Published online Jan 27, 2022. doi: 10.4240/wjgs.v14.i1.64
Peer-review started: May 18, 2021
First decision: June 15, 2021
Revised: June 29, 2021
Accepted: December 22, 2021
Article in press: December 22, 2021
Published online: January 27, 2022
Processing time: 245 Days and 18.7 Hours
Timing of invasive intervention such as operative pancreatic debridement (OPD) in patients with acute necrotizing pancreatitis (ANP) is linked to the degree of encapsulation in necrotic collections and controlled inflammation. Additional markers of these processes might assist decision-making on the timing of surgical intervention. In our opinion, it is logical to search for such markers among routine laboratory parameters traditionally used in ANP patients, considering simplicity and cost-efficacy of routine laboratory methodologies.
To evaluate laboratory variables in ANP patients in the preoperative period for the purpose of their use in the timing of surgery.
A retrospective analysis of routine laboratory parameters in 53 ANP patients undergoing OPD between 2017 and 2020 was performed. Dynamic changes of routine hematological and biochemical indices were examined in the preoperative period. Patients were divided into survivors and non-survivors. Survivors were divided into subgroups with short and long post-surgery length of stay (LOS) in hospital. Correlation analysis was used to evaluate association of laboratory variables with LOS. Logistic regression was used to assess risk factors for patient mortality.
Seven patients (15%) with severe acute pancreatitis (SAP) and 46 patients (85%) with moderately SAP (MSAP) were included in the study. Median age of participants was 43.2 years; 33 (62.3%) were male. Pancreatitis etiology included biliary (15%), alcohol (80%), and idiopathic/other (5%). Median time from diagnosis to OPD was ≥ 4 wk. Median postoperative LOS was at the average of 53 d. Mortality was 19%. Progressive increase of platelet count in preoperative period was associated with shortened LOS. Increased aspartate aminotransferase and direct bilirubin (DB) levels the day before the OPD along with weak progressive decrease of DB in preoperative period were reliable predictors for ANP patient mortality.
Multifactorial analysis of dynamic changes of routine laboratory variables can be useful for a person-tailored timing of surgical intervention in ANP patients.
Core Tip: This is a retrospective study to evaluate laboratory variables in patients with acute necrotizing pancreatitis in the preoperative period for their use in the timing of operative pancreatic debridement (OPD). We demonstrated that progressive increase in platelet counts correlate with shortened length of hospital stay. It can indicate granulation tissue formation, and can be considered as an additional marker for OPD timing. Persistent hepatic malfunction, which is indicated by a weak progressive decrease of the direct bilirubin and increased aspartate aminotransferase level can signify a high risk of post-operative mortality. Multifactorial analysis of dynamic changes of laboratory variables can be useful for person-tailored timing of OPD.
- Citation: Susak YM, Opalchuk K, Tkachenko O, Rudyk M, Skivka L. Routine laboratory parameters in patients with necrotizing pancreatitis by the time of operative pancreatic debridement: Food for thought. World J Gastrointest Surg 2022; 14(1): 64-77
- URL: https://www.wjgnet.com/1948-9366/full/v14/i1/64.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i1.64
Acute pancreatitis (AP) is the most prevalent and fairly unpredictable and potentially lethal gastrointestinal disease with an annual incidence ranging from 4.0 to 45 per 100000 persons[1,2]. About 20% of AP patients develop severe disease, and around 20% of them develop necrosis of the pancreas and peripancreatic tissues resulting in acute necrotizing pancreatitis (ANP). ANP development is associated with prolonged illness, organ failure and a high mortality rate, which can reach 30% in patients with infected pancreatic necrosis[3,4]. ANP patients usually need intensive care and frequent numerous procedures in the course of the treatment. Operative pancreatic debridement (OPD) is considered a gold standard treatment for ANP patients requiring surgical intervention. For a long time, this procedure was accompanied by significant morbidity and high mortality rates. Nowadays, refined operative techniques in combination with surgeon experience have allowed us to decrease perioperative mortality rates. In the past 10 years, minimally invasive techniques have been applied to the treatment of NP patients. Nevertheless, many ANP patients require a combination of minimally invasive techniques and OPD in order to achieve complete debridement. Moreover, OPD remains an important treatment approach for ANP patients who are refractory to minimally invasive treatment[5-7]. Considering the complicated ANP pathophysiology and highly variable clinical course, a person-tailored approach to intervention methods including OPD makes sense according to the specific conditions of patients. One of the key points in these patient-tailored approaches is the timing of surgical intervention, in order to gain the most beneficial result[8,9].
Timing on invasive intervention in ANP patients is often linked to the degree of encapsulation in necrotic collections. The degree of necrotic collections encapsulation is important because walling-off allows the immune system demarcation between viable and necrotic tissues, thereby facilitating effective debridement[10-12]. It is commonly admitted that the timing of encapsulation takes about 4 wk (after symptom onset) and this timescale is included in the Revised Atlanta Classification[13]. However, the pathophysiology and time course of necrotic collection walling-off are not fully understood and remain a topic of debate. According to clinical observations of van Grinsven et al[14], and opposed to common opinion, largely or fully encapsulated necrotic collections can be observed in ANP patients at every phase of the disease. Assessment of the degree of encapsulation of necrotic collections is influenced by imaging and clinical features. Additional markers of this process might assist decision-making on the timing of surgical intervention. The search for these markers should be based on current knowledge of the biology of necrotic tissue encapsulation. In our opinion, it is logical to search for such markers among routine laboratory parameters traditionally used in ANP patients, considering simplicity and cost-efficacy of routine laboratory methodologies. This study was aimed to evaluate distinctive features of routine biochemical and hematological parameters in patients with ANP by the time of OPD for the purpose of their use as additional markers for the timing of surgical intervention.
We conducted a retrospective analysis of a prospectively collected O.O. Bogomolets National Medical University (Kyiv, Ukraine) (Department of Surgery with a course of emergency and vascular surgery) database of 53 ANP patients who underwent OPD between 2017 and 2020 in Kyiv City Clinical Emergency Hospital, Ukraine. Approval was obtained from the Ethics Committee of Kyiv City Clinical Emergency Hospital (Protocol #25-15-60, from 20 November 2017), and consent was obtained from all subjects before the commencement of the study.
AP was diagnosed in all patients with clinical signs of acute abdominal pain and a three or more times increased level of serum amylase. AP severity was established according to the revised Atlanta classification and Marshall scoring system[13]. Pancreatic and peripancreatic necrosis was detected in the patients using ultrasound imaging and contrast-enhanced computed tomography.
All patients were treated according to the local treatment protocol that was clinically approved for AP patients from year 2014. After admission, patients were managed on the intensive care unit (ICU) using the “four catheters” rule[15]: Catheter for epidural anesthesia, installment of the feeding intestinal probe further than the Treitz ligament level, the central venous catheterization and the programmed laparocentesis. Median length of ICU stay was 3.2 d.
All patients were initially treated with a minimally invasive technique: laparocentesis, percutaneous drainage of the retroperitoneal space, pleural and abdominal cavities. Primarily, percutaneous drainage was used in all patients under ultrasound control of infected necrotic areas. Abdominal drainage was conducted on each patient two or more times.
Indications for necrosectomy were persisting organ failure and documented infected necrosis. Organ failure was defined as follows; Pulmonary insufficiency: PaO2 ≤ 60 mmHg in spite of receiving 4 L of oxygen per minute via a nasal tube or need for mechanical ventilation. Cardiocirculatory insufficiency: Systolic blood pressure ≤ 90 mmHg or necessity for catecholamine support. Renal failure: a serum creatinine level ≥150 μmol/L and/or necessity for hemofiltration/hemodialysis. Metabolic disorders: A serum calcium level ≤ 1.87 mmol/L or a platelet (PLT) count ≤ 100 × 109/L. Multiple organ failure (MOF) was established as failure of 2 or more organ systems. Infected pancreatic/peripancreatic necrosis was revealed according to the imaging (the presence of extraluminal gas in the pancreatic and/or peripancreatic tissues) and/or bacteriological (positive bacterial culture of aspiration and drainage content of pancreatic and/or peripancreatic tissues) findings. During laparotomy, blunt debridement of necrotic tissue and tissues of the retroperitoneal space was performed. Drainage PVC tubes were inserted through separate incisions (3-4 cm) on the lateral areas of the abdomen with their tips placed to the necrotic cavities under the colon. The abdomen was closed afterwards, and local continuous lavage was started.
Outcome variables were: (1) Total hospital length of stay (LOS); (2) Post-OPD LOS in survivors; (3) LOS between OPD and death (LOSOPD-D) in non-survivors; and (4) Hospital mortality.
For each enrolled patient, routine laboratory variables were measured for time period from the time of admission until surgical intervention (OPD). EDTA-anticoagulated venous blood samples for all laboratory tests were drawn between 7 am and 8 am in the morning, and laboratory indices were calculated within 1.5-2.5 h.
Routine biochemical parameters [serum level of total bilirubin (TB) direct bilirubin (DB), aspartate aminotransferase (AST), alanine aminotransferase (ALT), α-amylase (AML), as well as gamma-glutamyl transpeptidase (GGT), glucose, creatinine, blood urea nitrogen] were measured using automatic biochemical analyzer Olympus AU-800 (Olympus, Tokyo, Japan). Routine hematological parameters [hemoglobin (Hgb), hematocrit (HCT), total red blood cell count (RBC), total white blood cell count (WBC), PLT] were determined using automatic hematological analyzer Mindray BC-2800 (Mindray, Shenzhen, China).
The dynamic changes of all laboratory variables were calculated as follows: A - Day 1 (on admission); B - Day 3-7; ∆ (B-A); C - Day 12-16; ∆ (C-B); D - Day 21-24; ∆ (D-C); E - Day before the OPD; ∆ (E-D); ∆ (E-C); ∆ (E-D); ∆ (E-A); ∆ (A-E); A to E ratio (A/E).
Hematological and biochemical reference values in our hospital are as follows: Hgb, 130-160 g/L (male) and 120-140 g/L (female); HCT, 40%-48% (male) and 36%-46% (female); RBC, 4.5-5.9 × 1012/L (male) and 4.1-5.1 × 1012/L (female); WBC, 3.9-10 × 109/L; PLT, 180-320 × 109/L; TB, 2-21 μmol/L; DB, 0-5 μmol/L; ALT, 0.1-0.68 μkat/L; AST, 0.1-0.45 μkat/L; AML, 12-32 U/L; GGT, 9–48 U/L; glucose, 3.3-6.5 mmol/L; creatinine 71-106 μmol/L; blood urea nitrogen, 2.5-8.3 mmol/L. Permissible error of the assay was ≤ 5% of the total coefficient of variation according to the manufacturer statement.
Normally distributed variables were compared using Student’s t-test, non-normally distributed variables using Mann-Whitney U-test. Data are presented as means ± SD.
Spearman correlation test was used to determine the statistical relationships between the preoperative values of measured laboratory variables and different LOS indices. A 2-tailed P ≤ 0.05 was considered statistically significant in all analyses. The prognostic validities of measured laboratory variables values was analyzed using receiver operating characteristic (ROC) analysis.
To identify the variables associated with mortality, univariate and multivariate logistic regression analysis was conducted. Odds ratios (OR) are represented with their respective 95% confidence intervals (CI). The Hosmer-Lemeshow test was applied to verification the goodness-of-fit of the logistic regression. All tests were assessed by odds ratio OR and their 95%CI. Statistical analyses were performed by SPSS software (version 19.0; SPSS Inc., Chicago, IL, USA).
The statistical methods of this study were reviewed and approved by Vitaliy Gurianov, associate professor of Healthcare Management Department, Bogomolets National Medical University, Kyiv, Ukraine.
General characteristics of study participants are summarized in Table 1. Fifty-three ANP patients were enrolled during this study: 7 patients (15%) with severe AP (SAP) and 46 patients (85%) with moderately severe AP (MSAP). Thirty-three (62.3 %) were male and 20 (37.7%) were female. Median age of the patients was 43.2 years. Pancreatitis etiology included: Alcohol, biliary, posttraumatic, and idiopathic. Single and MOF included cardiocirculatory insufficiency, renal failure, and pulmonary insufficiency. Other complications included an omental abscess (n = 42), erosive bleeding (n = 7), a pancreatic fistula (n = 4), an intestinal fistula (n = 4), and a post-necrotic cyst (n = 7). The mean total LOS was 85 d. Median timing of the OPD was 30 d [range, 20-86 d] from the onset of the disease. Median post-surgical LOS was at the average of 53 d. Mortality rate was 19%.
| Characteristic | Value |
| Sex, age, severity scores | |
| Male, n (%) | 33 (62.3) |
| Female, n (%) | 20 (37.7) |
| Age, yr [range] | 43 [23-68] |
| APACHE II score | 8 |
| Marshall score | 4 |
| Mortality, % | 19 |
| Etiology, n (%) | |
| Alcohol | 42 (79) |
| Biliary | 4 (7) |
| Posttraumatic | 4 (7) |
| Idiopathic | 3 (5) |
| Comorbidity, n (%) | |
| Multiple organ failure | 5 (9) |
| Cardiovascular | 11 (20) |
| Renal | 4 (7) |
| Respiratory | 10 (18) |
| Pneumonia | 12 (22) |
| Necrosis infection | 53 (100) |
| Extrapancreatic infection | 53 (100) |
| Sepsis | 8 (15) |
| Preoperative interventions | |
| Laparocentesis | 53 |
| Thoracocentesis | 31 |
| Percutaneous drain | 147 |
| Endoscopic | 33 |
According to hospital mortality, 53 patients were divided into the survivor’s group (n = 43), and non-survivor’s group (n = 10). There were no significant differences with respect to age and gender between the two groups. It is necessary to note, that non-survivors were characterized by the increased sepsis rate [6 (60%) vs 4 (9%) in survivors] and MOF rate [3 (33.3%) vs 2 (4.7%) in survivor’s].
According to post-OPD LOS 43 survivors were divided into two subgroups: Post-OPD LOS ≤ 50 d (n = 12), and post-OPD LOS ≥ 50 d (n = 31). There were no significant differences with respect to age and severity scores between the two subgroups. It is necessary to point, that females prevailed in subgroup with post-OPD LOS ≤ 50 d.
The dynamic changes of laboratory variables in the survivors with different post-OPD LOS are summarized in Table 2. Baseline values (Day 1) of many of laboratory variables were not significantly different between survivors with different post-OPD LOS. Compared with patients with post-OPD LOS ≤ 50, patients with post-OPD LOS ≥ 50 had moderately higher Hgb (176.3 ± 31.2 vs 111.4 ± 12.1, P ≤ 0.05). Patients with post-OPD LOS ≥ 50 also tended to exhibit higher baseline ALT and AST (1.79 ± 1.31 vs 0.71 ± 0.52 and 0.99 ± 0.35 vs 0.56 ± 0.31 respectively). However, these values were characterized by significant individual variability. Significant differences were observed in PLT count in patients with different post-OPD LOS. PLT count increased progressively in the preoperative period in patients from both subgroups. However, in participants with post-OPD LOS ≥ 50, it did not go beyond the reference range, while in patients with post-OPD LOS ≤ 50 it exceeded the reference values by at least two times the day before OPD. Slightly increased WBC count was observed in all survivors until the OPD with significant individual variability, which indicates persistent inflammation. Initially increased DB levels decreased progressively in preoperative period without statistically significant differences between subgroups. AML levels remained higher than reference values the day before OPD in all survivors. There were no significant differences with respect to other measured laboratory variables (data not shown).
| Laboratory variable | Post-OPD LOS ≤ 50 d, n = 12 | post-OPD LOS ≥ 50 d, n = 31 |
| Hgb (g/L) | ||
| Day 1 (A) | 111.4 ± 12.1 | 176.3 ± 31.2a |
| Day 3-9 (B) | 93.6 ± 8.9 | 116.4 ± 26.6 |
| Δ (B-A) | -35.5 ± 12.9 | -46.4 ± 7.5 |
| Day before OPD (E) | 89.4 ± 7.8 | 83.6 ± 7.2 |
| Δ (E-A) | -22.0 ± 11.4 | -79.2 ± 12.0a |
| WBC (× 109/L) | ||
| Day 1 (A) | 9.6 ± 3.9 | 11.5 ± 1.7 |
| Day 3-9 (B) | 16.5 ± 9.8 | 13.1 ± 4.6 |
| Δ (B-A) | 3.6 ± 6.3 | -0.6 ± 6.6 |
| Day before OPD (E) | 10.1 ± 2.5 | 13.8 ± 4.7 |
| Δ (E-A) | 0.6 ± 2.4 | 2.4 ± 4.3 |
| PLT (× 109/L) | ||
| Day 1 (A) | 236.5 ± 57.8 | 223.5 ± 64.2 |
| Day 3-9 (B) | 453.5 ± 58.3 | 224.0 ± 44.5a |
| Δ (B-A) | 232.8 ± 50.9 | -7.5 ± 57.8a |
| Day before OPD (E) | 648.0 ± 74.7 | 360.2 ± 104.8a |
| Δ (E-A) | 430.5 ± 76.4 | 181.0 ± 48.7a |
| AST (μkat/L) | ||
| Day 1 (A) | 0.56 ± 0.31 | 0.99 ± 0.35 |
| Day 3-9 (B) | 0.44 ± 0.06 | 0.72 ± 0.13a |
| Δ (B-A) | -0.18 ± 0.27 | -0.84 ± 0.41 |
| Day before OPD (E) | 0.36 ± 0.11 | 0.42 ± 0.19 |
| Δ (E-A) | -0.19 ± 0.28 | -1.0 ± 1.0 |
| ALT (μkat/L) | ||
| Day 1 (A) | 0.71 ± 0.52 | 1.79 ± 1.31 |
| Day 3-9 (B) | 0.46 ± 0.18 | 1.02 ±0.52a |
| Δ (B-A) | -0.34 ± 0.43 | -1.32 ± 0.84 |
| Day before OPD (E) | 0.51 ± 0.22 | 0.51 ± 0.16 |
| Δ (E-A) | -0.21 ± 0.39 | -1.28 ± 1.24 |
| DB (μmol/L) | ||
| Day 1 (A) | 15.73 ± 19.79 | 14.95 ± 11.53 |
| Day 3-9 (B) | 3.21 ± 0.87 | 6.78 ± 4.37 |
| Δ (B-A) | -18.5 ± 22.19 | -10.02 ± 10.81 |
| Day before OPD (E) | 1.72 ± 1.01 | 2.55 ± 0.74 |
| Δ (E-A) | -14.02 ± 19.79 | -12.4 ± 11.53 |
| AML (U/L) | ||
| Day 1 (A) | 65.8 ± 48.07 | 56.3 ± 24.47 |
| Day 3-9 (B) | 26.62 ± 8.11 | 38.94 ± 27.03 |
| Δ (B-A) | -32.34 ± 43.11 | -17.36 ± 16.55 |
| Day before OPD (E) | 21.18 ± 4.85 | 27.46 ± 16.61 |
| Δ (E-A) | -44.62 ± 47.55 | -28.84 ± 41.51 |
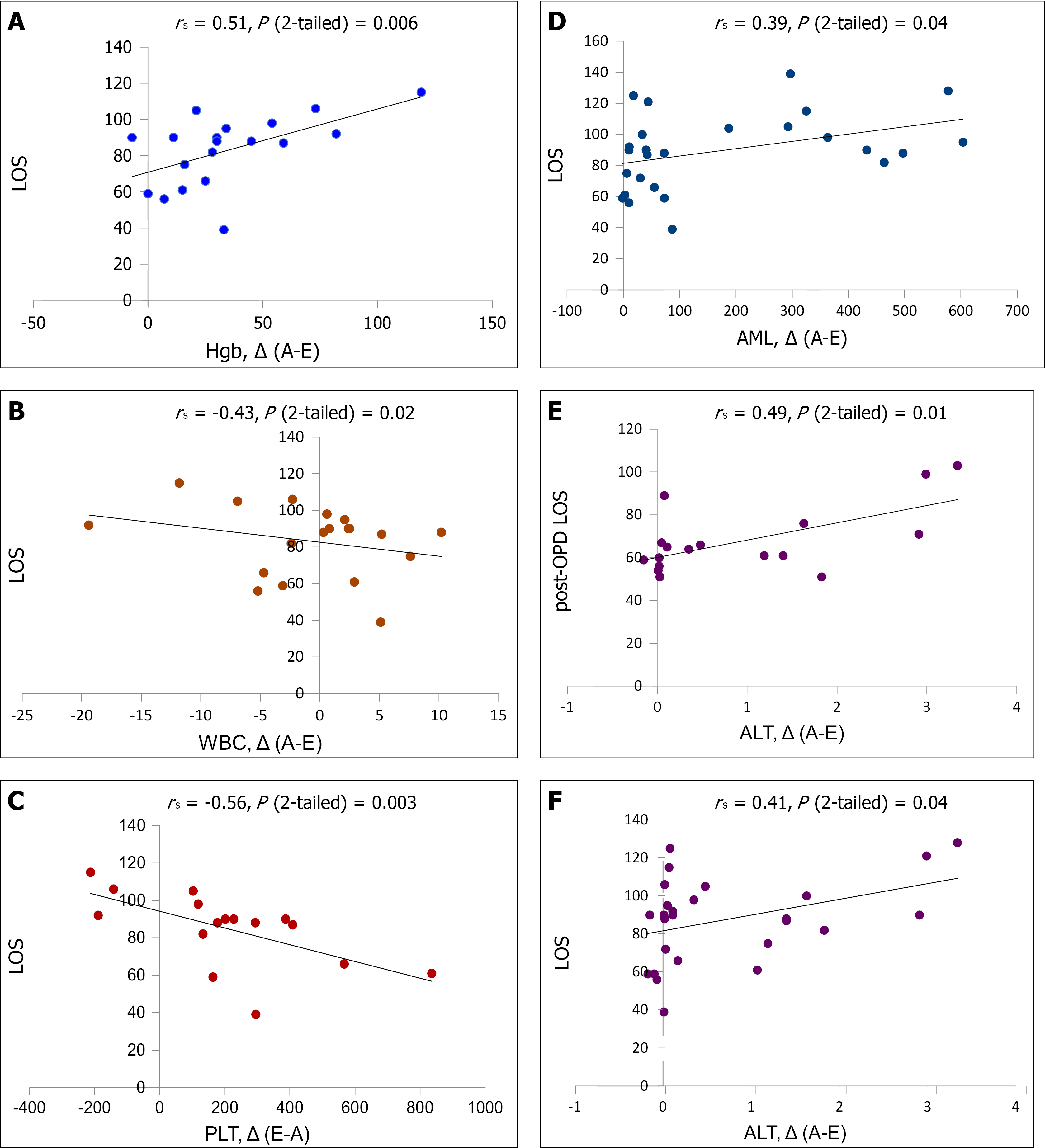
There was a significant correlation between total LOS and Hgb level ∆(A-E) (Figure 1A), indicating that a significant decrease of Hgb concentration is associated with prolonged total and post-surgical LOS. A significant inverse correlation was observed between total LOS and WBC count ∆(A-E) (Figure 1B), suggesting that a progressive decrease of WBC count during the pre-operative period till reference values is associated with shortened post-OPD LOS. A significant inverse correlation was also registered between total LOS and PLT count ∆(E-A) (Figure 1C), indicating that a substantial increase of PLT count before the surgery accompanies shortened post-surgery recovery. Moderate correlation was revealed between total LOS and AML ∆(A-E) (Figure 1D). Considering that AML values were near reference range in all survivors the day before surgery, this correlation suggests that a highly increased AML value on admission is associated with the disease severity, and as a result with prolonged pre- and post-surgery LOS. High values of ALT ∆(A-E) significantly correlated with both total LOS and post-OPD LOS (Figure 1E and F). Considering that ALT values did not exceed the reference range in all survivors the day before the OPD, these correlations indicate that increased baseline ALT value (as a marker of ongoing liver disease process[16]) is associated with disease severity and prolonged recovery.
We further performed univariate logistic regression analysis to find out potential risk factors associated with hospital mortality, as shown in Table 3. Four laboratory variables were associated with mortality, including AST, AML and DB serum levels the day before the surgery (E values), as well as E to A ratio for DB. Other measured laboratory parameters were unrelated to outcomes.
| Variable | OR | 95%CI | P value |
| AST (E), μkat/L | 1.0377 | 1.6514-1.3392 | 0.3612 |
| α-amylase (E), U/L | 0.8771 | 0.7657-1.0046 | 0.7543 |
| DB (E), μmol/L | 2.2201 | 1.0475-4.7051 | 0.6374 |
| DB (A/E) | 0.6941 | 0.4613-1.0445 | 0.5221 |
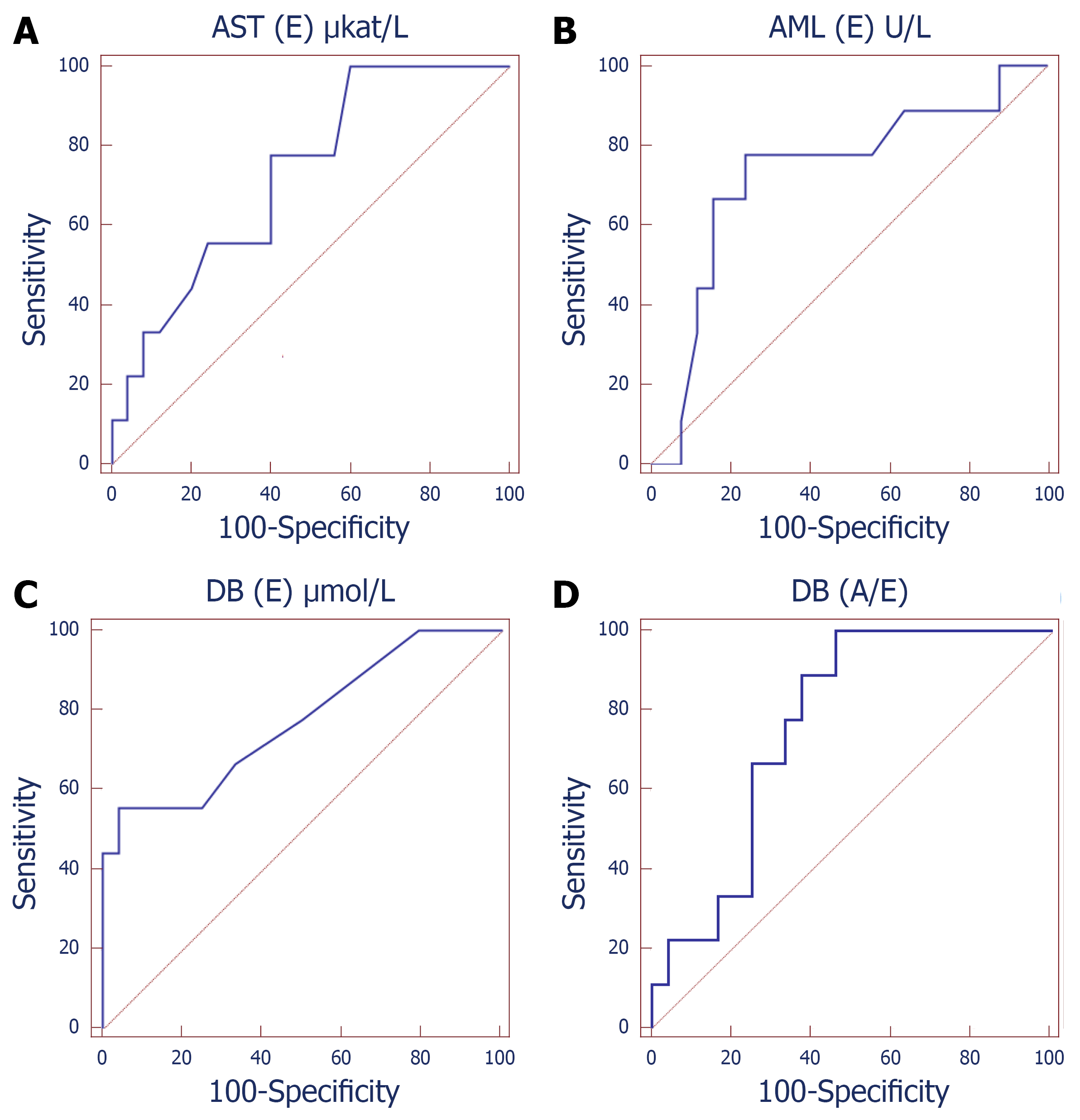
To investigate the predictive values of laboratory variables, ROC analysis was conducted (Table 4, Figure 2). The AUC of AML (E) (AUC: 0.729, 95%CI: 0.550-0.866, P < 0.032) was greater than the other biomarkers. The optimal cutoff value of AML (E) was ≤ 17.2 U/L, with 66.7% sensitivity, 84.0% specificity, 60.0% PPV and 87.5% NPV. In addition, a DB (E) value of > 4.2 μmol/L allowed discrimination between ANP survivors and non-survivors, with a sensitivity of 44.4% and a specificity of 100.0% (AUC: 0.782, 95%CI: 0.608-0.905, PPV: 100.0%, NPV: 83.3%, P < 0.001). The AUC of ΔPCT7 was 0.834 (95%CI: 0.759-0.906, P < 0.001), with 80.5% sensitivity, 81.6% specificity, 76.6% PPV and 88.2% NPV at the best threshold value of < 5.3 ng/mL. The predictive value of AST and DB (A/E) were less accurate with the sensitivity less than 50%. None of the other variables was useful to predict mortality in ANP patients (data not shown).
| Variables | Cutoff | Sensitivity | Specificity | AUC | 95%CI | PPV | NPV | P value |
| AST (E), μkat/L | > 0.53 | 33.3% | 92.0% | 0.727 | 0.547-0.865 | 60.0% | 79.3% | 0.016 |
| α-amylase (E), U/L | ≤ 17.2 | 66.7% | 84.0% | 0.729 | 0.550-0.866 | 60.0% | 87.5% | < 0.032 |
| DB (E), μmol/L | > 4.2 | 44.4% | 100.0% | 0.782 | 0.608-0.905 | 100.0% | 83.3% | < 0.001 |
| DB(A/E) | ≤ 1 | 22.2% | 95.8% | 0.764 | 0.584-0.894 | 66.7% | 76.7% | 0.0015 |
Next, we attempted to evaluate whether a combination of different laboratory variables could promote the predictive accuracy further (Table 5). Notably, the combination form of (AST(E) > 0.53 μkat/L + AML (E) ≤ 17.2 U/L + DB(E) > 4.2 μmol/L + DB (A/E) < 1) resulted in the greatest AUC (AUC: 0.935, P < 0.0005) than other variables, either alone or in combination.
| Multivariable model | AUC | 95%CI | P value |
| AST (E) + AML (E) | 0.791 | 0.618-0.911 | 0.016 |
| AST (E) + DB (E) | 0.784 | 0.610-0.906 | 0.0011 |
| AML (E) + DB (E) | 0.884 | 0.777-0.908 | 0.0002 |
| AST (E) + AML (E) + DB (E) | 0.884 | 0.728-0.968 | 0.003 |
| DB (E) + DB (A/E) | 0.87 | 0.708-0.961 | 0.0006 |
| AST (E) + DB (A/E) | 0.87 | 0.708-0.961 | 0.0016 |
| AML (E) + DB (A/E) | 0.84 | 0.674-0.945 | 0.0026 |
| AST (E) + AML (E) + DB (A/E) | 0.88 | 0.719-0.966 | 0.0023 |
| AST (E) + AML (E) + DB (E) + DB (A/E) | 0.935 | 0.792-0.991 | 0.0005 |
In this study, we monitored routine laboratory variables for the purpose of their use as additional markers to assist decision-making on the timing of surgical intervention in ANP patients. Hospital mortality, as well as total and post-OPD LOS were chosen as criteria, associated with optimal OPD timing. Routine laboratory variables and their dynamic changes were examined in the preoperative period in order to compare key hematological and biochemical indices and their changes in survivors and non-survivors, as well as in ANP patients with short and long post-surgical LOS at the recommended time point of surgical intervention (about 4 wk after symptom onset). Surprisingly, the AML value within the reference range the day before the OPD was quite a reliable predictor of hospital mortality in ANP patients. One can suggest, that discrepancy between clinical picture and normal value of this laboratory index can be considered as an alarming marker for disease outcome and surgery timing. Increased values of AST and DB the day before the OPD as well as the absence of a substantial decrease of DB level in the preoperative period (A/D ratio < 1) were also reliable predictors of hospital mortality. Taken in combination, these biomarkers provided greater predictive accuracy than individual markers. Hyperbilirubinemia including increased level of DB is considered as an independent risk factor for mortality in critically ill patients[17]. Liver malfunction represents a sometimes serious and fatal complication during the ANP progression, since the liver can mediate extra pancreatic organ impairment by releasing toxic substances[18]. Hepatic injury caused by inflammatory mediators generated in ANP patients cannot only aggravate the disease course, but also develop into severe hepatic failure and can cause patient death[19]. Increased AST the day before the OPD can indicate persistent severe hepatic dysfunction. Hyperbilirubinemia can be considered as a consequence of severe hepatic dysfunction, and additionally can be a risk factor of the impairment of the oxygen-dependent bactericidal activity of innate immunity cells and as a result the sepsis development[20]. The alteration trend of variables is an important component of multivariable predictive model. In the current study, we revealed that DB (A/E) had good prognostic capacity among other laboratory variables. The course of ANP is a rapidly-changing process which is too complicated to be estimated by a single measurement. The trend of laboratory indices alteration can reflect disease development more accurately, in particular when absolute baseline values are high. In this study, we emphasize the importance of combined analysis of absolute values and dynamic alterations of laboratory variables. Thus, according to our multivariable prognostic model, persistent hepatic failure along with a normal AML level should be taken into account in OPD timing as a predictive marker of a high mortality risk.
The estimated time of readiness of the ANP patient for surgery is the time period of the summation of the two most important events. First is the systemic inflammatory response syndrome (SIRS) down-regulation, since it is SIRS that is the most important cause of high mortality that accompanies surgical intervention in the early period after symptoms onset. The second is the necrotic collection encapsulation, since this phenomenon technically facilitates effective debridement. Therefore, the whole set of routine laboratory parameters should be viewed from the angle of these two events.
ANP course progresses in two phases. First phase is characterized by SIRS development with single or MOF. This phase continues at the average 10-14 d, and then consistently gives way to compensatory systemic anti-inflammatory syndrome. Inter alia, SIRS is usually characterized by persistent leukocytosis[21]. SIRS in ANP is commonly associated with the liver injury and, as a result with the rise of such routine laboratory indices as serum Alkaline Phosphatase, AST, ALT, TB, DB, AML and lipase levels. Therefore, routine laboratory variables such as WBC count and biochemical markers of liver injury can be indicative for the evaluation of SIRS and of Multiple Organ Dysfunction Syndrome in ANP patients.
Necrotic collection walling-off is, in effect, the development of a granulation tissue (GT) capsule around the necrotic area[22,23]. Primary function of the GT capsule is to prevent the systemic spread of inflammatory mediators (e.g., cytokines and eicosanoids) and signals danger for the immune system which originated from necrotic cells. Thus, this temporary barrier is aimed at compartmentalization of the inflammatory response[24]. Another important function of the GT capsule is to protect the encapsulated area from the infection. The basis of GT is usually composed of a fibrous capsule, and its core cell component is commonly represented by fibroblasts. Fibroblasts deposit fibronectin in a soft extracellular matrix. This matrix separates necrotic collection from the surrounding tissues and can then be used for the recruitment of other cells into GT[25]. Therefore, one can suppose, that fibroblast migration into the necrotic area is a crucial step of the encapsulation. Fibroblast recruitment into the necrotic area is orchestrated by the coordinated effect of numerous cytokines and growth factors. Among others, fibroblast growth factor and platelet-derived growth factor (PDGF) are the major cytokines that initiate and afterward support fibroblast proliferation and chemotactic activity resulting in the necrotic area encapsulation[26-28]. Clinical observations of Stojek et al[29] indirectly confirmed this assumption. According to findings of this scientific group, serum levels of PDGF-BB is significantly increased in patients with chronic pancreatitis, which is associated with chronic inflammation and fibrosis. Activated platelets represent one of the main sources of these growth factors[30,31]. Given the above, we assumed, that leukocytosis diminishing (as a marker of SIRS down-regulation) along with the increase of PLT count (as a marker of necrotic tissue encapsulation) could indicate a beneficial condition for OPD timing. In this study, a substantial progressive increase of PLT count along with moderate decrease of WBC count strongly correlated with shortened LOS. We suppose that progressive increase of PLT count in the preoperative period can be considered as one of the additional markers indicating the development of the GT capsule around the necrotic area.
There are several limitations in the present study. First, the number of patients was small, and further analysis needs to be done with a larger number of ANP patients to confirm its reproducibility. Second, comprehensive sex-centered evaluation would be more desirable considering the prevalence of female patients in the subgroup with shortened LOS. Third, it is desirable to complement the examination of the dynamic changes in PLT count with the determining of serum levels of cytokines involved in GT formation.
By focusing on dynamic changes of routine laboratory variables in the preoperative period in ANP patients, we demonstrated that a progressive increase in PLT count along with a decrease of leukocytosis correlates with a shortened LOS and can indicate GT formation, and can be considered as an additional marker for OPD timing. Whereas persistent hepatic malfunction, which is indicated by a weak progressive decrease of DB in the preoperative period and increased AST level can signify a high risk of post-operative mortality. Thus, multifactorial analysis of dynamic changes of routine laboratory variables can be useful for a person-tailored timing of surgical intervention in ANP patients.
Timing on invasive intervention in patients with acute necrotizing pancreatitis is linked to the degree of encapsulation in necrotic collections. Assessment of the degree of encapsulation of necrotic collections is influenced by imaging and clinical features. However, the pathophysiology and time course of necrotic collection walling-off are not fully understood and vary significantly between patients.
Additional markers of necrosis encapsulation might assist decision-making on the timing of surgical intervention. The search for these markers should be based on current knowledge of the biology of necrotic tissue encapsulation. In our opinion, it is logical to search for such markers among routine laboratory parameters traditionally used in acute necrotizing pancreatitis (ANP) patients, considering simplicity and cost-efficacy of routine laboratory methodologies.
To evaluate laboratory variables in ANP patients in the preoperative period for the purpose of their use for the timing of surgery.
This was a retrospective study of 53 ANP patients undergoing operative pancreatic debridement (OPD). Dynamic changes of routine hematological and biochemical indices were examined in the preoperative period. Patients were divided into survivors and non-survivors. Survivors were further divided into a subgroup with short and long post-surgery length of stay (LOS) in hospital. Correlation analysis was used to evaluate the association of laboratory variables with LOS. Logistic regression was used to assess risk factors for patient mortality.
Progressive increase of platelet count in the preoperative period was associated with shortened total and post-surgery LOS. Increased aspartate aminotransferase and direct bilirubin (DB) levels the day before the OPD as well as the absence of substantial decrease of DB level in preoperative period were reliable predictors for ANP patient mortality.
Multifactorial analysis of dynamic changes of routine laboratory variables can be useful for a person-tailored timing of surgical intervention in ANP patients.
Comprehensive sex-centered evaluation of routine laboratory variables should be performed considering sex differences in the course of inflammation. Dynamic changes of serum levels of cytokines associated with fibro granulation tissue formation should also be studied for the person-tailored invasive intervention timing.
The authors are very grateful to all participants recruited in this study. The authors express their sincere thanks to Dr. Olexander Gorbach for the technical support.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Ukraine
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Inal V, Mannan F S-Editor: Chang KL L-Editor: Filipodia P-Editor: Chang KL
| 1. | Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, van Santvoort HC, Besselink MG. Acute pancreatitis. Lancet. 2020;396:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 579] [Article Influence: 115.8] [Reference Citation Analysis (0)] |
| 2. | Cofaru FA, Nica S, FierbinȚeanu-Braticevici C. Assessment of severity of acute pancreatitis over time. Rom J Intern Med. 2020;58:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Aparna D, Kumar S, Kamalkumar S. Mortality and morbidity in necrotizing pancreatitis managed on principles of step-up approach: 7 years experience from a single surgical unit. World J Gastrointest Surg. 2017;9:200-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Leonard-Murali S, Lezotte J, Kalu R, Blyden DJ, Patton JH, Johnson JL, Gupta AH. Necrotizing pancreatitis: A review for the acute care surgeon. Am J Surg. 2021;221:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Maatman TK, Flick KF, Roch AM, Zyromski NJ. Operative pancreatic debridement: Contemporary outcomes in changing times. Pancreatology. 2020;20:968-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | van Santvoort HC, Bakker OJ, Bollen TL, Besselink MG, Ahmed Ali U, Schrijver AM, Boermeester MA, van Goor H, Dejong CH, van Eijck CH, van Ramshorst B, Schaapherder AF, van der Harst E, Hofker S, Nieuwenhuijs VB, Brink MA, Kruyt PM, Manusama ER, van der Schelling GP, Karsten T, Hesselink EJ, van Laarhoven CJ, Rosman C, Bosscha K, de Wit RJ, Houdijk AP, Cuesta MA, Wahab PJ, Gooszen HG; Dutch Pancreatitis Study Group. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 466] [Article Influence: 33.3] [Reference Citation Analysis (2)] |
| 7. | Wroński M, Cebulski W, Witkowski B, Jankowski M, Kluciński A, Krasnodębski IW, Słodkowski M. Comparison between minimally invasive and open surgical treatment in necrotizing pancreatitis. J Surg Res. 2017;210:22-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Yin T, Wang CY. [Rediscussion of the individualized surgical intervention and timing for necrotizing pancreatitis]. Zhonghua Wai Ke Za Zhi. 2019;57:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Garg PK, Zyromski NJ, Freeman ML. Infected Necrotizing Pancreatitis: Evolving Interventional Strategies From Minimally Invasive Surgery to Endoscopic Therapy-Evidence Mounts, But One Size Does Not Fit All. Gastroenterology. 2019;156:867-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Baron TH, DiMaio CJ, Wang AY, Morgan KA. American Gastroenterological Association Clinical Practice Update: Management of Pancreatic Necrosis. Gastroenterology. 2020;158:67-75.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 414] [Article Influence: 82.8] [Reference Citation Analysis (2)] |
| 11. | Leppäniemi A, Tolonen M, Tarasconi A, Segovia-Lohse H, Gamberini E, Kirkpatrick AW, Ball CG, Parry N, Sartelli M, Wolbrink D, van Goor H, Baiocchi G, Ansaloni L, Biffl W, Coccolini F, Di Saverio S, Kluger Y, Moore E, Catena F. 2019 WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg. 2019;14:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 538] [Cited by in RCA: 423] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 12. | Besselink MG, Verwer TJ, Schoenmaeckers EJ, Buskens E, Ridwan BU, Visser MR, Nieuwenhuijs VB, Gooszen HG. Timing of surgical intervention in necrotizing pancreatitis. Arch Surg. 2007;142:1194-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 223] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 13. | Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS; Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4932] [Cited by in RCA: 4323] [Article Influence: 360.3] [Reference Citation Analysis (45)] |
| 14. | van Grinsven J, van Brunschot S, van Baal MC, Besselink MG, Fockens P, van Goor H, van Santvoort HC, Bollen TL; Dutch Pancreatitis Study Group. Natural History of Gas Configurations and Encapsulation in Necrotic Collections During Necrotizing Pancreatitis. J Gastrointest Surg. 2018;22:1557-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 15. | Susak IaM, Tkachenko OA, Malysh IR, Dirda OO, Fedorchuk OH. [Prognostication of course and treatment of peripancreatic infiltrate in patients, suffering an acute necrotic pancreatitis]. Klin Khir. 2014;20-22. [PubMed] |
| 16. | Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC; Public Policy Committee of the American Association for the Study of Liver Disease. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 585] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 17. | Nagae M, Egi M, Kubota K, Makino S, Mizobuchi S. Association of direct bilirubin level with postoperative outcome in critically ill postoperative patients. Korean J Anesthesiol. 2018;71:30-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Miyahara S, Isaji S. Liver injury in acute pancreatitis and mitigation by continuous arterial infusion of an antibiotic via the superior mesenteric artery. Pancreas. 2001;23:204-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Zhang XP, Wang L, Zhang J. Study progress on mechanism of severe acute pancreatitis complicated with hepatic injury. J Zhejiang Univ Sci B. 2007;8:228-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Field E, Horst HM, Rubinfeld IS, Copeland CF, Waheed U, Jordan J, Barry A, Brandt MM. Hyperbilirubinemia: a risk factor for infection in the surgical intensive care unit. Am J Surg. 2008;195:304-6; discussion 306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Caserta S, Mengozzi M, Kern F, Newbury SF, Ghezzi P, Llewelyn MJ. Severity of Systemic Inflammatory Response Syndrome Affects the Blood Levels of Circulating Inflammatory-Relevant MicroRNAs. Front Immunol. 2017;8:1977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Kokosis G, Perez A, Pappas TN. Surgical management of necrotizing pancreatitis: an overview. World J Gastroenterol. 2014;20:16106-16112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Boškoski I, Costamagna G. Walled-off pancreatic necrosis: where are we? Ann Gastroenterol. 2014;27:93-94. [PubMed] |
| 24. | Cavaillon JM, Annane D. Compartmentalization of the inflammatory response in sepsis and SIRS. J Endotoxin Res. 2006;12:151-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Alhajj M, Bansal P, Goyal A. Physiology, Granulation Tissue. 2020 Nov 2. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. [PubMed] |
| 26. | Tu HJ, Zhao CF, Chen ZW, Lin W, Jiang YC. Fibroblast Growth Factor (FGF) Signaling Protects Against Acute Pancreatitis-Induced Damage by Modulating Inflammatory Responses. Med Sci Monit. 2020;26:e920684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | di Mola FF, Friess H, Riesle E, Koliopanos A, Büchler P, Zhu Z, Brigstock DR, Korc M, Büchler MW. Connective tissue growth factor is involved in pancreatic repair and tissue remodeling in human and rat acute necrotizing pancreatitis. Ann Surg. 2002;235:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Johnson CL, Weston JY, Chadi SA, Fazio EN, Huff MW, Kharitonenkov A, Köester A, Pin CL. Fibroblast growth factor 21 reduces the severity of cerulein-induced pancreatitis in mice. Gastroenterology. 2009;137:1795-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Stojek M, Adrych K, Rojek L, Smoczynski M, Sledzinski T, Szrok S, Swierczynski J. Decreased serum platelet derived growth factor BB levels in acute and increased in chronic pancreatitis. World J Gastroenterol. 2014;20:13127-13132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Eisinger F, Patzelt J, Langer HF. The Platelet Response to Tissue Injury. Front Med (Lausanne). 2018;5:317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 31. | Deptuła M, Karpowicz P, Wardowska A, Sass P, Sosnowski P, Mieczkowska A, Filipowicz N, Dzierżyńska M, Sawicka J, Nowicka E, Langa P, Schumacher A, Cichorek M, Zieliński J, Kondej K, Kasprzykowski F, Czupryn A, Janus Ł, Mucha P, Skowron P, Piotrowski A, Sachadyn P, Rodziewicz-Motowidło S, Pikuła M. Development of a Peptide Derived from Platelet-Derived Growth Factor (PDGF-BB) into a Potential Drug Candidate for the Treatment of Wounds. Adv Wound Care (New Rochelle). 2020;9:657-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |