Published online Jul 27, 2021. doi: 10.4240/wjgs.v13.i7.717
Peer-review started: March 29, 2021
First decision: May 28, 2021
Revised: June 4, 2021
Accepted: June 22, 2021
Article in press: June 22, 2021
Published online: July 27, 2021
Processing time: 116 Days and 0.3 Hours
Early oral feeding (EOF) is an important measure for early recovery of patients with gastrointestinal tumors after surgery, which has emerged as a safe and effective postoperative strategy for improving clinical outcomes.
To determine the safety and efficacy of early oral feeding in postoperative patients with upper gastrointestinal tumor.
This meta-analysis was analyzed using Review Manager version 5.3 and Stata version 14. All clinical studies that analyzed efficacy and safety of EOF for postoperative patients with upper gastrointestinal tumor were included.
Fifteen studies comprising 2100 adult patients met all the inclusion criteria. A significantly lower risk of pneumonia was presented in the EOF compared with TOF group [relative risk (RR) = 0.63, 95% confidence interval (CI): 0.44–0.89, P = 0.01]. Length of hospital stay was significantly shorter in the EOF group than in the TOF group [weighted mean difference (WMD) = -1.91, 95%CI: -2.42 to -1.40; P < 0.01]. Cost of hospitalization was significantly lower (WMD = -4.16, 95%CI: -5.72 to -2.61; P < 0.01), and CD4 cell count and CD4/CD8 cell ratio on postoperative day 7 were significantly higher in the EOF group than in the TOF group: CD4 count (WMD = 7.17, 95%CI: 6.48–7.85; P < 0.01), CD4/CD8 ratio (WMD = 0.29, 95%CI: 0.23–0.35; P < 0.01). There was no significant difference in risk of anastomotic leak and total postoperative complications.
EOF as compared with TOF was associated with lower risk of pneumonia, shorter hospital length of stay, lower cost of hospitalization, and significantly improved postoperative immune function of patients.
Core Tip: Postoperative early oral feeding (EOF) is safe and effective for improving clinical outcomes in patients with lower gastrointestinal tumor. To our knowledge, this study is the largest meta-analysis of randomized controlled trials to date, including 2100 participants, of whom 1042 received EOF protocols and 1058 received traditional oral feeding, to assess the safety and efficacy in postoperative patients with upper gastrointestinal tumor. Our review clarified that EOF results in accelerated convalescence, reduction of the risk of pneumonia, length of hospital and medical costs, and better immune status.
- Citation: Hao T, Liu Q, Lv X, Qiu J, Zhang HR, Jiang HP. Efficacy and safety of early oral feeding in postoperative patients with upper gastrointestinal tumor: A systematic review and meta-analysis. World J Gastrointest Surg 2021; 13(7): 717-733
- URL: https://www.wjgnet.com/1948-9366/full/v13/i7/717.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i7.717
China has a 30% and 40% higher mortality of cancer than the United Kingdom and United States, respectively, and 36.4% of the cancer-related deaths are from upper gastrointestinal tract cancers (stomach, liver, and esophagus), with poor prognosis[1]. At present, surgery is still the most effective treatment. However, most of the cancer patients have accompanying malnutrition, which increases the possibility of surgical complications. Thus, it is necessary to carry out perioperative nutritional support as early as possible. Fortunately, a large number of studies have proved that early enteral nutrition is beneficial and can speed up postoperative recovery. Enhanced Recovery After Surgery (ERAS) guidelines advocate early resumption of normal oral diet to decrease surgical stress response[2,3].
Re-establishment of oral feeding as early as possible after surgery is important in the multimodal ERAS nursing strategy, which is associated with reducing morbidity, length of stay and cost[4,5]. At present, early oral feeding (EOF), i.e. oral intake (water or nutrient solution) within 24 h after surgery, has been widely practiced in patients with lower gastrointestinal tract surgery, and has benefited from a large number of experimental studies and reliable evidence-based medicine. However, for patients with upper gastrointestinal tract tumor, according to our observations, surgeons have a conservative attitude towards EOF, and the current method is still placing a nutrition tube or an intestinal stoma, which undoubtedly adds additional trauma and economic pressure to the patient. Although there are many studies of early oral enteral nutrition after surgery of the upper gastrointestinal tract, the results have not been consistent, and most of them are not randomized controlled trials (RCTs).
The purpose of our study was to analyze the safety and efficacy of EOF in postoperative patients with upper gastrointestinal tumors (esophagus, stomach, duodenum, and/or pancreas). Although there have been meta-analyses of EOF in patients with upper gastrointestinal tumors[6], we collected updated evidence and only included RCTs of upper gastrointestinal tumors to make our results more reliable. This is believed to be the first meta-analysis of upper gastrointestinal tumors only including RCTs. We used postoperative complications and exhaust time as the main outcome indicators, and evaluated the changes in hospitalization time, hospitalization costs, and immune indicators after surgery.
The present systematic review was prepared and revised according to the PRISMA 2009 Checklist. We registered the protocol with PROSPERO (International Prospective Register of Systematic Reviews), registration number CRD42021225789 (http://www.crd.york.ac.uk/PROSPERO).
The research question was structured according to the PICOS (Population, Intervention, Comparator, Outcome and Study Design) criteria. Clinical studies that analyzed efficacy and safety of EOF for postoperative patients with upper gastrointestinal tumor were collected from PubMed, Embase, Web of Science, Cochrane Library, Chinese National Knowledge Infrastructure, Wanfang, and VIP databases until the end of December 2020. We used MeSH terms and keyword combinations when searching. The MeSH terms were: "Gastrointestinal Tract", "Upper Gastrointestinal Tract", "Esophagus", "Stomach", "Duodenum", "Pancreas" and "Neoplasms", "Anastomosis, Roux en Y", "Esophagectomy", "Esophagoplasty", "Gastrectomy", "Gastroenterostomy", "Pancreaticoduodenectomy", "Enteral Nutrition", "Nutritional Support", "Diet Therapy", "Nutrition Therapy", "Dietary Supplements", and "Feeding Methods". We also screened manually the reference lists of all included studies. Two independent researchers extracted the literature data, and the third researcher judged if there were any differences.
Inclusion criteria were: (1) Patients with upper gastrointestinal tumor (including esophageal, stomach, pancreatic or duodenal cancer) undergoing surgery; (2) EOF, including water or liquid, within 24 h after surgery; (3) RCTs; (4) Studies including one or more of the outcomes; (5) Control group was traditional oral feeding (TOF) or late oral feeding, including any form of enteral nutrition later than 24 h, or total parenteral nutrition; and (6) English or Chinese language. Exclusion criteria were: (1) Duplicate documents, abstract, review, case reports, animal research, and non-adult studies; (2) Non-RCTs and noncomparative studies; (3) Oral feeding after surgery later than 24 h; (4) Incomplete data or no full text; (5) Studies including non-tumor patients and lower gastrointestinal tumors; and (6) Other irrelevant research.
After identification of all potentially eligible studies, we evaluated the studies according to the quality evaluation criteria of the Cochrane System Reviewer Manual. The members of the research group clearly formulated the purpose of the analysis, the search procedures, and the source plan of the data. Two investigators independently extracted the literature data, and discussed with a third researcher to settle any discrepancies or divergences. The extracted content included study and baseline population characteristics (first author, publication year, country, sample size, research type, age, sex, operation type), intervention (time postoperative oral feeding started and the feeding program), comparison (time postoperative oral feeding started and the nutrition plan). Primary outcomes of interest were postoperative complications and time of gas passage. Secondary outcomes were length of postoperative hospital stay, cost of hospitalization, immune function indicators (CD4 cell count and CD4/CD8 cell ratio) (Tables 1 and 2).
| Ref. | Year | Sample size, n | Time of gas passage (mean ± SD, h) | LOS (mean ± SD, d) | Cost of hospitalization (mean ± SD, CNY, × 1000) | Pneumonia, n | Anastomotic leakage, n | ||||||
| EOF | TOF | EOF | TOF | EOF | TOF | EOF | TOF | EOF | TOF | EOF | TOF | ||
| Wang et al[15] | 2017 | 38 | 42 | 73.5 ± 6.3 | 80.1 ± 8.7 | 8.2 ± 1.6 | 9.5 ± 1.7 | 57.2 ± 5.1 | 63.1 ± 4.3 | 5%6 | 7%6 | 0 | 4.8%6 |
| Liu et al[12] | 2011 | 30 | 32 | 2.15 ± 0.431 | 2.97 ± 0.521 | NR | NR | NR | NR | 1 | 3 | 0 | 0 |
| Yang et al[18] | 2013 | 25 | 25 | 78.8 ± 8.4 | 87.1 ± 11.3 | 7.81 ± 2.58 | 9.62 ± 1.91 | 29.6 ± 4.2 | 35.2 ± 3.8 | NR | NR | 0 | 0 |
| Wang et al[14] | 2017 | 60 | 60 | 67.6 ± 7.5 | 85.2 ± 8.5 | 6.5 ± 1.8 | 9.2 ± 2.6 | NR | NR | 2 | 6 | 2 | 3 |
| Lin et al[13] | 2018 | 47 | 53 | 2.83 ± 0.961 | 3.56 ± 0.991 | 11.91 ± 1.43 | 12.68 ± 1.42 | 3.56 ± 0.615 | 3.93 ± 0.755 | 2 | 10 | 1 | 2 |
| Li et al[17] | 2014 | 50 | 50 | 67.3 ± 7.9 | 84.6 ± 8.7 | 6.8 ± 1.9 | 9.3 ± 2.5 | NR | NR | NR | NR | NR | NR |
| Yu et al[16] | 2016 | 72 | 67 | 2.1 ± 1.21 | 3.3 ± 1.51 | 6.0 ± 1.8 | 7.7 ± 2.5 | 1.5 ± 0.55 | 1.6 ± 0.85 | 1 | 3 | 0 | 1 |
| Li et al[12] | 2015 | 200 | 200 | 67.3 ± 7.9 | 84.6 ± 8.7 | 6.8 ± 1.9 | 9.3 ± 2.5 | NR | NR | NR | NR | NR | NR |
| Gao et al[10] | 2019 | 101 | 97 | 2.05 ± 0.711 | 2.50 ± 0.911 | NR | NR | NR | NR | NR | NR | 1 | 1 |
| Hur et al[20] | 2011 | 28 | 26 | 1.9 ± 1.21 | 2.9 ± 0.81 | 7.2 ± 1.7 | 8.5 ± 2.9 | 7749 ± 12504 | 8415 ± 29454 | 1 | 0 | 1 | |
| Berkelmans et al[19] | 2020 | 65 | 67 | NR | NR | NR | NR | NR | NR | 16 | 23 | 12 | 11 |
| Sun et al[22] | 2018 | 140 | 140 | 2 (2-3)1,2 | 3 (2-3)1,2 | 7 (7-8)2 | 10 (9-12)2 | NR | NR | 15 | 17 | 5 | 6 |
| Mahmoodzadeh et al[23] | 2015 | 54 | 55 | 3 (2-3)1,2 | 4 (3-4)1,2 | 6 (5.75-7)2 | 8 (7-9)2 | NR | NR | NR | NR | 2 | 1 |
| Shimizu et al[21] (DG) | 2018 | 70 | 84 | 2 (1-3)1,3 | 2 (1-6)1,3 | 10 (5-70)3 | 10 (5-31)3 | NR | NR | 0 | 2 | 5 | 2 |
| Shimizu et al[21] (TG) | 32 | 30 | 2 (1-4)1,3 | 3 (1-6)1,3 | 10 (7-16)3 | 12 (7-44)3 | NR | NR | 1 | 0 | 4 | 4 | |
| Mi et al[11] | 2012 | 30 | 30 | 79.9 ± 9.5 | 86.6 ± 8.7 | 7.83 ± 2.23 | 9.57 ± 1.96 | 30.22 ± 3.22 | 34.6 ± 3.21 | 0 | 1 | 0 | 0 |
| Postoperative complications, n | CD4_PreO, (%), mean ± SD | CD4_POD1, (%), mean ± SD | CD4_POD7, (%), mean ± SD | CD4/CD8_PreO, mean ± SD | CD4/CD8_POD1, mean ± SD | CD4/CD8_POD7, mean ± SD | |||||||
| EOF | TOF | EOF | TOF | EOF | TOF | EOF | TOF | EOF | TOF | EOF | TOF | EOF | TOF |
| 13%1 | 17%1 | 43 ± 4 | 43 ± 4 | 36 ± 3 | 34 ± 3 | 42 ± 4 | 36 ± 4 | 1.8 ± 0.2 | 1.7 ± 0.3 | 1.4 ± 0.3 | 1.5 ± 0.3 | 1.8 ± 0.2 | 1.4 ± 0.3 |
| 7 | 7 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| 3 | 4 | 43.2 ± 3.7 | 43.6 ± 4.1 | 35.3 ± 5.3 | 35.8 ± 3.6 | 40.6 ± 5.1 | 35.2 ± 3.8 | 1.68 ± 0.22 | 1.66 ± 0.27 | 1.52 ± 0.33 | 1.51 ± 0.42 | 1.77 ± 0.27 | 1.56 ± 0.31 |
| 7 | 17 | 43.4 ± 3.5 | 43.1 ± 3.1 | 30.6 ± 2.5 | 30.9 ± 2.4 | 42.4 ± 2.8 | 34.7 ± 2.3 | 1.9 ± 0.3 | 1.9 ± 0.2 | 1.5 ± 0.4 | 1.6 ± 0.3 | 1.8 ± 0.3 | 1.6 ± 0.3 |
| 5 | 16 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| NR | NR | 43.2 ± 3.7 | 42.9 ± 3.3 | 30.4 ± 2.7 | 30.7 ± 2.6 | 42.2 ± 3.0 | 34.5 ± 2.5 | 1.8 ± 0.3 | 1.7 ± 0.3 | 1.3 ± 0.4 | 1.4 ± 0.4 | 1.7 ± 0.3 | 1.4 ± 0.4 |
| 10 | 11 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| NR | NR | 43.5 ± 3.8 | 43.5 ± 3.6 | 31.5 ± 2.8 | 30.5 ± 2.5 | 42.1 ± 3.6 | 34.4 ± 2.4 | 1.6 ± 0.4 | 1.8 ± 0.3 | 1.6 ± 0.6 | 1.4 ± 0.3 | 1.7 ± 0.3 | 1.4 ± 0.8 |
| 11 | 10 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| 7 | 8 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| 48 | 56 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| 6 | 5 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| 17 | 8 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| 11 | 6 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| 4 | 5 | 42.2 ± 3.5 | 42.5 ± 3.6 | 36.4 ± 3.1 | 35.7 ± 2.9 | 40.6 ± 3.9 | 34.8 ± 3.1 | 1.76 ± 0.21 | 1.75 ± 0.22 | 1.40 ± 0.31 | 1.62 ± 0.45 | 1.76 ± 0.28 | 1.46 ± 0.23 |
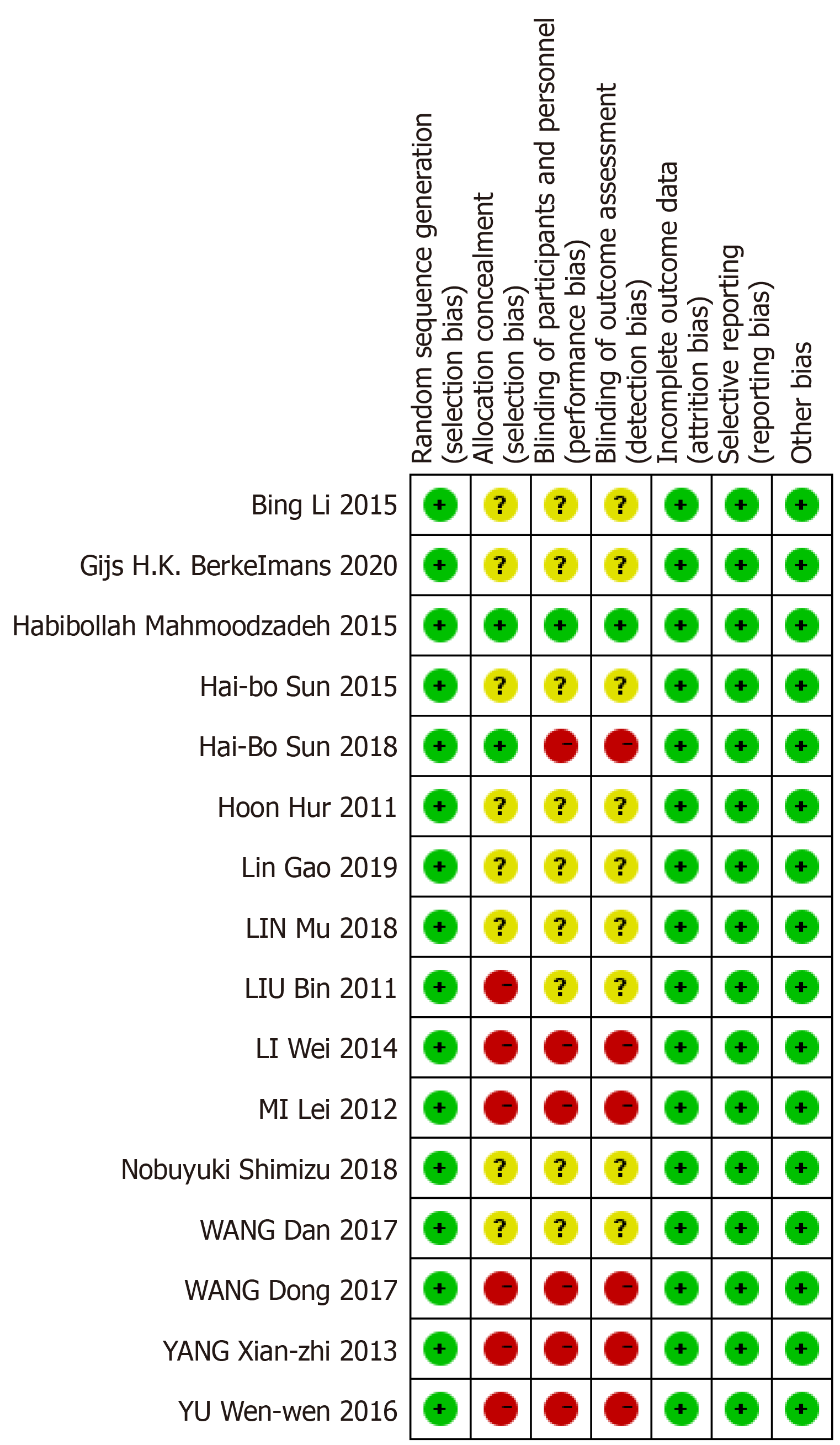
All included RCTs were evaluated by another two investigators separately using the risk of bias assessment tool recommended by the Cochrane Collaboration[7]. The main indicators included: (1) Randomization; (2) Allocation concealment; (3) Blinding of participants and personnel; (4) Blinding of outcome assessment; (5) Incomplete outcome data; (6) Selective outcome reporting; and (7) Other bias. Risk of bias for each included study was graded as high risk, low risk or unclear.
Statistical analysis was performed using Review Manager version 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark) and Stata version 14 (StataCorp LP, College Station, TX, United States). We invited an expert in biomedical statistics (Qingshan Chen, MD, PhD, Jinan University) to evaluate the statistical methods. The results were expressed with relative risk (RR) for the dichotomous variables and weighted mean difference (WMD) for the continuous variables, with 95% confidence intervals (CIs). If the study did not provide mean ± SD, they were obtained using an online calculator[8]. The I2 statistic was used to evaluate statistical heterogeneity. If I2 was > 50%, the data were regarded as having substantial heterogeneity. Thus, a random-effects model was used and we found the reason via sensitivity analysis; otherwise, a fixed-effects model was selected. Funnel scatterplot and Egger’s test were chosen to assess publication bias. P < 0.05 was statistically significant. Forest plots represented the pooled RR and 95%CIs. A funnel plot was drawn to detect publication bias.
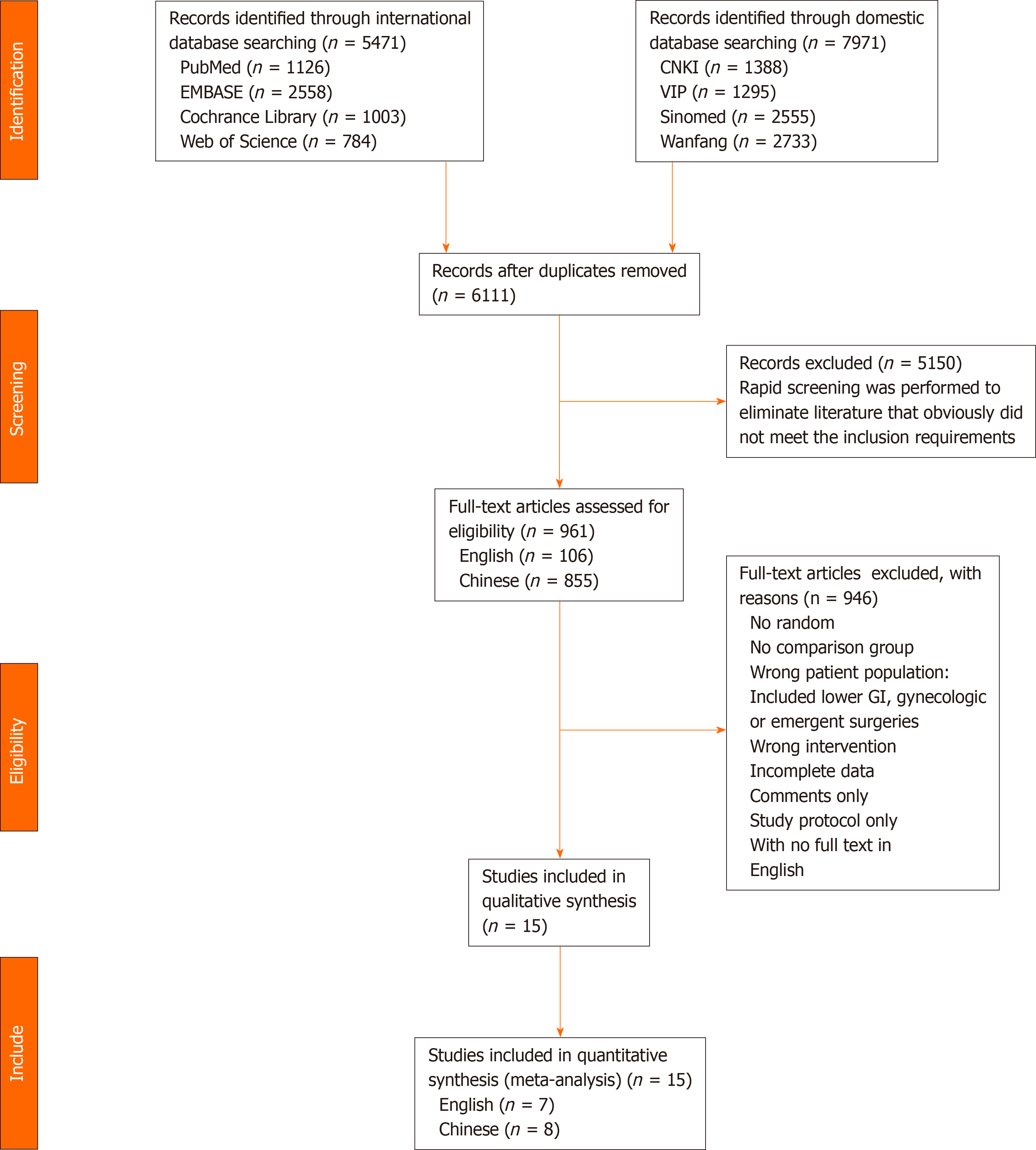
According to inclusion and exclusion criteria, we selected 13442 preliminary studies, including 5471 English and 7971 Chinese studies. After eliminating studies that did not meet the inclusion requirements and duplicates by rapid screening, we evaluated other studies, and removed those that did not meet the inclusion criteria and from which we could not extract data. Finally, we included 15 studies[9-23], of which seven were English and eight Chinese. The study selection process is outlined in the PRISMA flowchart (Figure 1). We evaluated the risk bias of the included studies using the Cochrane Collaboration’s tool.
All 15 studies reported on 2100 patients (1042 receiving EOF and 1058 TOF). There were 12 studies of gastric cancer, 2 of esophageal cancer, and 1 of both esophageal and gastric cancer. A study of pancreatic cancer and duodenum cancer did not include EOF. Table 3 presents the main characteristics of the included studies. Assessment of the risk of bias across all included studies is presented in Figure 2. The main risk of bias was blinding among these RCTs, as it was difficult to perform double blinding in such procedural trials.
| Ref. | Year | Country | Location of cancer | Sample size, n | Sex, male/female, n | Age in yr, mean ± SD, male/female | Outcomes | |||
| EOF | TOF | EOF | TOF | EOF | TOF | |||||
| Wang et al[15] | 2017 | China | Gastric | 38 | 42 | NR | NR | 58 ± 101 | ①②③④⑤⑥⑦⑧ | |
| Liu et al[12] | 2011 | China | Gastric | 30 | 32 | 12/18 | 19/13 | 56.34 | 57.54 | ①②④ |
| Yang et al[18] | 2013 | China | Gastric | 25 | 25 | 15/10 | 16/9 | 57.5 ± 13.7 | 56.8 ± 11.9 | ①④⑤⑥⑦⑧ |
| Wang et al[14] | 2017 | China | Gastric | 60 | 60 | 31/29 | 33/27 | 58.6 ± 7.8 | 54.2 ± 8.4 | ①②③④⑤⑦⑧ |
| Lin et al[13] | 2018 | China | Gastric | 47 | 53 | NR | NR | 52.2 ± 6.61 | ①②③④⑤⑥ | |
| Li et al[17] | 2014 | China | Gastric | 50 | 50 | 26/24 | 28/22 | 60.8 ± 5.9 | 56.0 ± 7.6 | ④⑤⑦⑧ |
| Yu et al[16] | 2016 | China | Gastric | 72 | 67 | 57/15 | 49/18 | 57.8 ± 13.1 | 60.1 ± 11.8 | ①②③④⑤⑥ |
| Li et al[12] | 2015 | China | Gastric | 200 | 200 | 104/96 | 112/88 | 60.8 ± 5.9 | 56.0 ± 7.6 | ④⑤⑦⑧ |
| Gao et al[10] | 2019 | China | Gastric | 101 | 97 | 68/33 | 55/42 | 56.3 ± 10.2 | 53.9 ± 11.6 | ①②③④ |
| Hur et al[20] | 2011 | South Korea | Gastric | 28 | 26 | 20/8 | 21/5 | NR (mean ± SD) | ①②③④⑤⑥ | |
| Berkelmans et al[19] | 2020 | Netherlands Sweden | Esophageal | 65 | 67 | 56/9 | 56/8 | 65 (59-70)2 | 65 (61-70)2 | ②③ |
| Sun et al[22] | 2018 | China | Esophageal | 140 | 140 | 92/48 | 103/37 | 62 (53-59)2 | 63 (58-69)2 | ①②③④⑤ |
| Mahmoodzadeh et al[23] | 2015 | Iran | Both | 54 | 55 | 29/25 | 29/26 | 64.2 ± 8.2 | 66.4 ± 7.7 | ①③④⑤ |
| Shimizu et al[21] (DG) | 2018 | Japan | Gastric | 70 | 84 | 36/34 | 54/30 | 64.5 (37-79)3 | 64 (25-79)3 | ①②③④⑤ |
| Shimizu et al[21] (TG) | 32 | 30 | 25/7 | 8/22 | 68.5 (48-78)3 | 68.5 (40-79)3 | ||||
| Mi et al[11] | 2012 | China | Gastric | 30 | 30 | 15/15 | 12/18 | 57.2 ± 9.5 | 60.0 ± 10.3 | ①②③④⑤⑥⑦⑧ |
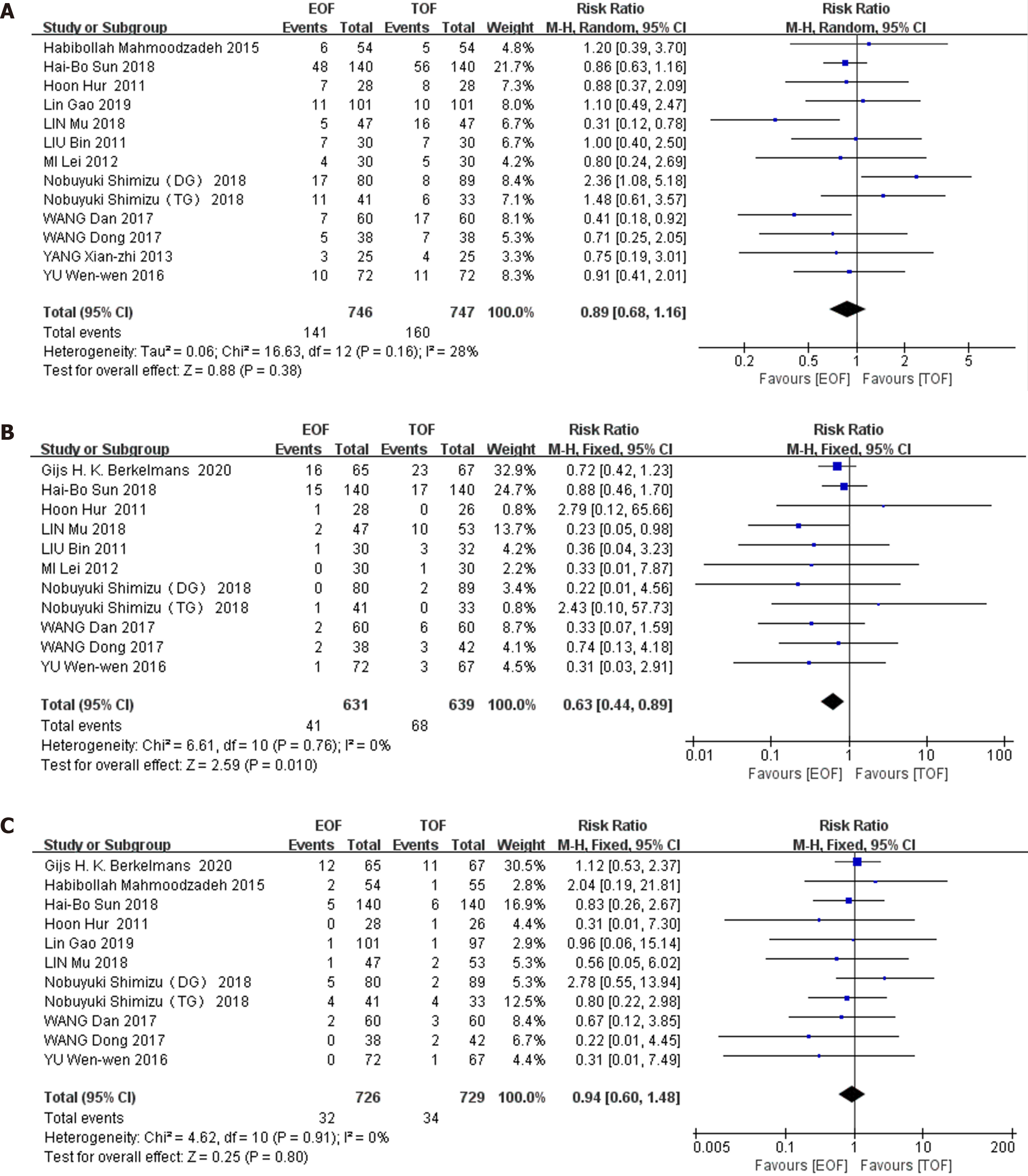
Primary outcomes: Twelve RCTs (involving 1493 patients) reported postoperative complications as dichotomous data. The incidence of postoperative complications in the EOF group was 141 (141/746, 18.9%) and 160 (160/747, 21.4%) in the group receiving TOF. Combined analysis showed that EOF did not increase the morbidity of postoperative complications compared with TOF (RR 0.89, 95%CI: 0.68–1.16, P = 0.38), and no significant heterogeneity was found among these trials (χ2 = 16.63; I2 = 28%; P = 0.16) (Figure 3A).
Eleven RCTs (involving 1270 patients) provided data regarding pneumonia: 6.5% (41/631 patients) in the EOF group and 11% (68/639) in the TOF group. Pooling analysis indicated that the incidence of pneumonia was significantly reduced in the EOF group (RR = 0.63, 95%CI: 0.44–0.89, P = 0.01), and no heterogeneity was found among these trials (χ2 = 6.61; I2 = 0%; P = 0.76) (Figure 3B).
11 RCTs (involving 1455 patients) reported anastomotic leakage, amounting to 4.4% (32/726 patients) in the EOF group and 4.7% (34/729) in the TOF group. Pooling the results suggested that EOF did not increase anastomotic leakage compared with TOF (RR = 0.94, 95%CI: 0.60–1.48, P = 0.80), and there was no heterogeneity observed in these studies (χ2 = 4.62; P = 0.91; I2 = 0%) (Figure 3C).
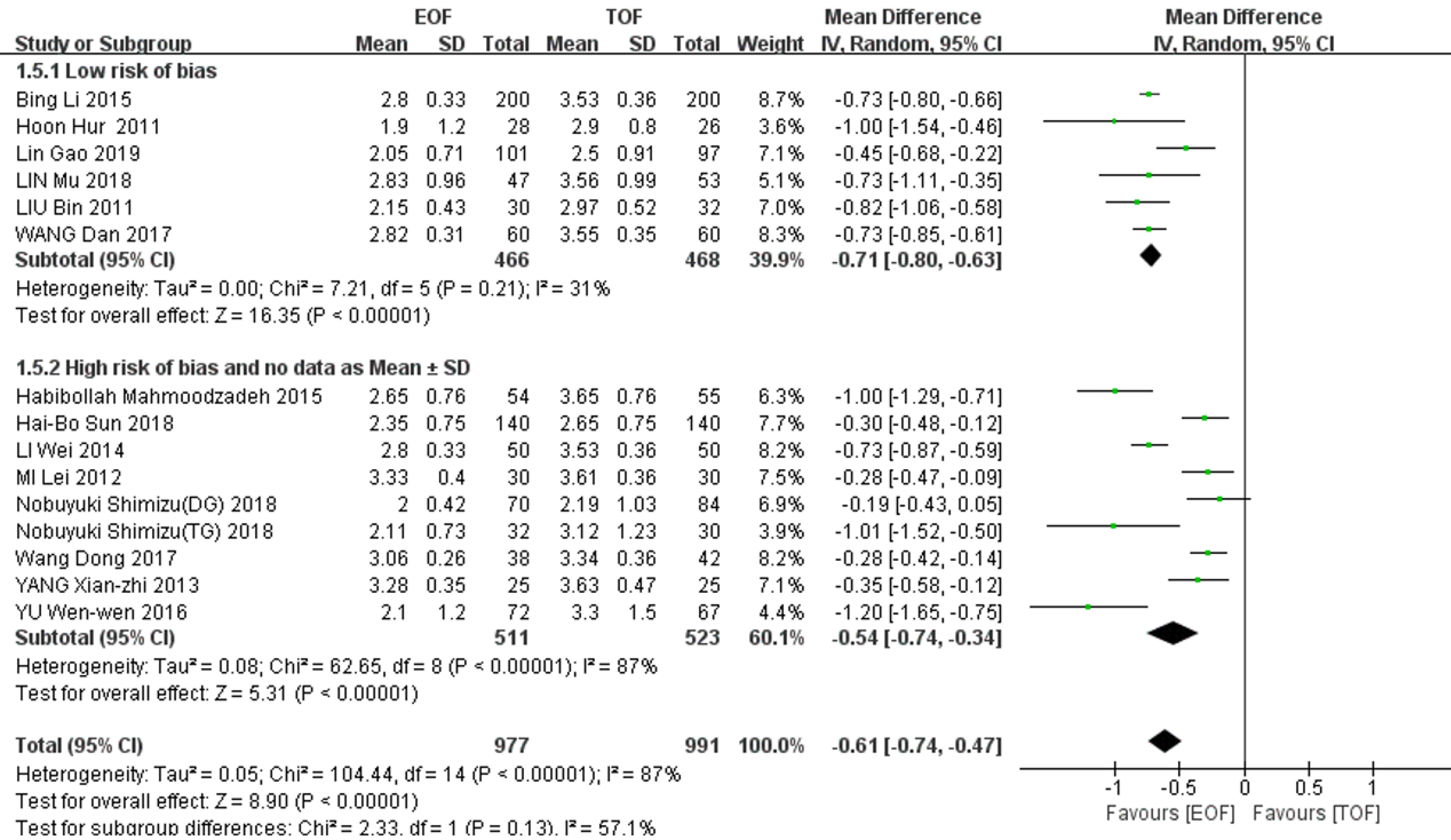
14 studies (1968 patients) reported postoperative exhaust time. There was significantly heterogeneity among the studies (χ2 = 104.44, I2 = 87%, P < 0.01), and a random-effects model was adopted for the pooled analysis. The postoperative exhaust time in the EOF group was significantly earlier than that in the TOF group (WMD = -0.61, 95%CI: -0.74--0.47]; P < 0.01). When we used sensitivity to analyze the sources of heterogeneity, we found that after eliminating the studies that did not directly provide mean ± SD and those with high risk bias (Table 4), the remaining data after combined analysis showed no significant heterogeneity (χ2 = 7.21, I2 = 31%, P = 0.21), and the results still suggested that the EOF group could significantly shorten the exhaust time (WMD = - 0.71, 95%CI: -0.80--0.63; P < 0.01) (Figure 4).
| Ref. | Year | Sample size, n | Postoperative exhaust time in h, mean ± SD | Eliminate reason | ||
| EOF | TOF | EOF | TOF | |||
| Wang et al[15] | 2017 | 38 | 42 | 73.5 ± 6.3 | 80.1 ± 8.7 | High risk of bias |
| Yang et al[18] | 2013 | 25 | 25 | 78.8 ± 8.4 | 87.1 ± 11.3 | High risk of bias |
| Mi et al[11] | 2012 | 30 | 30 | 79.9 ± 9.5 | 86.6 ± 8.7 | High risk of bias |
| Li et al[17] | 2014 | 50 | 50 | 67.3 ± 7.9 | 84.6 ± 8.7 | High risk of bias |
| Yu et al[16] | 2016 | 72 | 67 | 2.1 ± 1.21 | 3.3 ± 1.51 | No data as mean ± SD |
| Sun et al[22] | 2018 | 140 | 140 | 2 (2-3)1,2 | 3 (2-3)1,2 | No data as mean ± SD |
| Mahmoodzadeh et al[23] | 2015 | 54 | 55 | 3 (2-3)1,2 | 4 (3-4)1,2 | No data as mean ± SD |
| Shimizu et al[21] (DG) | 2018 | 70 | 84 | 2 (1-3)1,3 | 2 (1-6)1,3 | No data as mean ± SD |
| Shimizu et al[21] (TG) | 32 | 30 | 2 (1-4)1,3 | 3 (1-6)1,3 | No data as mean ± SD | |
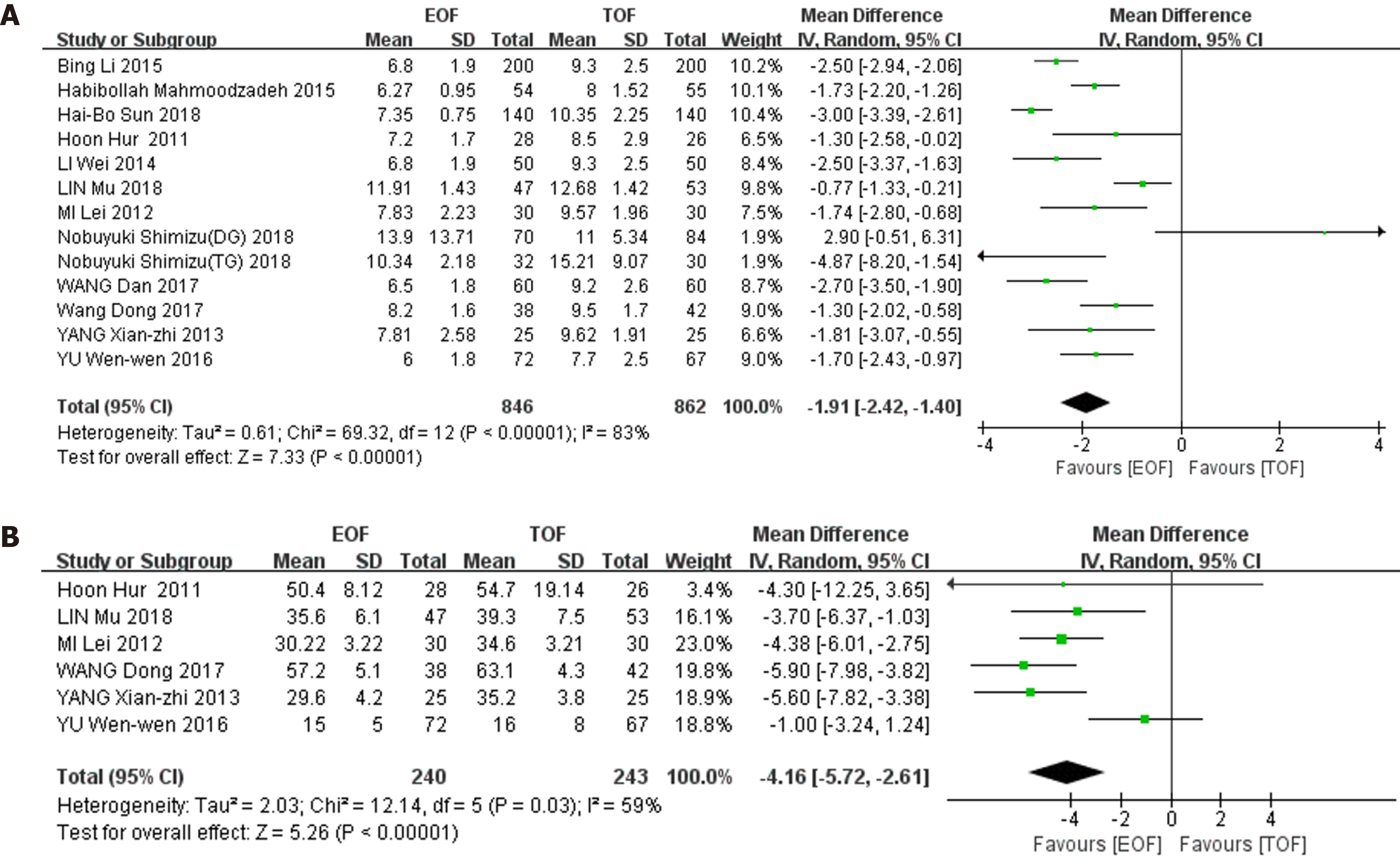
Secondary outcomes: 12 studies (1708 patients) reported the length of postoperative hospital stay. Heterogeneity was found among these studies (χ2 = 69.32, I2 = 83%, P < 0.01), and a random-effects model was used for the pooled analysis. The length of postoperative hospital stay in the EOF group was significantly shorter than that in the TOF group (WMD = -1.91, 95%CI: -2.42--1.40; P < 0.01) (Figure 5A).
6 studies (482 patients) reported the cost of hospitalization. Heterogeneity was present in these trials (χ2 = 12.14, I2 = 59%, P = 0.03), therefore, a random-effects model was chosen for the combined analysis. The cost of hospitalization was significantly lower in the EOF group than in the TOF group (WMD = -4.16, 95%CI: -5.72--2.61]; P < 0.01) (Figure 5B).
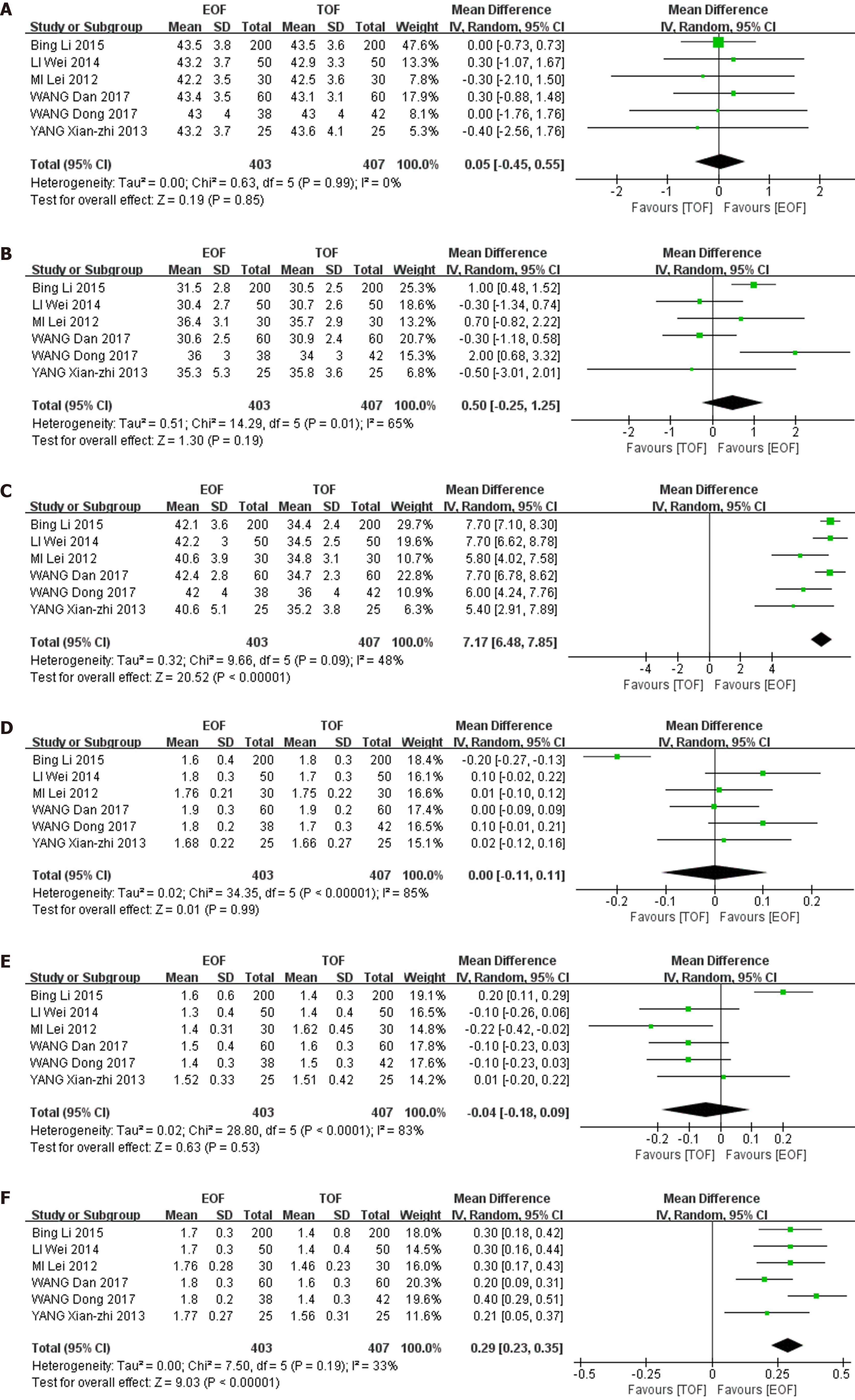
6 studies (810 patients) reported CD4 cell count and CD4/CD8 cell ratio. We performed a baseline consistency check on CD4 count and CD4/CD8 ratio the day before the operation and found that the baseline was consistent: CD4 (WMD = 0.05, 95%CI: -0.45-0.55; P = 0.85), CD4/CD8 (WMD = 0.00, 95%CI: -0.11-0.11; P = 0.99). We evaluated the results on postoperative day (POD) 1 and 7 after surgery and found that CD4 count and CD4/CD8 ratio in the EOF group were higher than in the control group on POD1, but not significantly: CD4 (WMD = 0.50, 95%CI: -0.25-1.25; P = 0.19), CD4/CD8 (WMD = 0.04, 95%CI: -0.18-0.09; P = 0.53). However, on POD7, CD4 and CD4/CD8 in the EOF group were significantly higher than those in the TOF group: CD4 (WMD = 7.17, 95%CI: 6.48–7.85; P < 0.01), CD4/CD8 (WMD = 0.29, 95%CI: 0.23–0.35; P < 0.01). No significant heterogeneity was present in CD4 and CD4/CD8 results on POD7: CD4 (χ2 = 9.66, I2 = 48%, P = 0.09), CD4/CD8 (χ2 = 7.50, I2 = 33%, P = 0.19) (Figure 6).
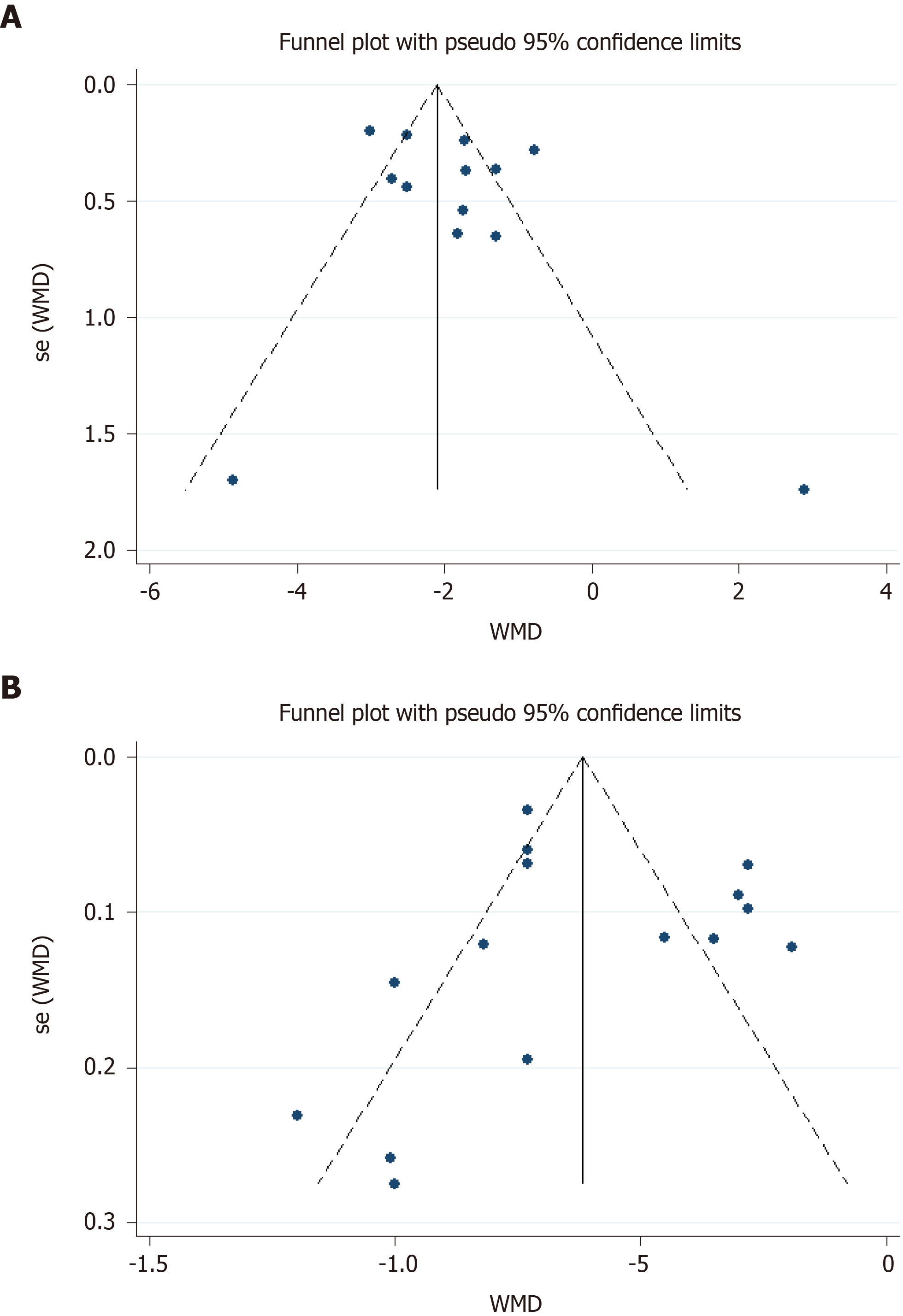
Due to the obvious heterogeneity of the data analysis after combining the length of postoperative hospital stay and exhaust time, we used the funnel plot and Egger’s test to detect publication bias. The analysis indicated that the publication bias was small (Figure 7).
During the past few decades, there have been many surgical practices to keep patients nil by mouth until the return of bowel function, especially in gastrointestinal surgery with resection and anastomosis, to avoid related complications[24]. However, in recent years this routine practice has been questioned. Delayed enteral nutrition could lead to atrophic changes in the intestinal mucosa, reduction in nutrient absorption, and impairment in intestinal immune function, which have been demonstrated in animal and human studies[25,26]. As a result, tissue injury at distant sites and the development of multiple organ failure can occur[27]. Therefore, a lot of research on early enteral nutrition has appeared in the last 10 years. In these studies, there are probably 3 methods of early postoperative enteral nutrition: Early oral, jejunostomy tube or nasojejunal tube feeding. Although a nasojejunal tube or a jejunostomy tube is used in most cases, which is the best way remains to be determined.
Han-Geurts et al[28] showed that early oral intake did not reduce the duration of postoperative intestinal obstruction, and recovery of gastrointestinal function did not affect tolerance of an oral diet. Other researchers have proposed that resuming oral diet as soon as possible can even promote the recovery of gastrointestinal function[29,30]. Therefore, a lot of studies on EOF have been implemented recently. In the past few decades, many high-quality studies have pointed out that the safety and benefit of EOF after colorectal surgery[31,32]. Recently, the same results appeared in patients undergoing upper gastrointestinal surgery, mainly gastric and esophageal surgery[23,33,34], while there have been few operations on the pancreas and duodenum. A study on early enteral nutrition after pancreatoduodenectomy has shown that early enteral nutrition increases postoperative complications, and is not recommended in terms of safety and feasibility[35]. However, another meta-analysis[36] of enteral nutrition after pancreatoduodenectomy showed that enteral nutrition is associated with a significantly shorter length of stay compared to parenteral nutrition. In our study, we only included RCTs on gastric and esophageal cancer, and concluded that the complication of pneumonia and length and cost of hospitalization were significantly decreased. This is similar to the results of a meta-analysis on the effects of EOF in the upper gastrointestinal tract[6]. However, the results of a Japanese study were different, which concluded that EOF does not reduce the length of hospital stay after distal gastrectomy and increases the risk of complications. We consider that this might be related to the research design. They divided gastric surgery into distal and total gastrectomy, and obtained inconsistent results. Our study included esophageal and gastric surgery, and did not group the procedures, which may have caused inconsistent results. Furthermore, we counted the changes in immune indicators after surgery. We measured CD4 cell count and CD4/CD8 cell ratio, showing that both indicators were significantly increased, indicating that EOF seems to enhance the immune system.
Meta-analyses of RCTs represent the best possible option to summarize the beneficial and harmful effects of interventions[37]. However, RCTs can have high levels of bias related to weak randomization methods, lack of blinding, and incomplete outcome data. There is no doubt that the current research had some limitations. First, although the total sample size of the study was > 2000, some of the included RCTs were smaller in size. Second, there was considerable heterogeneity in the included studies. No remarkable heterogeneity was found in the incidence of complications (including anastomotic leakage and pneumonia). However, there was significant heterogeneity in postoperative exhaust time, hospitalization costs, length of stay, and CD4 cell count and CD4/CD8 cell ratio. This significant heterogeneity may be attributed to clinical heterogeneity, including the technical status of each institution, the inclusion of standard surgical approaches, inconsistent outcome assessments, and different EOF procedures. Third, as this study included fewer studies on esophageal cancer, we did not conduct group assessments for esophageal and gastric cancer, which increased the bias to a certain extent. However, we included most relevant RCTs and obtained positive results, which have contributed to the advancement of the application of EOF in upper gastrointestinal surgery.
The present updated meta-analysis and systematic review demonstrate that application of EOF after esophageal and gastric cancer surgery is safe and effective. EOF can significantly reduce the incidence of pneumonia, reduce hospitalization time and hospitalization costs, and significantly improve the postoperative immune function of patients. However, due to the heterogeneity of the included trials, further high-quality, large-sample and multicenter RCTs with long-term follow-up are needed. Finally, we believe that with the advancement of medical technology, EOF will be commonly used in upper gastrointestinal surgery.
Early oral feeding (EOF) has emerged as a safe and effective postoperative strategy for improving clinical outcomes in patients with lower gastrointestinal tumor. However, controversies exist with regard to EOF practice in postoperative patients with upper gastrointestinal tumor.
The purpose of this systematic and meta-analysis was to evaluate the role and importance of EOF in postoperative patients with upper gastrointestinal tumor.
By comparing the safety and efficacy of EOF and TOF, it provided a valuable evidence and safe choice for early rehabilitation of patients in the future.
PubMed, EMBASE, Web of Science, Cochrane Library, Chinese National Knowledge Infrastructure, Wanfang, and VIP databases were searched up to December 2020 for all available randomized controlled trials (RCTs) comparing EOF and traditional oral feeding (TOF) of postoperative patients with upper gastrointestinal tumors. Fifteen RCTs, with a total of 2100 participants, were analyzed in this study, of whom 1042 underwent EOF and 1058 TOF protocols.
In the meta-analysis of postoperative pneumonia and anastomotic leak, there was no significant heterogeneity (I2 = 0%); therefore, a fixed-effect model was applied. A significantly lower risk of pneumonia was presented (RR = 0.63, 95%CI: 0.44–0.89, P = 0.01). In the meta-analysis of postoperative exhaust time, there was significant heterogeneity among the studies (I2 = 87%). But, after eliminating the studies that did not directly provide mean ± SD and those with high risk bias, the remaining data after combined analysis showed no significant heterogeneity (I2 = 31%), and the results suggested that the EOF group could significantly shorten the exhaust time (WMD = 0.71, 95%CI: 0.80-0.63; P < 0.01). No significant heterogeneity was present in CD4 cell count and CD4/CD8 cell ratio results on POD7: CD4 count (I2 = 48%,), CD4/CD8 (I2 = 33%); accordingly, a fixed-effect model was applied. On POD7, CD4 count and CD4/CD8 in the EOF group were significantly higher than those in the TOF group: CD4 count (WMD = 7.17, 95%CI: 6.48–7.85; P < 0.01), CD4/CD8 ratio (WMD = 0.29, 95%CI: 0.23–0.35; P < 0.01).
Our unit has been committed to early postoperative rehabilitation for more than 10 years. According to our experience, this meta-analysis is consistent with the clinical situation; therefore, we suggest that EOF can be used for patients with upper gastrointestinal tumors after surgery.
Early recovery after surgery has always been an important point for patients with gastrointestinal tumors. The present updated meta-analysis and systematic review demonstrate that application of EOF after esophageal and gastric cancer surgery is safe and effective. We consider that choosing appropriate patients and precise surgical operations will help the implementation of EOF. Additionally, we should conduct further high-quality, large-sample and multicenter RCTs with long-term follow-up.
We would like to thank Dr. Qing-Shan Chen, a member of the Biostatistics Service from the Department of Medical Statistics, Jinan University, for reviewing the statistical methods in this study.
Manuscript source: Unsolicited manuscript
Specialty type: Nutrition and dietetics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Laoveeravat P S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Li JH
| 1. | Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond). 2019;39:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 1122] [Article Influence: 187.0] [Reference Citation Analysis (1)] |
| 2. | Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, McNaught CE, MacFie J, Liberman AS, Soop M, Hill A, Kennedy RH, Lobo DN, Fearon K, Ljungqvist O; Enhanced Recovery After Surgery Society. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr. 2012;31:783-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 464] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 3. | Scott MJ, Baldini G, Fearon KC, Feldheiser A, Feldman LS, Gan TJ, Ljungqvist O, Lobo DN, Rockall TA, Schricker T, Carli F. Enhanced Recovery After Surgery (ERAS) for gastrointestinal surgery, part 1: pathophysiological considerations. Acta Anaesthesiol Scand. 2015;59:1212-1231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 258] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 4. | Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, Laviano A, Ljungqvist O, Lobo DN, Martindale R, Waitzberg DL, Bischoff SC, Singer P. ESPEN guideline: Clinical nutrition in surgery. Clin Nutr. 2017;36:623-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 859] [Cited by in RCA: 1060] [Article Influence: 132.5] [Reference Citation Analysis (0)] |
| 5. | Greco M, Capretti G, Beretta L, Gemma M, Pecorelli N, Braga M. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg. 2014;38:1531-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 629] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 6. | Willcutts KF, Chung MC, Erenberg CL, Finn KL, Schirmer BD, Byham-Gray LD. Early Oral Feeding as Compared With Traditional Timing of Oral Feeding After Upper Gastrointestinal Surgery: A Systematic Review and Meta-analysis. Ann Surg. 2016;264:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24818] [Article Influence: 1772.7] [Reference Citation Analysis (3)] |
| 8. | Shi J, Luo D, Weng H, Zeng XT, Lin L, Chu H, Tong T. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods. 2020;11:641-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 285] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 9. | Li B, Liu HY, Guo SH, Sun P, Gong FM, Jia BQ. The postoperative clinical outcomes and safety of early enteral nutrition in operated gastric cancer patients. J BUON. 2015;20:468-472. [PubMed] |
| 10. | Gao L, Zhao Z, Zhang L, Shao G. Effect of early oral feeding on gastrointestinal function recovery in postoperative gastric cancer patients: a prospective study. J BUON. 2019;24:194-200. [PubMed] |
| 11. | MI L, Zhang B, Zhang D, Zhou Y, Wang D. Efect of early oral enteral nutrition on clinical outcomes after gastric cancer surgery. Zhonghua Weichang Waike Zazhi. 2012;15:464-467. [DOI] [Full Text] |
| 12. | Liu B, Chen J, Huang S. Application of early oral feeding after curative surgery for distal gastric cancer. Huaxi Yixue. 2011;26:1666-1668. |
| 13. | Lin M. Application of early oral feeding in the treatment of postoperative gastric cancer. Zhongguo Shiyong Yiyao. 2018;13:113-115. [DOI] [Full Text] |
| 14. | Wang D, Zhong B, Liu Z, Li D, Wang D. Effect of early oral enteral nutrition on postoperative recovery of gastric cancer patients. Zhonghua Putong Waike Zazhi. 2017;32:883-884. [DOI] [Full Text] |
| 15. | Wang D, Zhang L, Cheng X, Zhang W. The influence of early enteral nutrition or parenteral nutrition therapy on the immune function and nutritional status of gastric cancer patients with radical surgery. Linchuang Zhongliuxue Zazhi. 2017;22:423-426. [DOI] [Full Text] |
| 16. | Yu W, Tao R, Yan K, Han X, Li H, Liu H. Clinical Study of Early Oral Feeding aft er Laparoscopic Radical Distal Gastrectomy. Zhongguo Puwai Jichu Yu Linchuang Zazhi. 2016;23:1339-1343. [DOI] [Full Text] |
| 17. | Li W, Zhou L, Sun H, Chen G, Chen L. Influence of Early Enteral Nutrition on Postoperative Clinical Effects of Gastric Cancer Patients Treated with Radical Operations. Shiyong Aizheng Zazhi. 2014;(1):56-58, 61. [DOI] [Full Text] |
| 18. | Yang XZ, Ge H. Observation of early oral eternal nutrition on clinical outcomes after gastric cancer surgery. Sichuan Yixue. 2013;34:1329-1331. |
| 19. | Berkelmans GHK, Fransen LFC, Dolmans-Zwartjes ACP, Kouwenhoven EA, van Det MJ, Nilsson M, Nieuwenhuijzen GAP, Luyer MDP. Direct Oral Feeding Following Minimally Invasive Esophagectomy (NUTRIENT II trial): An International, Multicenter, Open-label Randomized Controlled Trial. Ann Surg. 2020;271:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 20. | Hur H, Kim SG, Shim JH, Song KY, Kim W, Park CH, Jeon HM. Effect of early oral feeding after gastric cancer surgery: a result of randomized clinical trial. Surgery. 2011;149:561-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Shimizu N, Oki E, Tanizawa Y, Suzuki Y, Aikou S, Kunisaki C, Tsuchiya T, Fukushima R, Doki Y, Natsugoe S, Nishida Y, Morita M, Hirabayashi N, Hatao F, Takahashi I, Choda Y, Iwasaki Y, Seto Y. Effect of early oral feeding on length of hospital stay following gastrectomy for gastric cancer: a Japanese multicenter, randomized controlled trial. Surg Today. 2018;48:865-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Sun HB, Li Y, Liu XB, Zhang RX, Wang ZF, Lerut T, Liu CC, Fiorelli A, Chao YK, Molena D, Cerfolio RJ, Ozawa S, Chang AC; written on behalf of the AME Thoracic Surgery Collaborative Group. Early Oral Feeding Following McKeown Minimally Invasive Esophagectomy: An Open-label, Randomized, Controlled, Noninferiority Trial. Ann Surg. 2018;267:435-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 23. | Mahmoodzadeh H, Shoar S, Sirati F, Khorgami Z. Early initiation of oral feeding following upper gastrointestinal tumor surgery: a randomized controlled trial. Surg Today. 2015;45:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Gabor S, Renner H, Matzi V, Ratzenhofer B, Lindenmann J, Sankin O, Pinter H, Maier A, Smolle J, Smolle-Jüttner FM. Early enteral feeding compared with parenteral nutrition after oesophageal or oesophagogastric resection and reconstruction. Br J Nutr. 2005;93:509-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Buchman AL, Moukarzel AA, Bhuta S, Belle M, Ament ME, Eckhert CD, Hollander D, Gornbein J, Kopple JD, Vijayaroghavan SR. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. JPEN J Parenter Enteral Nutr. 1995;19:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 274] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 26. | Nguyen NQ, Besanko LK, Burgstad C, Bellon M, Holloway RH, Chapman M, Horowitz M, Fraser RJ. Delayed enteral feeding impairs intestinal carbohydrate absorption in critically ill patients. Crit Care Med. 2012;40:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | McClave SA, Lowen CC, Martindale RG. The 2016 ESPEN Arvid Wretlind lecture: The gut in stress. Clin Nutr. 2018;37:19-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Han-Geurts IJ, Hop WC, Kok NF, Lim A, Brouwer KJ, Jeekel J. Randomized clinical trial of the impact of early enteral feeding on postoperative ileus and recovery. Br J Surg. 2007;94:555-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 29. | Zhang K, Cheng S, Zhu Q, Han Z. [Early vs traditional postoperative oral feeding in patients undergoing elective colorectal surgery: a meta-analysis of safety and efficacy]. Zhonghua Wei Chang Wai Ke Za Zhi. 2017;20:1060-1066. [PubMed] |
| 30. | Zhuang CL, Ye XZ, Zhang CJ, Dong QT, Chen BC, Yu Z. Early vs traditional postoperative oral feeding in patients undergoing elective colorectal surgery: a meta-analysis of randomized clinical trials. Dig Surg. 2013;30:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 31. | Hartsell PA, Frazee RC, Harrison JB, Smith RW. Early postoperative feeding after elective colorectal surgery. Arch Surg. 1997;132:518-20; discussion 520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Reissman P, Teoh TA, Cohen SM, Weiss EG, Nogueras JJ, Wexner SD. Is early oral feeding safe after elective colorectal surgery? Ann Surg. 1995;222:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 257] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 33. | Jo DH, Jeong O, Sun JW, Jeong MR, Ryu SY, Park YK. Feasibility study of early oral intake after gastrectomy for gastric carcinoma. J Gastric Cancer. 2011;11:101-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Lassen K, Kjaeve J, Fetveit T, Tranø G, Sigurdsson HK, Horn A, Revhaug A. Allowing normal food at will after major upper gastrointestinal surgery does not increase morbidity: a randomized multicenter trial. Ann Surg. 2008;247:721-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 209] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 35. | Perinel J, Mariette C, Dousset B, Sielezneff I, Gainant A, Mabrut JY, Bin-Dorel S, Bechwaty ME, Delaunay D, Bernard L, Sauvanet A, Pocard M, Buc E, Adham M. Early Enteral Versus Total Parenteral Nutrition in Patients Undergoing Pancreaticoduodenectomy: A Randomized Multicenter Controlled Trial (Nutri-DPC). Ann Surg. 2016;264:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 36. | Adiamah A, Ranat R, Gomez D. Enteral vs parenteral nutrition following pancreaticoduodenectomy: a systematic review and meta-analysis. HPB (Oxford). 2019;21:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Hernandez AV, Marti KM, Roman YM. Meta-Analysis. Chest. 2020;158:S97-S102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |