Published online Nov 27, 2022. doi: 10.4240/wjgs.v14.i11.1272
Peer-review started: July 13, 2022
First decision: July 31, 2022
Revised: August 20, 2022
Accepted: October 12, 2022
Article in press: October 12, 2022
Published online: November 27, 2022
Processing time: 135 Days and 0.5 Hours
Gallbladder cancer (GBC) is one of the leading and aggressive cancers in this region of India. It is very difficult to diagnose in the early stage, as it lacks typical early signs and symptoms; thus, the diagnosis is often in the advanced stage, which ultimately leads to a poor 5-year survival outcome. Tumor markers including carbohydrate antigen 19-9 (CA 19-9), carcinoembryonic antigen (CEA), CA 125, CA 242, and alpha fetoprotein are used as indicators in the diagnosis and prognosis of GBC.
To compare tumor marker levels between GBC and benign GB diseases (GBDs) and to assess the combined use of tumor markers to increase the diagnostic accuracy for GBC.
Patients of either sex aged ≥ 18 years, with suspected GBC (GB polyp, irregular thick GB wall, GB mass, porcelain GB) on the basis of radiological imaging were included in this study. GB wall thickness using ultrasonography and tumor markers CEA, CA 125, CA 19-9, and CA 242 in all patients were recorded. All cases after surgical intervention were divided into two groups, GBC and benign GBD, according to histopathological examination findings. The cases were followed up and clinical findings, radiological findings, and levels of tumor markers were assessed.
A total of 200 patients were included in this study, of whom 80 patients had GBC and 120 patients had benign GBD. The median (interquartile range) age was 52.0 (41.0-60.0) years and the majority of patients (132, 66.0%) were women. Tumor markers including CA 19-9, CA 125, CEA, and CA 242 were significantly elevated in patients with GBC (P < 0.001). There was a significant reduction in tumor markers at 3 and 6 mo from baseline (P < 0.001). The mean survival of patients with normal and elevated levels of tumor markers CA 125, CA 19-9, and CEA was comparable; however lymph node metastasis and CA 242 expression level were independent prognostic factors.
Serum levels of tumor markers including CA 19-9, CA 125, CEA, and CA 242 were significantly associated with GBC. However, no significant association was observed between the presence of elevated levels of any tumor marker with respect to survival. Tumor marker assessment during follow-up may represent a treatment response.
Core Tip: Gallbladder cancer (GBC) is one of the leading and aggressive cancers, which is often diagnosed in the advanced and metastatic stage as it lacks typical early signs and symptoms. This study assessed the different tumor markers separately and in combination, to determine the diagnostic accuracy of these markers and prognostic significance in GBC. The level of tumor markers was significantly elevated in GBC. There was no association between the presence of elevated levels of any marker and survival; however, it showed response to treatment with a significant reduction in tumor markers at 3 mo and 6 mo.
- Citation: Sinha SR, Prakash P, Singh RK, Sinha DK. Assessment of tumor markers CA 19-9, CEA, CA 125, and CA 242 for the early diagnosis and prognosis prediction of gallbladder cancer. World J Gastrointest Surg 2022; 14(11): 1272-1284
- URL: https://www.wjgnet.com/1948-9366/full/v14/i11/1272.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i11.1272
Gallbladder cancer (GBC) is one of the leading and most aggressive cancers in the north and north-east region of India. There is a high prevalence of GBC in the northern region of India, especially in women (11.8/100000 population) and the north-east region (17.1/100000 population)[1].
It is very difficult to diagnose GBC in the early stage as it lacks typical clinical early manifestations leading to poor 5-year survival outcomes[2-4]. It is critical to diagnose GBC as early as possible, as most patients present in the advanced stage and thus have a low chance of radical treatment and prolonged survival.
Presently, the diagnosis of GBC mainly depends on radiological imaging such as ultrasonography (USG), computed tomography (CT) scan, magnetic resonance imaging, positron emission tomography scan, and invasive examination such as fine-needle aspiration cytology, core biopsy, and laparoscopy. In spite of these, there is no single tumor marker that can be used to diagnose and prognosticate GBC[5-7].
Tumor markers such as carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA 125), CA 242, and CA 19-9 have been widely used for the diagnosis of various types of cancer. CEA and CA 19-9 have traditionally been used as tumor markers for GBC, although they are not very sensitive. Despite their low sensitivity, it has been found that when these markers are used individually to diagnose GBC, inconsistent results are obtained[8-11]. Currently, only one study from China has reported the combined use of these tumor markers to increase the diagnostic specificity and sensitivity for GBC[12].
The present study compared tumor marker levels between GBC and benign GB diseases (GBDs) and assessed the combined use of tumor markers to increase the diagnostic sensitivity and specificity for GBC.
This was an observational study conducted at the Department of Biochemistry in collaboration with the Department of General Surgery, Surgical Gastroenterology, and the State Cancer Institute, Indira Gandhi Institute of Medical Sciences, Patna from September 2018 to August 2020. The study was approved by the institutional ethics committee (Vide Letter No. 479/IEC/2018/IGIMS), and the study procedure was in accordance with the principles of the Declaration of Helsinki.
Patients of either sex aged ≥ 18 years and patients with high suspicion of GBC (irregular thick GB wall, GB mass, GB polyp, porcelain GB) on the basis of radiological imaging were included in this study. Patients with a GB mass with surgical obstructive jaundice, disseminated GBC, those already receiving chemotherapy or radiotherapy, and those who presented with synchronous second primary cancer were excluded from the study. A venous blood sample was collected from each patient in the fasting state. The data of all patients regarding age at presentation, weight, body mass index, biochemical parameters such as complete blood count, liver function test, kidney function test, tumor markers CEA, CA 125, CA 19-9, CA 242, and GB wall thickness using USG were recorded. All patients who were included in the study underwent surgical management and the surgical specimen was sent for histopathological examination (HPE). Cancer staging was performed according to the 8th edition of the American Joint Committee on Cancer TNM staging system for GBC (8th ed, 2017). All cases were divided into the GBC group and the benign GBD group according to HPE findings. Patients in the GBC group were evaluated at 3 and 6 mo. During each follow-up, clinical findings, radiological findings, the level of tumor markers, and other laboratory parameters were recorded.
Tumor markers including CA 125, CA 19-9, and CEA were estimated by the chemiluminescence immunoassay principle using the Beckman-Coulter Access 2 Immunoassay System, maintaining all quality control precautions using the Calibrator and Reagent Kit provided by Beckman Coulter with reference range (CA 125, 0-35 U/mL; CA19-9, 0-35 U/mL; CEA, 0-3 ng/mL). Tumor marker CA 242 was estimated with an enzyme-linked immunosorbent assay kit with reference range 0-20 U/mL.
The survival time for each patient was defined as the interval between the date of definitive resection and the date of last follow-up or death. Disease-free interval was defined as the interval between completion of surgical resection and diagnosis of recurrence.
Data were analyzed using the Statistical Package for Social Sciences software, version 23.0. Qualitative data are presented as numbers and percentages, whereas quantitative data are presented as the mean ± standard deviation or median (range), depending on the normal or skewed distribution of data. The normal distribution of quantitative data was assessed by the Shapiro-Wilk test. The independent sample t-test or the Mann-Whitney U-test was used for the continuous variables and the chi-square (χ2) test for the categorical variables. The Cox regression model was used to determine the correlation between mortality and liver function test. Hazards ratios (HRs) and 95% confidence intervals (CIs) were computed. Kaplan-Meier event-free survival was computed and plotted. P < 0.05 was considered statistically significant.
A total of 200 patients were included in this study, of whom 80 patients had GBC and 120 patients had benign GBD. The median (interquartile range [IQR]) age was 52.0 (41.0-60.0) years and 132 (66.0%) patients were women. The laboratory parameters are summarized in Table 1.
| Parameters | GBC, n = 80 | Benign GB disease, n = 120 | Total, n = 200 | P value |
| Age, yr (n = 200) | 57.0 (50.2-66.5) | 47.0 (34.0-56.0) | 52.0 (41.0-60.0) | < 0.001 |
| Sex (n = 200), n (%) | 0.951 | |||
| Men | 27 (33.8) | 41 (34.2) | 68 (34.0) | |
| Women | 53 (66.3) | 79 (65.8) | 132 (66.0) | |
| BMI in kg/m2, (mean) | 27.06 ± 4.46 | 26.50 ± 5.6 | 26.8 ± 4.98 | 0.229 |
| Hemoglobin in g/dL | 11.8 (10.8-12.6) | 11.6 (10.2-12.8) | 11.75 (10.6-12.70) | 0.523 |
| TLC in cells/μL | 8075.0 (6759.0-9801.0) | 7830.0 (6705.0-8800.0) | 7846.0 (6745.0-9440.0) | 0.094 |
| Lymphocytes in cells/μL | 27.4 (21.2-31.0) | 26.9 (22.0-31.0) | 27.0 (22.0-31.0) | 0.421 |
| Monocytes in cells/mm3 | 5.7 (3.4-7.9) | 6.0 (4.0-7.0) | 6.0 (4.0-7.30) | 0.604 |
| Neutrophils in cells/mm3 | 63.1 (58.2-69.6) | 63.0 (59.0-67.0) | 63.0 (58.92-67.77) | 0.816 |
| Eosinophils, % | 3.0 (1.2-4.0) | 3.5 (2.0-4.4) | 3.0 (2.0-4.10) | 0.023 |
| Basophils in cells/μL | 0.5 (0.3-1.0) | 0.4 (0.1-1.0) | 0.50 (0.20-1.0) | 0.351 |
| Bilirubin in mg/dL | ||||
| Total | 0.9 (0.6-1.2) | 0.8 (0.6-1.1) | 0.87 (0.64-1.12) | 0.251 |
| Direct | 0.3 (0.2-0.5) | 0.3 (0.2-0.5) | 0.30 (0.20-0.52) | 0.621 |
| Indirect | 0.6 (0.4-0.7) | 0.4 (0.3-0.6) | 0.50 (0.36-0.65) | 0.015 |
| ALP in IU/L | 119.5 (80.5-163.2) | 109.0 (76.2-136.2) | 111.0 (78.0-146.50) | 0.019 |
| SGOT in U/L | 34.0 (27.2-43.0) | 28.0 (24.0-34.0) | 31.0 (25.0-36.0) | 0.001 |
| SGPT in U/L | 27.0 (21.0-36.5) | 23.0 (21.0-30.5) | 24.0 (21.0-34.0) | 0.012 |
| INR | 1.1 (1.0-1.1) | 1.1 (1.0-1.1) | 1.12 (1.10-1.12) | 0.158 |
| Serum creatinine in mg/dL | 0.8 (0.7-0.9) | 0.8 (0.7-0.9) | 0.80 (0.68-0.97) | 0.459 |
| BUN in mg/dL | 12.3 (9.0-14.4) | 12.3 (9.9-14.5) | 12.30 (5.0-12.0) | 0.479 |
| GB wall thickness in mm | 12.0 (9.2-15.1) | 6.0 (4.0-8.0) | 8.0 (5.0-12.0) | < 0.001 |
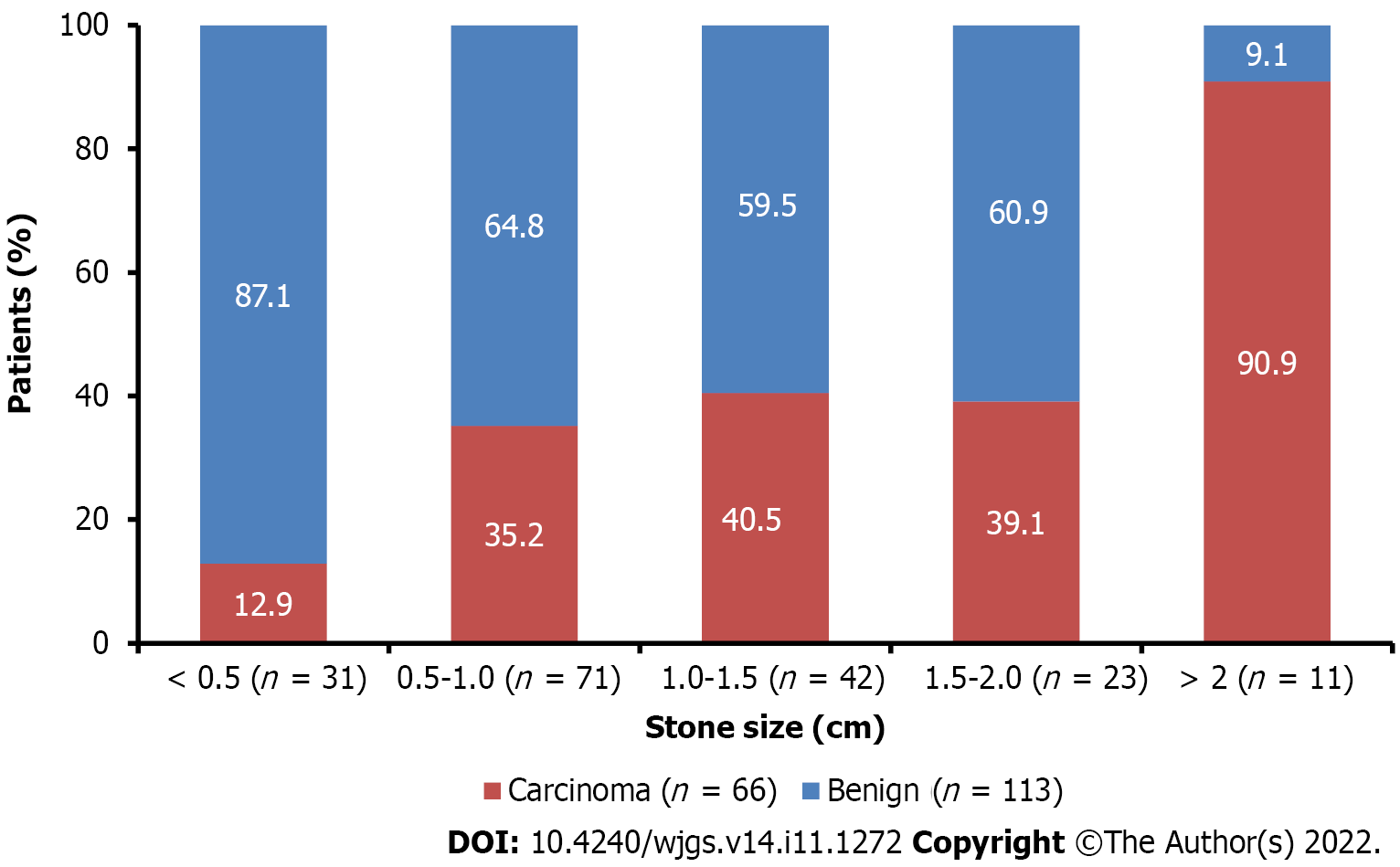
Although, IQR indirect bilirubin was significantly higher in patients with GBC compared to patients with benign GBD (0.6 mg/dL vs 0.4 mg/dL; P = 0.015), and the median levels of serum glutamic oxaloacetic transaminase (SGOT) (P = 0.001) and serum glutamic pyruvic transaminase (SGPT) (P = 0.012) were significantly higher in the GBC group than in the benign group, all values were within the normal range in both groups. GB wall thickness on USG was increased by twofold in patients from the GBC group (Table 1). The majority of patients (n = 71) had a stone size between 0.5 and 1 cm. In patients with benign GBD, the majority of patients had a stone size in the range of < 0.5-2.0 cm compared to the patients with GBC. However, the majority of patients with GBC had a stone size > 2 cm compared to the patients with benign GBD (Figure 1).
Tumor markers including CA 19-9, CA 125, CEA, and CA 242 were significantly elevated in patients with GBC (P < 0.001). CA 19-9 was elevated in 71.3%, CEA in 64.4%, and CA 242 in 86.3% of patients with GBC (Table 2).
| All parameters | Benign, n = 120 | Carcinoma, n = 80 | P value |
| CA 19-9 in U/mL | 3.1 (1.4-19.4) | 112.9 (23.3-318.8) | < 0.001 |
| CA 19-9, n (%) | |||
| Normal | 108 (90.0) | 23 (28.7) | < 0.001 |
| Elevated | 12 (10.0) | 57 (71.3) | |
| CA 125 in U/mL | 8.6 (3.1-15.1) | 24.5 (12.0-53.3) | < 0.001 |
| CA 125, n (%) | |||
| Normal | 112 (93.3) | 49 (61.3) | < 0.001 |
| Elevated | 8 (6.7) | 31 (38.8) | |
| CEA in µg/L | 2.3 (1.2-3.1) | 3.1 (1.8-4.5) | < 0.003 |
| CEA, n (%) | |||
| Normal | 114 (94) | 60 (75) | < 0.003 |
| Elevated | 6 (5.9) | 20 (25) | |
| CA 242 in U/mL | 2.8 (1.5-9.8) | 55.5 (32.7-96.5) | < 0.001 |
| CA 242, n (%) | |||
| Normal | 108 (90.0) | 11 (13.7) | < 0.001 |
| Elevated | 12 (10.0) | 69 (86.3) |
Serum levels of CA 19-9, CA 125, and CA 242 were significantly associated with age (P < 0.05). However, there was no significant association of tumor markers with presence of gallstones and sex of the patient (Table 3).
| Characteristics | CA 19-9 | P value | CA 125 | P value | CEA | P value | CA 242 | P value | ||||
| Normal, n = 131 | Elevated, n = 69 | Normal, n = 161 | Elevated, n = 39 | Normal, n = 181 | Elevated, n = 19 | Normal, n = 119 | Elevated,n = 81 | |||||
| Age, yr | 49.0 (39.0-59.0) | 55.0 (45.0-63.5) | 0.009 | 50.0 (39.0-59.0) | 56.0 (50.0-69.0) | 0.001 | 50.0 (40.0-59.5) | 56.0 (45.0-65.0) | 0.093 | 48.0 (34.0-56.0) | 56.0 (47.5-64.5) | < 0.001 |
| Sex | ||||||||||||
| Male | 46 (35.1) | 22 (31.9) | 0.754 | 51 (31.7) | 17 (43.6) | 0.112 | 62 (34.3) | 6 (31.6) | > 0.05 | 41 (34.5) | 27 (33.3) | 0.881 |
| Female | 85 (64.9) | 47 (68.1) | 110 (68.3) | 22 (56.4) | 119 (65.7) | 13 (68.4) | 78 (65.5) | 54 (66.7) | ||||
| Gallstones | ||||||||||||
| Absent | 12 (9.2) | 10 (14.5) | 0.181 | 19 (11.8) | 3 (7.7) | 0.578 | 21 (11.6) | 1 (5.3) | 0.701 | 7 (5.9) | 15 (18.5) | 0.010 |
| Present | 119 (90.8) | 59 (85.5) | 142 (88.2) | 36 (92.3) | 160 (88.4) | 18 (94.7) | 112 (94.1) | 66 (81.5) | ||||
The sensitivity of CA 19-9 and CA 242 was comparatively higher than CEA and CA 125 in different stages of GBC (Table 4).
| Clinical stages | Patients, n = 80 | CA 19-9 | CEA | CA 125 | CA 242 |
| I | 15 (18.6) | 10 (66.7) | 1 (6.7) | 5 (33.3) | 13 (86.7) |
| IIA | 13 (16.3) | 12 (92.3) | 1 (7.7) | 5 (38.5) | 12 (92.3) |
| IIB | 4 (5.0) | 4 (100.0) | 0 | 2 (50.0) | 4 (100.0) |
| IIIA | 4 (5.0) | 4 (100.0) | 0 | 2 (50.0) | 4 (100.0) |
| IIIB | 44 (55.0) | 27 (61.3) | 8 (18.1) | 17 (38.6) | 36 (81.8) |
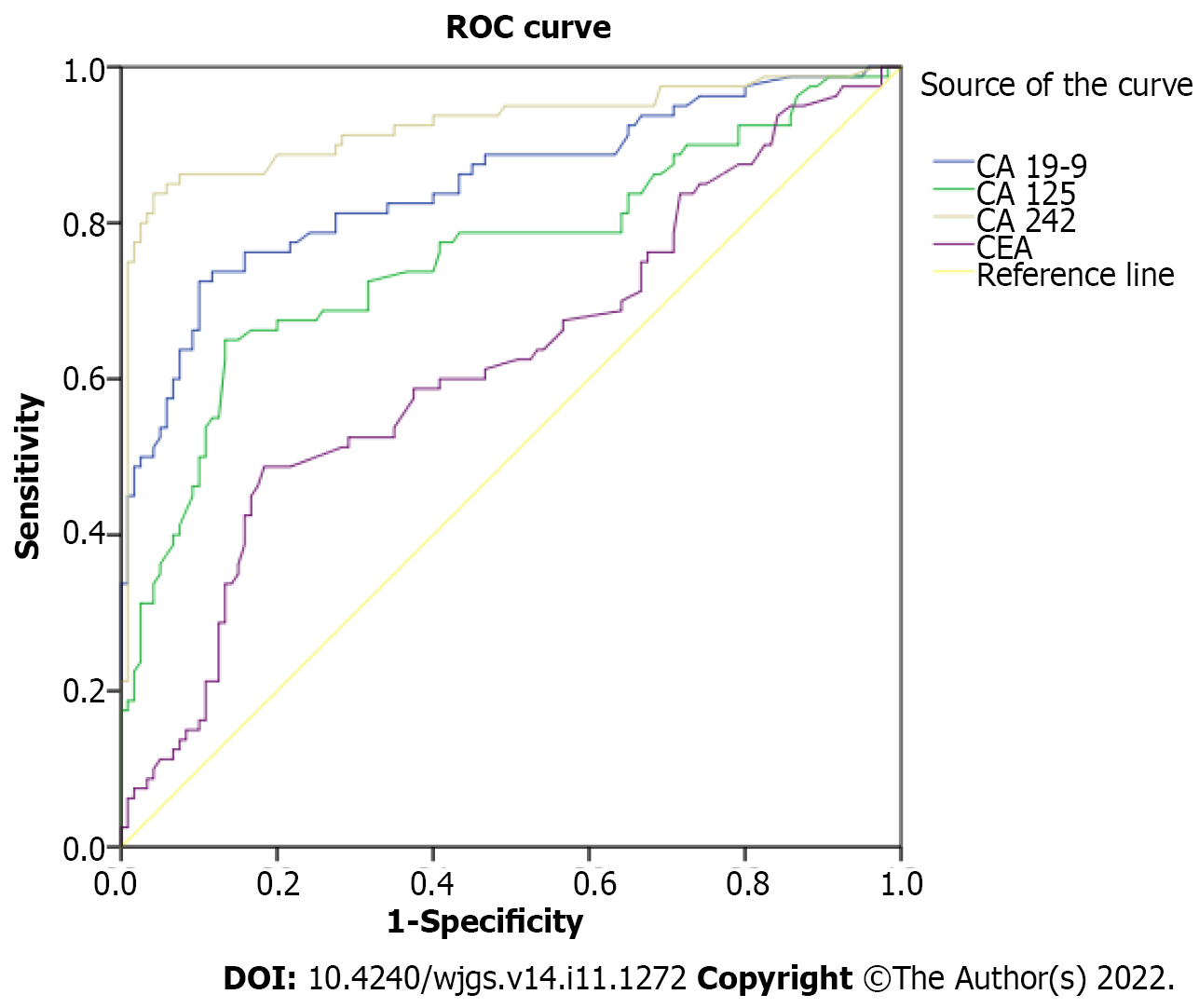
The sensitivity was 3.8% when all four markers exceeded the critical values. These results suggested that diagnosis of GBC based on combined detection of the tumor markers could increase the specificity, but not the sensitivity of diagnosis (Table 5). CA 242 had the highest sensitivity of 86.3%, and CA 125 had the highest specificity of 93.3% for the diagnosis of GBC (Table 6). Receiver operating characteristic curves are shown in Figure 2.
| Group | n | 1 marker | 2 markers | 3 markers | 4 markers |
| Benign GB disease | 120 | 29 (24.2) | 6 (5.0) | 1 (0.8) | 0 |
| GBC | 80 | 14 (17.5) | 27 (33.7) | 25 (31.3) | 3 (3.8) |
| Positive likelihood rate | 0.5% | 4.5% | 25% | 100% |
| Variable | Sensitivity, % | Specificity, % | PPV, % | NPV, % | AUC (95%CI); P value |
| CA 19-9 (cutoff: 39.21 by ROC) | 71.3 | 90.0 | 82.6 | 82.4 | 0.849 (0.791-0.907); < 0.001 |
| CA 125 (cutoff: 36.00 by ROC) | 38.8 | 93.3 | 79.5 | 69.6 | 0.758 (0.686-0.831); < 0.001 |
| CEA (cutoff: 10.36 by ROC) | 12.5 | 92.5 | 52.6 | 61.3 | 0.623 (0.542-0.703); 0.003 |
| CA 242 (cutoff: 15.10 by ROC) | 86.3 | 90.0 | 85.2 | 90.8 | 0.925 (0.881-0.969); < 0.001 |
A combination of CA 19-9 and CA 242 had the highest sensitivity of 83.2%, and a combination of ≥ 3 markers had the highest specificity of 100.0% for the diagnosis of GBC (Table 7).
| Variable | Sensitivity, % | Specificity, % | PPV, % | NPV, % |
| Combination of any 2 markers | 63.5 | 95.0 | 84.6 | 85.7 |
| Combination of markers CA 19-9 and CA 242, n = 26 | 83.2 | 93.3 | 96.2 | 83.5 |
| Combination of ≥ 3 markers | 35.0 | 100.0 | 100.0 | 69.8 |
Serum CEA, CA 125, CA 19-9, and CA 242 levels in GBC patients with and without lymph node metastasis (LNM) were compared. Serum CA 125, CA 19-9, CEA, and CA 242 levels were comparable between patients with LNM and patients without LNM (Table 8).
| Marker level | No LNM, n = 36 | LNM, n = 44 | P value |
| CA 19-9 in U/mL | 110.5 (54.2- 176.7) | 221.8 (14.9-753.0) | < 0.05 |
| CEA in µg/L | 3.2 (1.4-4.0) | 3.37 (1.9-6.2) | > 0.05 |
| CA 125 in U/mL | 23.0 (21.5-47.3) | 33.0 (7.4-64.2) | > 0.05 |
| CA 242 in U/mL | 48.5 (36.1-84.7) | 92.0 (25.8-112.0) | < 0.05 |
Multivariate survival analyses using the Cox proportional hazards model showed that LNM and CA 242 expression level were independent prognostic factors (Table 9).
| Prognostic factor | Parameter estimate | Wald χ2 | P value | Hazard ratio | 95% CI |
| CA 19-9 | 0 | 0.152 | 0.697 | 1 | 0.999-1.001 |
| CEA | -0.137 | 1.415 | 0.234 | 0.872 | 0.696-1.093 |
| CA 125 | 0.001 | 0.211 | 0.464 | 1.001 | 0.995-1.008 |
| CA 242 | 0.017 | 10.422 | 0.001 | 1.017 | 1.007-1.027 |
| LNM | -2.06 | 6.001 | 0.014 | 0.127 | 0.024-0.662 |
| Age | -0.05 | 2.814 | 0.093 | 0.951 | 0.897-1.009 |
| Sex | -0.264 | 0.098 | 0.755 | 0.768 | 0.146-4.027 |
| BMI | 0.038 | 0.478 | 0.489 | 1.038 | 0.933-1.155 |
| GB wall thickness | -0.076 | 2.096 | 0.148 | 0.927 | 0.837-1.027 |
| Stone size | -0.318 | 2.114 | 0.146 | 0.728 | 0.474-1.117 |
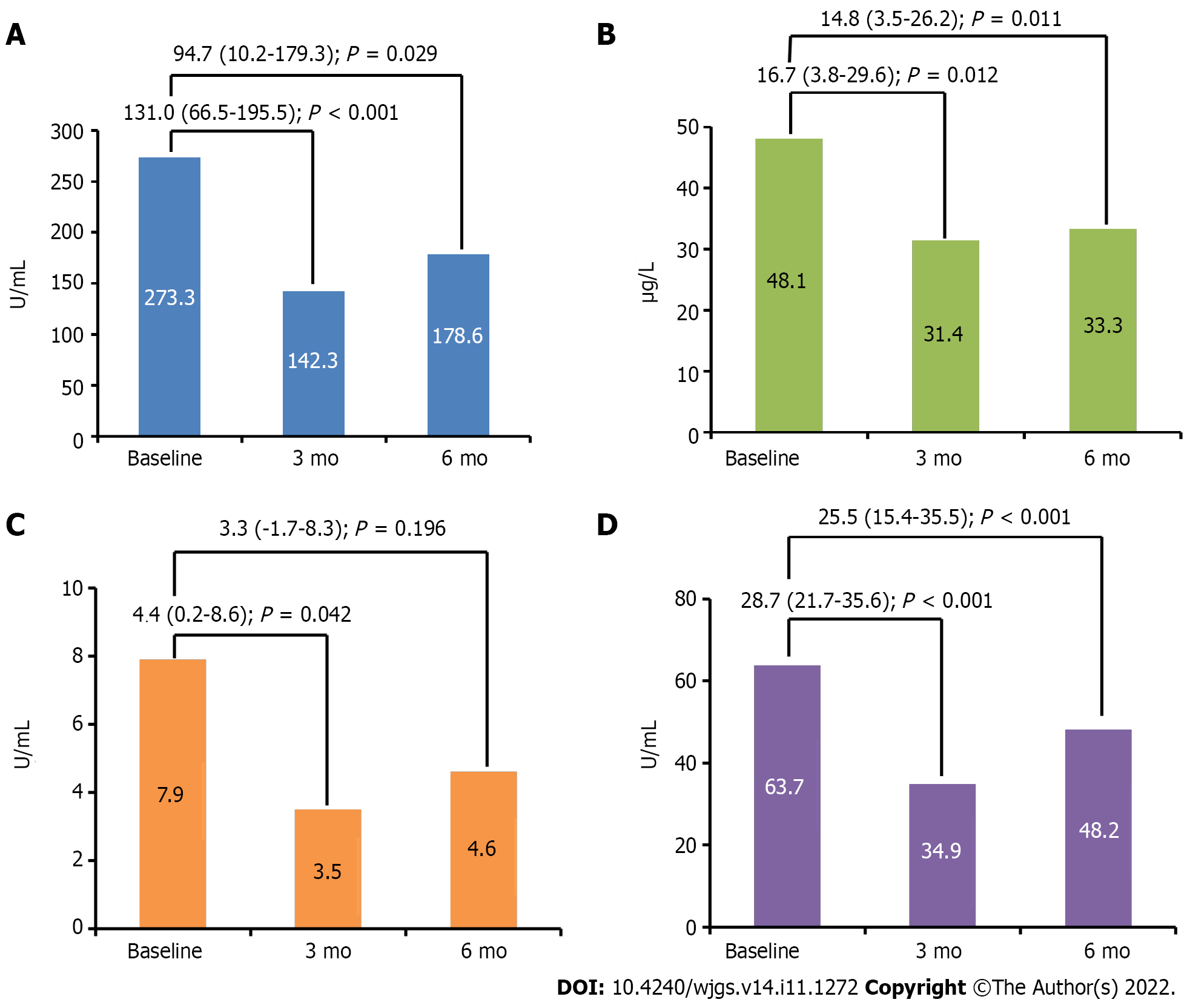
The CA 19-9 marker showed a significant reduction from baseline at the 3- and 6-mo follow-up (P < 0.001 and P = 0.029, respectively). CA 125 marker levels were also significantly reduced at 3 mo (P = 0.012) and 6 mo (P = 0.011). The CEA marker showed a significant reduction at 3 mo (P = 0.042); however, reduction from baseline at the 6-mo follow-up was insignificant (P = 0.196). CA 242 showed a significant reduction, both at the 3- and 6-mo follow-up (P < 0.001 and P = 0.001, respectively) (Figure 3).
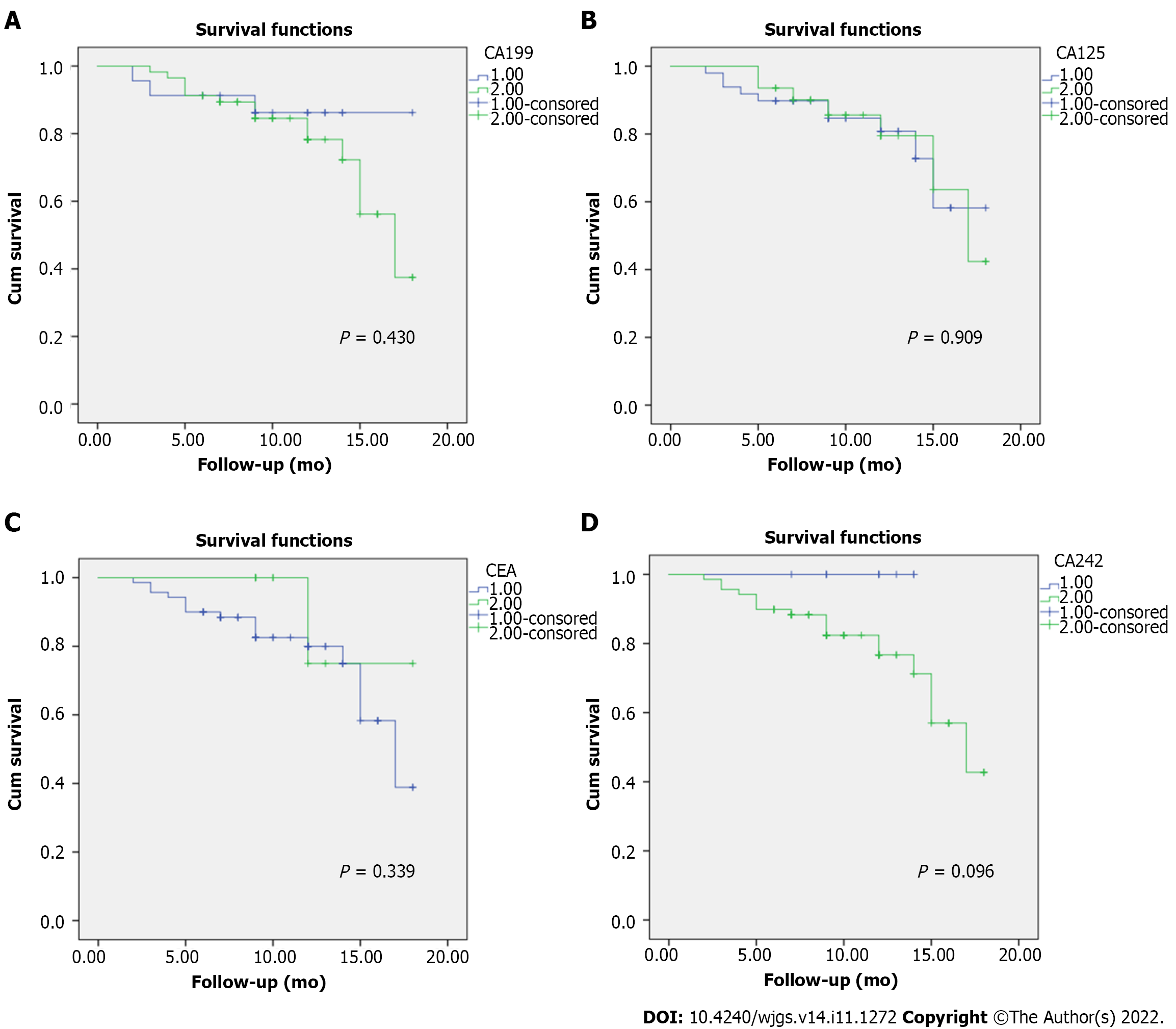
The mean survival of patients between normal and elevated levels for CA 125, CA 19-9, and CEA markers were comparable. There was no significant difference in terms of survival in patients with different levels of tumor markers, suggesting no significant association of the elevated levels of any marker and survival (Figure 4). Overall, there were 6 cases of recurrence with a mean disease-free interval of 9.2 mo.
This study was conducted in patients with suspected GBC to assess different tumor markers separately and in combination, to determine their diagnostic accuracy and prognosis of GBC. The key findings indicated that the IQR age was 52.0 (41.0-60.0) years and 132 (66.0%) patients were women. Although median levels of SGOT (P = 0.001) and SGPT (P = 0.012) were significantly higher in the GBC group than in the benign GBD group, they were within the normal range in both groups. GB wall thickness was increased twofold in patients with GBC. Tumor markers including CA 19-9, CA 125, CEA, and CA 242 were significantly elevated in patients with GBC (P < 0.001). Serum levels of CA 19-9, CA 125, and CA 242 were significantly associated with age (P < 0.05). The sensitivity of CA 19-9 and CA 242 was comparatively higher than CEA and CA 125 in different stages of GBC. The sensitivity was 3.8% when all four markers exceeded the critical values. CA 242 had the highest sensitivity of 86.3%, and CA 125 had the highest specificity of 93.3% for the diagnosis of GBC. There was a significant reduction in tumor markers at 3 and 6 mo from baseline (P < 0.001).
A total of 200 patients were included in this study, of whom 80 patients had GBC and 120 patients had benign GBD. Tumor markers CEA, CA 19-9, CA 125 and CA 242 have been used for the diagnosis and prognosis of various types of cancer including liver, gastric, colorectal, and pancreatic[9,13]. In this study, the serum levels of tumor markers CA 19-9, CA 125, CEA, and CA 242 were significantly higher in patients with GBC (P < 0.001) than in patients with benign GBD. This is in accordance with previous studies where all these tumor markers were evaluated as therapeutic and diagnostic markers[12-15].
In the present study, it was observed that CA 242 had the highest sensitivity of 86.3% and CA 125 had the highest specificity of 93.3% for the diagnosis of GBC. A recent study of 71 patients diagnosed with GBC showed that CA 19-9 had the highest sensitivity of 85% and CA 125 had the highest specificity of 81.8%[16]. A prospective study by Sachan et al[17] reported that CA 19-9 had better sensitivity and specificity (52% and 80%, respectively) than CEA (51% and 72%, respectively) for the prediction of tumor burden in patients with GBC. Another study by Wang et al[12] reported that CA 19-9 and CA 242 had the highest sensitivity and specificity of 71.7% and 98.7%, respectively. GBC can be detected using serum CA 19-9, which had moderate sensitivity and good specificity[18]. In a meta-analysis by Zhou[18], it was noted that GBC can be detected using serum CA 19-9, which had moderate sensitivity and good specificity. These findings suggest that the sensitivity and specificity of tumor markers were inconsistent when used individually for the diagnosis of GBC; however, better sensitivity was observed when the markers were used in combination[19-21]. In the current study, sensitivity was 3.8% when all four markers exceeded the critical values. This is in accordance with a previous study with a sensitivity of 8.9% and a diagnostic accuracy that was better when CA 19-9, CA 125, and CA 242 were used in combination. These results suggest that the diagnosis of GBC based on combined detection of the tumor markers could increase the sensitivity and specificity of the diagnosis.
Serum levels of CA 19-9, CA 125, and CA 242 were significantly associated with age (P < 0.05). However, there was no association of tumor markers with the presence of gallstones and sex of the patient. In accordance with this, a prospective exploratory study conducted at a tertiary care center in Lucknow, did not find any association of CA 242 with tumor stage, presence of jaundice, gallstones and sex of the patient[14].
The difference between mean survival with respect to normal vs elevated levels of tumor markers was not significant in this study. These findings may be explained by the inclusion criteria, as in the present study, only early and suspicious cases of GBC were included. In accordance with this, a previous study by Agarwal et al[14] explained that CA 19-9 and CA 242 are not recommended as prognostic markers. By contrast, Agarwal et al[16] reported the prognostic role of tumor markers in terms of overall survival rate.
The present study had a few limitations. It was a non-randomized observational study with a relatively small sample size and a short follow-up duration of only 6 mo. The study included only operable and suspicious cases of GBC to determine early indications of malignancy by assessing different tumor markers in resource-constrained countries. Further studies with a large number of patients with longer duration of follow-up are required to validate our results.
The present study suggested that serum levels of tumor markers including CA 19-9, CA 125, CEA and CA 242 were significantly associated with GBC. Significant reductions in tumor markers during follow-up show their importance as one of the criteria for assessment of treatment response. However, no significant association was observed between the presence of elevated levels of any marker and survival.
Tumor markers such as carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA 125), CA 242, and CA 19-9 have been widely used for the diagnosis of various types of cancer. Many researchers have focused on gallbladder cancer (GBC) and CEA or CA125, but no research has been carried out on all four markers together, especially in India.
This study focuses on the assessment of tumor markers CA 19-9, CEA, CA 125, and CA 242 for the early diagnosis and prognosis prediction of GBC.
The present study included patients with suspected GBC to assess different tumor markers separately and in combination, to determine their diagnostic accuracy and prognosis of GBC.
This observational study was conducted in patients of either sex aged ≥ 18 years, with suspected GBC (GB polyp, irregular thick GB wall, GB mass, porcelain GB) on the basis of radiological imaging. All cases after surgical intervention were divided and grouped into two groups, the GBC group and benign GB disease group, according to histopathological examination findings. The cases were followed up and clinical findings, radiological findings, and tumor markers were assessed.
The key findings indicated that the median (interquartile range) age was 52.0 (41.0-60.0) years and 132 (66.0%) patients were women. The median levels of serum glutamic oxaloacetic transaminase (SGOT) (P = 0.001) and serum glutamic pyruvic transaminase (SGPT) (P = 0.012) were significantly higher in the GBC group than in the benign GBD group but were within the normal range in both groups. GB wall thickness was increased twofold in patients with GBC. Tumor markers including CA 19-9, CA 125, CEA, and CA 242 were significantly elevated in patients with GBC (P < 0.001). Serum levels of CA 19-9, CA 125, and CA 242 were significantly associated with age (P < 0.05). The sensitivity of CA 19-9 and CA 242 was comparatively higher than CEA and CA 125 in different stages of GBC. The sensitivity was 3.8% when all four markers exceeded the critical values. CA 242 had the highest sensitivity of 86.3%, and CA 125 had the highest specificity of 93.3% for the diagnosis of GBC. There was a significant reduction in tumor markers at 3 and 6 mo from baseline (P < 0.001).
All four markers were important but in this study, CA 242 followed by CA 19-9 was most sensitive for the detection of GBC while CA125 was most specific for the diagnosis of GBC; however, CA 242 and CA 19-9 in combination were more specific and sensitive.
Currently, there is only one study from China that has reported the combined use of these tumor markers to increase the diagnostic specificity and sensitivity for GBC. This study was conducted to make an early diagnosis of GBC on the basis of tumor markers, which itself will lead to better survival outcomes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Peng XC, China; Zhou Z, China S-Editor: Chen YL L-Editor: Webster JR P-Editor: Chen YL
| 1. | National Cancer registry programme. Consolidated report of population based cancer registries: 2012-14 [Internet]. Available online: http://ncdirindia.org/NCRP/ALL_NCRP_REPORTS/PBCR_REPORT_2012_2014/index.htm. [Accessed on 24 Nov 2020]. |
| 2. | Hu L, Wang B, Liu X, Lv Y. Unsuspected gallbladder cancer: a clinical retrospective study. Arch Iran Med. 2013;16:631-635. [PubMed] |
| 3. | Peng HH, Zhang YD, Gong LS, Liu WD, Zhang Y. Increased expression of microRNA-335 predicts a favorable prognosis in primary gallbladder carcinoma. Onco Targets Ther. 2013;6:1625-1630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Liu TY, Tan ZJ, Jiang L, Gu JF, Wu XS, Cao Y, Li ML, Wu KJ, Liu YB. Curcumin induces apoptosis in gallbladder carcinoma cell line GBC-SD cells. Cancer Cell Int. 2013;13:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Okada K, Kijima H, Imaizumi T, Hirabayashi K, Matsuyama M, Yazawa N, Dowaki S, Tobita K, Ohtani Y, Tanaka M, Inokuchi S, Makuuchi H. Clinical significance of wall invasion pattern of subserosa-invasive gallbladder carcinoma. Oncol Rep. 2012;28:1531-1536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Eil R, Hansen PD, Cassera M, Orloff SL, Sheppard BC, Diggs B, Billingsley KG. Bile duct involvement portends poor prognosis in resected gallbladder carcinoma. Gastrointest Cancer Res. 2013;6:101-105. [PubMed] |
| 7. | Zhai G, Yan K, Ji X, Xu W, Yang J, Xiong F, Su J, McNutt MA, Yang H. LAPTM4B allele *2 is a marker of poor prognosis for gallbladder carcinoma. PLoS One. 2012;7:e45290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Ghosh M, Sakhuja P, Singh S, Agarwal AK. p53 and beta-catenin expression in gallbladder tissues and correlation with tumor progression in gallbladder cancer. Saudi J Gastroenterol. 2013;19:34-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Zhang D, Yu M, Xu T, Xiong B. Predictive value of serum CEA, CA19-9 and CA125 in diagnosis of colorectal liver metastasis in Chinese population. Hepatogastroenterology. 2013;60:1297-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 10. | He CZ, Zhang KH, Li Q, Liu XH, Hong Y, Lv NH. Combined use of AFP, CEA, CA125 and CAl9-9 improves the sensitivity for the diagnosis of gastric cancer. BMC Gastroenterol. 2013;13:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 11. | Zur B, Holdenrieder S, Walgenbach-Brünagel G, Albers E, Stoffel-Wagner B. Method comparison for determination of the tumor markers AFP, CEA, PSA and free PSA between Immulite 2000 XPI and Dimension Vista 1500. Clin Lab. 2012;58:97-105. [PubMed] |
| 12. | Wang YF, Feng FL, Zhao XH, Ye ZX, Zeng HP, Li Z, Jiang XQ, Peng ZH. Combined detection tumor markers for diagnosis and prognosis of gallbladder cancer. World J Gastroenterol. 2014;20:4085-4092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 88] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (2)] |
| 13. | Hatzaras I, Schmidt C, Muscarella P, Melvin WS, Ellison EC, Bloomston M. Elevated CA 19-9 portends poor prognosis in patients undergoing resection of biliary malignancies. HPB (Oxford). 2010;12:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Agarwal A, Tiwari V, Kumari S, Husain N. Prognostic value of CA19-9 and CA242 in gallbladder cancer - an exploratory study. Int J Contemporary Med Res. 2019;6:F4-F7. [DOI] [Full Text] |
| 15. | Kumar N, Rajput D, Gupta A, Popuri V, Kundal A, Sharma J, Puliyath N, Shasheendran. Utility of triple tumor markers CA19-9, CA125 and CEA in predicting advanced stage of carcinoma gallbladder: a retrospective study. Int Surg J. 2020;7:2527-2531. [DOI] [Full Text] |
| 16. | Agrawal S, Gupta A, Gupta S, Goyal B, Siddeek RAT, Rajput D, Chauhan U, Kishore S, Gupta M, Kant R. Role of carbohydrate antigen 19-9, carcinoembryonic antigen, and carbohydrate antigen 125 as the predictors of resectability and survival in the patients of Carcinoma Gall Bladder. J Carcinog. 2020;19:4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Sachan A, Saluja SS, Nekarakanti PK, Nimisha, Mahajan B, Nag HH, Mishra PK. Raised CA19-9 and CEA have prognostic relevance in gallbladder carcinoma. BMC Cancer. 2020;20:826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Zhou X. Meta-analysis of the diagnostic performance of serum carbohydrate antigen 19-9 for the detection of gallbladder cancer. Int J Biol Markers. 2022;37:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Strom BL, Maislin G, West SL, Atkinson B, Herlyn M, Saul S, Rodriguez-Martinez HA, Rios-Dalenz J, Iliopoulos D, Soloway RD. Serum CEA and CA 19-9: potential future diagnostic or screening tests for gallbladder cancer? Int J Cancer. 1990;45:821-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 42] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Shukla VK, Gurubachan, Sharma D, Dixit VK, Usha. Diagnostic value of serum CA242, CA 19-9, CA 15-3 and CA 125 in patients with carcinoma of the gallbladder. Trop Gastroenterol. 2006;27:160-165. [PubMed] |
| 21. | Liska V, Treska V, Skalicky T, Fichtl J, Bruha J, Vycital O, Topolcan O, Palek R, Rosendorf J, Polivka J, Holubec L. Evaluation of Tumor Markers and Their Impact on Prognosis in Gallbladder, Bile Duct and Cholangiocellular Carcinomas - A Pilot Study. Anticancer Res. 2017;37:2003-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |