Published online May 27, 2021. doi: 10.4240/wjgs.v13.i5.419
Peer-review started: January 12, 2021
First decision: February 14, 2021
Revised: February 19, 2021
Accepted: April 26, 2021
Article in press: April 26, 2021
Published online: May 27, 2021
Processing time: 126 Days and 13 Hours
In recent years, we created and employed a new anastomosis method, “bridging” pancreaticogastrostomy, to treat patients with extremely severe pancreatic injury. This surgery has advantages such as short length of surgery, low secondary trauma, rapid construction of shunts for pancreatic fluid, preventing second surgeries, and achieving good treatment outcomes in clinical practice. However, due to the limited number of clinical cases, there is a lack of strong evidence to support the feasibility and safety of this surgical procedure. Therefore, we carried out animal experiments to examine this procedure, which is reported here.
To examine the feasibility and safety of a new rapid method of pancreaticogastrostomy, “bridging” pancreaticogastrostomy.
Ten Landrace pigs were randomized into the experimental and control groups, with five pigs in each group. “Bridging” pancreaticogastrostomy was performed in the experimental group, while routine mucosa-to-mucosa pancreaticogastrostomy was performed in the control group. After surgery, the general condition, amylase levels in drainage fluid on Days 1, 3, 5, and 7, fasting and 2-h postprandial blood glucose 6 mo after surgery, fasting, 2-h postprandial peripheral blood insulin, and portal vein blood insulin 6 mo after surgery were assessed. Resurgery was carried out at 1 and 6 mo after the former one to examine the condition of the abdominal cavity and firmness and tightness of the pancreaticogastric anastomosis and pancreas.
After surgery, the general condition of the animals was good. One in the control group did not gain weight 6 mo after surgery, whereas significant weight gain was present in the others. There were significant differences on Days 1 and 3 after surgery between the two groups but no differences on Days 5 and 7. There were no differences in fasting and 2-h postprandial blood glucose and fasting and 2-h insulin values of postprandial peripheral blood and portal vein blood 6 mo after surgery between the two groups. One month after surgery, the sinus tract orifice/anastomosis was patent in the two groups. Six months after surgery, the sinus tract orifice/anastomosis was sealed, and pancreases in both groups presented with chronic pancreatitis.
“Bridging” pancreaticogastrostomy is a feasible and safe a means of damage control surgery during the early stage of pancreatic injury.
Core Tip: This is a basic experimental research paper on the damage control surgery for pancreatic trauma, "bridging" pancreaticogastrostomy. To examine the feasibility and safety of this technique in Landrace pigs, we designed a series of research experiments. We found “bridging” pancreaticogastrostomy can be used to establish rapidly a channel and has similar short- and long-term results compared with the routine mucosa-mucosa pancreaticogastrostomy.
- Citation: Feng J, Zhang HY, Yan L, Zhu ZM, Liang B, Wang PF, Zhao XQ, Chen YL. Feasibility and safety of “bridging” pancreaticogastrostomy for pancreatic trauma in Landrace pigs. World J Gastrointest Surg 2021; 13(5): 419-428
- URL: https://www.wjgnet.com/1948-9366/full/v13/i5/419.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v13.i5.419
In emergency surgery for severe pancreatic trauma, controlling damage and preventing secondary damage is a problem that deserves attention[1-4]. There are not many options for surgeons to deal with this problem. Examples include emergent pancreaticoduodenectomy and pancreatic repair surgery[5-7]. The aims of the surgery are to shunt rationally pancreatic fluid, bile, and digestive juices in addition to hemostasis and to repair organ damage in the cavity. In recent years, we created and employed a new anastomosis method, “bridging” pancreaticogastrostomy, to treat patients with an extremely severe pancreatic injury. This surgery has advantages such as short length of surgery, low secondary trauma, rapid construction of shunts for pancreatic fluid, preventing second surgeries, and achieving good treatment outcomes in clinical practice. However, due to the limited number of clinical cases, there is a lack of strong evidence to support the feasibility and safety of this surgical procedure. Therefore, we carried out animal experiments to examine this procedure, which is reported here.
Ten healthy Landrace pigs weighing 20-25 kg were selected with no gender restrictions. The pigs were provided by the Experimental Animal Center of Chinese PLA General Hospital. All animals were reared in single cages and periodically fed with refined standard pig feed. Animals were fasted 1 d before surgery and in the first 2 d after surgery but allowed to drink water. Feeding was gradually resumed from Day 3 onwards. Pigs were randomized into the experimental and control groups, with five pigs in each one. “Bridging” pancreaticogastrostomy was performed in the experimental group (Group A), while routine mucosa-to-mucosa pancreaticogastrostomy was performed in the control group (Group B). The operational procedures for the animal experiments were approved by the Institutional Animal Care and Use Committee of PLA General Hospital.
Animal anesthesia: Sedazine II (xylazine hydrochloride injection) + midazolam injection (volume ratio: 1:1) was used for anesthesia induction by 0.5 mL/kg intramuscular injection. After endotracheal intubation was successful, the ventilator was first connected and then adjusted so that tidal volume was 10 mL/kg, respiratory rate was 16/min, and FIO2 was 0.4. Positive end-expiratory pressure was maintained at 4 cm H2O to maintain good oxygenation in animals. Isoflurane (0.8%) inhalation anesthesia combined with intravenous injection of 2-5 mg/kg fentanyl citrate through the ear vein was used for anesthesia maintenance. Intravenous infusion of rocuronium bromide (20-50 mg/pig) was used as the muscle relaxant. The external jugular vein was dissected to establish venous access. The carotid artery was dissected for connection to the arterial monitoring machine. During surgery, balanced solutions were used to supplement the physiological needs and intraoperative fluid loss in animals. During surgery, blood pressure, heart rate, and temperature were monitored.
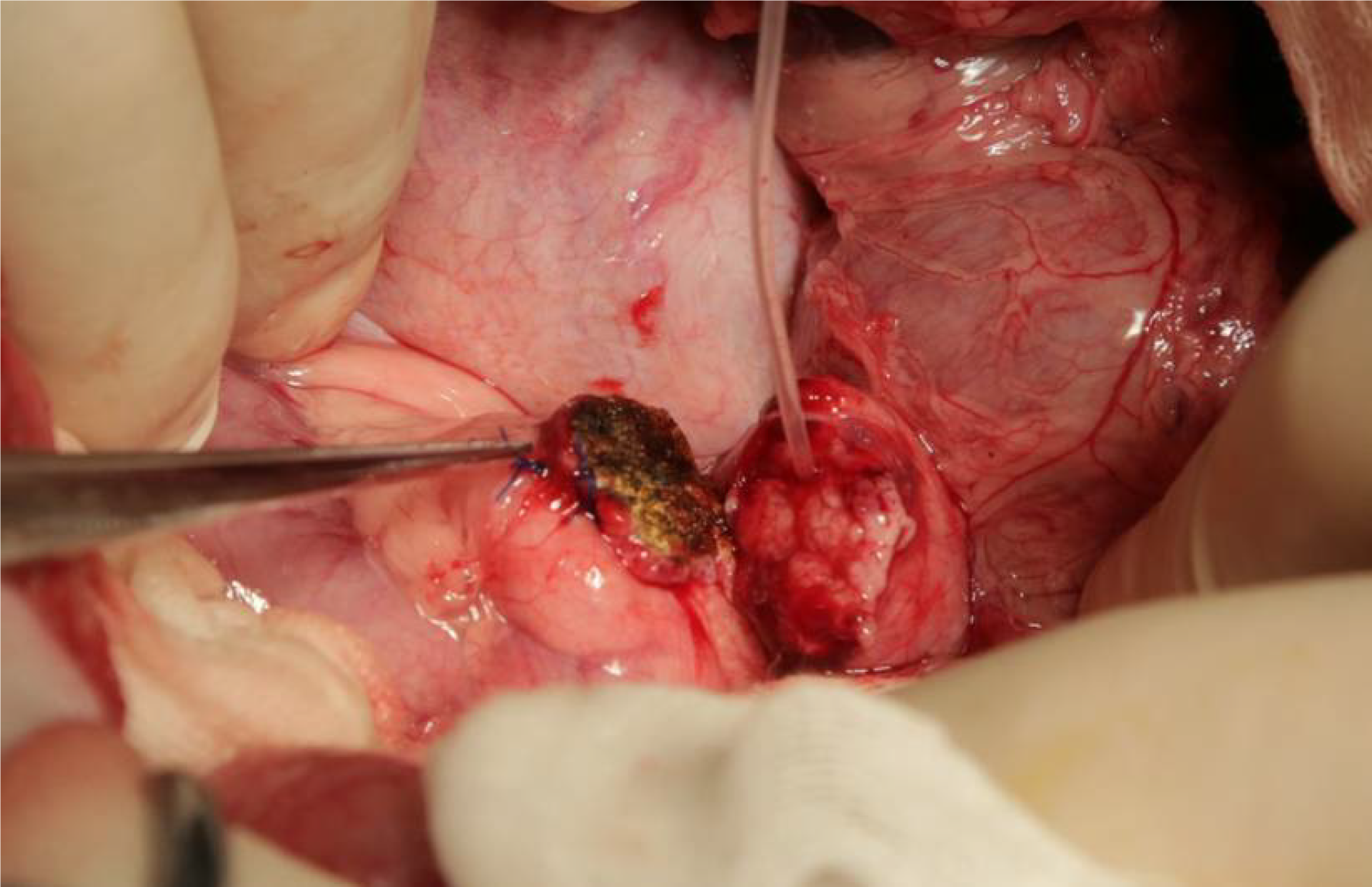
Surgery procedures: After anesthesia, an abdominal midline incision was made up to the second row of nipples and down to 5 cm above the navel. The total length was approximately 12 cm, and the abdomen was accessed layer by layer. The duodenum was palpated along the stomach and pylorus, and the duodenum was pulled. A thin pink pancreatic head could be seen to be pulled up. The pancreatic head was dissected, the proximal pancreas was retained, and a cross U-type was made using 5-0 prolene sutures (Figure 1). A 0.6 mm or 0.8 mm pancreatic duct drainage tube was inserted into the distal end.
A “bridging” pancreaticogastrostomy was performed in the experimental group: The gastric wall near the pancreatic stump (generally, the posterior gastric wall at the greater curvature near the pylorus is selected) was used for perforation in an avascular region before the pancreatic duct was inserted into the gastric cavity. At the insertion site, a purse-string suture of the serosa was carried out. Approximately 2 cm of the pancreatic duct was retained between the pancreas and the stomach (Figure 2). A large volume of physiological saline was used to rinse the abdominal cavity. A rubber drainage tube was retained at the abdominal wall and secured. Another hole was made beside the incision for drainage. Approximately 2 cm of drainage tube was retained at the outer end. The pig was carefully examined for the absence of pancreatic fistula and bleeding before the abdomen was closed layer by layer.
Routine pancreaticogastrostomy was performed in the control group: Mucosa-to-mucosa pancreaticogastrostomy was carried out at the gastric wall at the pancreatic stump side (Figure 3). Other procedures were the same as the experimental group.
Observation indicators included the general condition of animals, such as feeding, bowel movements, activity, and weight changes. Pancreatic fistula evaluation: Drainage fluid was collected on Days 1, 3, 5, and 7 after surgery to measure amylase levels. The animals were euthanized in two batches: The first batch was euthanized 1 mo after surgery, and two pigs each were randomly selected from the experimental and control groups. The second batch was euthanized 6 mo after surgery, and three pigs each were randomly selected from the experimental and control groups. Resurgery was performed before euthanasia in the animals, and the anesthesia procedure was the same as above. Surgery was performed to examine the abdominal cavity, including the presence/absence of walled-off necrosis, inflammation in the residual pancreas (color, texture), and patency of the sinus tract between the pancreas and stomach. Fasting and 2-h insulin values of postprandial venous blood were collected before euthanasia for animals that were euthanized 6 mo after surgery. Blood samples were used for blood glucose and insulin measurements. During surgery, portal vein blood was collected to measure insulin levels.
Statistical analysis was performed with SPSS 19.0 (Armonk, NY, United States). Continuous variables were presented as mean ± standard error or median and 95% confidence interval and compared by using Student’s t-test, the Mann-Whitney U test, or Wilcoxon’s signed-rank test, as appropriate. P < 0.05 was considered statistically significant.
All animals in both groups survived the surgery, and no perioperative death occurred. Among animals that were continuously observed for 6 mo, one from the control group did not gain weight, which was the same as before surgery. The weight of the remaining animals increased from 30 kg before surgery to 86-90 kg.
From postoperative amylase measurements (Table 1), it can be seen that amylase decreased to normal levels 5 d after surgery in most animals in the experimental and control groups, and only one animal from the control group showed a rebound in amylase level, which increased on Day 7 after surgery. Amylase levels were significantly higher in the experimental group than in the control group on Day 1 after surgery, but there were no differences between the two groups after Day 3. Fasting and 2-h postprandial blood glucose was measured 6 mo after surgery (Table 2). The comparison showed that there was no statistically significant difference between the two groups. Fasting and 2-h peripheral blood insulin and portal vein blood insulin levels were measured 6 mo after surgery (Table 3). The comparison showed that there was no statistically significant difference between the two groups.
| Day | Experimental group | Control group | P value | ||||||||
| No. 1 | No. 2 | No. 3 | No. 4 | No. 5 | No. 6 | No. 7 | No. 8 | No. 9 | No. 10 | ||
| 1 | 32916 | 11278 | 10557 | 24107 | 13576 | 7653 | 8804 | 2516 | 13865 | 658 | 0.04 |
| 3 | 2329 | 8088 | 3760 | 7089 | 4632 | 591 | 1685 | 2610 | 7538 | 216 | 0.15 |
| 5 | 278 | 200 | 178 | 232 | 485 | 517 | 108 | 955 | 4629 | 58 | 0.31 |
| 7 | 1006 | 14 | 79 | 23 | 77 | 39256 | 3237 | 16 | 0.35 | ||
| Experimental group | Control group | P value | |||||
| No. 5 | No. 6 | No. 7 | No. 8 | No. 9 | No. 10 | ||
| Fasting blood glucose | 5.57 | 5.2 | 5.88 | 4.49 | 5.23 | 5.41 | 0.21 |
| 2-h postprandial blood glucose | 5.52 | 7.57 | 7.12 | 7.03 | 6.94 | 5.77 | 0.84 |
| Experimental group | Control group | P value | |||||
| No. 5 | No. 6 | No. 7 | No. 8 | No. 9 | No. 10 | ||
| Fasting peripheral blood insulin | 23.6 | 18.6 | 27.4 | 14.3 | 17.3 | 21.3 | 0.16 |
| 2-h postprandial peripheral blood insulin | 31.2 | 25.3 | 31.7 | 26.7 | 37.9 | 35.4 | 0.38 |
| Portal vein blood insulin | 74.9 | 54.3 | 62.1 | 57.1 | 52.4 | 51.5 | 0.18 |
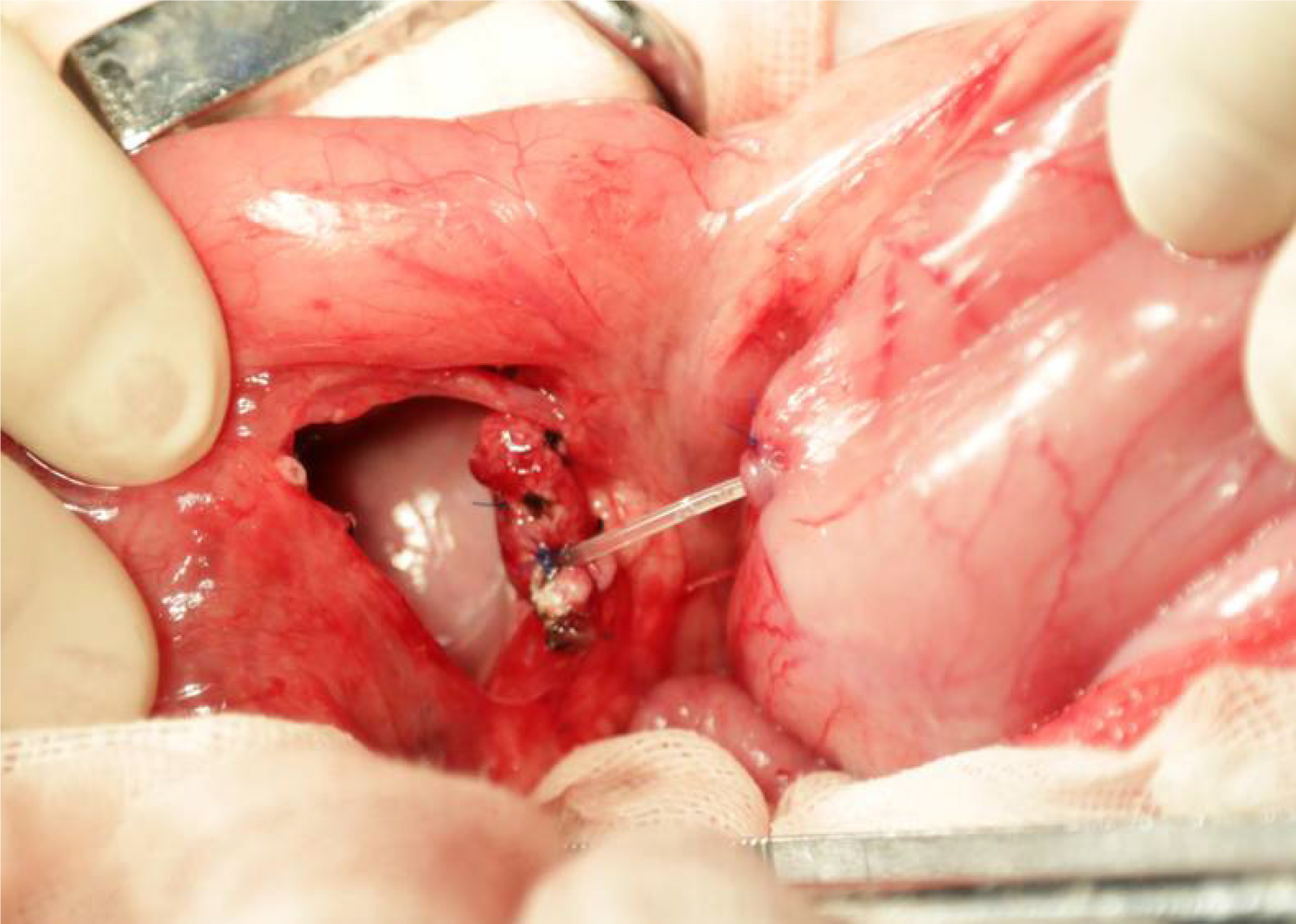
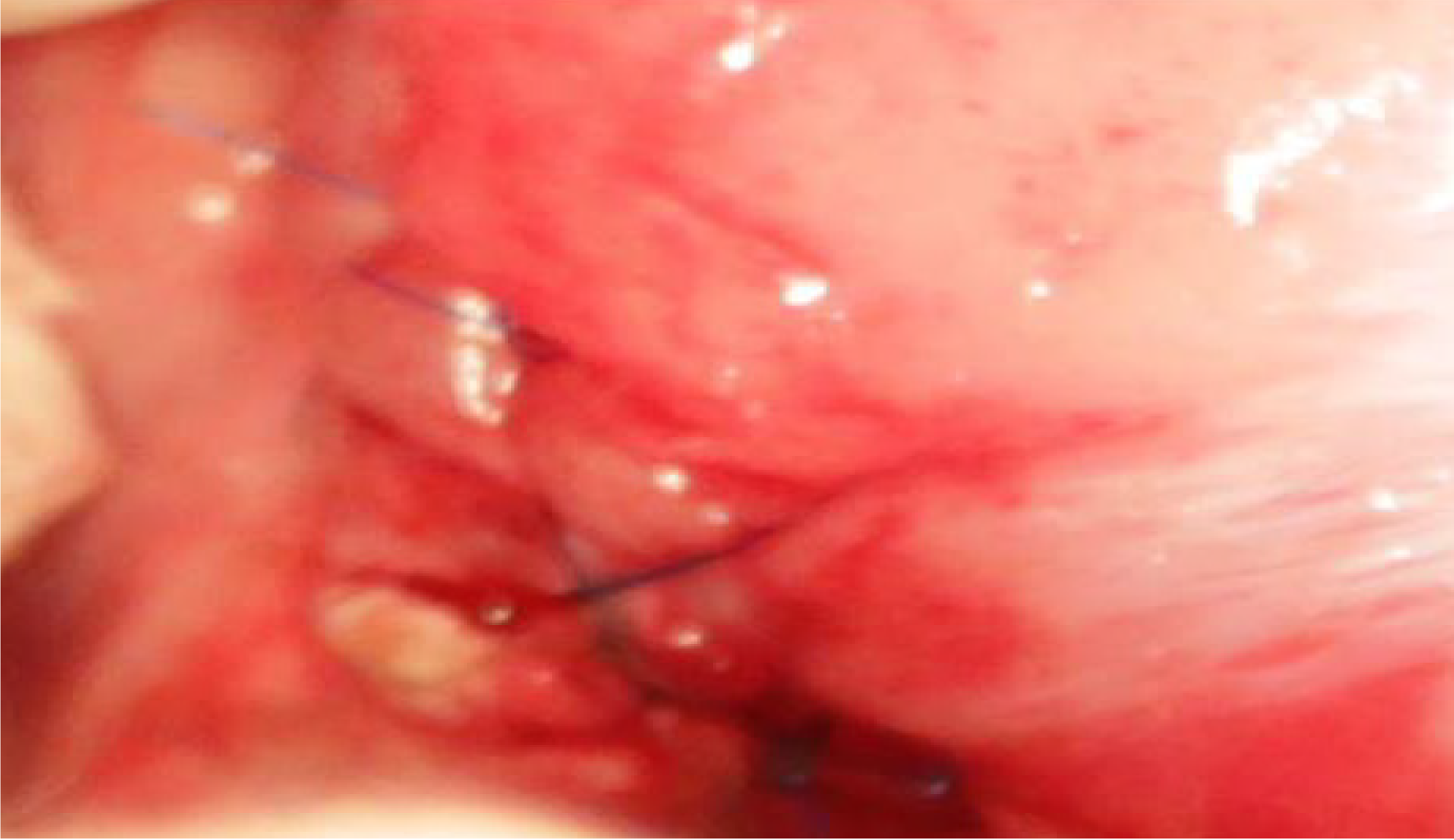
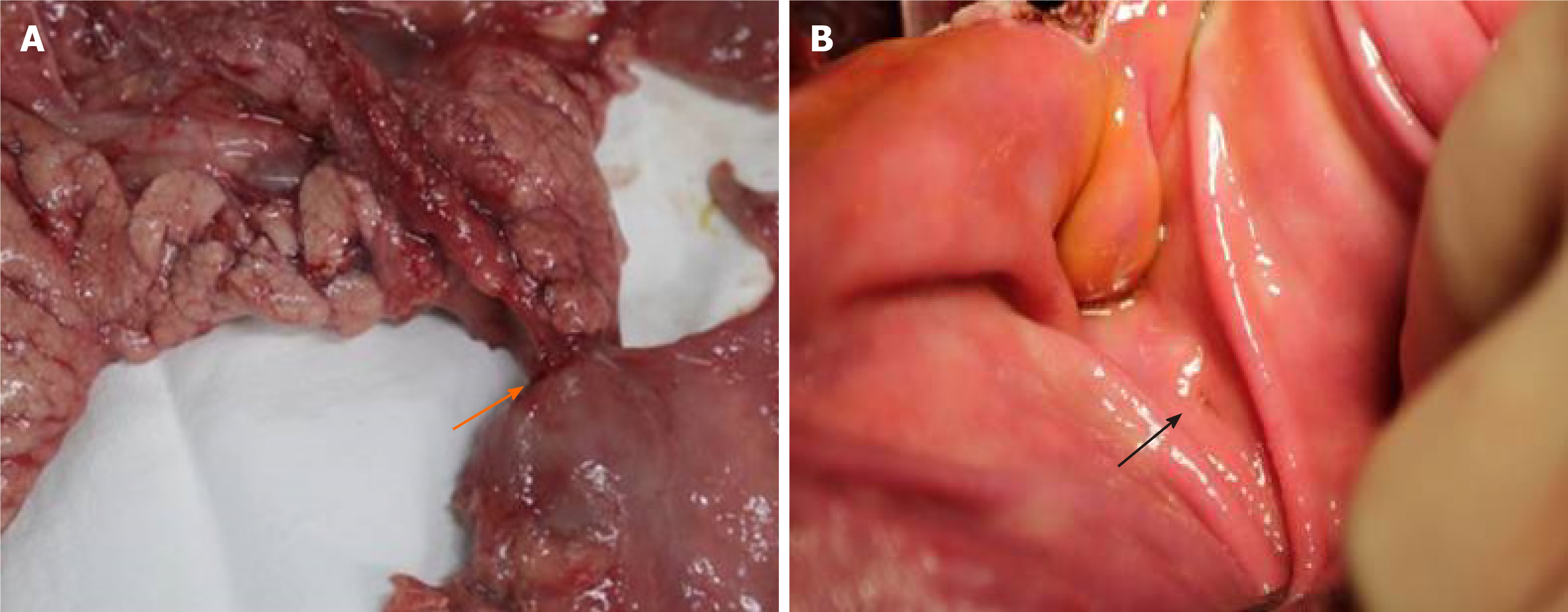
During the resurgery 1 mo after the surgery, the drainage tube spontaneously fell out. After open abdomen surgery was carried out, we found that there were severe intra-abdominal adhesions. The sinus tract in the experimental group was intact, and the intragastric sinus tract opening was clearly seen (Figure 4). The pancreaticogastric anastomosis in the control group showed good healing, and the intragastric anastomosis was clearly seen (Figure 5).
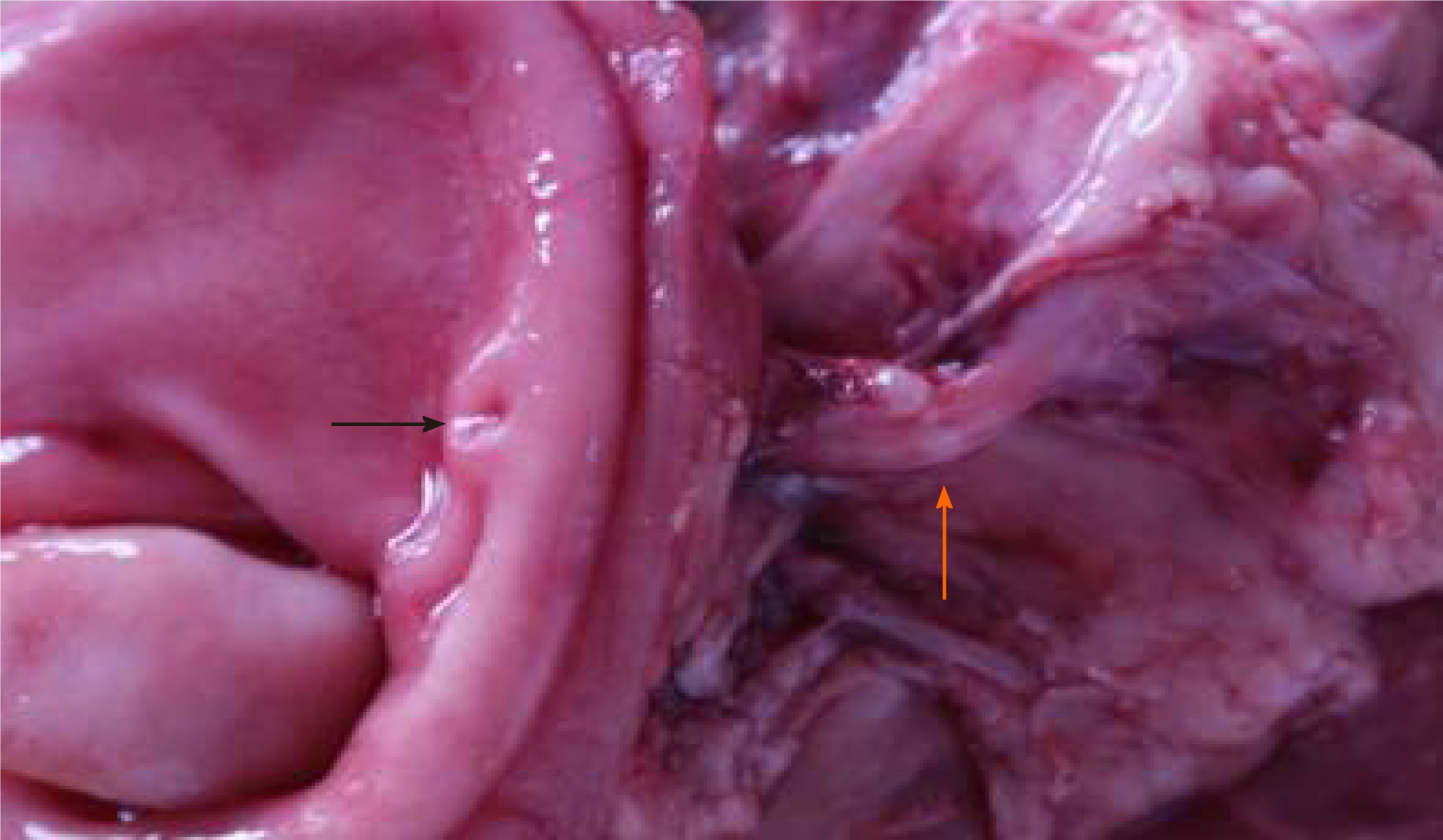
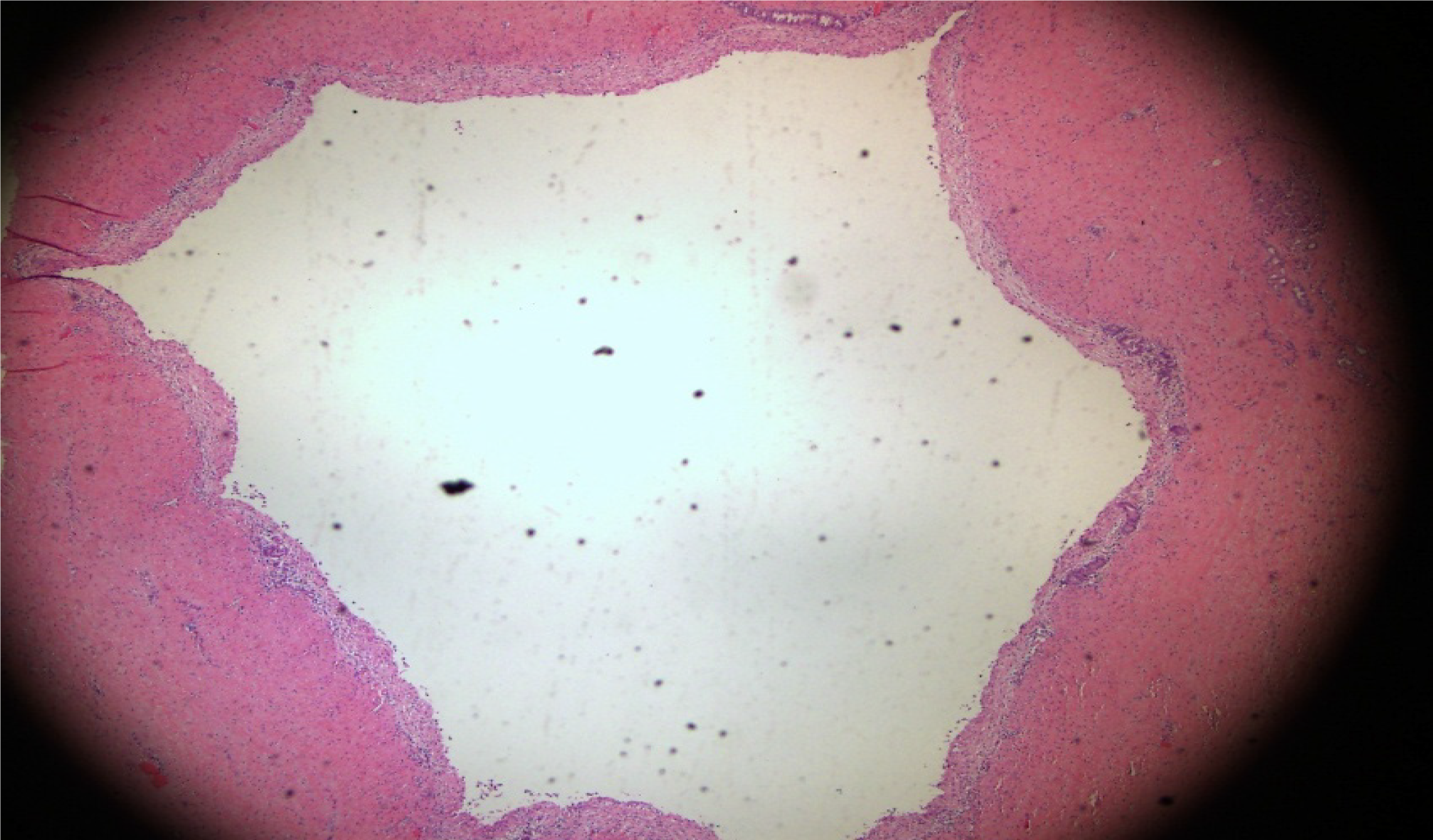
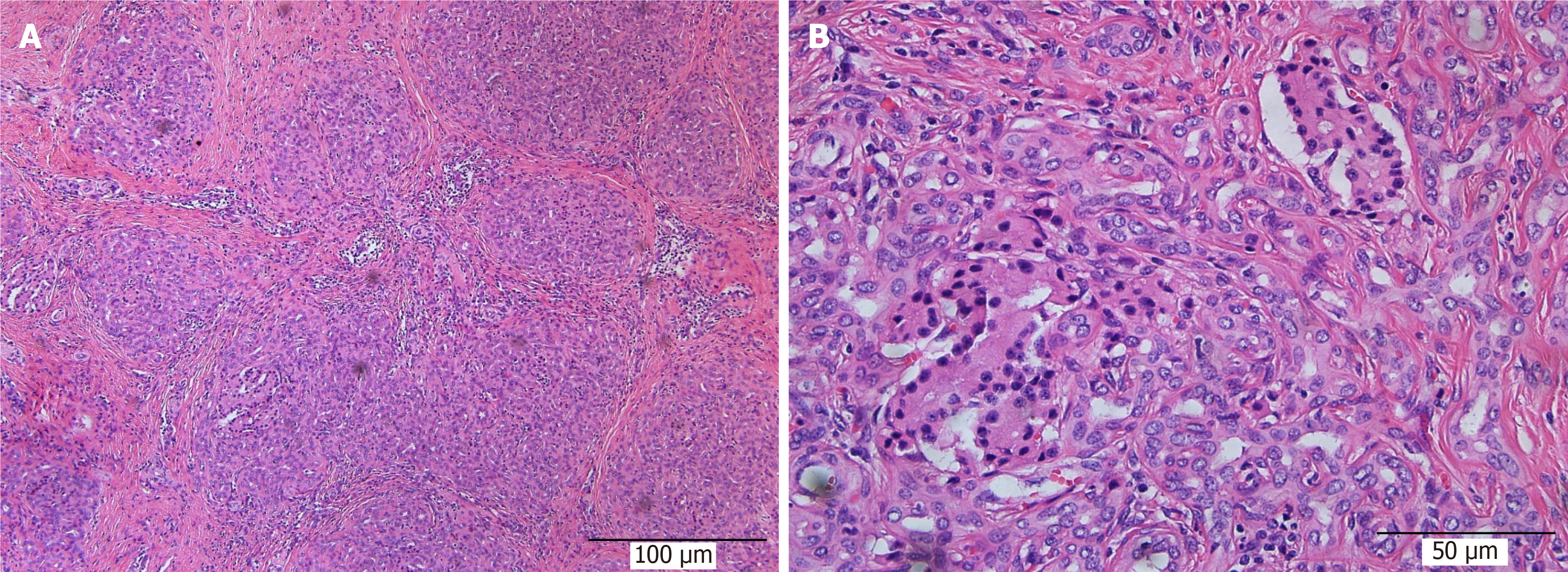
During resurgery 6 mo after surgery, intra-abdominal adhesions were reduced. Multiple walled-off necroses were present in the abdominal cavity in one from the control group, and no necrotic lesions were present in the abdominal cavity of the other animals. Careful autopsy of the stomach did not find sinus tract opening or anastomosis in the experimental and control groups. The texture of the pancreas in all animals was hard, and the pancreatic duct was significantly dilated, which denotes the presentation of chronic pancreatitis. The pancreas and sinus tract were used for pathological tests. The results were the same between the experimental and the control groups. Hematoxylin and eosin staining showed that the pancreas was filled with large amounts of fibrous tissues and acinar atrophy and there was a drastic decrease in acinar cells and pancreatic islets (Figures 6 and 7).
The drainage of pancreatic fluid while controlling damage during emergent surgical treatment of severe pancreatic trauma (American Association for the Surgery of Trauma grade 4 or 5) is extremely important[8-10]. Even though pancreatic fluid drainage can be achieved in conventional surgeries, such as the Whipple procedure and middle pancreatectomy followed by pancreaticojejunal Roux-en-Y anastomosis, surgical trauma is a factor that cannot be overlooked. How do we solve the problem of pancreatic fluid drainage while complying with the principle of damage control? We innovatively proposed a ”bridge“ between the pancreas and stomach in which only intubation is carried out, which is not an anastomosis in the traditional sense. We termed this technology ”bridging” pancreaticogastrostomy. This procedure can rapidly solve the problem of pancreatic fluid drainage without simultaneously increasing surgical trauma. After we inserted a silicon tube with the same diameter as the pancreatic duct into the pancreatic duct at the damaged site, the silicone tube was passed through the posterior gastric wall and the anterior gastric wall to the outside of the body to establish a ”bridging“ anastomosis. Three to four months after surgery, gastroscopy could be used to cut the drainage tube in the gastric cavity and to pull out the outer part; the connection between the pancreas and the stomach forms a channel.
Techniques similar to ”bridging” anastomosis have been reported. Xu et al[11] used pancreaticoenteric dissection bridge drainage to treat two patients with severe pancreatic fistula and intra-abdominal bleeding after Whipple surgery. During resurgery, the pancreatic stump was resutured, and a drainage tube was inserted into the pancreatic duct in the distal end. The jejunal loop was sealed, and the drainage tube in the pancreatic duct was inserted through another hole into the jejunal loop. Around 10 cm of the internal drainage tube was present in the abdominal cavity to create internal drainage. After surgery, patients recovered and were discharged. No related complications occurred during the 18-mo follow-up. In 2010, Kent et al[12] used bridging stents to treat seven patients with severe pancreatic fistula after Whipple surgery, of which success was achieved in five patients. Their method was similar to the method of Xu et al[11], the aforementioned methods mainly focused on the management of severe pancreatic fistula after Whipple surgery. There are no reports on the treatment of severe pancreatic trauma at present.
“Bridging” pancreaticogastrostomy mentioned in this article has the following strengths: (1) Direct insertion of a tube to create an internal fistula to achieve pancreatic fluid drainage; (2) The drainage tube passing through the posterior gastric wall and anterior gastric wall to the outside of the body to form external drainage to facilitate observation of pancreatic fluid drainage; in the later stage, gastroscopy can be used for resection and removal of the lateral side, and internal drainage is formed in the medial side; (3) Short operation duration and rapid repair, which minimize the length of surgery; (4) Retaining pancreatic function; (5) No traditional anastomosis present; therefore, fistula due to poor anastomosis healing would not occur; and (6) Preventing resurgery for pancreatic fluid sinus tract or pancreaticojejunostomy.
This experiment is a supplement to clinical studies and validates the feasibility of the new method of pancreatic fluid drainage through “bridging.” In particular, we carried out short- and long-term observations of “bridging” pancreaticogastrostomy. From the study results, we found that severe pancreatic leakage did not occur at the early stages of “bridging” anastomosis. In the late stages, the main problem is the detachment of the pancreatic duct drainage tube and drainage sinus tract stenosis, resulting in the pancreatic stump becoming harder and pancreatic atrophy. This is similar to traditional pancreaticogastrostomy. Pancreaticogastric anastomosis was first reported in 1946[13]. Many researchers believed that pancreaticogastrostomy is a simple and safe anastomosis method. The incidence of the early pancreatic fistula for pancreaticogastric anastomosis was lower than that for pancreaticojejuno
In conclusion, ”bridging“ pancreaticogastrostomy is completely feasible as a means of damage control surgery during the early stage of pancreatic injury. However, it will lead to pancreaticogastric anastomosis stenosis at the late stage, and pancreatic endocrine and exocrine functions are worthy of further examination. The greatest limitations of this study are that pancreatic duct support tubes were all detached in the two groups, strong muscle contractions in the gastric wall resulted in sinus tract/anastomosis closure, and chronic pancreatitis occurred at the late stage.
In recent years, a new anastomosis method, “bridging” pancreaticogastrostomy, to treat patients with an extremely severe pancreatic injury was created and employed. This surgery has advantages such as short length of surgery, low secondary trauma, rapid construction of shunts for pancreatic fluid, preventing second surgeries, and achieving good treatment outcomes in clinical practice.
There is a lack of strong evidence to support the feasibility and safety of this surgical procedure. We carried out an animal experiment to examine this procedure.
This study aimed to examine the feasibility and safety of a new rapid method of pancreaticogastrostomy, “bridging” pancreaticogastrostomy.
In total, 10 Landrace pigs were randomized into the experimental and control groups. “Bridging” pancreaticogastrostomy was performed in the experimental group, while routine mucosa-to-mucosa pancreaticogastrostomy was performed in the control group. The resurgery was carried out at 1 and 6 mo after the first surgery to examine the condition of the abdominal cavity and firmness and tightness of the pancreaticogastric anastomosis and pancreas.
One animal in the control group did not gain weight 6 mo after surgery, whereas significant weight gain was present in the others. There were significant differences on Days 1 and 3 after surgery between the two groups but no differences on Days 5 and 7. One month after surgery, the sinus tract orifice/anastomosis was patent in the two groups. Six months after surgery, the sinus tract orifice/anastomosis was sealed, and pancreases in both groups presented with chronic pancreatitis.
"Bridging" pancreaticogastrostomy is completely feasible as a means of damage control surgery during the early stage of pancreatic injury.
“Bridging” pancreaticogastrostomy can be used to establish rapidly a channel and has similar short- and long-term results as conventional pancreaticogastrostomy.
Manuscript source: Unsolicited manuscript
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koshy A, Sato H S-Editor: Wang JL L-Editor: Filipodia P-Editor: Li JH
| 1. | Morgan K, Mansker D, Adams DB. Not just for trauma patients: damage control laparotomy in pancreatic surgery. J Gastrointest Surg. 2010;14:768-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Schmiegelow AF, Stockholm JH, Burgdorf SK. [Traumatic pancreatic lesions]. Ugeskr Laeger. 2019;181. [PubMed] |
| 3. | Krige JEJ, Kotze UK, Setshedi M, Nicol AJ, Navsaria PH. Management of pancreatic injuries during damage control surgery: an observational outcomes analysis of 79 patients treated at an academic Level 1 trauma centre. Eur J Trauma Emerg Surg. 2017;43:411-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Lee KJ, Kwon J, Kim J, Jung K. Management of blunt pancreatic injury by applying the principles of damage control surgery: experience at a single institution. Hepatogastroenterology. 2012;59:1970-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Paulino J, Vigia E, Cunha M, Amorim E. Two-stage pancreatic head resection after previous damage control surgery in trauma: two rare case reports. BMC Surg. 2020;20:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Krige JE, Navsaria PH, Nicol AJ. Damage control laparotomy and delayed pancreatoduodenectomy for complex combined pancreatoduodenal and venous injuries. Eur J Trauma Emerg Surg. 2016;42:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Seamon MJ, Kim PK, Stawicki SP, Dabrowski GP, Goldberg AJ, Reilly PM, Schwab CW. Pancreatic injury in damage control laparotomies: Is pancreatic resection safe during the initial laparotomy? Injury. 2009;40:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Degiannis E, Glapa M, Loukogeorgakis SP, Smith MD. Management of pancreatic trauma. Injury. 2008;39:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Ohshio G, Hamashima Y, Manabe T, Miyashita T, Suzuki T, Tobe T. [DU-PAN-2 antigen in the serum of patients with tumors of the pancreas or biliary tract]. Nihon Geka Gakkai Zasshi. 1987;88:359. [PubMed] |
| 10. | Kaman L, Iqbal J, Pall M, Bhukal I, Behera A, Singh G, Singh R. Current management of pancreatic trauma. Trop Gastroenterol. 2012;33:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Xu J, Dai X, Bu X, Gao F, Zhang X. Pancreaticojejunal bridge-anastomosis: a novel option for surgeon to preserve pancreatic body and tail in urgent reoperation for intra-abdominal massive hemorrhage after pancreaticoduodenectomy. World J Surg. 2010;34:2457-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Kent TS, Callery MP, Vollmer CM Jr. The bridge stent technique for salvage of pancreaticojejunal anastomotic dehiscence. HPB (Oxford). 2010;12:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Waugh JM, Clagett OT. Resection of the duodenum and head of the pancreas for carcinoma; an analysis of thirty cases. Surgery. 1946;20:224-232. [PubMed] |
| 14. | Qin H, Luo L, Zhu Z, Huang J. Pancreaticogastrostomy has advantages over pancreaticojejunostomy on pancreatic fistula after pancreaticoduodenectomy. A meta-analysis of randomized controlled trials. Int J Surg. 2016;36:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Daamen LA, Smits FJ, Besselink MG, Busch OR, Borel Rinkes IH, van Santvoort HC, Molenaar IQ; Dutch Pancreatic Cancer Group. A web-based overview, systematic review and meta-analysis of pancreatic anastomosis techniques following pancreatoduodenectomy. HPB (Oxford). 2018;20:777-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Benini L, Gabbrielli A, Cristofori C, Amodio A, Butturini G, Cardobi N, Sozzi C, Frulloni L, Mucelli RP, Crinò S, Bassi C, Marchegiani G, Andrianello S, Malleo G, Salvia R. Residual pancreatic function after pancreaticoduodenectomy is better preserved with pancreaticojejunostomy than pancreaticogastrostomy: A long-term analysis. Pancreatology. 2019;19:595-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Ishikawa O, Ohigashi H, Eguchi H, Yokoyama S, Yamada T, Takachi K, Miyashiro I, Murata K, Doki Y, Sasaki Y, Imaoka S. Long-term follow-up of glucose tolerance function after pancreaticoduodenectomy: comparison between pancreaticogastrostomy and pancreaticojejunostomy. Surgery. 2004;136:617-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Amano H, Takada T, Ammori BJ, Yasuda H, Yoshida M, Uchida T, Isaka T, Toyota N, Kodaira S, Hijikata H, Takada K. Pancreatic duct patency after pancreaticogastrostomy: long-term follow-up study. Hepatogastroenterology. 1998;45:2382-2387. [PubMed] |