings and surgical procedures for reoperation are summarized in Table 2. All patients had disturbed oral intake. X-ray examination showed poor passage of contrast medium at the hiatus, jejunal stenosis (approximately 1 cm in length) at the hiatus and dilatation of the distal esophagus (Figure 1A). Bending of the jejunal limb was also suggested or suspected in all patients. Body weight decreased markedly after the first LTG in all patients (Table 3). The mean weight loss was 26% (range 14%–33%) at the time of reoperation. Two patients experienced aspiration pneumonia, which was confirmed with CT examination. In all five patients, endoscopy showed intact anastomosis, but the jejunal limb was bent and sometimes seemed to be tortuous (Figure 1B). However, the 1-cm diameter endoscope could be passed through the bent portion in all patients. Endoscopic balloon dilatation was tried in all cases but did not achieve permanent results. No cancer recurrence was observed in any patient in any of several diagnostic modalities.
| Case | 1 | 2 | 3 | 4 | 5 |
| Age | 69 | 67 | 80 | 76 | 66 |
| Interval from the 1st operation (mo) | 7 | 9 | 8 | 5 | 21 |
| Preoperative endoscopy | |||||
| Anastomotic stricture | - | - | - | - | - |
| Recurrence | - | - | - | - | - |
| Efferent scope passage | + | + | + | + | + |
| Bending or tortuous | + | + | + | + | + |
| Preoperative UGI series | |||||
| Esophagus dilatation | + | + | + | + | + |
| Length of jejunal stricture | 1.5 cm | 0.8 cm | occluded | 1.0 cm | 1.0 cm |
| Bending or tortuous | + | + | + | + | + |
| Preoperative CT | |||||
| Recurrence | - | - | - | - | - |
| Pneumonia | + | - | - | + | - |
| Approach | Laparoscopic | Laparoscopic | Laparoscopic | Laparoscopic | Laparoscopic |
| Operation time (min) | 66 | 255 | 118 | 203 | 113 |
| Blood loss (mL) | 3 | 223 | 45 | 36 | 10 |
| Number of ports | 4 | 5 | 5 | 5 | 5 |
| Adhesiolysis | + | ++ | + | + | + |
| Intraoperative endoscopy | + | + | + | -1 | + |
| Fixation of jejunum | - | + | + | + | + |
| Left crus cutting | + | + | + | + | + |
| Resume of oral intake (POD) | 1 | 3 | 2 | 2 | 1 |
| Length of hospital stay (d) | 5 | 7 | 22 | 9 | 6 |
| Postoperative complications | - | - | - | - | - |
| Case | 1 | 2 | 3 | 4 | 5 | mean |
| Height (cm) | 169.4 | 164.0 | 171.5 | 154.7 | 145.2 | 161.0 |
| BW before the first LTG (kg) | 66.3 | 71.3 | 65.1 | 57.5 | 54.8 | 63.0 |
| BWLR at the discharge of the first LTG (%) | -8.0% | -7.0% | -4.6% | -4.2% | -6.9% | -6.1% |
| BWLR before the reoperation (%) | -25.3% | -25.5% | -13.8% | -33.0% | -32.3% | -26.0% |
| BWLR at the discharge of the reoperation (%) | -24.0% | -23.6% | -13.2% | -28.9% | -32.5% | -24.4% |
| Maximal BWLR from the reoperation (%) | -17.0% | -23.6% | -5.7% | -21.7% | -21.5% | -17.9% |
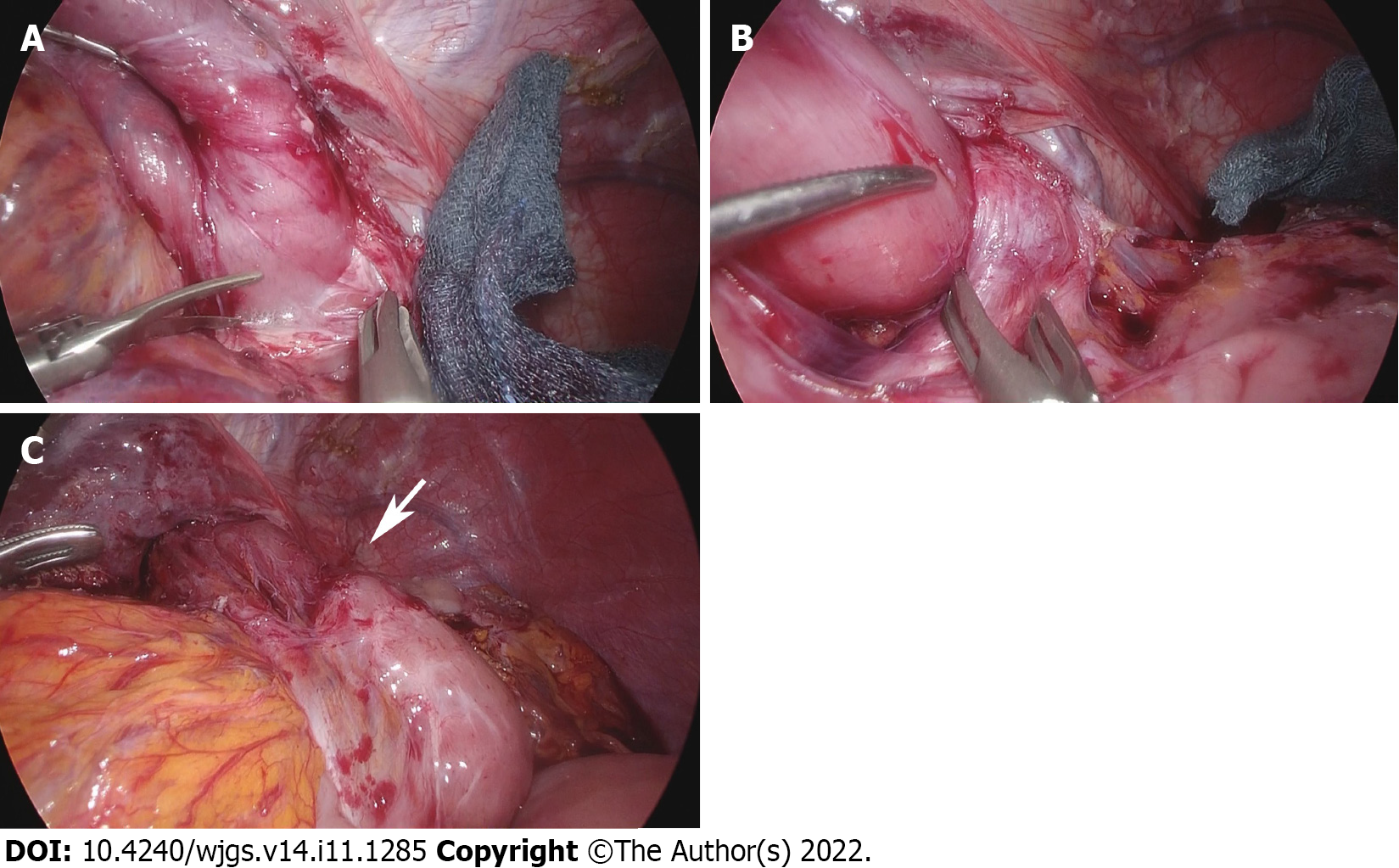
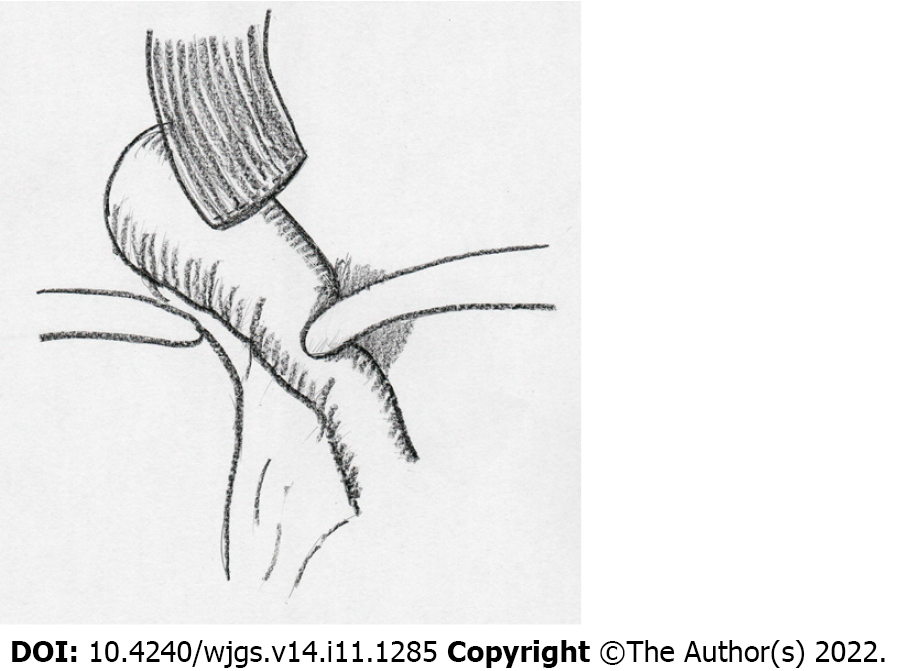
Laparoscopic adhesiolysis around the hiatus was planned. Adhesiolysis was performed via the previous five port sites, except for one patient who could be treated via four ports. In all patients, adhesions between the liver and suprapancreatic portion were very strong, and adhesiolysis at the esophageal stump was also challenging because of scar formation at the staple line. However, adhesions at other locations were released easily in four patients, who had all undergone previous surgery by laparoscope. In the one patient who had undergone prior open distal gastrectomy before LTG, adhesiolysis was challenging in the upper abdominal cavity. However, adhesions were mild around the hiatus, which had been newly manipulated during LTG. On the left crus of the diaphragm, the jejunal limb was bent and had fibrous adhesions (Figure 2A and B). The anastomosis was elevated far above the hiatus after adhesiolysis up to the level of anastomosis in all cases. The jejunal limb above the hiatus was slightly shifted to the left side in the mediastinum. As shown in Figure 3, a schema based on these findings, the jejunal limb stricture resulted from the shortened remnant esophagus and jejunal bending resulted from loose and fibrous adhesions on the left crus at the esophageal hiatus. This was confirmed by the presence of a pressure mark on the jejunum after completion of adhesiolysis (Figure 2C). During reoperation, the hiatus was enlarged by dividing the left crus in all patients to obtain a better operative view and to prevent jejunal stricture. The jejunal limb was fixed to the right side of the hiatus or other abdominal structures to achieve a straight line after intraoperative endoscopic luminal examination (Figure 4A).
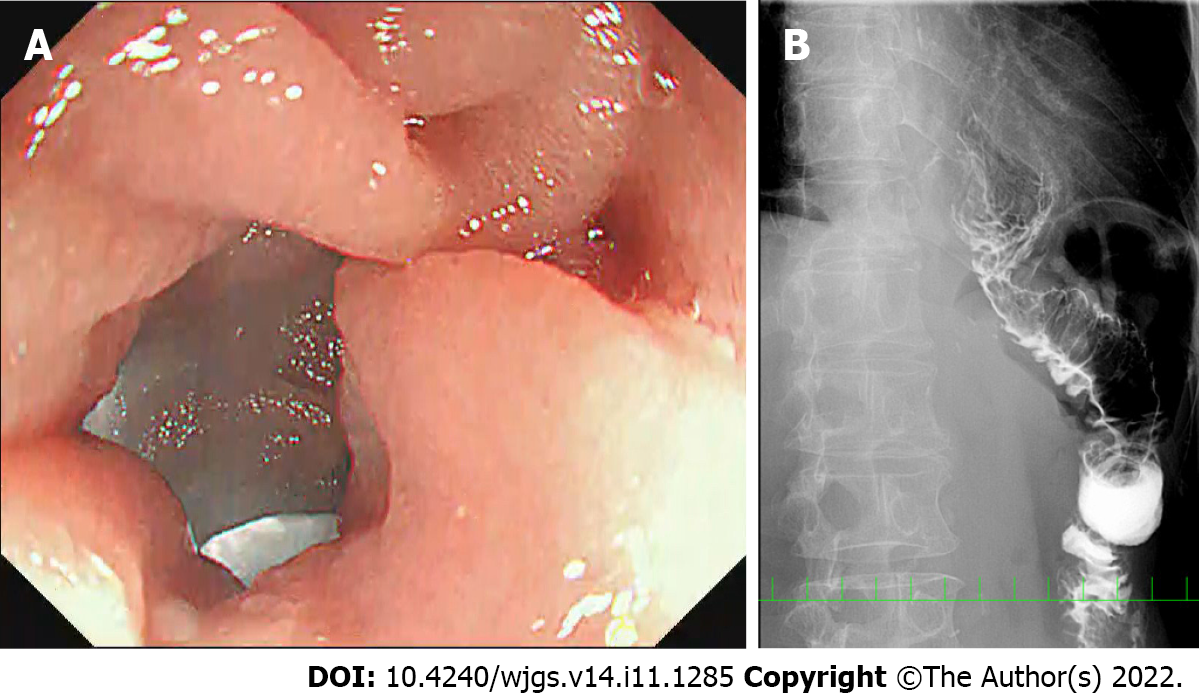
Postoperative X-ray examinations after reoperation showed no disturbed passage or bending of the jejunal limb in any of the five patients (Figure 4B). There were no operative morbidities after the reoperation for adhesiolysis. All patients gained body weight after the reoperation (Table 3). After a mean duration of 47 mo (range 11–82 mo) after reoperation for adhesiolysis, all patients were well and had usual oral intake.
In this report, we describe unusual disturbed passage through the jejunal limb near the esophageal hiatus that occurred in five patients (2.4%) after purely LTG following by OEJ. This serious complication did not result from anastomotic stenosis after alimentary tract reconstruction or from hiatal stenosis caused by scar formation; the jejunal limb stenosis was caused by a shortened remnant esophagus and excessive mobilization of the jejunal limb, which produced bending or tortuosity and loose fibrous adhesions on the left crus at the hiatus. Because balloon dilatation did not successfully resolve this disorder, surgical treatment for adhesiolysis, division of the left crus and rearrangement of the jejunal limb were performed.
Anastomotic stenosis is a well-known postoperative complication after esophagojejunostomy following total gastrectomy. In early reports, Tsujimoto et al[8] summarized that the complication occurred in 0% to 3.8% of patients undergoing LTG following by OEJ; this rate was lower than that after circular-stapled anastomosis and the functional end-to-end method. In recent reports, the rate of anastomotic stenosis is similarly low, ranging from 0% to 4.6%[3,9,16-18]. However, little is known about disturbed passage of the jejunal limb near the esophageal hiatus. In our series, 2.4% of patients had this uncommon disorder. Huang et al[19] reported difficulty with solid food intake in some patients after LTG and OEJ, according to clinical queries of constituent items of pain on the European Organisation for Research and Treatment of Cancer 30-item Core Quality of Life Questionnaire (EORTC-QLQ-C30) and dysphagia on the EORTC-QLQ 22-item Stomach assessment tool. Therefore, it is possible that this postoperative change might happen to some extent in every patient after LTG and OEJ. In that case, this disorder might not be an independent category of postoperative complications. We initially thought that we encountered the five patients in whom severe symptoms developed. However, the patients’ serious complaints would not have improved without surgical treatment. Therefore, we summarize below the characteristics of this complicated disorder in our five patients to help others avoid missing the timing for reoperation.
First, severe symptoms developed in the relatively early period after LTG. Next, oral intake was seriously disturbed and weight loss was severe. Aspiration pneumonia developed in some patients. Disturbed oral intake could be verified by X-ray examination, which showed poor passage of contrast medium resulting from jejunal stenosis and bending of the jejunal limb near the hiatus, in addition to dilatation of the distal esophagus. No anastomotic stenosis was seen on endoscopic luminal examination. Moreover, the scope could be passed through the bent jejunal limb and endoscopic balloon dilatation was unsuccessful in permanently resolving the disorder. Finally, several cancer surveillance processes revealed no recurrence of gastric carcinoma.
We think that disturbed oral intake, continued weight loss or aspiration pneumonia suggest the need for surgical treatment of this disorder after LTG. Total gastrectomy is often associated with reduced oral intake and weight loss. Okabe et al[3] reported that the patients had lost 7.2% of initial body weight at 2 years after laparoscopic distal gastrectomy and 13.9% at 2 years after laparoscopic total gastrectomy. In our five patients, weight loss reached 26% of body weight, which was much greater compared with the 13.9% reported by Okabe et al[3]. Moreover, LTG is not directly associated with aspiration pneumonia. Two patients in our series developed aspiration pneumonia, which should be considered a serious sign for the advanced stage of this disorder. Because endoscopic balloon dilatation was unsuccessful in all patients, surgical treatment for adhesiolysis should be considered as soon as possible. We did not hesitate to perform laparoscopic surgical treatment because postoperative intraabdominal adhesions are relatively easy to release when the prior surgery has been performed by laparoscopy[20]. One patient previously had an open distal gastrectomy before LTG, which should be called laparoscopic complete gastrectomy. Even in this patient, adhesions at the newly manipulated surgical sites around the hiatus were not very strong at reoperation for adhesiolysis.
Commonly observed findings in our series enable us to speculate on the mechanisms of disturbed passage through the jejunal limb near the hiatus after LTG. Dense and tough adhesions were not observed around the esophageal hiatus, except on the staple line at the esophageal stump, even if the esophageal hiatus had been divided and enlarged. Therefore, uncommon severe scar formation was an unlikely cause of jejunal stricture near the hiatus. It is also unlikely to the drainage tube placed during LTG was responsible for these strictures. Our speculations are described below.
First, the remnant esophagus was short, and the anastomotic site was elevated at reoperation in all patients. Approximately 5 cm of the remnant esophagus had to be isolated to perform the overlapped method. A prepared and isolated esophagus easily shrinks[21]. Because the remnant esophagus was not fixed at the hiatus, the anastomotic site moved upward into the mediastinum, elevating the jejunal limb to the level of the hiatus.
Next, bending or tortuosity of the jejunal limb and adhesions on the left crus at the hiatus might play an important role. Generally, the jejunal limb is prepared so that it is easily approximated to the esophageal stump during LTG. This is because the tension is not easily assessed because of the decreased tactile sensation using laparoscopic forceps and high tension is associated with anastomotic leakage. Excessive mobilization of the raised jejunal limb might result in higher elevation of the anastomosis when the anastomosis is not fixed. Bending of the jejunal limb might occur at the hiatus because of excessive mobilization of the jejunal limb. The left crus of the diaphragm, which is a left side component of the esophageal hiatus, is commonly prepared to isolate the esophagus or dissect the left paraesophageal lymph nodes during total or proximal gastrectomy for gastric or esophagogastric junctional cancer. Therefore, this portion generally is stripped of serosa after total or proximal gastrectomy, resulting in some adhesion formation even after laparoscopic surgery. Finally, jejunal limb stricture at the hiatus might be produced through this process. As the operative findings showed, fibrous adhesions were always observed at this portion and were suspected to be responsible for bending of the jejunal limb. The jejunal stricture length of approximately 1 cm was consistent with the thickness of the crus which sometimes made a pressure mark on the jejunal limb. To prevent these conditions, the anastomosis must be fixed firmly around the hiatus; however, fixation cannot always be performed if the remnant esophagus is short. In this case, arrangement of the jejunal limb may help to avoid adhesions between the jejunal limb and the left crus that cause bending. We consider that the jejunal limb, except for the mesenteric component, should be fixed to the right side of the hiatus or other abdominal structures by a couple of stitches using nonabsorbable sutures to achieve a linear alimentary tract near the hiatus.
The direction of the esophageal transection and the anastomotic side on the esophageal stump did not account for this complication. We considered that the flexible organs would move easily with gravity to the wide left subphrenic space after total gastrectomy. Laparoscopic surgery results in few postoperative adhesions, which facilitates this movement[20]. If anastomosis is made on the left side of the esophageal stump that is transected horizontally[3,4,9,16,17,22,23], the jejunal limb will fall into the left subphrenic space after the anastomosis. The jejunal limb could then become largely tortuous unless the limb is fixed to other abdominal structures to avoid torsion. However, we previously believed that flexibility of the jejunal limb should not be disturbed by fixation to promote better peristalsis. Indeed, this concern was consistent with a previous report that jejunal elevation could cause intractable stenosis after LTG with circular-stapled esophagojejunostomy, depending on the side of the afferent loop[24].To prevent stenosis, the anastomosis should be created on the right side[8,18] or the posterior side[13,25] of the esophageal stump. In these cases, torsion will be minimal, even if the jejunal limb falls into the vacant space under the left diaphragm. We now consider it important to arrange the jejunal limb in a straight line without excessive mobilization after OEJ, regardless of where the anastomosis is created on the esophageal stump. In addition, enlargement of the hiatus by division of the left crus might be useful. In all five of our patients, the left crus was cut to arrange the jejunal limb in a straight-line during reoperation.
In conclusion, disturbed passage through the jejunal limb near the esophageal hiatus can occur in the relatively early period after OEJ following LTG, and surgical treatment for adhesiolysis, division of the left crus and rearrangement of the jejunal limb is required to treat this complication. Depending on the speculated cause of jejunal limb stricture, prevention of this complication may be possible during the original LTG procedure.
Overlapped esophagojejunostomy (OEJ) is a secure purely laparoscopic reconstruction after laparoscopic total gastrectomy (LTG). Very few cases of postoperative anastomotic leakage or stenosis have been reported in patients treated with OEJ after LTG.
We have experienced five unusual cases of jejunal limb stricture near the esophageal hiatus without anastomotic stenosis during long-term observation after LTG with OEJ.
The objectives in this paper are mechanisms and prevention for this complication are described.
From April 2009 until May 2020, 211 patients who underwent LTG following by OEJ for reconstruction of the alimentary tract were enrolled in this study.
We describe the characteristics of this complication to assist treatment and prevention.
We had experienced five cases, all patients needed reoperation. We needed to know the mechanism of this complication.
LTG was widely used for gastric carcinoma. OEJ is a secure purely laparoscopic reconstruction method. Postoperative complications were very low. However, we had experienced unusual cases of jejunal limb stricture.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hosogi H, Japan; Jiang X, China S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Matsui H, Uyama I, Sugioka A, Fujita J, Komori Y, Ochiai M, Hasumi A. Linear stapling forms improved anastomoses during esophagojejunostomy after a total gastrectomy. Am J Surg. 2002;184:58-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Inaba K, Satoh S, Ishida Y, Taniguchi K, Isogaki J, Kanaya S, Uyama I. Overlap method: novel intracorporeal esophagojejunostomy after laparoscopic total gastrectomy. J Am Coll Surg. 2010;211:e25-e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 3. | Okabe H, Obama K, Tanaka E, Nomura A, Kawamura J, Nagayama S, Itami A, Watanabe G, Kanaya S, Sakai Y. Intracorporeal esophagojejunal anastomosis after laparoscopic total gastrectomy for patients with gastric cancer. Surg Endosc. 2009;23:2167-2171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Shinohara T, Kanaya S, Taniguchi K, Fujita T, Yanaga K, Uyama I. Laparoscopic total gastrectomy with D2 Lymph node dissection for gastric cancer. Arch Surg. 2009;144:1138-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Bracale U, Marzano E, Nastro P, Barone M, Cuccurullo D, Cutini G, Corcione F, Pignata G. Side-to-side esophagojejunostomy during totally laparoscopic total gastrectomy for malignant disease: a multicenter study. Surg Endosc. 2010;24:2475-2479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Greenleaf EK, Sun SX, Hollenbeak CS, Wong J. Minimally invasive surgery for gastric cancer: the American experience. Gastric Cancer. 2017;20:368-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Etoh T, Honda M, Kumamaru H, Miyata H, Yoshida K, Kodera Y, Kakeji Y, Inomata M, Konno H, Seto Y, Kitano S, Hiki N. Morbidity and mortality from a propensity score-matched, prospective cohort study of laparoscopic versus open total gastrectomy for gastric cancer: data from a nationwide web-based database. Surg Endosc. 2018;32:2766-2773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Tsujimoto H, Uyama I, Yaguchi Y, Kumano I, Takahata R, Matsumoto Y, Yoshida K, Horiguchi H, Aosasa S, Ono S, Yamamoto J, Hase K. Outcome of overlap anastomosis using a linear stapler after laparoscopic total and proximal gastrectomy. Langenbecks Arch Surg. 2012;397:833-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Morimoto M, Kitagami H, Hayakawa T, Tanaka M, Matsuo Y, Takeyama H. The overlap method is a safe and feasible for esophagojejunostomy after laparoscopic-assisted total gastrectomy. World J Surg Oncol. 2014;12:392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Noshiro H, Miyasaka Y, Akashi M, Iwasaki H, Ikeda O, Uchiyama A. Minimally invasive esophagogastrectomy for esophagogastric junctional cancer. Ann Thorac Surg. 2012;93:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Kanaya S, Haruta S, Kawamura Y, Yoshimura F, Inaba K, Hiramatsu Y, Ishida Y, Taniguchi K, Isogaki J, Uyama I. Video: laparoscopy distinctive technique for suprapancreatic lymph node dissection: medial approach for laparoscopic gastric cancer surgery. Surg Endosc. 2011;25:3928-3929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Sano T, Sasako M, Mizusawa J, Yamamoto S, Katai H, Yoshikawa T, Nashimoto A, Ito S, Kaji M, Imamura H, Fukushima N, Fujitani K; Stomach Cancer Study Group of the Japan Clinical Oncology Group. Randomized Controlled Trial to Evaluate Splenectomy in Total Gastrectomy for Proximal Gastric Carcinoma. Ann Surg. 2017;265:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 235] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 13. | Son SY, Cui LH, Shin HJ, Byun C, Hur H, Han SU, Cho YK. Modified overlap method using knotless barbed sutures (MOBS) for intracorporeal esophagojejunostomy after totally laparoscopic gastrectomy. Surg Endosc. 2017;31:2697-2704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K; ACTS-GC Group. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 1942] [Article Influence: 107.9] [Reference Citation Analysis (0)] |
| 15. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4397] [Article Influence: 549.6] [Reference Citation Analysis (4)] |
| 16. | Lee TG, Lee IS, Yook JH, Kim BS. Totally laparoscopic total gastrectomy using the overlap method; early outcomes of 50 consecutive cases. Surg Endosc. 2017;31:3186-3190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Huang CM, Huang ZN, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Lu J, Chen QY, Cao LL, Lin M, Tu RH. An Isoperistaltic Jejunum-Later-Cut Overlap Method for Esophagojejunostomy Anastomosis After Totally Laparoscopic Total Gastrectomy: A Safe and Feasible Technique. Ann Surg Oncol. 2017;24:1019-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Kawamura H, Ohno Y, Ichikawa N, Yoshida T, Homma S, Takahashi M, Taketomi A. Anastomotic complications after laparoscopic total gastrectomy with esophagojejunostomy constructed by circular stapler (OrVil™) versus linear stapler (overlap method). Surg Endosc. 2017;31:5175-5182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Huang ZN, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Lu J, Chen QY, Cao LL, Lin M, Tu RH, Lin JL. Digestive tract reconstruction using isoperistaltic jejunum-later-cut overlap method after totally laparoscopic total gastrectomy for gastric cancer: Short-term outcomes and impact on quality of life. World J Gastroenterol. 2017;23:7129-7138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Tsuruta A, Itoh T, Hirai T, Nakamura M. Multi-layered intra-abdominal adhesion prophylaxis following laparoscopic colorectal surgery. Surg Endosc. 2015;29:1400-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Siu KF, Cheung HC, Wong J. Shrinkage of the esophagus after resection for carcinoma. Ann Surg. 1986;203:173-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Ko CS, Gong CS, Kim BS, Kim SO, Kim HS. Overlap method versus functional method for esophagojejunal reconstruction using totally laparoscopic total gastrectomy. Surg Endosc. 2021;35:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Kitagami H, Morimoto M, Nakamura K, Watanabe T, Kurashima Y, Nonoyama K, Watanabe K, Fujihata S, Yasuda A, Yamamoto M, Shimizu Y, Tanaka M. Technique of Roux-en-Y reconstruction using overlap method after laparoscopic total gastrectomy for gastric cancer: 100 consecutively successful cases. Surg Endosc. 2016;30:4086-4091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Tokuhara T, Nakata E, Tenjo T, Kawai I, Kondo K, Ueda H, Tomioka A. Stenosis after esophagojejunostomy with the hemi-double-stapling technique using the transorally inserted anvil (OrVil™) in Roux-en-Y reconstruction with its efferent loop located on the patient's left side following laparoscopic total gastrectomy. Surg Endosc. 2019;33:2128-2134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Nagai E, Ohuchida K, Nakata K, Miyasaka Y, Maeyama R, Toma H, Shimizu S, Tanaka M. Feasibility and safety of intracorporeal esophagojejunostomy after laparoscopic total gastrectomy: inverted T-shaped anastomosis using linear staplers. Surgery. 2013;153:732-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |