Published online Nov 27, 2022. doi: 10.4240/wjgs.v14.i11.1260
Peer-review started: September 19, 2022
First decision: October 21, 2022
Revised: October 30, 2022
Accepted: November 16, 2022
Article in press: November 16, 2022
Published online: November 27, 2022
Processing time: 66 Days and 23.6 Hours
Chronic liver disease (CLD) related thrombocytopenia increases the risk of bleeding and poor prognosis. Many liver disease patients require invasive pro
To evaluate the efficacy of rhTPO in the treatment of patients with CLD-associated thrombocytopenia undergoing invasive procedures.
All analyses were based on the retrospective collection of clinical data of patients with CLD who were treated in the Department of Infectious Diseases at The First Affiliated Hospital of Soochow University between June 2020 and December 2021. Fifty-nine male and 41 female patients with liver disease were enrolled in this study to assess the changes in platelet counts and parameters before and after the use of rhTPO for thrombocytopenia. Adverse events related to treatment, such as bleeding, thrombosis, and disseminated intravascular coagulation, were also investigated.
Among the enrolled patients, 78 (78%) showed a platelet count increase after rhTPO use, while 22 (22%) showed no significant change in platelet count. The mean platelet count after rhTPO treatment in all patients was 101.53 ± 81.81 × 109/L, which was significantly improved compared to that at baseline (42.88 ± 16.72 × 109/L), and this difference was statistically significant (P < 0.001). In addition, patients were further divided into three subgroups according to their baseline platelet counts (< 30 × 109/L, 30-50 × 109/L, > 50 × 109/L). Subgroup analyses showed that the median platelet counts after treatment were significantly higher (P < 0.001, all). Ninety (90%) patients did not require platelet transfusion partially due to an increase in platelet count after treatment with rhTPO. No serious adverse events related to rhTPO treatment were observed. Overall, rhTPO demonstrated good clinical efficacy for treating CLD-associated thrombocytopenia.
rhTPO can improve platelet count, reduce the risk of bleeding, and decrease the platelet transfusion rate, which may promote the safety of invasive procedures and improve overall survival of patients with CLD.
Core Tip: Recombinant human thrombopoietin (rhTPO), commonly used to treat primary immune thrombocytopenic purpura and thrombocytopenia caused by solid tumor chemotherapy, has not been extensively investigated in the treatment of chronic liver disease (CLD)-related thrombocytopenia, where there is an increased risk of bleeding and a poor prognosis, especially in patients undergoing invasive procedures or surgery. Our retrospective study evaluates the efficacy of rhTPO in the treatment of patients with CLD-associated thrombocytopenia undergoing invasive procedures. Overall, rhTPO demonstrated good clinical efficacy by improving platelet count, reducing bleeding risk and decreasing the platelet transfusion rate, which can promote the probability of tolerance to receive invasive management and improve overall survival of patients with CLD.
- Citation: Ding JN, Feng TT, Sun W, Cai XY, Zhang Y, Zhao WF. Recombinant human thrombopoietin treatment in patients with chronic liver disease-related thrombocytopenia undergoing invasive procedures: A retrospective study. World J Gastrointest Surg 2022; 14(11): 1260-1271
- URL: https://www.wjgnet.com/1948-9366/full/v14/i11/1260.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i11.1260
A platelet count of < 150 × 109/L in circulation is defined as thrombocytopenia[1]. The major causes of thrombocytopenia include hematological diseases, bone marrow suppression after chemotherapy for malignant tumors, drug-induced thrombocytopenia, and chronic liver disease (CLD). The incidence rate of thrombocytopenia caused by CLD varies in different studies, with the average morbidity ranging from 6%-78%[2]. As CLD progresses, the degree of thrombocytopenia worsens. Patients with end-stage liver disease often experience serious complications. An extremely low platelet count aggravates the risk of bleeding and has a poor prognosis[3]. Many patients with CLD require invasive procedures or surgeries, such as liver biopsy, endoscopic variceal ligation, endoscopic injection sclerotherapy and transjugular intrahepatic portosystemic shunt for varices, splenectomy for hypersplenism, hepatectomy for liver cancer, and non-liver surgery. The risk of bleeding during invasive procedures in patients with CLD is associated with platelet count, coagulopathy status, and the type of procedure. An increased risk of bleeding with invasive procedures has been reported in patients with CLD[3], and there is no defined treatment modality for CLD-induced thrombocytopenia. Re
Clinical data of 100 patients with CLD treated in the Department of Infectious Diseases at The First Affiliated Hospital of Soochow University between June 2020 and December 2021 were retrospectively collected. The inclusion and exclusion criteria were based on consensus and guidelines[4,5] for the diagnosis and treatment of chronic viral hepatitis, alcoholic liver disease, autoimmune hepatitis, cirrhosis, and hepatocellular carcinoma. The inclusion criteria were as follows: (1) Patients over 18 years of age with CLD, cirrhosis, or liver cancer caused by different factors; (2) Platelet count < 50 × 109/L or requiring increase based on clinical judgment; and (3) an rhTPO dose of 300 U/kg per day with a medication duration of at least five days. The exclusion criteria were as follows: (1) Thrombocytopenia caused by platelet inhibitors, linezolid, chloramphenicol, vancomycin, sulfonamides, fluoroquinolones, or other drugs; (2) Thrombocytopenia caused by tumor chemotherapy; (3) Thrombocytopenia caused by severe infection; (4) Thrombocytopenia caused by hematological diseases; (5) Tseudothrombocytopenia and idiopathic thrombocytopenia, such as when blood samples are collected in ethylenediaminetetraacetic acid tubes; and (6) Thrombotic disease in the past six months, including pulmonary embolism, portal vein thrombosis, and deep venous thrombosis. The study was reviewed by the ethics committee of The First Affiliated Hospital of Soochow University, and ethical approval was obtained (2020 Ethics Approval No. 216).
Clinical data were collected retrospectively, including sex; age; etiology of liver diseases; routine blood tests, such as hemoglobin levels, platelet (PLT) count, platelet crit (PCT), platelet volume (MPV), and platelet distribution width (PDW); routine biochemical tests such as for total bilirubin (TBIL), serum albumin; routine blood coagulation tests, such as prothrombin time (PT), fibrinogen; other indicators, such as changes in vital signs during treatment; complications such as hepatic encephalopathy and ascites; and platelet transfusion rate. Adverse events related to treatment such as bleeding, thrombosis, and disseminated intravascular coagulation were also collected. All patients underwent Child-Pugh scoring according to laboratory examination results, imaging data, and clinical manifestations. We also performed subgroup analyses according to different baseline platelet levels, Child-Pugh grades, and medication duration.
All data were analyzed using SPSS version 25.0. Normally distributed data are expressed as mean ± SD. Non-normally distributed data are expressed as median and quartile ranges. To compare measurement data between two groups, the t-test or Wilcoxon rank sum test was used depending on whether data conformed to a normal distribution. The paired t-test or Wilcoxon signed rank test was used to compare changes in intra-group variables. Counting data are expressed as frequency and percentage, and the Pearson χ2 test or Fisher’s exact probability test was used for comparison between two groups. Unless otherwise stated, all treatment effect tests were performed at a bilateral significance level of 0.05. P < 0.05 was considered statistically significant.
A total of 100 patients were reviewed in this study, including 59 men and 41 women, with a mean age of 58.48 ± 13.90 years. Analysis of the etiology of CLD among the patients were shown in Table 1. Ninety-five patients had already been diagnosed with liver cirrhosis (LC) before enrollment. The mean duration of CLD was 11.54 years. Among the enrolled patients, the mean hemoglobin and serum albumin levels were 93.44g/L and 30.23 g/L, and the median of TBIL, fibrinogen and PT levels were 61 μmol/L, 1.50 g/L and 16.20 s, respectively, suggesting that CLD patients always accompany with liver dysfunction. As for complications related to liver disease, 21 (21%) patients had hepatic encephalopathy and 56 (56%) had ascites. During the treatment period, 90 (90%) patients did not receive PLT transfusions. From baseline to post-treatment, 4 (4%) patients had anorexia and fatigue and 2 (2%) had low-grade fever (temperature < 38 °C). No serious adverse events related to rhTPO treatment, such as infection, bleeding, or thromboembolism were observed (Table 1).
| Characteristic | Count (%) |
| Male | 59 (59.00) |
| Age | 58.48 ± 13.90 |
| Etiology | |
| Hepatitis B related CLD | 38 (38.00) |
| Hepatitis C related CLD | 3 (3.00) |
| Schistosome related CLD | 16 (16.00) |
| Autoimmune liver disease | 14 (14.00) |
| Alcoholic liver disease | 5 (5.00) |
| Liver tumors | 14 (14.00) |
| Drug induced CLD | 2 (2.00) |
| Liver abscess | 1 (1.00) |
| Chronic liver failure | 1 (1.00) |
| Budd Chiari syndrome | 1 (1.00) |
| CLD of unknown origin | 5 (5.00) |
| Child-Pugh grades | |
| Grade A (5-6 points) | 8 (8.00) |
| Grade B (7-9 points) | 48 (48.00) |
| Grade C (10-15 points) | 44 (44.00) |
| Different platelet counts (× 109/L) | |
| Group I (< 30) | 25 (25.00) |
| Group II (30-50) | 43 (43.00) |
| Group III (> 50) | 32 (32.00) |
| Medication duration | |
| Group A ( 7 d) | 31 (31.00) |
| Group B (8-14 d) | 38 (38.00) |
| Group C (15-21 d) | 22 (22.00) |
| Group D (22-28 d) | 9 (9.00) |
| No platelet transfusion | 90 (90.00) |
| Side effect | |
| Fever | 2 (2.00) |
| Fatigue and anorexia | 4 (4.00) |
Routine blood test results at baseline and post-treatment (within 10 d after drug withdrawal) were analyzed. PLT count increased significantly after treatment compared with that at baseline (42.88 ± 16.72 vs 101.53 ± 81.81 × 109/L, Table 2), and PLT count increased on average by 58.65 ± 79.24 × 109/L. Among the enrolled patients, 78 (78%) showed PLT count increased after rhTPO, and 22 (22%) showed no significant change in PLT count. The paired sample t-test was used to further analyze the data of the two groups. The PLT count and PCT levels increased significantly after treatment (P < 0.001, Table 2).
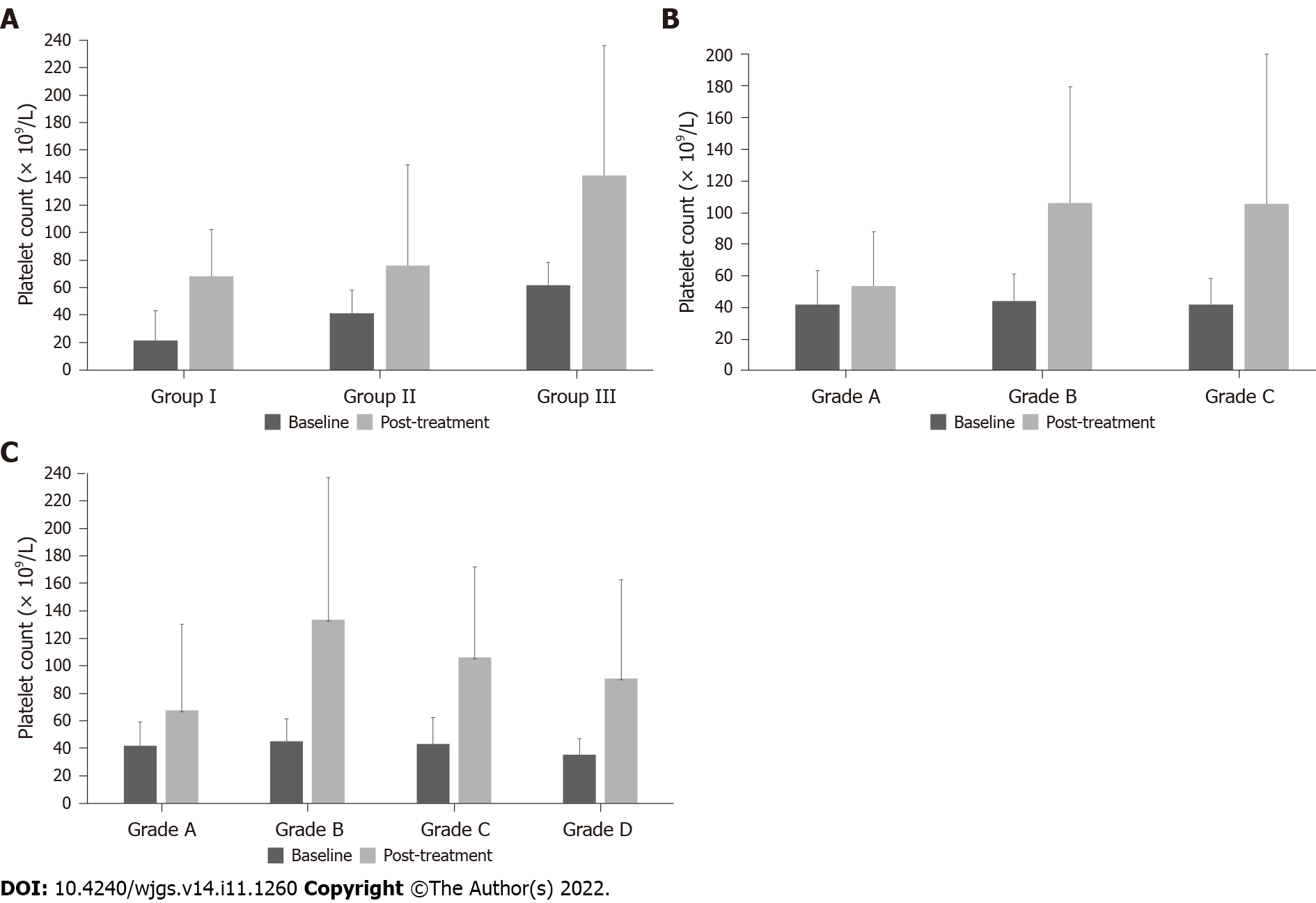
Subgroup analysis was performed based on baseline PLT counts. The overall population was divided into three groups according to the baseline PLT count, with 25 patients with PLT counts < 30 × 109/L in group I, 43 with PLT counts of 30-50 × 109/L in group II, and 32 with PLT counts of > 50 × 109/L in group III. Changes in PLT count and PCT before and after treatment were analyzed (Tables 3 and 4, Figure 1A). Regardless of baseline PLT count, the overall PLT count increased in post-treatment compared to that before treatment, and the difference was statistically significant (P < 0.05).
| Baseline (× 109/L) | Post-treatment (× 109/L) | Change (× 109/L) | P value1 | |
| Group I (n = 25) | < 0.001 | |||
| mean ± SD | 21.60 ± 7.22 | 68.28 ± 57.52 | 46.68 ± 56.77 | |
| Median | 21.00 | 55.00 | 35.00 | |
| IQR | 16.50-28.00 | 30.50-82.50 | 4.50-56.50 | |
| Min, max | 6.00, 30.00 | 7.00, 235.00 | -4.00, 214.00 | |
| Group II (n = 43) | < 0.001 | |||
| mean ± SD | 41.19 ± 5.81 | 96.23 ± 80.58 | 55.05 ± 79.80 | |
| Median | 41.00 | 76.00 | 35.00 | |
| IQR | 35.00-46.00 | 54.00-133.00 | 12.00-93.00 | |
| Min, max | 31.00, 50.00 | 9.00, 489.00 | -31.00, 448.00 | |
| Group III (n = 32) | < 0.001 | |||
| mean ± SD | 61.78 ± 8.28 | 141.53 ± 86.99 | 79.75 ± 87.04 | |
| Median | 61.00 | 127.50 | 70.00 | |
| IQR | 55.00-67.00 | 53.25-214.25 | -4.75-146.50 | |
| Min, max | 51.00, 86.00 | 7.00, 307.00 | -53.00, 255.00 |
| Baseline (%) | Post-treatment (%) | Change (%) | P value1 | |
| Group I (n = 25) | 0.0182 | |||
| mean ± SD | 0.02 ± 0.01 | 0.08 ± 0.06 | 0.06 ± 0.05 | |
| Median | 0.02 | 0.08 | 0.05 | |
| IQR | 0.01-0.03 | 0.02-0.12 | 0.01-0.10 | |
| Min, max | 0.01, 0.03 | 0.01, 0.18 | 0.00, 0.15 | |
| Group II (n = 43) | < 0.0013 | |||
| mean ± SD | 0.05 ± 0.04 | 0.13 ± 0.09 | 0.08 ± 0.11 | |
| Median | 0.05 | 0.09 | 0.05 | |
| IQR | 0.04-0.05 | 0.07-0.16 | 0.03-0.11 | |
| Min, max | 0.03, 0.26 | 0.01, 0.51 | -0.19, 0.46 | |
| Group III (n = 32) | < 0.0014 | |||
| mean ± SD | 0.07 ± 0.01 | 0.17 ± 0.09 | 0.10± 0.10 | |
| Median | 0.07 | 0.17 | 0.07 | |
| IQR | 0.06-0.08 | 0.09-0.25 | 0.01-0.18 | |
| Min, max | 0.05, 0.09 | 0.04, 0.36 | -0.04, 0.29 |
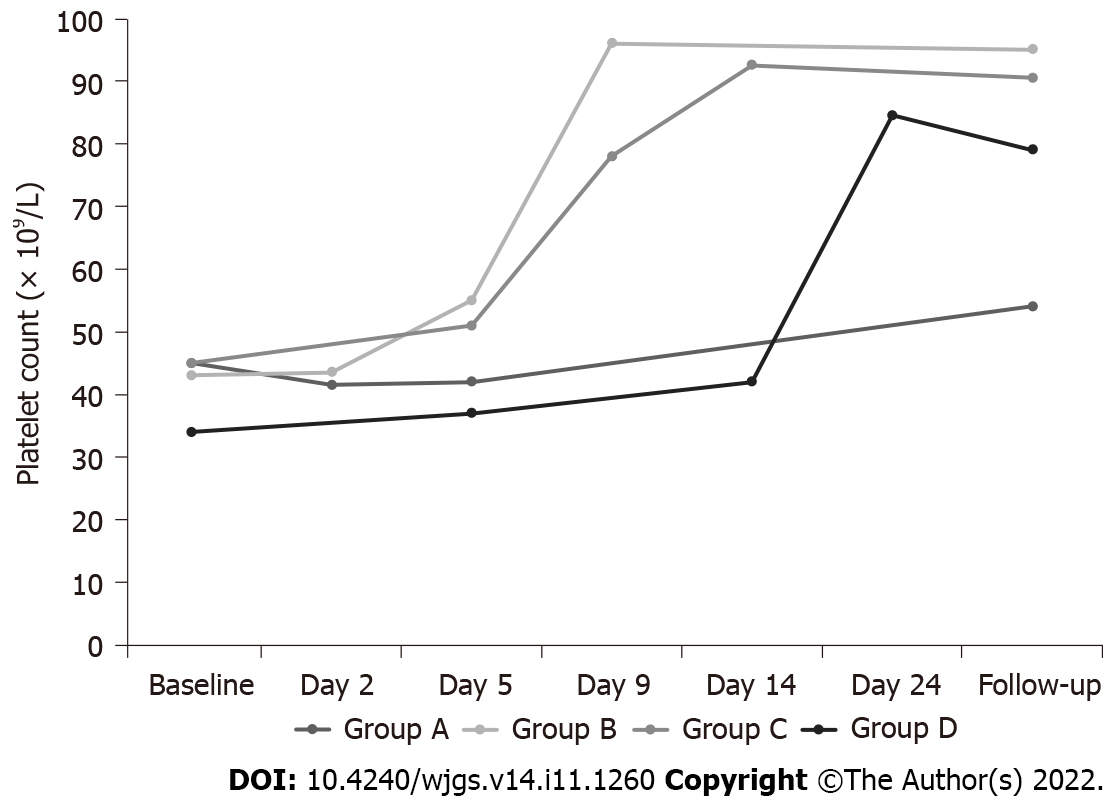
The efficacy of treatment was evaluated according to the different Child-Pugh Grades. The PLT count after rhTPO treatment was higher than that before treatment, regardless of the Child-Pugh grades (Table 5, Figure 1B). The average medication duration of Child-Pugh grade A, B and C patients were 6.75 ± 1.99, 11.81 ± 5.84, and 13.14 ± 6.73 d, respectively. All patients were grouped according to the medication duration, with 31 patients with seven days in group A, 38 patients with 8-14 d in group B, 22 patients with 15-21 d in group C and 9 patients with 22-28 d in group D. The mean treatment duration with rhTPO was 12 d in all enrolled patients. Patients in each group were analyzed at baseline, during treatment, and post-treatment periods (Table 6-9, Figure 1C), focusing on PLT count and treatment-related adverse events. The PLT count of patients with CLD showed an overall upward trend following rhTPO treatment (Figure 2).
| Baseline (× 109/L) | Post-treatment (× 109/L) | Change (× 109/L) | |
| Child-Pugh A (n = 8) | |||
| mean ± SD | 41.75 ± 21.68 | 53.63 ± 34.13 | 11.88 ± 33.00 |
| Median | 47.00 | 48.50 | 3.00 |
| IQR | 23.00-55.75 | 39.25-62.25 | -6.00-38.50 |
| Min, max | 6.00, 73.00 | 7.00, 127.00 | -34.00, 71.00 |
| Child-Pugh B (n = 48) | |||
| mean ± SD | 44.08 ± 17.01 | 105.88 ± 73.34 | 61.79 ± 69.53 |
| Median | 46.50 | 82.00 | 50.50 |
| IQR | 30.00-55.75 | 49.25-175.00 | 5.00-124.75 |
| Min, max | 10.00, 80.00 | 7.00, 266.00 | -53.00, 214.00 |
| Child-Pugh C (n = 44) | |||
| mean ± SD | 42.00 ± 16.36 | 105.52 ± 94.30 | 63.52 ± 91.48 |
| Median | 40.50 | 72.50 | 37.50 |
| IQR | 33.25-51.75 | 47.00-139.75 | 6.25-104.00 |
| Min, max | 9.00, 86.00 | 15.00, 489.00 | -36.00, 448.00 |
| Baseline | Day 2 | Day 5 | Post-treatment | Change | |
| n | 31 | 10 | 26 | 31 | 31 |
| mean ± SD (× 109/L) | 41.87 ± 17.40 | 42.90 ± 29.41 | 42.04 ± 27.39 | 67.74 ± 62.81 | 25.87 ± 57.67 |
| Median (× 109/L) | 45.00 | 41.50 | 42.00 | 54.00 | 10.00 |
| IQR (× 109/L) | 33.00-55.00 | 17.25-54.00 | 19.75-54.25 | 28.00-80.00 | -7.00-44.00 |
| Min, max (× 109/L) | 6.00, 73.00 | 15.00,111.00 | 9.00, 127.00 | 7.00, 303.00 | -34.00, 244.00 |
| Baseline | Day 2 | Day 5 | Day 9 | Post-treatment | Change | |
| n | 38 | 20 | 29 | 26 | 38 | 38 |
| mean ± SD (× 109/L) | 45.42 ± 16.27 | 46.10 ± 14.34 | 60.79 ± 33.18 | 108.19 ± 65.18 | 133.85 ± 103.23 | 81.97 ± 90.29 |
| Median (× 109/L) | 43.00 | 43.50 | 55.00 | 96.00 | 95.00 | 55.00 |
| IQR (× 109/L) | 33.50-55.50 | 33.75-57.75 | 37.00-70.00 | 49.00-166.75 | 58.00-190.00 | 21.25-127.25 |
| Min, max (× 109/L) | 9.00, 80.00 | 25.00, 71.00 | 18.00, 170.00 | 28.00, 261.00 | 21.00, 489.00 | -27.00, 448.00 |
| Baseline | Day 5 | Day 9 | Day 14 | Post-treatment | Change | |
| n | 22 | 17 | 18 | 18 | 22 | 22 |
| mean ± SD (× 109/L) | 43.36 ± 19.23 | 47.47 ± 19.60 | 69.50 ± 33.25 | 107.61 ± 65.53 | 106.32 ± 65.97 | 62.95 ± 64.96 |
| Median (× 109/L) | 45.00 | 51.00 | 78.50 | 92.50 | 90.50 | 47.50 |
| IQR (× 109/L) | 27.75-59.25 | 31.00-54.50 | 32.50-85.75 | 55.25-181.25 | 55.25-148.00 | 16.5-128.25 |
| Min, max (× 109/L) | 10.00, 86.00 | 19.00, 95.00 | 20.00, 138.00 | 7.00, 235.00 | 7.00, 222.00 | -53.00, 179.00 |
| Baseline | Day 5 | Day 14 | Day 24 | Post-treatment | Change | |
| n | 9 | 9 | 7 | 8 | 9 | 9 |
| mean ± SD (× 109/L) | 35.56 ± 11.72 | 41.22 ± 17.41 | 50.29 ± 33.76 | 83.88 ± 34.87 | 90.89 ± 71.85 | 55.33 ± 63.15 |
| Median (× 109/L) | 34.00 | 37.00 | 42.00 | 84.50 | 79.00 | 45.00 |
| IQR (× 109/L) | 27.50-38.00 | 33.00-51.50 | 39.00-45.00 | 47.75-118.00 | 36.50-126.50 | 5.50-93.00 |
| Min, max (× 109/L) | 25.00, 64.00 | 16.00, 78.00 | 18.00, 124.00 | 42.00, 127.00 | 29.00, 254.00 | -12.00, 190.00 |
A common complication of CLD in the blood is thrombocytopenia. The incidence of thrombocytopenia caused by CLD varies across different studies. The average prevalence of thrombocytopenia in CLD is about 6%; however, when the disease progresses to LC, the morbidity can reach 78%[2]. Apart from viral hepatitis, the incidence of alcoholic liver disease and non-alcoholic fatty liver disease are gradually increasing, and immune hepatitis and drug-induced liver dysfunction also account for some cases of CLD. In this study, the mean duration of liver disease in enrolled patients was 11.54 years, and 95% of them had already progressed from CLD to LC. Viral hepatitis is the most common cause of cirrhosis-induced thrombocytopenia. In our study, 41 (41%) cases of CLD with thrombocytopenia were caused by hepatitis B or C viral infection.
CLD-associated thrombocytopenia has complicated mechanisms, and the reduced production, excessive destruction, and abnormal distribution of PLT are all involved. A decrease in thrombopoietin (TPO) levels is the leading cause of thrombocytopenia. TPO is a hematopoietic growth factor that exerts its biological effects by binding to specific c-Mpl receptors on the surface of megakaryocytes and PLT; it regulates the proliferation, differentiation, and internal replication of megakaryocytes and modulates PLT-specific proteins and circulating PLT concentration[6]. Thus, TPO can stimulate PLT production and increase peripheral blood PLT count. In addition, TPO acts on hematopoietic stem cells to protect and regulate the hematopoietic stem cell pool. It cooperates with erythropoietin, stem cell factor, interleukin-3, and granulocyte colony stimulating factor to promote the proliferation of erythroid and granulocyte progenitor cells and stem cells to enter the proliferation cycle[7,8]. TPO is mainly synthesized in liver parenchymal and sinusoidal cells and also in the bone marrow and kidney[9]. TPO level in peripheral blood decreases with liver malfunction persists, which is particularly manifested in CLD and LC[2,10]. Hypersplenism is another classic cause of thrombocytopenia in patients with LC. The larger the spleen, the more blood cells are retained in the spleen and the more obvious the decrease in blood cell count in peripheral circulation.
In patients with CLD and LC, mild (50-100 × 109/L) thrombocytopenia is often not complicated by serious bleeding risk. Treatment may be suspended temporarily without the need for invasive operations or the occurrence of complications, such as esophagogastric varices. Moderate (20-50 × 109/L) and severe (< 20 × 109/L) thrombocytopenia are independent risk factors for poor prognosis in advanced CLD[11]. Oliver et al[12] compared the mortality of patients with CLD undergoing non-liver surgery to that of patients without CLD, and found that the odds of mortality were 1.8–3.3 times higher in patients with CLD [odds ratio of bleeding 2.0 (1.8-2.3)]. Owing to the high risk of bleeding, symptomatic treatment, such as PLT transfusion and the use of TPO analogs and TPO receptor agonists, is often required according to the etiology and changes in the patient’s condition. PLT transfusion carries the risk of PLT antibody production, which results in resistance to subsequent PLT transfusion[13]. Moreover, PLT transfusion still has potential risks[14], such as infectious diseases caused by blood transfusion, fever, allergic reactions, and hemolytic reactions. Besides, PLT transfusion has a limited effect on CLD, with PLT counts increasing by approximately 10 × 109/L after transfusion[15,16], while PLT transfusion can increase PLT count by 30 × 109/L in healthy patients[17]. In this study, 90% patients did not receive PLT transfusion, partially due to an increase in their PLT count after rhTPO treatment. Romiplostim and eltrombopag are widely used TPO receptor agonists. However, owing to the risk of thromboembolic adverse events[18], these drugs are not suitable for patients with CLD. rhTPO is a full-length glycosylated TPO expressed by Chinese hamster ovary cells and purified via gene recombination technology. Because its characteristics are similar to those of endogenous TPO, rhTPO has similar pharmacological effects on PLT levels. The drug was approved for use in China for the treatment of thrombocytopenia caused by chemotherapy for solid tumors and ITP. In our study, rhTPO had a positive effect on CLD associated thrombocytopenia, and no serious adverse effects were observed. The results suggest that rhTPO could improve the platelet count and reduce the risk of bleeding in patients with CLD, also increase the probability of tolerance to receive invasive management, such as liver surgery, liver biopsy, and artificial extracorporeal liver support, to improve clinical benefits in patients. The PLT count increased in 78 patients after treatment with rhTPO, and there was no significant change in the PLT count of 22 patients, including 13 with end-stage LC, six with liver cancer, and three with severe liver dysfunction. Due to the ineffectiveness of rhTPO in these patients, specific mechanisms were speculated: (1) Bone marrow suppression caused by CLD. CLD caused by hepatitis viruses [such as the hepatitis C virus (HCV)] inhibits PLT production in the bone marrow, resulting in thrombocytopenia[2]. In a study by Zhang et al[19], the core envelope of HCV was highly homologous with the PLT membrane glycoprotein GPIIIa49-66 and induced thrombocytopenia in the form of molecular modeling, which may be the reason for HCV-related LC associated thrombocytopenia. Recently, a retrospective analysis[20] also showed a significant increase in PLT count after virus elimination in patients with HCV-related CLD or LC. In addition, alcohol inhibits the formation of hematopoietic cells, increases damage, and changes the morphology and function of hematopoietic cells through direct toxicity to the bone marrow and peripheral blood[21]; (2) CLD-induced production of PLT antibodies, such as PLT-associated immunoglobulin G (IgG) antibodies and autoantibodies against PLT membrane proteins. PLT-associated IgG and PLT glycoprotein autoantibody levels are increased in patients with LC[22]. In patients with liver disease, autoantibodies against PLT surface antigens accelerate the consumption of PLTs in the spleen and trigger rapid destruction[23]. Kajihara et al[24] found that patients with LC or ITP had similar anti-PLT membrane Glycoprotein Ⅱb Ⅲa (GP Ⅱb Ⅲa) antibody responses. The frequency of stimulation of GP IIb IIIa antibody to produce B cells in patients with LC is even higher than that in patients with ITP, suggesting that autoantibody-mediated PLT destruction is partly involved in LC-related thrombocytopenia. Similarly, Wada et al[23] found that B cells produced by anti-GP Ⅱb IIIa antibodies may predict the efficacy of TPO agonists in patients with CLD or LC; and (3) thrombocytopenia caused by CLD is complicated by infection. Decreased platelet count produced by megakaryocytes in the bone marrow is the main cause in CLD-related thrombocytopenia[2]. Patients with CLD exhibit impaired immune function and are immunocompromised. Various factors, such as liver dysfunction, intestinal bacterial translocation, and increased portal and systemic shunt, increase the risk of infection[25], especially with tumors and end-stage LC, making patients prone to severe infection and even sepsis. Moreover, infection can promote disease progression and increase mortality in patients with CLD. Bone marrow suppression caused by infectious agents is common in clinics. Sepsis accounts for approximately 50% of all cases of thrombocytopenia in severe patients[26]. In patients with sepsis, the pathogenesis of thrombocytopenia is often related to an imbalance in the host response[27], such as an increase in cytokine levels, enhancement of vascular endothelial cell activity, and serious loss of vascular integrity. PLTs are activated by inflammatory factors and bacterial products, causing a cascade reaction of coagulation and promoting the excessive consumption of PLTs.
Additionally, this study showed that rhTPO significantly improved PLT counts and PCT levels in enrolled patients compared with the values at baseline levels, regardless of the duration of medication or the Child-Pugh grade. PCT level can be used as a parameter to predict advanced fibrosis and cirrhosis. It mainly refers to the percentage of PLT in the peripheral blood volume[28,29]. In this study, PCT levels increased after rhTPO administration compared to those before administration. A possible reason is that PCT can be expressed as the product of PLT count and MPV and increases with PLT count. MPV and PDW reflect PLT size and function, respectively. They can be used as indicators of inflammatory responses in vivo and reflect PLT activation. Large PLTs are more likely to produce inflammatory factors and prethrombotic substances, which promote inflammatory reactions and thrombosis in the body. In this study, the differences of MPV and PDW between baseline and post-treatment were not statistically significant, suggesting that rhTPO mainly affected PLT counts and had little effect on MPV and PDW indices.
In conclusion, rhTPO was effective in the treatment of CLD-associated thrombocytopenia, with no serious adverse events related to treatment, suggesting good medication safety and providing a new approach for the treatment of CLD-related thrombocytopenia. As such, rhTPO can prevent hemorrhagic events and provide opportunities for safer invasive procedures or other non-liver surgeries, which can improve the overall survival for patients with CLD. Moreover, the administration of rhTPO could reduce the need for PLT transfusion and the risks associated with it[30]. This study has some limitations. Firstly, it was a retrospective study. The sample size was small and limited to one region. The follow-up duration was short; thus, we could not assess the long-term effects. Owing to the generally low PLT count in the enrolled patients, there was a lack of data on the collection of PLT parameters. In future studies, more comprehensive data are needed, including body mass index, medication history, and long-term follow-up evaluation after treatment, to further prove the efficacy and safety of rhTPO in CLD-related thrombocytopenia.
Thrombocytopenia is a common complication in chronic liver disease (CLD), promoting a high risk of bleeding and a poor prognosis, especially in patients undergoing invasive procedures or surgeries.
Recombinant human thrombopoietin (rhTPO) is commonly used to treat primary immune thrombocytopenic purpura and thrombocytopenia caused by solid tumor chemotherapy, and has not been extensively investigated in the treatment of CLD-related thrombocytopenia.
This study aimed to evaluate the efficacy of rhTPO in the treatment of patients with CLD-associated thrombocytopenia undergoing invasive procedures.
This retrospective analysis of clinical data of patients with CLD assessed the changes in platelet counts and parameters before and after the use of rhTPO for thrombocytopenia. Subgroup analysis was performed according to different characteristics, such as baseline platelet count levels. Adverse events related to treatment were investigated.
Among the enrolled patients, 78 (78%) showed an elevation in platelet count after rhTPO use. The mean platelet count after rhTPO treatment in all patients was 101.53 ± 81.81 × 109/L, which was significantly improved compared to that at baseline (42.88 ± 16.72 × 109/L), and this difference was statistically significant (P < 0.001). Subgroup analysis also showed the same result. Ninety (90%) patients did not require platelet transfusion partially due to an increase in platelet count after treatment with rhTPO.
rhTPO was effective in the treatment of CLD-associated thrombocytopenia with good medication safety, promoting the safety of invasive procedures and improving overall survival of patients with CLD.
rhTPO could be a new approach for the treatment of CLD-related thrombocytopenia that will promote clinical benefits in patients with CLD who are undergoing invasive procedures.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nio M, Japan; Wagner-Skacel J, Austria S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Dieterich DT, Bernstein D, Flamm S, Pockros PJ, Reau N. Review article: a treatment algorithm for patients with chronic liver disease and severe thrombocytopenia undergoing elective medical procedures in the United States. Aliment Pharmacol Ther. 2020;52:1311-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Peck-Radosavljevic M. Thrombocytopenia in chronic liver disease. Liver Int. 2017;37:778-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 187] [Article Influence: 23.4] [Reference Citation Analysis (1)] |
| 3. | Terrault N, Chen YC, Izumi N, Kayali Z, Mitrut P, Tak WY, Allen LF, Hassanein T. Avatrombopag Before Procedures Reduces Need for Platelet Transfusion in Patients With Chronic Liver Disease and Thrombocytopenia. Gastroenterology. 2018;155:705-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 168] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 4. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6059] [Article Influence: 865.6] [Reference Citation Analysis (3)] |
| 5. | Chinese Society of Hepatology; Chinese Medical Association. [Chinese guidelines on the management of liver cirrhosis]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:846-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 6. | Kuter DJ. Managing thrombocytopenia associated with cancer chemotherapy. Oncology (Williston Park). 2015;29:282-294. [PubMed] |
| 7. | Mosaad YM. Hematopoietic stem cells: an overview. Transfus Apher Sci. 2014;51:68-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Seita J, Weissman IL. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med. 2010;2:640-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 594] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 9. | Qian S, Fu F, Li W, Chen Q, de Sauvage FJ. Primary role of the liver in thrombopoietin production shown by tissue-specific knockout. Blood. 1998;92:2189-2191. [PubMed] |
| 10. | Rauber P, Lammert F, Grotemeyer K, Appenrodt B. Immature platelet fraction and thrombopoietin in patients with liver cirrhosis: A cohort study. PLoS One. 2018;13:e0192271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Bleibel W, Caldwell SH, Curry MP, Northup PG. Peripheral platelet count correlates with liver atrophy and predicts long-term mortality on the liver transplant waiting list. Transpl Int. 2013;26:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Oliver JB, Merchant AM, Koneru B. The Impact of Chronic Liver Disease on Postoperative Outcomes and Resource Utilization. J Invest Surg. 2021;34:617-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Slichter SJ. Evidence-based platelet transfusion guidelines. Hematology Am Soc Hematol Educ Program. 2007;172-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Lange NW, Salerno DM, Berger K, Cushing MM, Brown RS Jr. Management of Hepatic Coagulopathy in Bleeding and Nonbleeding Patients: An Evidence-Based Review. J Intensive Care Med. 2021;36:524-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Furuichi Y, Takeuchi H, Yoshimasu Y, Kasai Y, Abe M, Itoi T. Thrombopoietin receptor agonist is more effective than platelet transfusion for chronic liver disease with thrombocytopenia, shown by propensity score matching. Hepatol Res. 2020;50:1062-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Hidaka H, Kurosaki M, Tanaka H, Kudo M, Abiru S, Igura T, Ishikawa T, Seike M, Katsube T, Ochiai T, Kimura K, Fukuhara T, Kano T, Nagata T, Tanaka K, Kurokawa M, Yamamoto K, Osaki Y, Izumi N, Imawari M. Lusutrombopag Reduces Need for Platelet Transfusion in Patients With Thrombocytopenia Undergoing Invasive Procedures. Clin Gastroenterol Hepatol. 2019;17:1192-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 17. | Yoshiji H, Ueno Y, Kurosaki M, Torimura T, Hatano E, Yatsuhashi H, Yamakado K. Treatment algorithm for thrombocytopenia in patients with chronic liver disease undergoing planned invasive procedures. Hepatol Res. 2021;51:1181-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Afdhal NH, Giannini EG, Tayyab G, Mohsin A, Lee JW, Andriulli A, Jeffers L, McHutchison J, Chen PJ, Han KH, Campbell F, Hyde D, Brainsky A, Theodore D; ELEVATE Study Group. Eltrombopag before procedures in patients with cirrhosis and thrombocytopenia. N Engl J Med. 2012;367:716-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 241] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 19. | Zhang W, Nardi MA, Borkowsky W, Li Z, Karpatkin S. Role of molecular mimicry of hepatitis C virus protein with platelet GPIIIa in hepatitis C-related immunologic thrombocytopenia. Blood. 2009;113:4086-4093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Saab S, Brown RS Jr. Management of Thrombocytopenia in Patients with Chronic Liver Disease. Dig Dis Sci. 2019;64:2757-2768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Ballard HS. Hematological complications of alcoholism. Alcohol Clin Exp Res. 1989;13:706-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Zermatten MG, Fraga M, Moradpour D, Bertaggia Calderara D, Aliotta A, Stirnimann G, De Gottardi A, Alberio L. Hemostatic Alterations in Patients With Cirrhosis: From Primary Hemostasis to Fibrinolysis. Hepatology. 2020;71:2135-2148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 23. | Wada N, Uojima H, Satoh T, Okina S, Iwasaki S, Shao X, Takiguchi H, Arase Y, Itokawa N, Atsukawa M, Miyazaki K, Hidaka H, Kako M, Kagawa T, Iwakiri K, Horie R, Suzuki T, Koizumi W. Impact of Anti-GPIIb/IIIa Antibody-Producing B Cells as a Predictor of the Response to Lusutrombopag in Thrombocytopenic Patients with Liver Disease. Dig Dis. 2021;39:234-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Kajihara M, Kato S, Okazaki Y, Kawakami Y, Ishii H, Ikeda Y, Kuwana M. A role of autoantibody-mediated platelet destruction in thrombocytopenia in patients with cirrhosis. Hepatology. 2003;37:1267-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Xu JH, Yu YY, Xu XY. [Emphasizing the clinical diagnosis and treatment of liver disease-associated infections]. Zhonghua Gan Zang Bing Za Zhi. 2021;29:721-724. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Levi M, Opal SM. Coagulation abnormalities in critically ill patients. Crit Care. 2006;10:222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 206] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 27. | Claushuis TA, van Vught LA, Scicluna BP, Wiewel MA, Klein Klouwenberg PM, Hoogendijk AJ, Ong DS, Cremer OL, Horn J, Franitza M, Toliat MR, Nürnberg P, Zwinderman AH, Bonten MJ, Schultz MJ, van der Poll T; Molecular Diagnosis and Risk Stratification of Sepsis Consortium. Thrombocytopenia is associated with a dysregulated host response in critically ill sepsis patients. Blood. 2016;127:3062-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 226] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 28. | Michalak A, Cichoż-Lach H, Guz M, Kozicka J, Cybulski M, Jeleniewicz W. Plateletcrit and Mean Platelet Volume in the Evaluation of Alcoholic Liver Cirrhosis and Nonalcoholic Fatty Liver Disease Patients. Biomed Res Int. 2021;2021:8867985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Wang J, Xia J, Yan X, Yang Y, Wei J, Xiong Y, Wu W, Liu Y, Chen Y, Jia B, Chen Z, Zhang Z, Ding W, Huang R, Wu C. Plateletcrit as a potential index for predicting liver fibrosis in chronic hepatitis B. J Viral Hepat. 2020;27:602-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Khemichian S, Terrault NA. Thrombopoietin Receptor Agonists in Patients with Chronic Liver Disease. Semin Thromb Hemost. 2020;46:682-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |