Published online Nov 27, 2022. doi: 10.4240/wjgs.v14.i11.1219
Peer-review started: July 22, 2022
First decision: September 26, 2022
Revised: October 10, 2022
Accepted: October 19, 2022
Article in press: October 19, 2022
Published online: November 27, 2022
Processing time: 126 Days and 2.1 Hours
Few studies compared the oncological and biological characteristics between ampullary carcinoma (AC) and cancer of the second portion of the duodenum (DC-II), although both tumors arise from anatomically close locations.
To elucidate differences in clinicopathological characteristics, especially the patterns of lymph node metastasis (LNM), between AC and DC-II.
This was a retrospective cohort study of 80 patients with AC and 27 patients with DC-II who underwent pancreaticoduodenectomy between January 1998 and December 2018 in two institutions. Clinicopathological factors, LNM patterns, and prognosis were compared between the two groups.
The patients with AC and DC-II did not exhibit significant differences in 5-year overall survival (66.0% and 67.1%, respectively) and 5-year relapse-free survival (63.5% and 62.2%, respectively). Compared to the patients with DC-II, the rate of preoperative biliary drainage was higher (P = 0.042) and the rates of digestive symptoms (P = 0.0158), ulcerative-type cancer (P < 0.0001), large tumor diameter (P < 0.0001), and advanced tumor stage (P = 0.0019) were lower in the patients with AC. The LNM rates were 27.5% and 40.7% in patients with AC and DC-II, respectively, without significant difference (P = 0.23). The rates of LNM to hepatic nodes (N-He) and pyloric nodes (N-Py) were significantly higher in patients with DC-II than in those with AC (metastasis to N-HE: 18.5% and 5% in patients with DC-II and AC, respectively; P = 0.0432; metastasis to N-Py: 11.1% and 0% in patients with DC-II and AC, respectively; P = 0.0186)
Although there were no significant differences in the prognosis and recurrence rates between the two groups, metastases to N-He and N-Py were more frequent in patients with DC-II than in those with AC.
Core Tip: Few studies compared the oncological and biological characteristics between ampullary carcinoma (AC) and cancer of the second portion of the duodenum (DC-II), although both tumors arise from anatomically close locations. Here, we found that the rate of preoperative biliary drainage was significantly higher and the rates of digestive symptoms, ulcerative-type cancer, large tumor diameter, and advanced tumor stage were significantly lower in AC than in DC-II. There were no significant differences in prognosis, recurrence, and lymph node metastasis rates between the two groups, although hepatic and pyloric lymph node metastases were more frequent in DC-II than in AC.
- Citation: Nishio K, Kimura K, Murata A, Ohira G, Shinkawa H, Kodai S, Amano R, Tanaka S, Shimizu S, Takemura S, Kanazawa A, Kubo S, Ishizawa T. Comparison of clinicopathological characteristics between resected ampullary carcinoma and carcinoma of the second portion of the duodenum. World J Gastrointest Surg 2022; 14(11): 1219-1229
- URL: https://www.wjgnet.com/1948-9366/full/v14/i11/1219.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i11.1219
Ampullary carcinoma (AC) accounts for 0.2% of all gastrointestinal cancers and 7% of all periampullary cancers[1]. In contrast to other periampullary carcinomas, AC is associated with higher resection rates and better prognosis because of its earlier presentation due to the anatomical characteristics[2]. The reported rates of resection and 5-year survival after resection of AC are approximately 50%[3] and 30%-52%[4,5], respectively, whereas primary duodenal cancer (DC) accounts for approximately 0.3% of all gastrointestinal cancers[6] and 30%-45% of all small intestinal cancers[7]. The reported rates of resection and 5-year survival after resection of DC are 39%[8] and 37%-67%[9-12], respectively. The only curative treatment for both AC and DC, especially DC located in the second portion of the duodenum (DC-II), is surgical resection with regional lymph node dissection using pancreaticoduodenectomy. The National Comprehensive Cancer Network (NCCN) guidelines recommend pancreaticoduodenectomy with en bloc removal of regional lymph nodes for resectable DC-II and state that pyloric preservation is acceptable in the absence of a hereditary condition[13]. In contrast, there are no NCCN guidelines for AC. The lymph node metastasis (LNM) patterns and the optimal range of lymph node dissection in DC-II and AC remain controversial. The present study aimed to compare the oncological and biological characteristics between DC-II and AC.
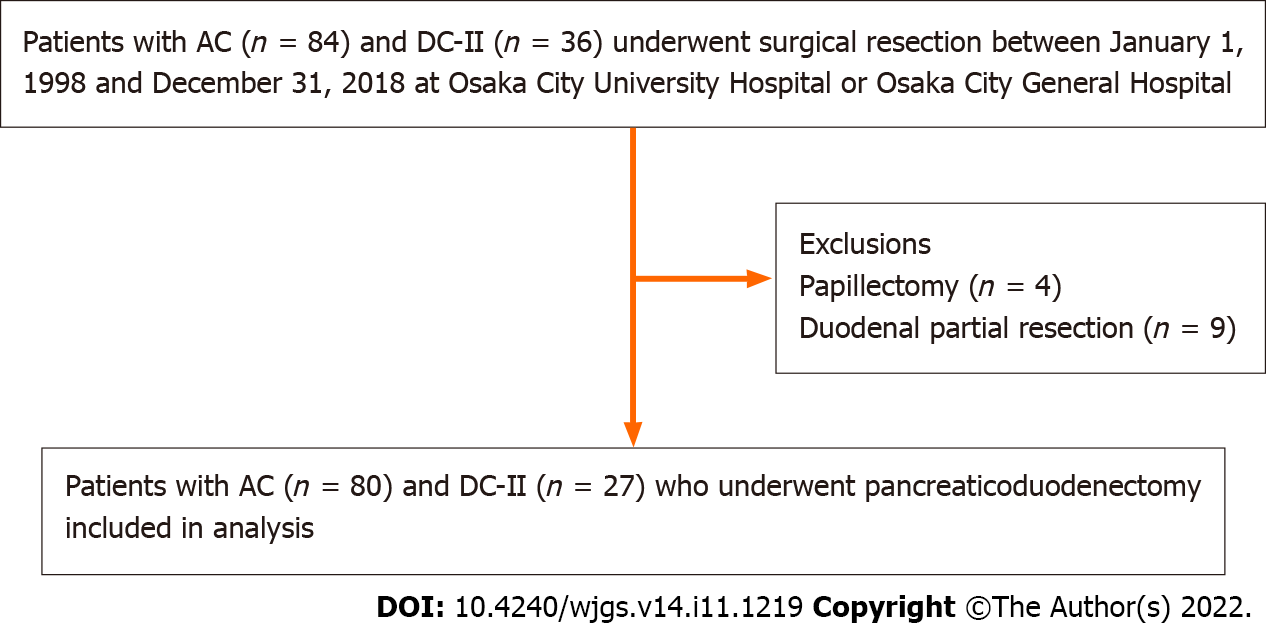
Eighty-four patients with AC and thirty-six patients with DC-II who underwent surgical resection in Osaka City University Hospital or Osaka City General Hospital between January 1, 1998 and December 31, 2018. After the exclusion of patients who underwent duodenal partial resection (n = 9) and papillectomy (n = 4), the remaining 80 patients with AC and 27 patients with DC-II who underwent pancreaticoduodenectomy were included in the present retrospective cohort study (Figure 1). All patients were followed for survival, and the median follow-up period was 36.5 (range, 2.3-227.3) months. Recurrence was defined when the tumor was detected again by imaging modalities, such as enhanced CT. Surgical approaches included classical pancreaticoduodenectomy (Whipple procedure) in 50 patients (12 patients with DC-II and 38 patients with AC), subtotal stomach-preserving pancreaticoduodenectomy in 49 patients (14 patients with DC-II and 35 patients with AC), and pylorus-preserving pancreaticoduodenectomy in 8 patients (1 patient with DC-II and 7 patients with AC). As adjuvant chemotherapy, 33 patients, including 8 patients with DC-II and 25 patients with AC, received S-1 (4 patients with DC-II and 14 patients with AC), tegafur-uracil (3 patients with DC-II and 8 patients with AC), and gemcitabine (1 patient with DC-II and 3 patients with AC). There were no definitive criteria for the administration of adjuvant chemotherapy.
The demographic and clinical variables included age, sex, preoperative body mass index, preoperative modified Glasgow prognostic score, tumor size, gross appearance, preoperative biliary drainage, preoperative symptoms, preoperative serum carbohydrate antigen level, preoperative serum carcinoembryonic antigen level, operative procedure, duration of operation, volume of intraoperative blood loss, histological grade, Union for International Cancer Control (UICC) classification, LNM, lymphatic invasion, venous invasion, postoperative complications, and adjuvant chemotherapy.
The TNM classification and the pathological stage of all tumor specimens were determined using the 7th edition of the UICC TNM classification[14]. Tumor differentiation was classified into well differentiated, moderately differentiated, poorly differentiated, and undifferentiated adenocarcinoma, according to the World Health Organization classification[15]. Regional lymph nodes were classified into superior pancreaticoduodenal lymph nodes (N-SP), inferior pancreaticoduodenal lymph nodes (N-IP), pyloric lymph nodes (N-Py), hepatic lymph nodes (N-He), and superior mesenteric lymph nodes (N-SM) according to AJCC Cancer Staging 7th edition[16]. The initial recurrent sites were classified into liver, lungs, distant lymph nodes, peritoneum, local, and others.
The clinicopathological factors were compared between the patients with DC-II and AC. Categorical variables were compared using the χ2 or Fisher’s exact test. Continuous variables were compared using Mann–Whitney U tests. Survival was calculated using the Kaplan–Meier method, and comparisons between the groups were performed using the log-rank test. P values of < 0.05 were considered to indicate statistical significance. All statistical analyses were performed using JMP® version 12 (SAS Institute, Cary, NC, United States).
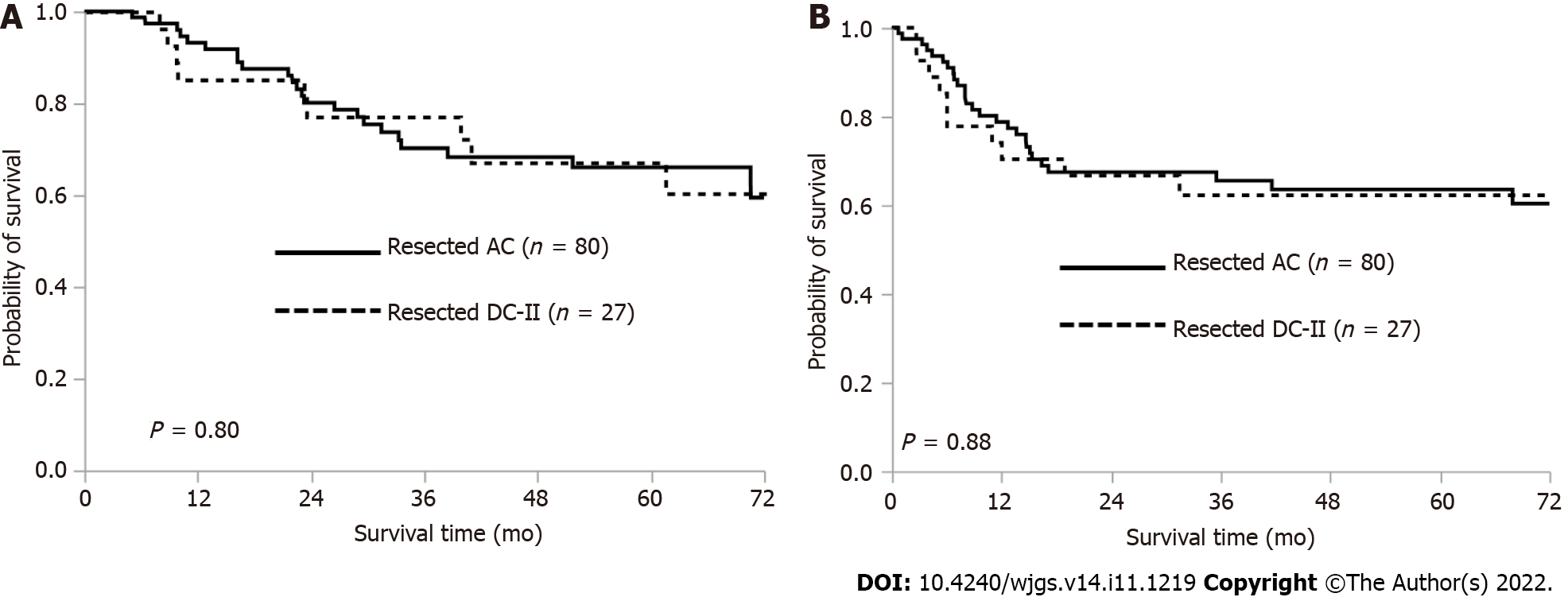
The 5-year overall survival (OS) rate was 66.0% in the patients with AC and 67.1% in those with DC-II (P = 0.80) (Figure 2A). The 5-year RFS rate was 63.5% in the patients with AC and 62.2% in those with DC-II (P = 0.88) (Figure 2B).
Table 1 shows the results of the comparative analysis of the clinicopathological factors between the patients with DC-II and AC. Briefly, the rate of preoperative biliary drainage was significantly higher in the patients with AC than in those with DC-II (P = 0.042). Conversely, the rates of digestive symptoms i.e., vomiting, nausea or abdominal pain (P = 0.0158), ulcerative-type tumor (P < 0.0001), large tumor diameter (P < 0.0001), and advanced tumor invasion (P = 0.0019) were significantly higher in the patients with DC-II than in those with AC. The LNM rate was 27.5% in the patients with AC and 40.7% in those with DC-II, without significant difference (P = 0.23).
| Variable | Comparison | DC-II (n = 27), % | AC (n = 80) | P value | |
| Sex | Male | 15 (55.6) | 49 (61.3) | 0.65 | |
| Female | 12 (44.4) | 31 (38.7) | |||
| Age | Median (range) | 69 (41-85) | 64 (37-84) | 0.35 | |
| Preoperative BMI (kg/m2) | Median (range) | 22.1 (16.9-27.3) | 21.7 (15.8-31.3) | 0.59 | |
| Preoperative mGPS | 0 | 17 | 47 | - | |
| 1 | 5 | 18 | - | ||
| 2 | 5 | 14 | - | ||
| 0 | 17 (63.0) | 47 (58.8) | 0.82 | ||
| 1-2 | 10 (37.0) | 32 (40.0) | |||
| Preoperative biliary drainage | No | 21 (77.8) | 44 (55.0) | 0.042 | |
| Yes | 6 (22.2) | 36 (45.0) | |||
| Preoperative symptoms | Absent | 8 (29.6) | 31 (38.7) | 0.49 | |
| Present | 19 (70.4) | 49 (61.3) | |||
| Digestive symptoms | Absent | 13 (48.1) | 60 (75.0) | 0.0158 | |
| Present | 14 (51.9) | 20 (25.0) | |||
| Anemia or tarry stool | Absent | 23 (85.2) | 77 (96.3) | 0.06 | |
| Present | 4 (14.8) | 3 (3.7) | |||
| Preoperative CA19-9 (U/mL) | Normal | 19 (70.4) | 56 (70.0) | 1 | |
| Elevated | 8 (29.6) | 24 (30.0) | |||
| Preoperative CEA (ng/mL) | Normal | 25 (92.6) | 66 (82.5) | 0.35 | |
| Elevated | 2 (7.4) | 13 (16.3) | |||
| Surgery | PD | 12 | 38 | - | |
| SSPPD | 14 | 35 | - | ||
| PpPD | 1 | 7 | - | ||
| Operation time (min) | Median (range) | 451 (287-837) | 446.5 (266-736) | 0.44 | |
| Intraoperative blood loss volume (mL) | Median (range) | 685 (80-4110) | 652 (150-9015) | 0.48 | |
| Gross appearance | Protruding type | 8 (29.6) | 59 (73.8) | < 0.0001 | |
| Ulcerative-type | 19 (70.4) | 21 (26.2) | |||
| Histological grade | Pap | 1 | 3 | - | |
| Well | 10 | 42 | - | ||
| Mod | 13 | 31 | - | ||
| Por | 1 | 4 | - | ||
| Muc | 2 | 0 | - | ||
| Pap/well | 11 (40.7) | 45 (56.3) | 0.19 | ||
| Mod/por/muc | 16 (59.3) | 35 (43.7) | |||
| Tumor diameter (mm) | Median (range) | 35 (14-65) | 18 (5-84) | < 0.0001 | |
| T category1 | Tis | 5 | 23 | - | |
| T1 (1a, 1b) | 5 (4, 1) | 9 | - | ||
| T2 | 1 | 28 | - | ||
| T3 | 5 | 16 | - | ||
| T4 | 11 | 4 | - | ||
| T0–T2 | 11 (40.7) | 60 (75.0) | 0.0019 | ||
| T3–T4 | 16 (59.3) | 20 (25.0) | |||
| N factor | N0 | 16 | 58 | - | |
| N1 | 5 | 22 | - | ||
| N2 | 6 | x | - | ||
| Lymph node metastasis | Absent | 16 (59.3) | 58 (72.5) | 0.23 | |
| Present | 11 (40.7) | 22 (27.5) | |||
| Number of lymph nodes with metastasis | Median (range) | 0 (0–6) | 0 (0–12) | 0.13 | |
| M factor | M0 | 24 | 78 | 0.1 | |
| M1 | 3 | 2 | - | ||
| Stage | 0 | 5 | 22 | - | |
| I (A, B) | 6 | 29 (11, 18) | - | ||
| II A | 2 | 4 | - | ||
| II B | 3 | 19 | - | ||
| III (A, B) | 8 (5, 3) | 4 | - | ||
| IV | 3 | 2 | - | ||
| Lymphatic invasion | 0 | 15 | 50 | - | |
| 1 | 4 | 12 | - | ||
| 2 | 7 | 15 | - | ||
| 3 | 1 | 2 | - | ||
| X | 0 | 1 | - | ||
| 0 | 15 (55.6) | 50 (62.5) | 0.5 | ||
| 1-3 | 12 (44.4) | 29 (36.3) | |||
| Venous invasion | 0 | 20 | 69 | - | |
| 1 | 5 | 8 | - | ||
| 2 | 2 | 2 | - | ||
| 3 | 0 | 0 | - | ||
| X | 0 | 1 | - | ||
| 0 | 20 (74.1) | 69 (86.3) | 0.13 | ||
| 1-3 | 7 (25.9) | 10 (12.5) | |||
| Postoperative complication (≥ CD III) | No | 18 (66.7) | 42 (52.5) | 0.26 | |
| Yes | 9 (33.3) | 38 (47.5) | |||
| Adjuvant chemotherapy | No | 19 (70.4) | 55 (68.8) | 1 | |
| Yes | 8 (29.6) | 25 (31.2) | |||
Table 2 shows the results of the comparative analysis of the affected sites and the frequency of LNM to specific sites between the patients with DC-II and AC. In summary, the rates of LNM to the N-He and the N-Py were significantly higher in the patients with DC-II than in those with AC (metastasis to N-He: 18.5% and 5% in patients with DC-II and AC, respectively; P = 0.0432; metastasis to N-Py: 11.1% and 0% in patients with DC-II and AC, respectively; P = 0.0186). There were no significant differences in the rates of metastases to the N-SP, N-IP, and N-SM between the patients with DC-II and AC.
| Variable | Comparison | DC-II (n = 27), % | AC (n = 80), % | P value |
| N-Pya | present | 3(11.1) | 0 (0) | 0.0186 |
| absent | 23 (85.2) | 73 (100) | ||
| N-He | present | 5 (18.5) | 4 (5) | 0.0432 |
| absent | 22 (81.5) | 76 (95) | ||
| N-SP | present | 7 (25.9) | 14 (17.5) | 0.40 |
| absent | 20 (74.1) | 66 (82.5) | ||
| N-IP | present | 3 (11.1) | 10(12.5) | 1.00 |
| absent | 24 (88.9) | 70 (87.5) | ||
| N-SM | present | 2 (7.4) | 5 (6.2) | 1.00 |
| absent | 25 (92.6) | 75 (93.8) |
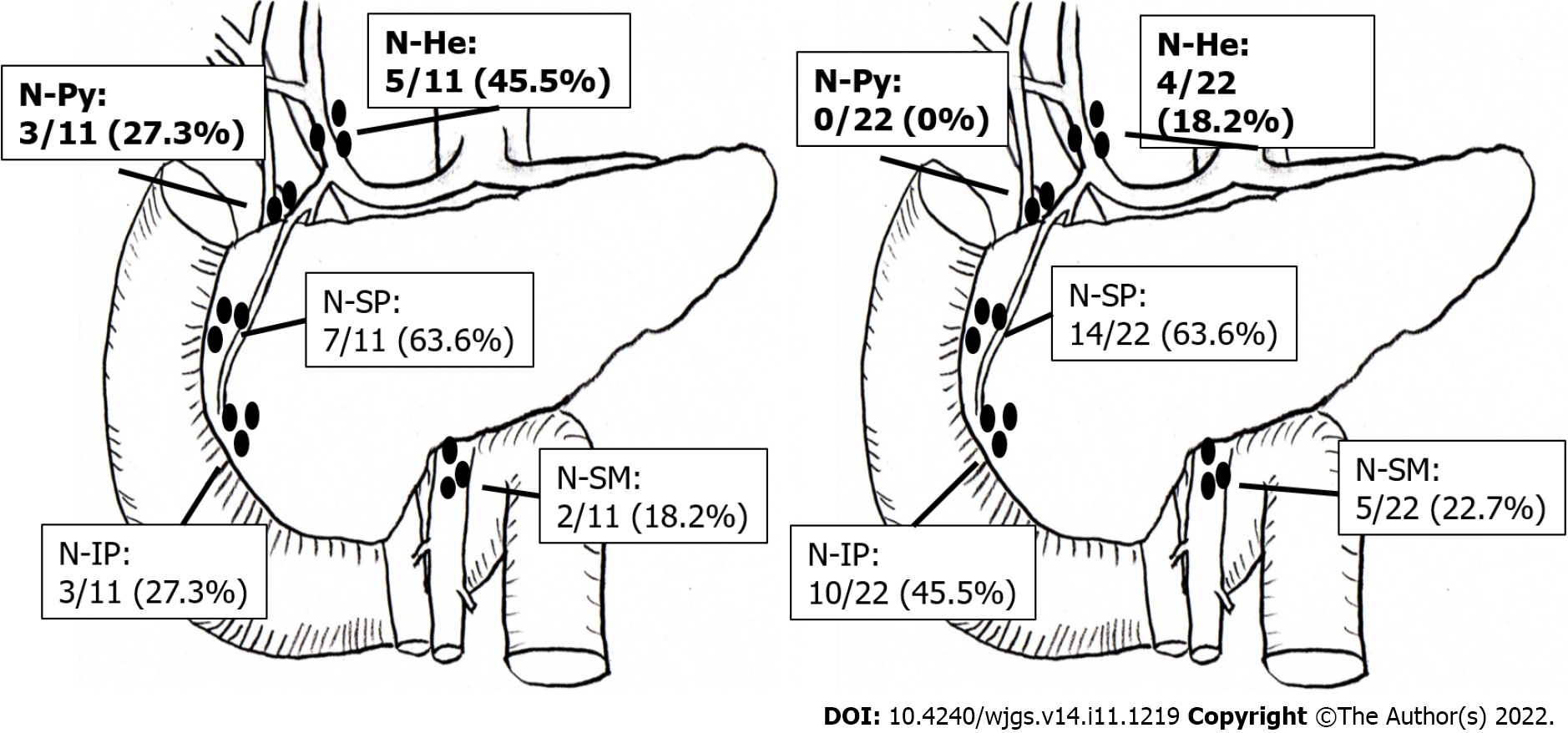
Figure 3 shows the LNM distribution in patients with DC-II and AC. Briefly, LNM was found in 11 of the 27 patients (40.7%) with DC-II, including metastases to N-SP, N-He, N-Py, N-IP, and N-SM in 7 (63.6%), 5 (45.5%), 3 (27.3%), 3 (27.3%), and 2 (18.2%) patients, respectively. Meanwhile, LNM was found in 22 of the 80 patients (27.5%) with AC, including metastases to N-SP, N-IP, N-SM, and N-He in 14 (63.6%), 10 (45.5%), 5 (22.7%), and 4 (18.2%) patients, respectively. Metastasis to N-Py was not found in any of the patients with AC (0%).
Table 3 shows the comparison of the initial recurrent sites of DC-II and AC. Initial recurrence was observed in 28 patients with AC and 10 patients with DC-II. Specifically, 10 (35.7%), 6 (21.4%), 6 (21.4%), and 5 patients (17.9%) with AC experienced recurrence in distant lymph nodes, lungs, liver, and local sites, respectively. Meanwhile, 5 (50%), 3 (30%), and 2 (20%) patients with DC-II experienced recurrence in distant lymph nodes, lungs, and liver, respectively, with no local recurrence observed in any of the patients with DC-II. There was no significant difference in the recurrence pattern between the patients with AC and DC-II.
| Initial recurrent site | DC-II (n = 10), % | AC (n = 28), % | P value |
| Liver | 2 (20.0) | 6 (21.4) | 1.00 |
| Lungs | 3 (30.0) | 6 (21.4) | 0.67 |
| Distant lymph nodes | 5 (50.0) | 10 (35.7) | 0.47 |
| Peritoneal dissemination | 1 (10.0) | 3 (10.7) | 1.00 |
| Local | 0 (0) | 5 (17.9) | 0.29 |
| Others | 1 (10.0) | 2 (7.1) | 1.00 |
The present study results indicated that metastases to N-He and N-Py were more frequent in patients with DC-II than in those with AC. The NCCN guidelines indicate that pancreatoduodenectomy with en bloc removal of regional lymph nodes, including retropancreatic, hepatic artery, inferior pancreaticoduodenal, and superior mesenteric lymph nodes, should be performed for resectable DC-II [13]. Furthermore, the guidelines state that pyloric preservation is acceptable in the absence of a hereditary condition[13]. The 7th edition of the UICC TNM classification of malignant tumors include N-Py as regional lymph nodes[14]. Sakamoto et al[17] indicated that the rate of metastasis to N-Py and N-He was significantly higher in patients with duodenal bulbs tumors and DC-II than in those with tumors in the third or fourth portion of the duodenum. Kato et al[18] reported that metastasis was detected in infrapyloric lymph nodes in 11.4% of patients with DC in the 1st-4th portion, and the location of the LNM did not exhibit a significant correlation with the primary site of DC. In the present study, metastasis to N-Py was found in 11.1% of patients with DC-II. In contrast, there are no NCCN guidelines for AC, and the 7th edition of the UICC TNM classification of malignant tumors include N-Py in the regional lymph nodes in patients with AC[14]. The General Rules for Clinical and Pathological Studies on Cancer of the Biliary Tract (6th edition) by the Japanese Society of Hepato-Biliary-Pancreatic Surgery include N-Py in the list of regional lymph nodes in patients with AC, although N-Py dissection is not mandatory[19]. Kayahara et al[20] reported that metastasis to N-Py was absent in patients with resected AC. Similarly, no patient with resected AC had metastasis to N-Py in the present study cohort. Mu et al[21] reported that the rate of metastasis to N-Py was 2.5% in patients with AC. Lee et al[22] also reported that LNM of AC first spread to the posterior pancreaticoduodenal lymph nodes followed by spread to the anterior pancreaticoduodenal nodes, and metastasis to N-Py and N-He was limited in patients with AC. Several studies on AC reported that lymphatic spread mainly extended from the posterior pancreaticoduodenal region to the superior mesenteric lymph nodes[20,23,24]. Furthermore, another study suggested that the papilla of Vater was derived from the ventral pancreas with not many communicating lymphatic vessels between the ventral and dorsal pancreas[25]; therefore, it was speculated that most of the LNM of AC moved toward N-SM via the inferior pancreaticoduodenal artery. However, we also speculated that lymphatic spread not only extended from the posterior pancreaticoduodenal region to the superior mesenteric node but also from the anterior pancreaticoduodenal region to N-Py and N-He via the gastric duodenal artery in DC-II. These anatomical considerations might be associated with the higher rates of metastases to N-He and N-Py in patients with DC-II than in those with AC.
In the current study, the rates of cases with large tumor diameter and advanced tumor invasion were higher in patients with DC-II than in those with AC. These differences might be due to the earlier appearance of symptoms, such as jaundice, in patients with AC than in those with DC-II, leading to the earlier diagnosis of AC. We did not observe significant differences in OS and RFS between the patients with AC and DC-II despite the more advanced tumor invasion observed in the patients with DC-II. These results might suggest that even in DC with more advanced tumor invasion than AC, the prognosis equivalent to AC could be obtained if pancreaticoduodenectomy with regional lymph node dissection as well as AC was performed. Riall et al[26] reported that the 5-year overall survival rate after pancreaticoduodenectomy was 37% in patients with AC and 51% in those with DC and that the prognosis of DC was significantly better than that of AC. Other studies reported that there was no significant difference in OS between the patients with resected AC and DC[27,28]. However, these studies were small in scale and retrospective in design; therefore, large-scale cohort studies are warranted for the accurate comparison of prognosis between the patients with DC and AC.
The present study results also revealed that distant lymph nodes were the most common sites of initial recurrence in both DC-II and AC. Several studies reported that the most common site of recurrence was liver in patients with AC undergoing curative resection[29,30]. Conversely, Cecchini et al[31] reported that 45% of the patients with resected DC had recurrence and that the first sites of recurrence were distant, locoregional, and both in 21%, 19%, and 5% of the patients. Onkendi et al[32] reported that approximately 60% of all recurrences were locoregional of paients with resected DC. However, these studies included segmental resection in addition to pancreaticoduodenectomy, which were considered as the cause of the high locoregional recurrence rate. In a study including patients undergoing pancreaticoduodenectomy for AC or DC, Bowitz et al[33] reported that the recurrence patterns of AC and DC were similar, with first recurrence to isolated distant sites in most patients with AC and DC (73.9%; AC, 69.2%; DC, 80.6%); the authors also reported that liver was the most affected distant site of recurrence (33.8%; AC, 28.8%; DC, 36.1%). In the present study, pancreaticoduodenectomy with regional lymph node dissection was performed in both the patients with AC and DC-II and the rate of recurrence at local sites such as the regional lymph nodes was lower than the rate of recurrence in distant lymph nodes. These results suggested that pancreaticoduodenectomy with regional lymph node dissection was effective not only in AC but also in DC-II.
The major limitations of the present study were the small sample size and the retrospective study design. Additionally, standard surgical procedures were not performed in some patients and the adjuvant chemotherapy indications and regimens were not standardized. Multicenter prospective studies with larger cohorts are necessary to clarify the prognosis and the LNM patterns in patients with DC-II and AC for the selection of appropriate surgical procedures with the best outcomes.
There were no significant differences in prognosis and recurrence rate between the patients with DC-II and AC despite the more advanced tumor invasion in patients with DC-II than in those with AC. Metastases to N-He and N-Py were more frequent in patients with DC-II than in those with AC.
Few studies have compared the oncological and biological characteristics between ampullary carcinoma (AC) and cancer of the second portion of the duodenum (DC-II), although both tumors arise from anatomically close locations.
The lymph node metastasis (LNM) patterns and the optimal range of lymph node dissection in DC-II and AC remain controversial.
The present study aimed to elucidate differences in clinicopathological characteristics, especially the patterns of LNM, between AC and DC-II.
This was a retrospective cohort study of 80 patients with AC and 27 patients with DC-II who underwent pancreaticoduodenectomy between January 1998 and December 2018 in two institutions. Clinicopathological factors, LNM patterns, and prognosis were compared between the two groups.
The rate of preoperative biliary drainage was significantly higher and the rates of digestive symptoms, ulcerative-type cancer, large tumor diameter, and advanced tumor stage were significantly lower in patients with AC than DC-II. There were no significant differences in prognosis, recurrence, and lymph node metastasis rates between the two groups, although hepatic and pyloric lymph node metastases were more frequent in DC-II than in AC.
Although there were no significant differences in the prognosis and recurrence rates between the two groups, metastases to N-He and N-Py were more frequent in patients with DC-II than in those with AC.
Lymph node dissection to N-He and N-Py may be omitted for AC, that is unlikely for DC-II.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Nah YW, South Korea; Sripongpun P, Thailand S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8283] [Cited by in RCA: 8225] [Article Influence: 483.8] [Reference Citation Analysis (0)] |
| 2. | Ahn DH, Bekaii-Saab T. Ampullary cancer: an overview. Am Soc Clin Oncol Educ Book. 2014;112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 3. | Rostain F, Hamza S, Drouillard A, Faivre J, Bouvier AM, Lepage C. Trends in incidence and management of cancer of the ampulla of Vater. World J Gastroenterol. 2014;20:10144-10150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Moekotte AL, Lof S, Van Roessel S, Fontana M, Dreyer S, Shablak A, Casciani F, Mavroeidis VK, Robinson S, Khalil K, Gradinariu G, Mowbray N, Al-Sarireh B, Fusai GK, Roberts K, White S, Soonawalla Z, Jamieson NB, Salvia R, Besselink MG, Abu Hilal M. Histopathologic Predictors of Survival and Recurrence in Resected Ampullary Adenocarcinoma: International Multicenter Cohort Study. Ann Surg. 2020;272:1086-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | Shinkawa H, Takemura S, Kiyota S, Uenishi T, Kaneda K, Sakae M, Urata Y, Ohata K, Nozawa A, Kubo S. Long-term outcome of surgical treatment for ampullary carcinoma. Hepatogastroenterology. 2012;59:1010-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 6. | Spira IA, Ghazi A, Wolff WI. Primary adenocarcinoma of the duodenum. Cancer. 1977;39:1721-1726. [PubMed] [DOI] [Full Text] |
| 7. | Kaklamanos IG, Bathe OF, Franceschi D, Camarda C, Levi J, Livingstone AS. Extent of resection in the management of duodenal adenocarcinoma. Am J Surg. 2000;179:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 90] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Buchbjerg T, Fristrup C, Mortensen MB. The incidence and prognosis of true duodenal carcinomas. Surg Oncol. 2015;24:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Zhang S, Cui Y, Zhong B, Xiao W, Gong X, Chao K, Chen M. Clinicopathological characteristics and survival analysis of primary duodenal cancers: a 14-year experience in a tertiary centre in South China. Int J Colorectal Dis. 2011;26:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Cloyd JM, Norton JA, Visser BC, Poultsides GA. Does the extent of resection impact survival for duodenal adenocarcinoma? Ann Surg Oncol. 2015;22:573-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Jiang QL, Huang XH, Chen YT, Zhang JW, Wang CF. Prognostic Factors and Clinical Characteristics of Patients with Primary Duodenal Adenocarcinoma: A Single-Center Experience from China. Biomed Res Int. 2016;2016:6491049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Lee CHA, Shingler G, Mowbray NG, Al-Sarireh B, Evans P, Smith M, Usatoff V, Pilgrim C. Surgical outcomes for duodenal adenoma and adenocarcinoma: a multicentre study in Australia and the United Kingdom. ANZ J Surg. 2018;88:E157-E161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen SA, Cooper HS, Deming DA, Garrido-Laguna I, Grem JL, Hoffe SE, Hubbard J, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen KS, Saltz LB, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Johnson-Chilla A, Gregory KM, Gurski LA. Small Bowel Adenocarcinoma, Version 1.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:1109-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 14. | Karseladze AI. [WHO histological classification of ovarian tumors. Geneva, 1999 (R.E.Scully, L.H.Sobin]. Arkh Patol. 2005;Suppl:1-64. [PubMed] |
| 15. | WHO Classification of Tumours Editoral Board: [Dilani Lokuhetty VAW, Reiko Watanabe, Ian A. Cree]. Digestive system tumours Lyon, France: International Agency for Research on Cancer; 2019. |
| 16. | AJCC cancer staging atlas second edition chicago: American joint committee on cancer; 2012. |
| 17. | Sakamoto T, Saiura A, Ono Y, Mise Y, Inoue Y, Ishizawa T, Takahashi Y, Ito H. Optimal Lymphadenectomy for Duodenal Adenocarcinoma: Does the Number Alone Matter? Ann Surg Oncol. 2017;24:3368-3375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Kato Y, Takahashi S, Kinoshita T, Shibasaki H, Gotohda N, Konishi M. Surgical procedure depending on the depth of tumor invasion in duodenal cancer. Jpn J Clin Oncol. 2014;44:224-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Japanese Society of Hepato-Biliary-Pancreatic Surgery. GeneralRules for Clinical and Pathological Studies on Cancer of the BiliaryTract, 6th edition Tokyo: Kanehara& Co.,Ltd; 2013. |
| 20. | Kayahara M, Nagakawa T, Ohta T, Kitagawa H, Miyazaki I. Surgical strategy for carcinoma of the papilla of Vater on the basis of lymphatic spread and mode of recurrence. Surgery. 1997;121:611-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Mu DQ, Peng YS, Wang FG, Xu QJ. Significance of perigastric lymph node involvement in periampullary malignant tumor. World J Gastroenterol. 2004;10:614-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Lee JH, Lee KG, Ha TK, Jun YJ, Paik SS, Park HK, Lee KS. Pattern analysis of lymph node metastasis and the prognostic importance of number of metastatic nodes in ampullary adenocarcinoma. Am Surg. 2011;77:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Yoshida T, Matsumoto T, Shibata K, Yokoyama H, Morii Y, Sasaki A, Kitano S. Patterns of lymph node metastasis in carcinoma of the ampulla of Vater. Hepatogastroenterology. 2000;47:880-883. [PubMed] [DOI] [Full Text] |
| 24. | Shirai Y, Ohtani T, Tsukada K, Hatakeyama K. Patterns of lymphatic spread of carcinoma of the ampulla of Vater. Br J Surg. 1997;84:1012-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Deki H, Sato T. An anatomic study of the peripancreatic lymphatics. Surg Radiol Anat. 1988;10:121-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Riall TS, Cameron JL, Lillemoe KD, Winter JM, Campbell KA, Hruban RH, Chang D, Yeo CJ. Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery. 2006;140:764-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 182] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 27. | Shaib WL, Sharma R, Brutcher E, Kim S, Maithel SK, Chen Z, Kooby DA, Kauh J, Landry J, El-Rayes BF. Treatment utilization and surgical outcome of ampullary and duodenal adenocarcinoma. J Surg Oncol. 2014;109:556-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Zenali M, Overman MJ, Rashid A, Broaddus RB, Wang H, Katz MH, Fleming JB, Abbruzzese JL. Clinicopathologic features and prognosis of duodenal adenocarcinoma and comparison with ampullary and pancreatic ductal adenocarcinoma. Hum Pathol. 2013;44:2792-2798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Narang AK, Miller RC, Hsu CC, Bhatia S, Pawlik TM, Laheru D, Hruban RH, Zhou J, Winter JM, Haddock MG, Donohue JH, Schulick RD, Wolfgang CL, Cameron JL, Herman JM. Evaluation of adjuvant chemoradiation therapy for ampullary adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Radiat Oncol. 2011;6:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Jin Z, Hartgers ML, Sanhueza CT, Shubert CR, Alberts SR, Truty MJ, Muppa P, Nagorney DM, Smyrk TC, Hassan M, Mahipal A. Prognostic factors and benefits of adjuvant therapy after pancreatoduodenectomy for ampullary adenocarcinoma: Mayo Clinic experience. Eur J Surg Oncol. 2018;44:677-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 31. | Cecchini S, Correa-Gallego C, Desphande V, Ligorio M, Dursun A, Wargo J, Fernàndez-del Castillo C, Warshaw AL, Ferrone CR. Superior prognostic importance of perineural invasion vs. lymph node involvement after curative resection of duodenal adenocarcinoma. J Gastrointest Surg. 2012;16:113-20; discussion 120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Onkendi EO, Boostrom SY, Sarr MG, Farnell MB, Nagorney DM, Donohue JH, Kendrick ML, Reid-Lombardo KM, Harmsen WS, Que FG. 15-year experience with surgical treatment of duodenal carcinoma: a comparison of periampullary and extra-ampullary duodenal carcinomas. J Gastrointest Surg. 2012;16:682-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Bowitz Lothe IM, Kleive D, Pomianowska E, Cvancarova M, Kure E, Dueland S, Gladhaug IP, Labori KJ. Clinical relevance of pancreatobiliary and intestinal subtypes of ampullary and duodenal adenocarcinoma: Pattern of recurrence, chemotherapy, and survival after pancreatoduodenectomy. Pancreatology. 2019;19:316-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |